1. Introduction
Many personal electronic devices (PEDs) such as smartphones, laptops, e-cigarettes, digital cameras, consumer drones and higher-energy-demanding applications such as electric vehicles, electric bikes, electric power tools and grid applications use lithium–ion batteries (LIBs) [
1]. The increasing growth in the adoption of the LIB in rechargeable batteries can be attributed to its light weight and size, low self-discharge, higher energy density, higher power density as well as its longer life span and efficiency when compared to nickel metal hydride (Ni-MH), nickel–cadmium (Ni-Cd) and lead–acid batteries, which had an earlier start [
2,
3].
After their first use, rather than dispose of them for recycling, some may still have reasonable capacity and can be used in a secondary application. Some used batteries taken from electric vehicles and reused in grid applications have been found to have performance similar to new lithium batteries at a reduced cost [
4,
5,
6]. This may be the case for LIBs in PEDs with the potential for a second life in other electrical systems. With the staggering number of discarded PEDs, the second-life LIB may have valuable capacity for miniature renewable systems such as <100 Wh solar systems. Other examples of secondary use can be in off-grid low-energy-intensity applications such as phone chargers, home lighting, flash lights and radios. Despite the supposed benefit of a second life, old cells must be assessed to ascertain whether they still have some useful capacity.
The assessment for the second life includes a physical examination, a review of historical records and performance-based tests [
7]. Physical examination checks for any deformity, cracks, corrosion or leakages that could immediately condemn the cells for recycling if present. Historical examination checks for the calendar year, cycling time and specific operating conditions in the past that can damage the cells. The records in the Battery Management System (BMS) have been used to study how the cycle life and calendar life affect the degradation of the battery [
8]. Unfortunately, some BMS data have proprietary laws protecting them, and historical data of some BMS do not exist. Another challenge to assessing the LIBs with a chance of being re-used is the unavailability of information on the technical specifications of many installed LIBs. The reason for this is that many battery manufacturers directly supply electric device manufacturers, so details on LIB specification are known between the companies but not available to the public or prospective second-life assessors. As observed in opened battery packs, internal codes or model numbers printed on the LIB do not provide enough details for second-life assessment. This makes physical and performance-based examination of the spent cells expedient.
Consequently, understanding the failure modes gives some valuable information for proper assessment. During manufacturing, the moisture content of the cell electrolytes and electrodes can result in early ageing [
9]. After production, LIBs have been found to experience capacity fades during the charge and discharge cycle and even when stored for a long time. The temperature, the current during charge and discharge, the operational voltage (overcharge and over-discharge), depth of discharge and the state of charge during storage affect the battery’s overall health [
10]. The mechanisms that lead to the degradation may result from growth in the solid electrolyte interphase (SEI) and decomposition of the binder and electrolyte [
11]. Other mechanisms include lithium deposition, structural disordering, electrode particle cracking, corrosion of current collectors and transition metal dissolution. These mechanisms can lead to loss of active material (LAM) and loss of lithium inventory (LLI) and may increase the battery’s internal resistance. The various degradations lead to both capacity and power fade.
The power fades in spent LIB cells is related to the increase in the internal resistance of the cells [
12,
13]. Moreover, the fade in the capacity of a spent LIB, which can be described as the state of health (SOH), is the ratio of the battery’s present capacity compared to its nominal rated capacity [
10].
where
Q is the current capacity and
is the nominal capacity. A benchmark on the SOH can then be used to decide whether it can have a second life. For example, ref. [
7] says that retired batteries with an SOH of value less than 75% when charged and discharged at 0.3 C at room temperature are not appropriate to use for a second time. Generally, the intended second life application would determine the tolerable SOH limit, while the SOH level where the cell shows signs of rapid degradation becomes the hard limit for reuse [
14].
Therefore, for the SOH calculation, a capacity test is carried out first by fully charging the LIB to the maximum cut-off voltage with constant current (CC) and then with constant voltage (CV) until the cut-off current is reached [
7]. This is 100% state of charge (SOC). Subsequently, the cell is discharged at a constant current and ambient temperature until the cell terminal voltage reaches the low cut-off voltage. The discharge capacity obtained by Coulomb’s counting in the discharge phase is the present capacity of the cell.
where
is the time at full charge,
is the time at low cut-off voltage and
is the discharge current at time,
t. This value depends on the temperature and the current. Unfortunately, carrying out a full charge and discharge of a spent lithium–ion cell will take considerable time with attendant resources.
Other methods used in estimating the SOH include data-driven methods, model-based methods, experimental methods and a combination of two or more methods [
15,
16]. The model-based methods include the equivalent circuit model (ECM), electrochemical models, Kalman filters and empirical models. The data-driven techniques use machine learning, including neural networks, autoregressive-moving average models and support vector machines. Unfortunately, the limitation of the model-based and data-driven methods for assessing the SOH of spent LIB is the lack of historical data from the BMS for prospective second-life companies until the regulatory framework for full disclosure of battery data is developed and enforced [
6]. Having a repository of model parameters of all the available commercial cells to assess old cells for a second life is a daunting challenge.
Another experimental method, besides the full capacity check for estimating the SOH, is the electrochemical impedance spectroscope (EIS). It can be used to study LIB characteristics [
17]. This method involves applying a small sinusoidal perturbation signal, which can be voltage or current, to the cell and measuring the corresponding sinusoidal response. The gain in amplitude and phase change shows the characteristic behaviour of the cell over a wide frequency range. Over the frequency spectrum, some relationship exists between EIS measurement results and battery ageing [
18,
19,
20,
21]. For example, ref. [
22] carried out EIS measurements on commercial 18650-format LiCoO
2 cells and found that the impedance response at 316 Hz is reflective of the SOH and is less affected by the change in the SOC at that frequency. Essential factors to consider in EIS measurements are the nature (current or voltage) and magnitude of disturbance to optimise the signal-to-noise ratio while sustaining a linear system response [
17]. Unfortunately, these are difficult to standardise in spent cells, where original settings or historical measurements that can serve as a reference may not be available.
Likewise, incremental capacity (IC) and differential voltage (DV) have been applied in studying various degradation modes together with estimating and predicting the SOH [
23]. From the measurement in the CC charge of the LIB, the IC curve is derived by differentiating the cell’s capacity with respect to terminal voltage and plotting against the open circuit voltage. At the same time, the DV is the inverse of IC, whereby the terminal voltage is differentiated with respect to capacity. The various peaks and valleys in the IC and DV decrease and increase, respectively, as the cell degrades; this correlates with the SOH. However, special filtering needs to be implemented to minimise the effect of noise in the IC/DV methods for proper estimation.
Another research used the CV phase to determine the SOH for nickel–manganese–cobalt (NMC), nickel–cobalt–aluminium (NCA) and lithium–cobalt–oxide (LCO) batteries. The cells were subjected to calendar ageing, and the first-order response of the current during the CV phase of charging was used to calculate a parameter that strongly correlates with the SOH [
24]. In the same paper, the CV duration for the lithium–iron–phosphate (LFP) battery had a simple linear relationship with the SOH. From the above result, the CV phase behaves differently for different chemistry types, so different algorithms must be adopted for different cell types. In another work, the integrated voltage was used to carry out online SOH estimation on an LIB in which the voltage was integrated during a CC charge between 3.85 V and 4.2 V [
25]. It was tested on an accelerated-aged cell, and a strong correlation between the integral and the SOH was observed as the cell degraded. However, the test was not carried out with varying currents to see the impact of the current on the correlation with the voltage integral value. Many battery testing and charging systems measure current, voltage and temperature and find the cell’s capacity using the simple integration of current measurement with respect to time [
25,
26,
27]. This can be easily adapted to the voltage integral whereby a simple addition of the algorithm for computing the voltage integral, similar to Coulomb’s counting for the charge capacity, can be made. Using an existing system with less retrofitting of new hardware or electronics motivated the research into the relationship between the SOH and the voltage integral values when the spent cells are charged at constant current and temperature.
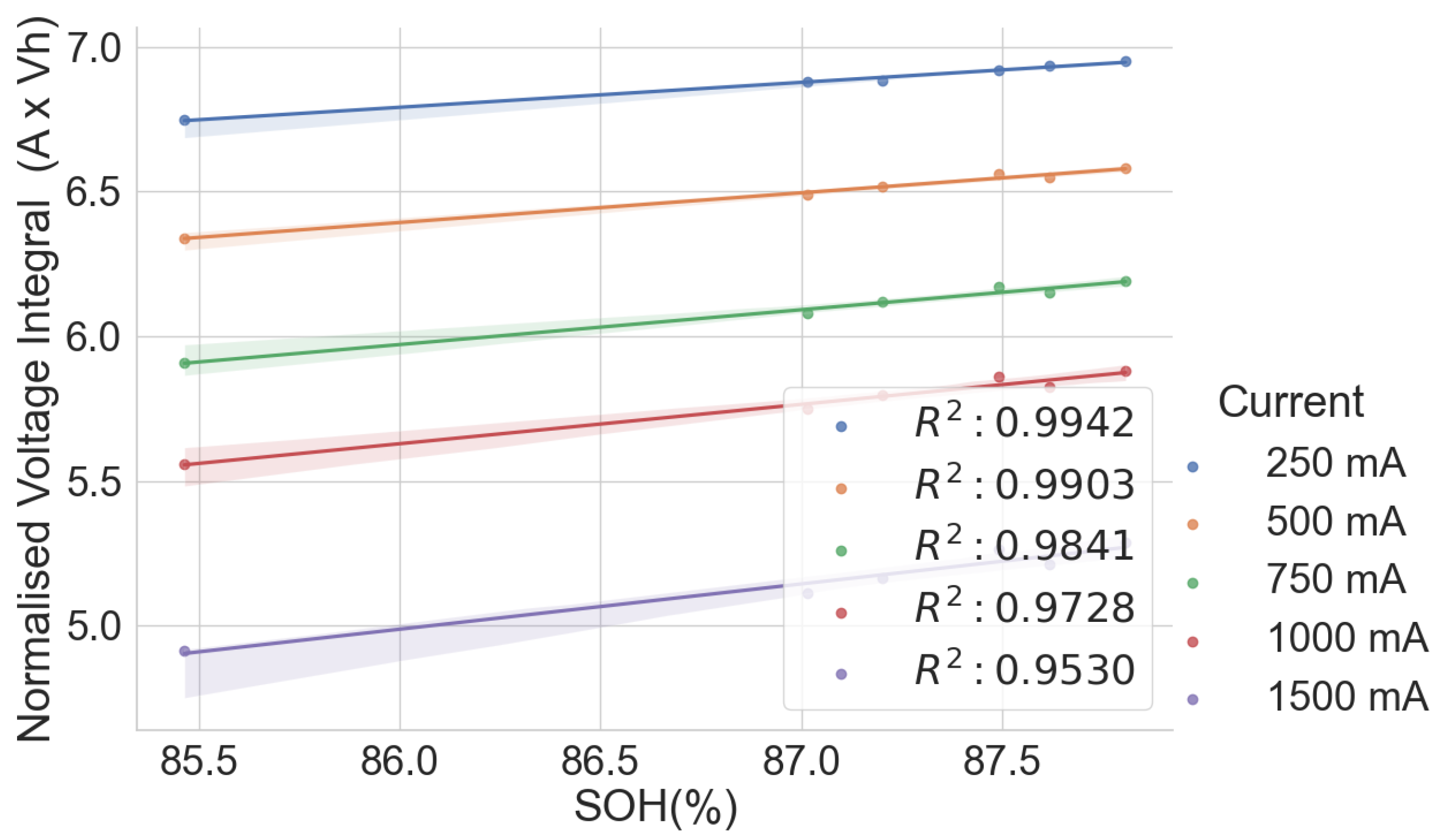
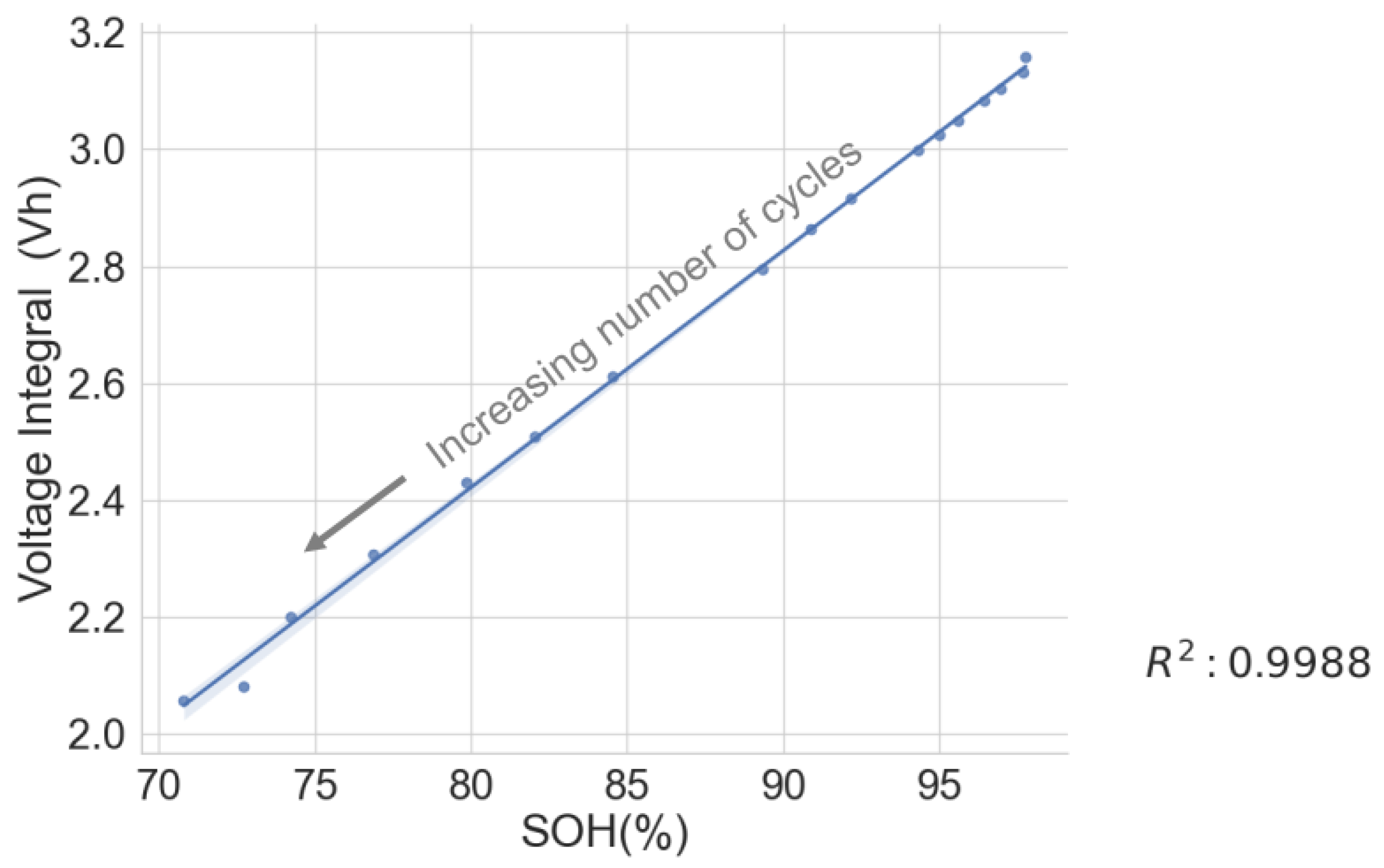
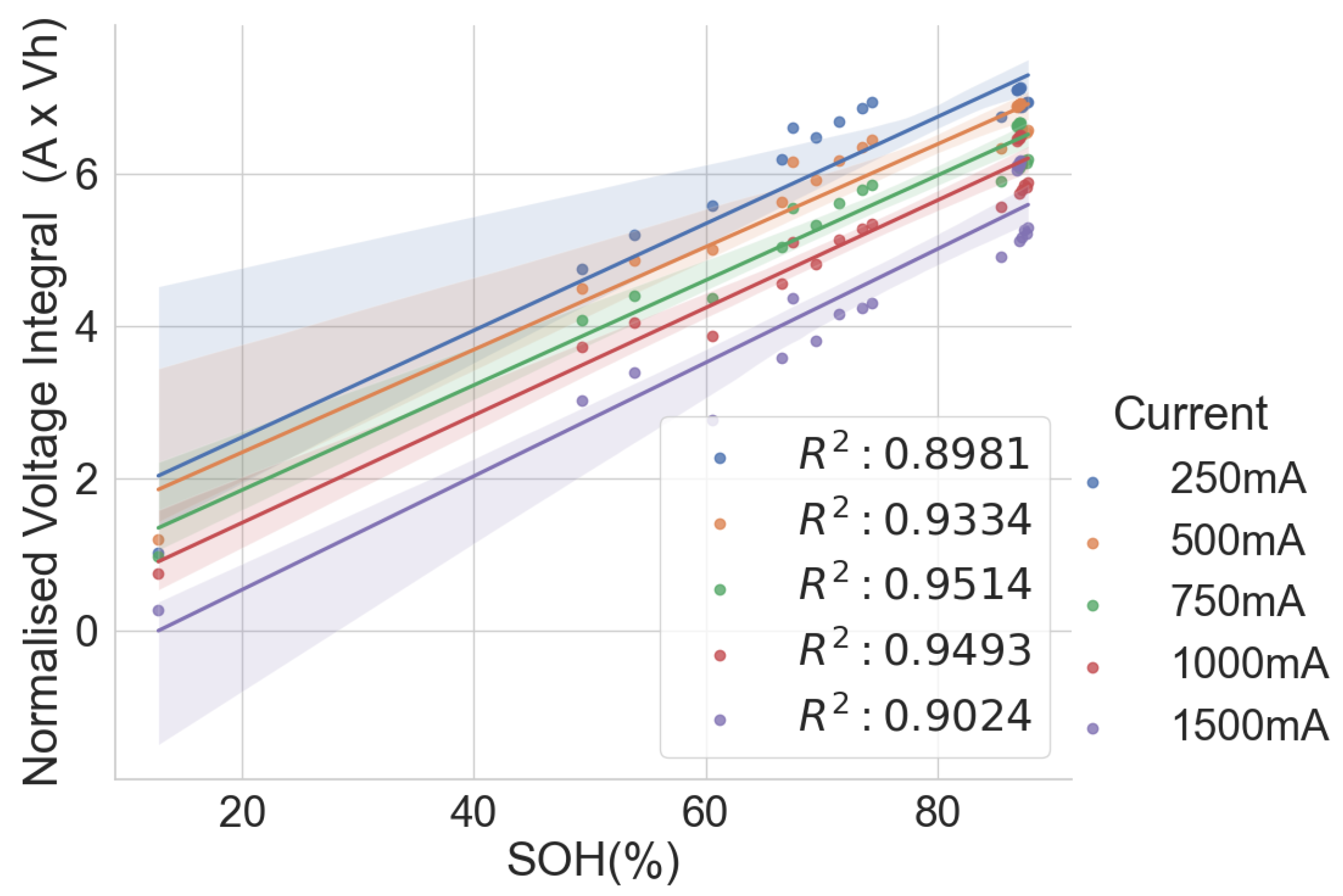
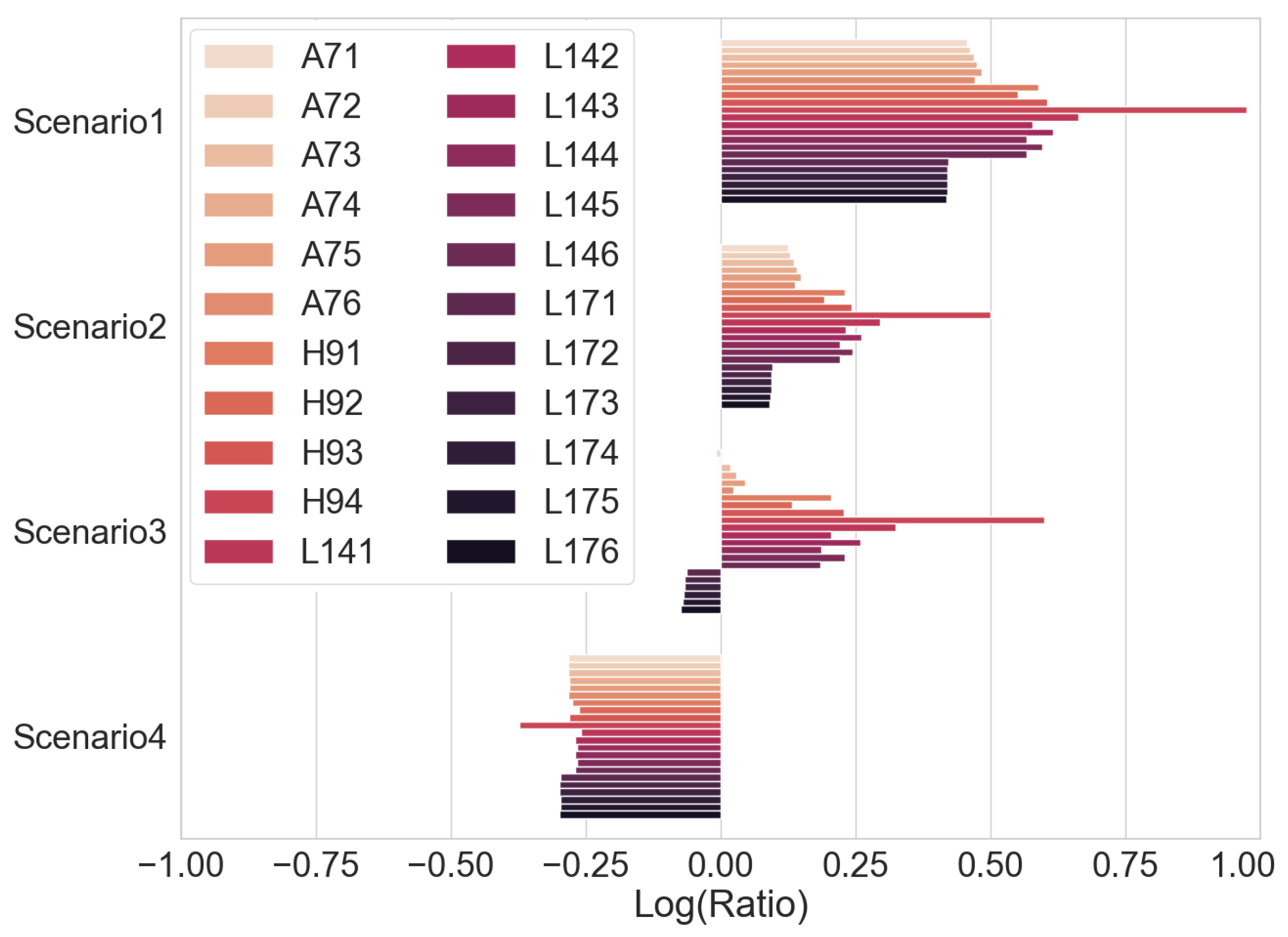
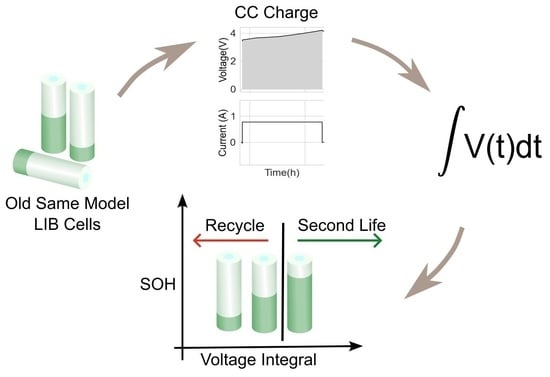
Thus, this work examines the relationship between the voltage integral and the SOH while considering the entire voltage range during the CC charge for a spent LIB. In addition, various charge currents were applied to see the current’s effect on the integrated voltage. The voltage integral,
, or the integrated voltage, as in Equation (
1), is expressed as the integration of the terminal voltage across the cell with respect to time at a particular constant current charge and constant ambient temperature.
where
is the time at low cut-off voltage or beginning of CC charge,
is the time at high cut-off voltage or end of CC charge and
is the terminal voltage at the time
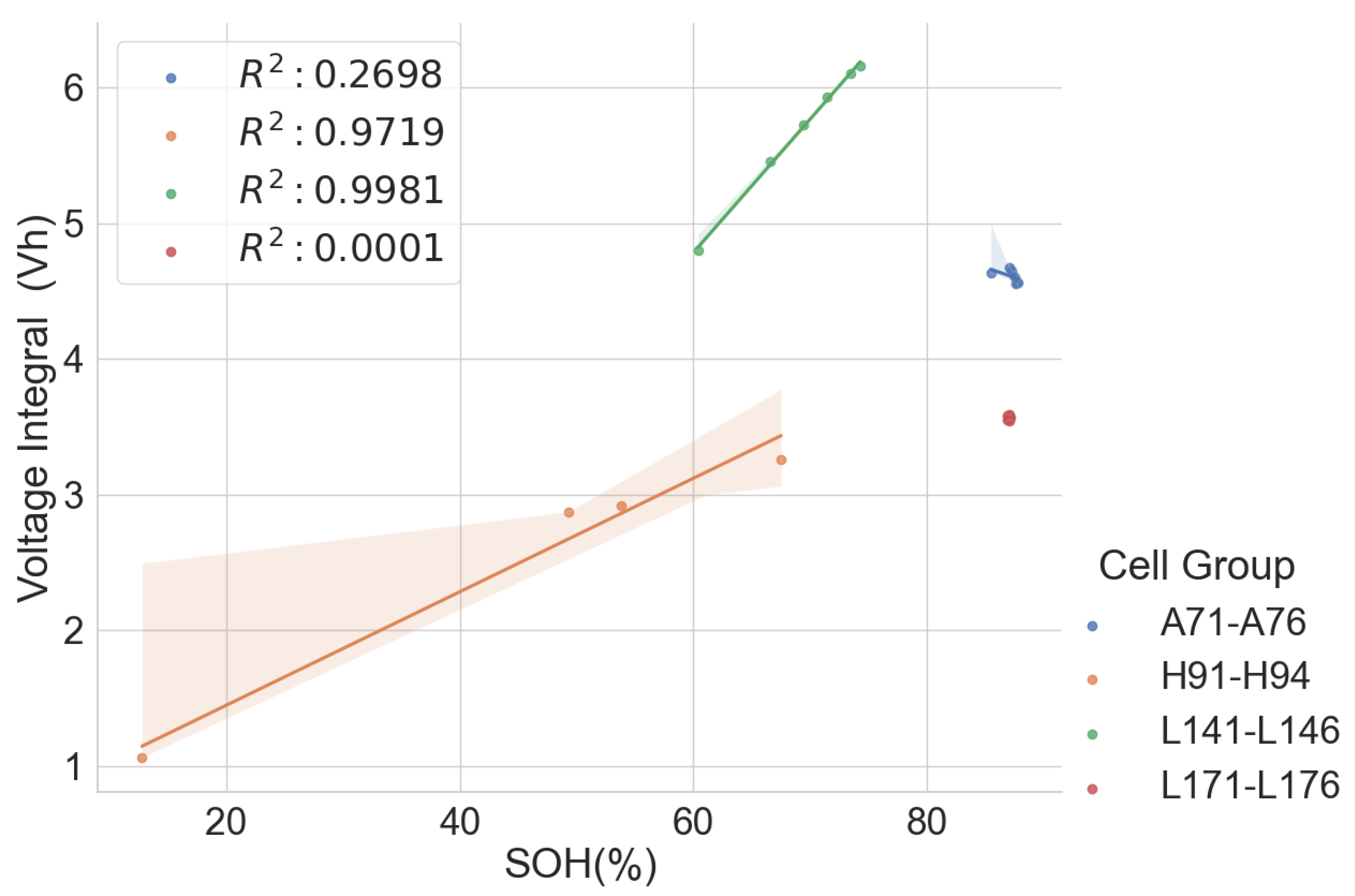
t. The value of the voltage integral was compared with the SOH of spent cells, and the result is presented here.
Section 2 on the materials and methods describes the test cells and the method adopted in the setup.
Section 3 shows the results of the experiments, while the discussion of the results is in
Section 4. The conclusion is provided in
Section 5.
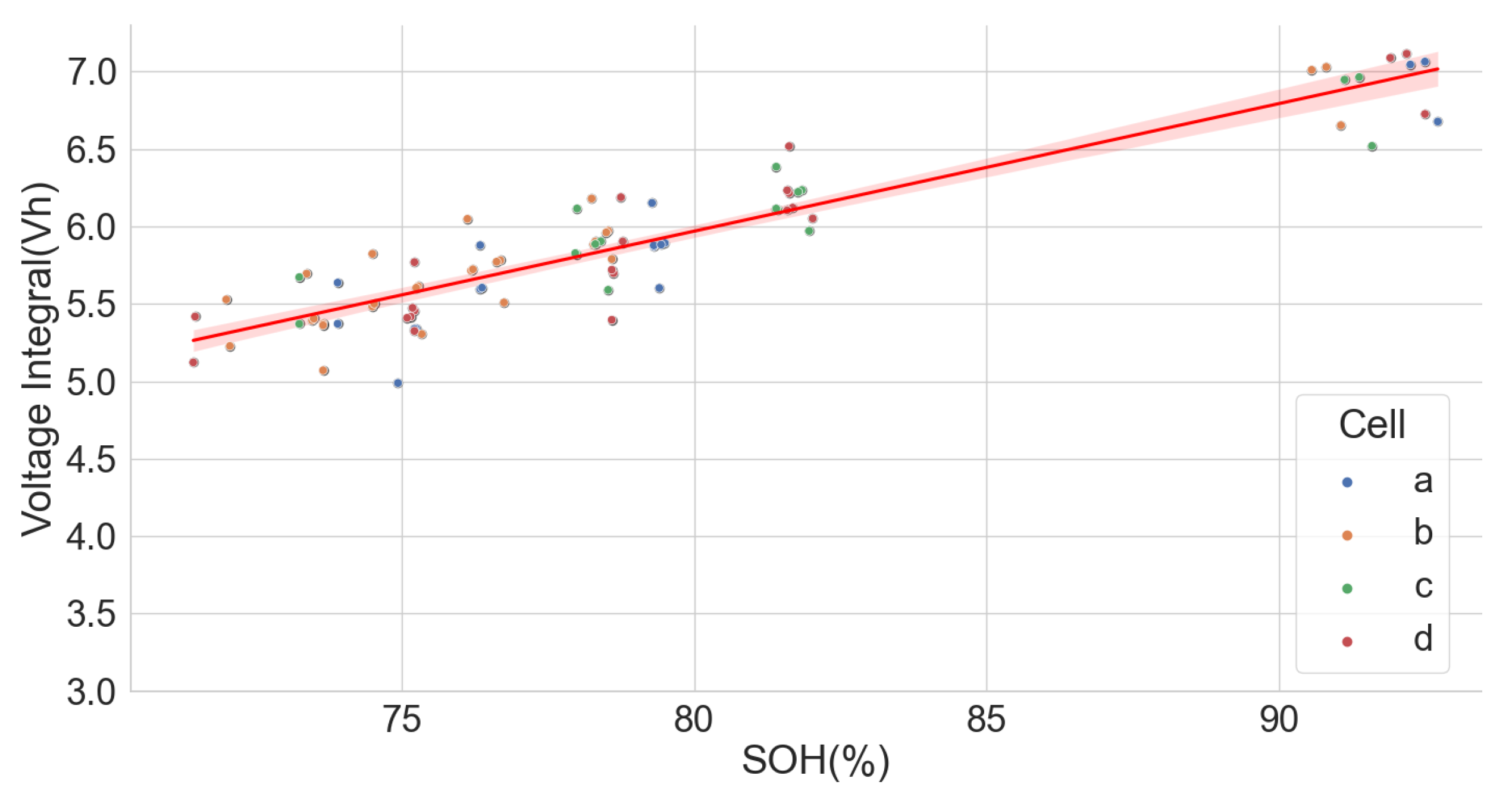
5. Conclusions
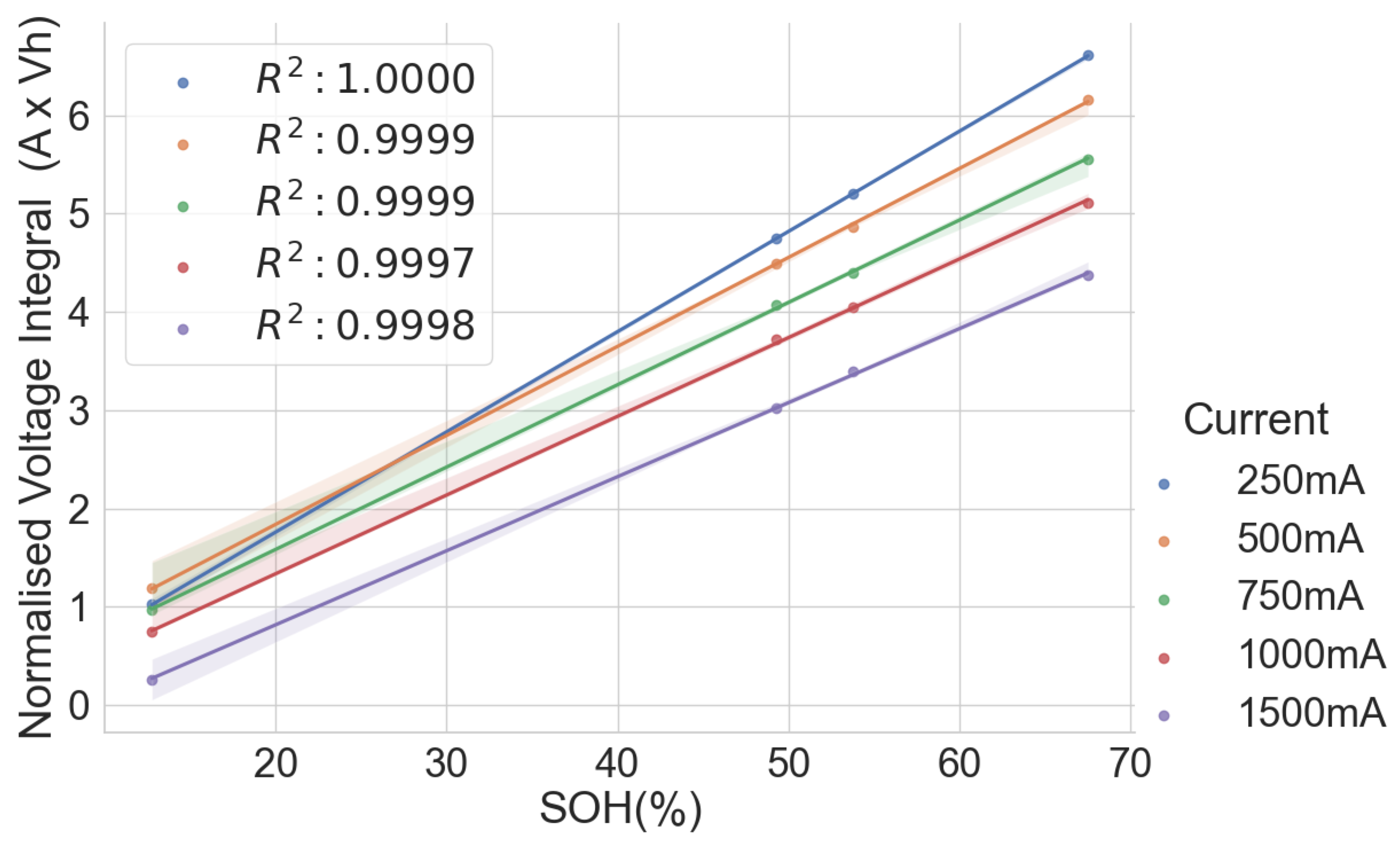
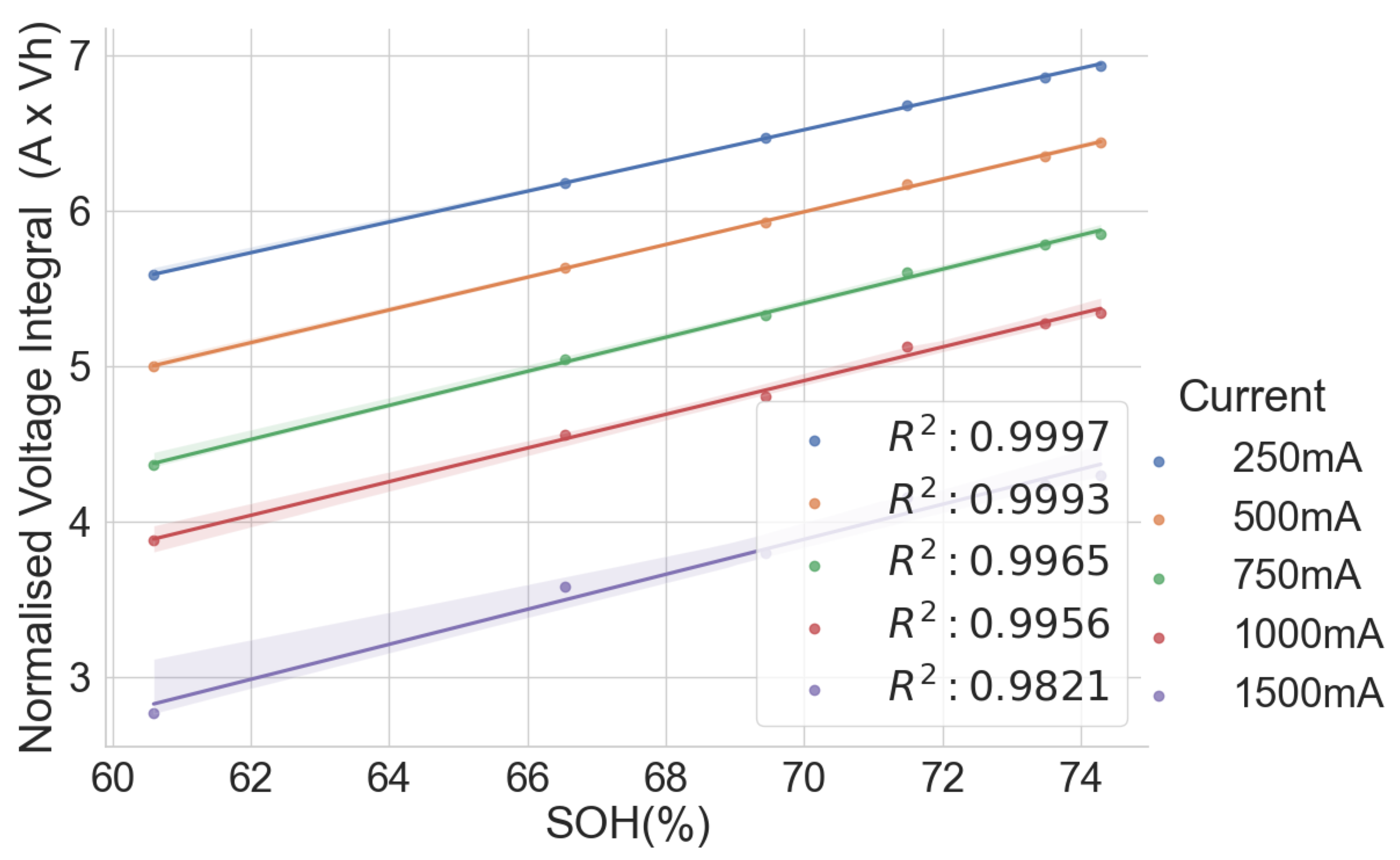
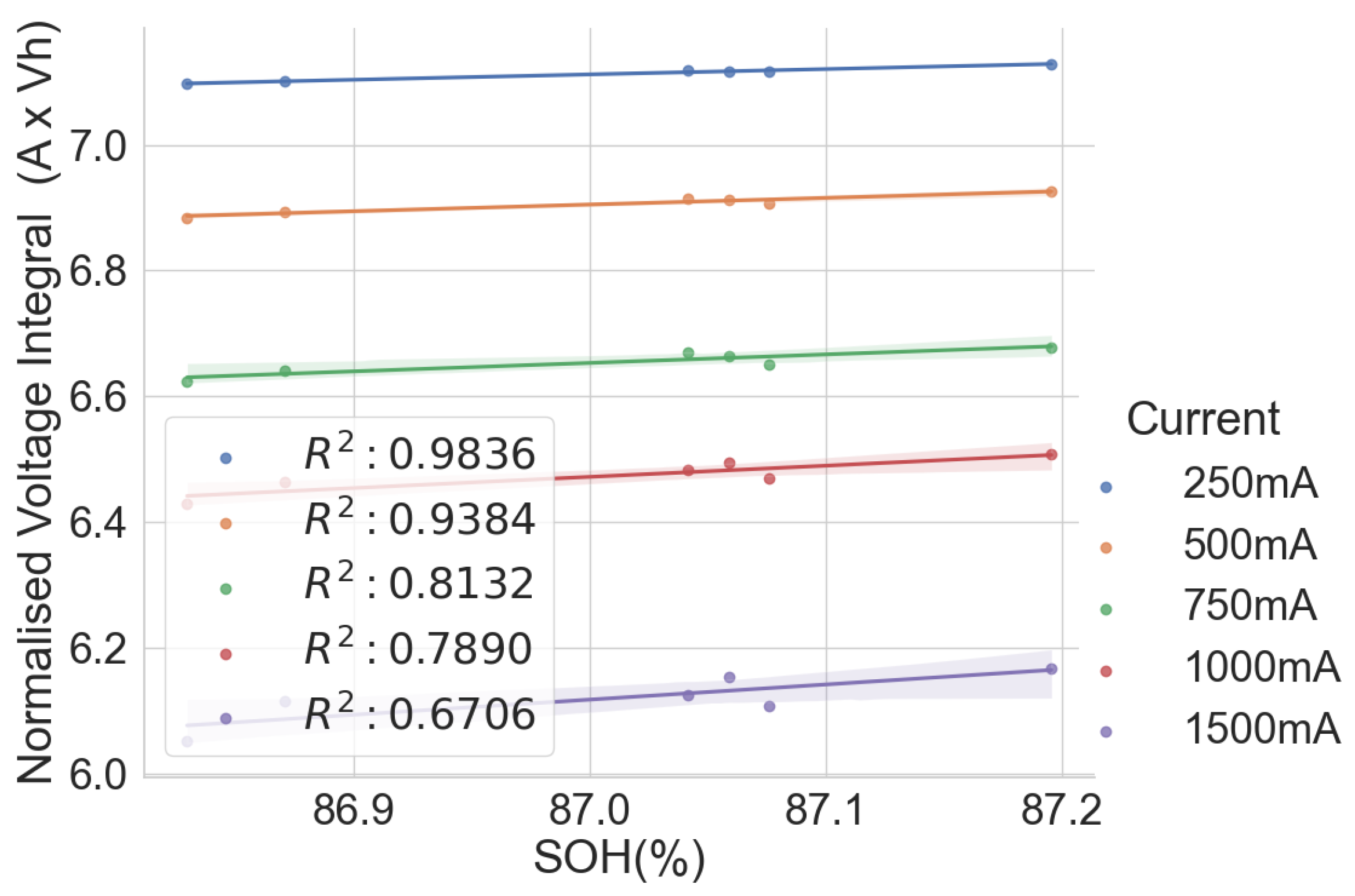
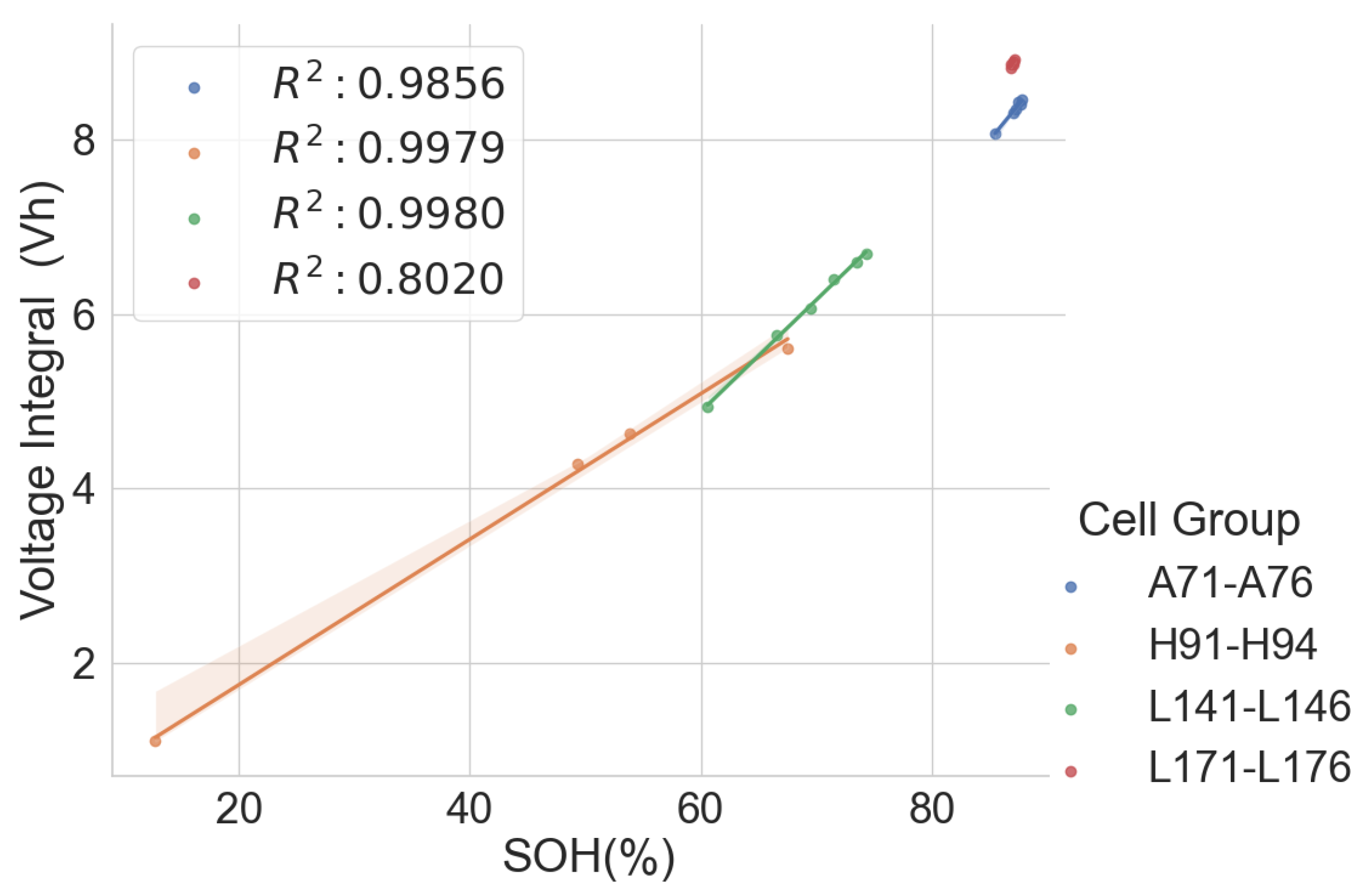
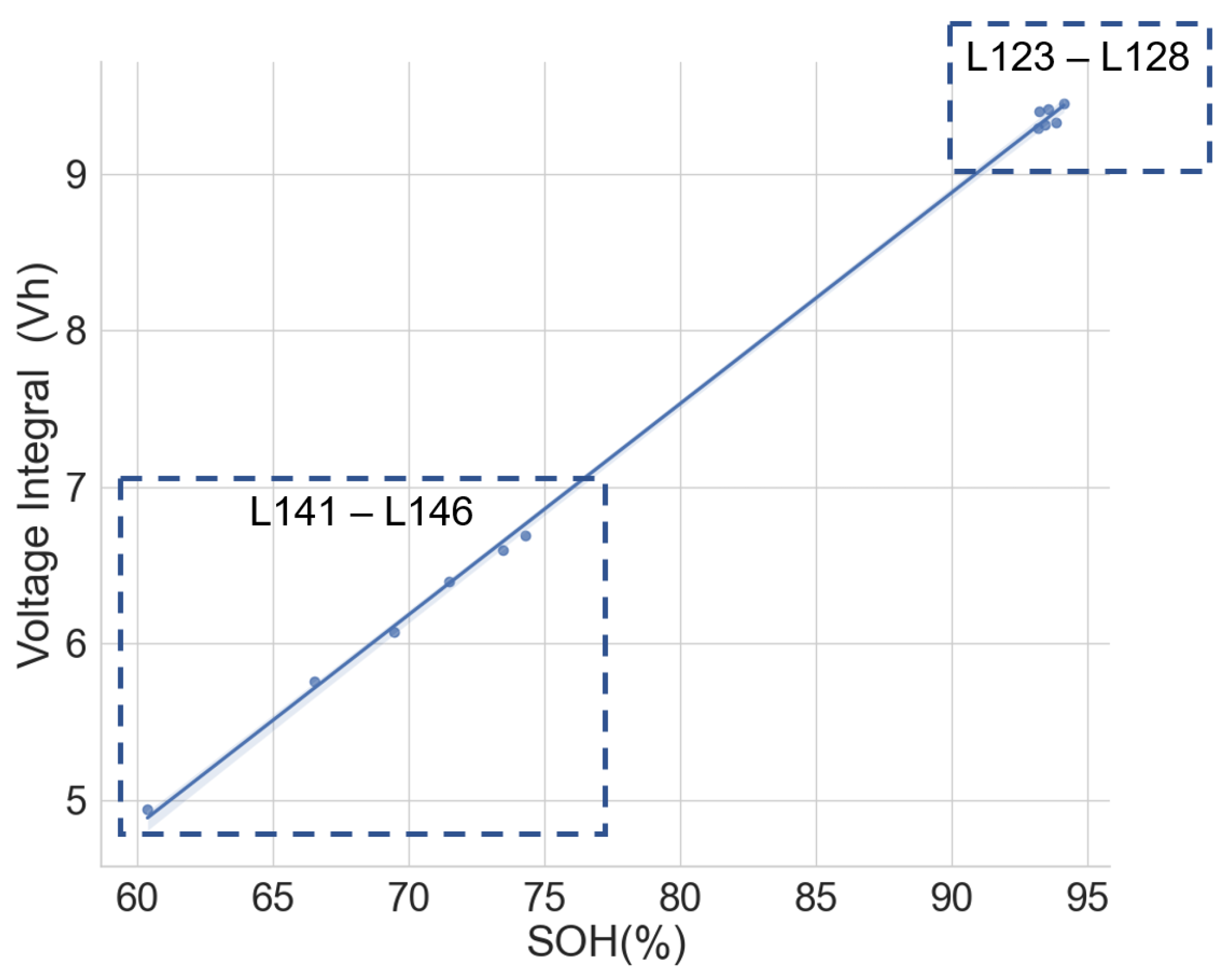
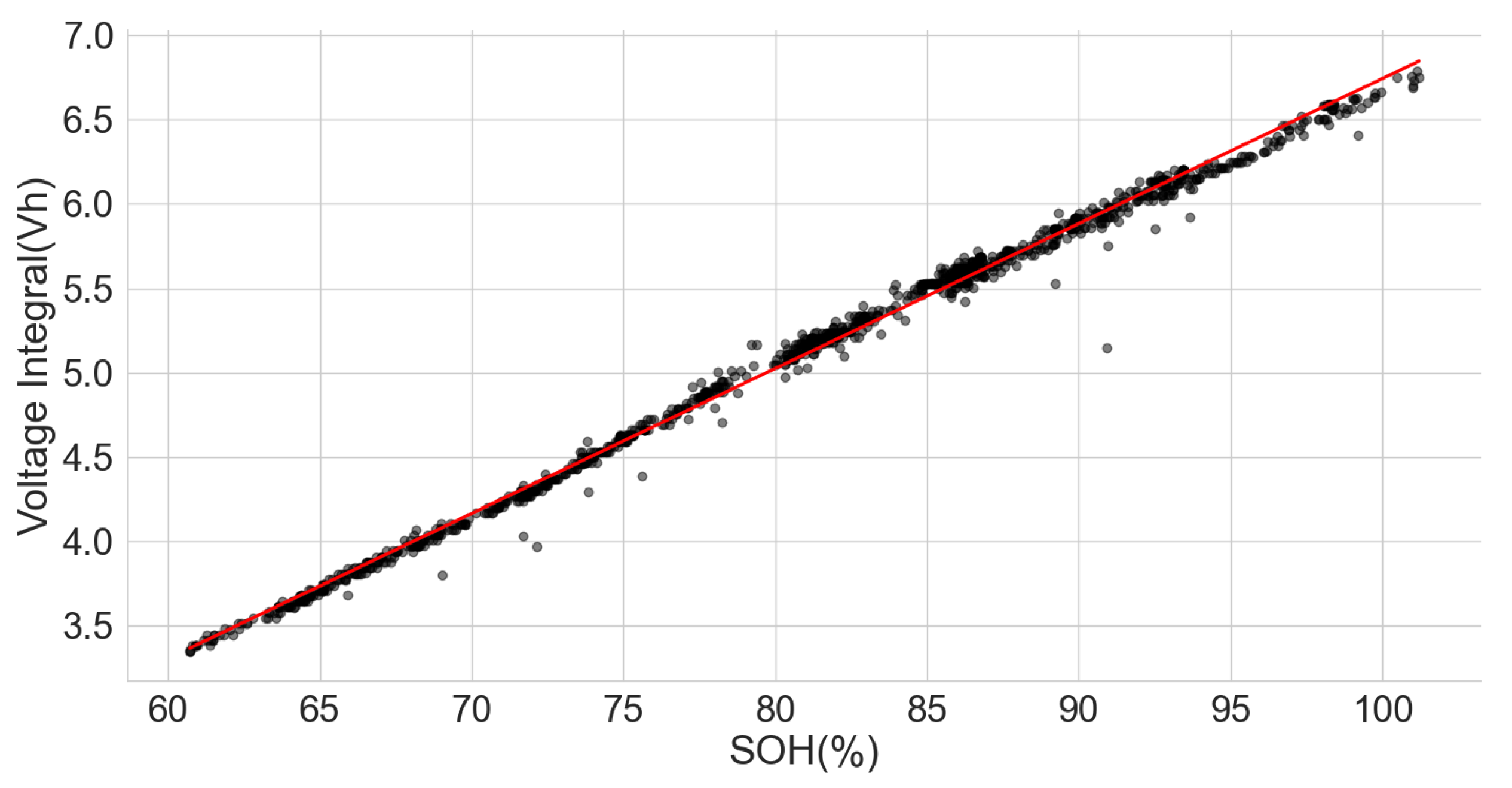
The linear relationship between the SOH and the voltage integral of cells of the same model provides a good indication for assessing spent lithium–ion cells’ potential for second life without carrying out a full charge and discharge on each cell. In addition, a dataset of ageing tests on cells with known chemistry, namely LFP, NMC, LCO and NCA, was analysed, and the result also shows a good correlation. Therefore, for first-life cells with unknown historical data, complete cycle charge and discharge can be carried out on a few similar cells with apparent differences in easily measurable variables such as resistance and open-circuit voltage to obtain the relationship between SOH and voltage integral during CC charge. This can be used to provide a simple degradation equation from which the SOH of the other cells can be estimated by substituting the voltage integral, which is obtained for the other cells by charging only from 0% SOC at low cut-off voltage to the high cut-off voltage using the same charge current and ambient temperature as the model cells. This would result in a significant reduction in the time required for testing all the cells. This method is also less complicated that only an adaptation of the existing algorithm, for an SOC check-up to voltage integral during the CC charge is sufficient to estimate the SOH and make a selection decision for second-life applications.
A particular voltage integral limit may be decided such that, during the CC charge, any cell that does not reach the limit at 4.2 V is discarded because it may not have a good capacity for reuse. Moreover, manufacturers can perform an ageing test on their models and provide such linear plots as performance information to end-users who can, in turn, use it in the online estimation of the SOH of the cells provided from the voltage integral, which was calculated from the measurements obtained at the same current and ambient temperature.
The recommendation from the result of this paper is to include the voltage integral in the evaluation methods for assessing the SOH of lithium–ion batteries. Further work is needed to investigate its viability in battery packs.