Differentiating Cyclability and Kinetics of Na+ Ions in Surface-Functionalized and Nanostructured Graphite Using Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Materials, Methods, and Characterization
2.1. Materials
2.2. Electrode Preparation
2.3. Cell Assembly
2.4. Characterization Methods
2.5. Electrochemical Impedance Spectroscopy
- The ohmic resistance of the cell comprises ionic and electronic resistances from the electrodes, flow fields, current collectors, and contact resistances. As the impedance of a resistor includes only the real part of a complex number, the resistances arising from different physicochemical processes are readily observable from the Nyquist plot.
- The non-ideal electrode surface analysis is conducted using a constant phase element (CPE), and the impedance arising can be formulated as follows:
- Inductive behavior at high frequency is observed due to electron movement in the potentiostat cables, and impedance arising can be determined from the following equation:
- The diffusional transport of electroactive species can be denoted by Warburg impedance, determined by the equation below:
3. Results and Discussion
3.1. Microscopic Analysis
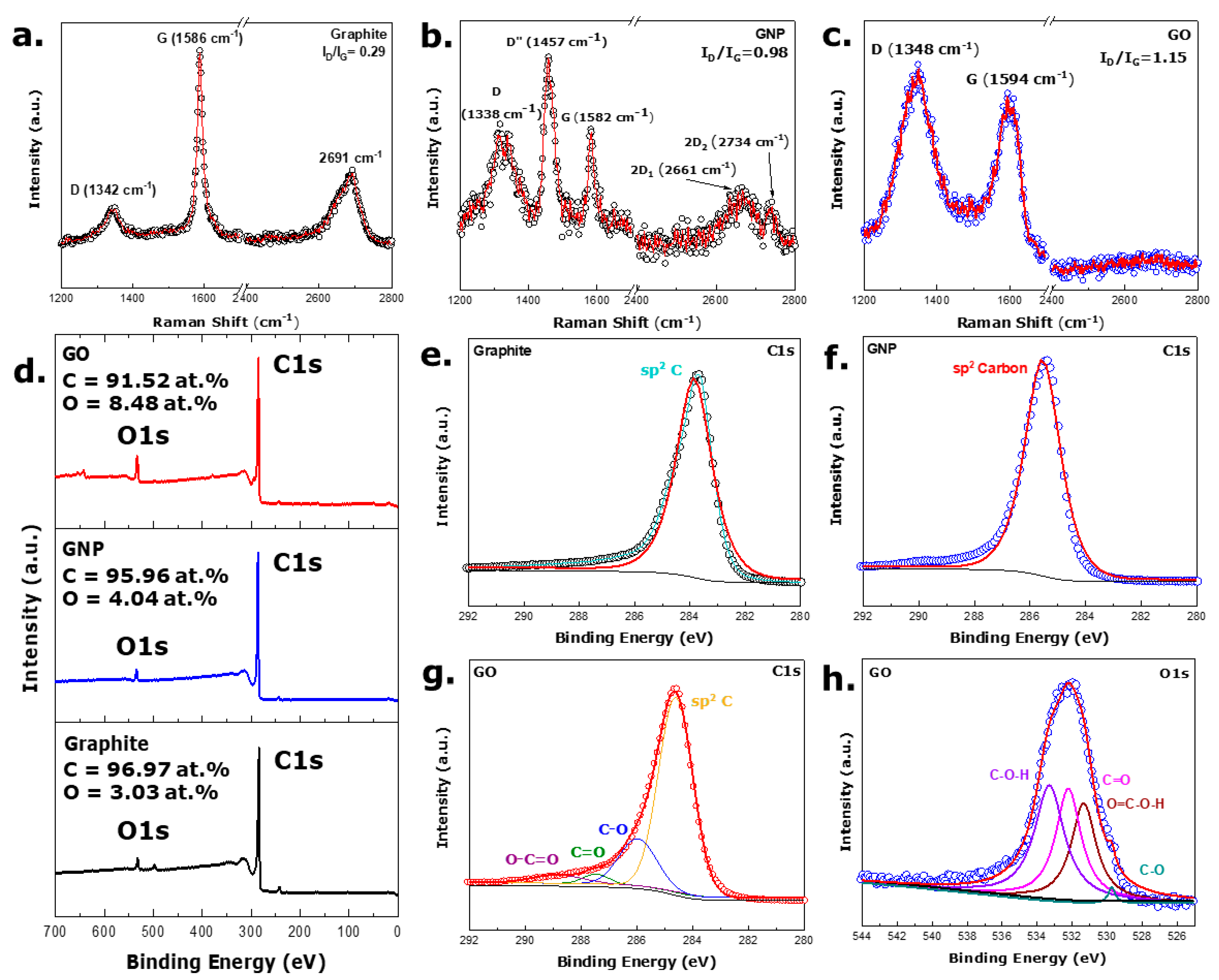
3.2. Spectroscopic Analysis
3.3. Reflection Analysis
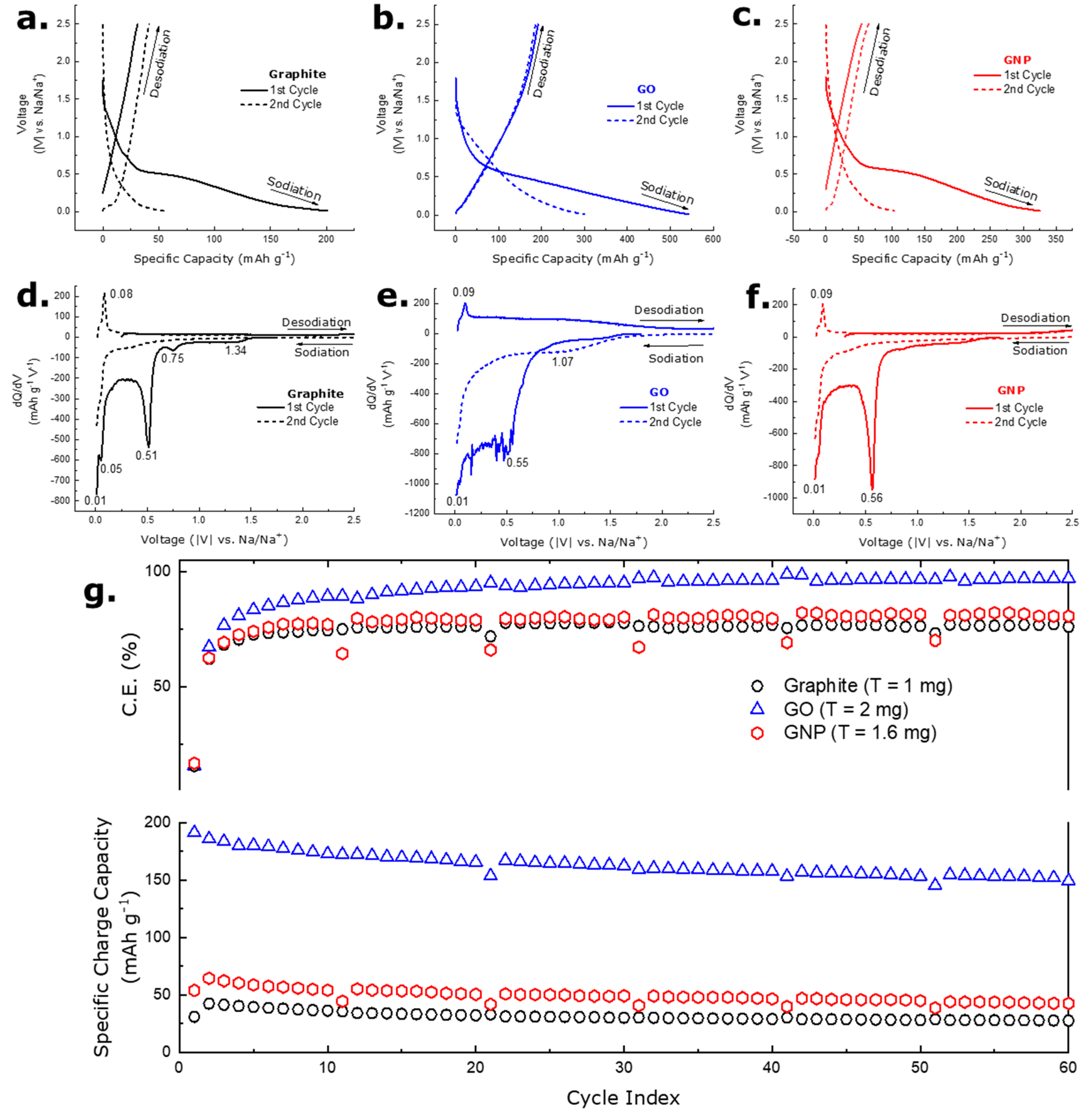
3.4. Electrochemical Analysis
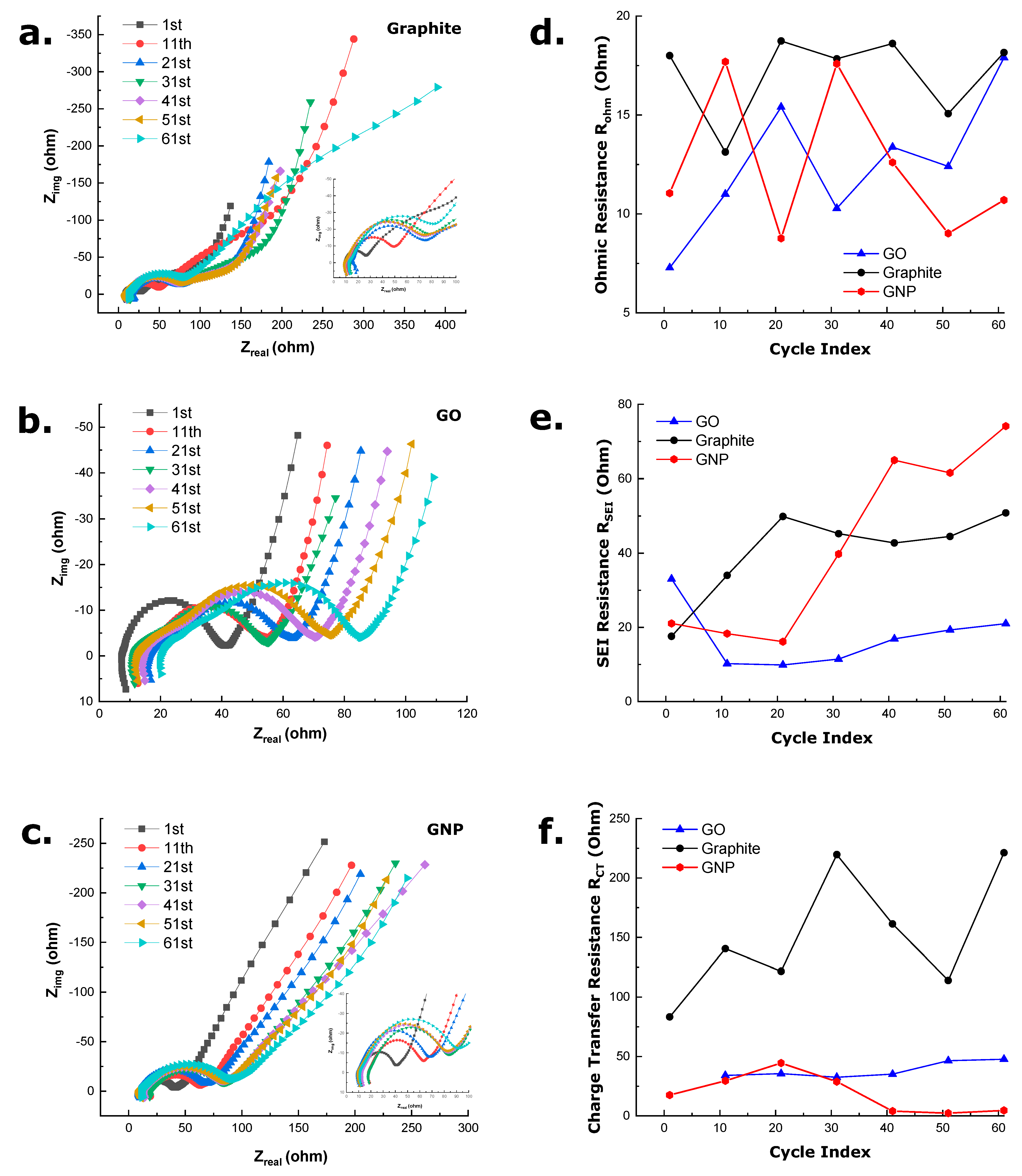
3.5. Electrochemical Impedance Spectra Analysis
- The inductive behavior observed in the high-frequency range of all the spectra can be primarily ascribed to the measurement system (wires connecting the half-cell to the potentiostat) or due to cell geometry or cell windings. Although, as in all spectra, the inductive loop is visible, in this study, it is assigned to be arising from the measurement system. The high-frequency intercept of the real impedance axis with the inductive circle corresponds to the sum of internal ohmic resistance, including the electrolyte, active material, current collectors, and electrical contacts [67].
- A prominent depressed semicircle appears in the medium frequency range. It is fitted using an R|Q element in the equivalent circuit. It denotes resistance arising from the solid electrolyte interface (SEI) formation (i.e., the desolvation of Na+ ions and their incorporation into the SEI). Specifically, this semicircle is absent in the first cycle. Still, it progresses throughout the cycling conditions as the formation of SEI layers passivates the anodes and prevents delamination of individual layers while leading to continuous capacity decay [68]. The significance of SEI growth is illustrated further in the following section.
- Another small flat semicircle, which reflects the charge transfer resistance and interfacial capacitance fitted with an R|Q element, appears in the low-frequency region. As the electrodes utilized in this investigation are neither ultrathin nor thick, a moderate semicircle is observed in the case of all three carbon allotropes. This semicircle can again be correlated to the time constant of the charge transfer being coupled with a double-layer type interfacial capacitance (Cdl). Several other factors, such as interparticle electronic resistance and porosity of the electrode, may influence the diameter of the semicircle further [69].
- A sloping line or tail at very low frequencies indicates Na+ ion diffusion in the active material of the cell electrodes. The Warburg element (W) fits the tail at the low-frequency region, establishing a connection to the mid-frequency responses modeled with R|Q elements. In this study, the fitting is conducted, placing the Warburg element in series with the double layer capacitance as the impedance arising due to SEI formation is well separated from the charge transfer process, and impedance contribution from various processes can be well separated in this way [70]. Previous literature has modeled this tail generalizing the Warburg element as a Q or CPE with N different from −0.5. However, in the Nyquist plots obtained, the tail inclination is higher than 45°, which a CPE generalization cannot define. This non-ideal behavior can be ascribed to the anisotropic diffusion in the particles with variable size distribution [71,72]—which is more prominent in the case of the GNP electrode as the electrode was prepared using sonication.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Niu, Y.; Ouyang, X.; Guo, C.; Han, P.; Zhou, R.; Heydari, A.; Zhou, Y.; Ikkala, O.; Tigranovich, G.A.; et al. Progress of organic, inorganic redox flow battery and mechanism of electrode reaction. Nano Res. Energy 2023, 2, e9120081. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible solid-state lithium-sulfur batteries based on structural designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Soares, D.M.; Ren, Z.; Mujib, S.B.; Mukherjee, S.; Martins Real, C.G.; Anstine, M.; Zanin, H.; Singh, G. Additive Manufacturing of Electrochemical Energy Storage Systems Electrodes. Adv. Energy Sustain. Res. 2021, 2, 2000111. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Bommier, C.; Zhu, H.; Ji, X.; Hu, L. Na-Ion Battery Anodes: Materials and Electrochemistry. Acc. Chem. Res. 2016, 49, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tian, Y.; Mei, Y.; Ni, L.; Wang, H.; Liu, H.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Robust NASICON-type iron-based Na4Fe3(PO4)2(P2O7) cathode for high temperature sodium-ion batteries. Chem. Eng. J. 2023, 458, 141385. [Google Scholar] [CrossRef]
- Jeon, I.; Yang, D.; Yadav, D.; Seo, J.; Zhang, H.; Yin, L.; Ahn, H.S.; Cho, C.-R. Sodium storage behavior and long cycle stability of boron-doped carbon nanofibers for sodium-ion battery anodes. Electrochim. Acta 2023, 439, 141730. [Google Scholar] [CrossRef]
- David, L.; Shareef, K.M.; Abass, M.A.; Singh, G. Three-dimensional polymer-derived ceramic/graphene paper as a Li-ion battery and supercapacitor electrode. RSC Adv. 2016, 6, 53894–53902. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Kulkarni, G.; Pahwa, S.; Zhong, Z.; Singh, G. Synthesis of Graphene Films by Rapid Heating and Quenching at Ambient Pressures and Their Electrochemical Characterization. ACS Appl. Mater. Interfaces 2013, 5, 546–552. [Google Scholar] [CrossRef]
- Dey, S.; Mujib, S.B.; Singh, G. Enhanced Li-Ion Rate Capability and Stable Efficiency Enabled by MoSe2 Nanosheets in Polymer-Derived Silicon Oxycarbide Fiber Electrodes. Nanomaterials 2022, 12, 553. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, L.; Tan, J.; Yan, Z.; Huang, Z.; Wang, X. Synthesis of lithium titanate nanorods as anode materials for lithium and sodium ion batteries with superior electrochemical performance. J. Power Source 2015, 283, 243–250. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous Doped Silicon Nanowires for Lithium Ion Battery Anode with Long Cycle Life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef]
- David, L.; Singh, G. Reduced Graphene Oxide Paper Electrode: Opposing Effect of Thermal Annealing on Li and Na Cyclability. J. Phys. Chem. C 2014, 118, 28401–28408. [Google Scholar] [CrossRef]
- Xia, J.-L.; Yan, D.; Guo, L.-P.; Dong, X.-L.; Li, W.-C.; Lu, A.-H. Hard Carbon Nanosheets with Uniform Ultramicropores and Accessible Functional Groups Showing High Realistic Capacity and Superior Rate Performance for Sodium-Ion Storage. Adv. Mater. 2020, 32, 2000447. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, G. Sodium and potassium ion storage in cation substituted 2D MoWSe2: Insights into the effects of upper voltage cut-off. Nanotechnology 2023, 34, 385401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yuge, R.; Nakahara, K.; Tamura, N.; Miyamoto, S. KOH etched graphite for fast chargeable lithium-ion batteries. J. Power Source 2015, 284, 258–263. [Google Scholar] [CrossRef]
- Mujib, S.B.; Vessalli, B.; Bizzo, W.A.; Mazon, T.; Singh, G. Cassava-and bamboo-derived carbons with higher degree of graphitization for energy storage. Nanomater. Energy 2020, 9, 54–65. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803. [Google Scholar] [CrossRef]
- Zhang, X.-R.; Yang, J.-Y.; Ren, Z.-Y.; Xie, K.-Y.; Ye, Q.; Xu, F.; Liu, X.-R. In-situ observation of electrolyte-dependent interfacial change of the graphite anode in sodium-ion batteries by atomic force microscopy. New Carbon Mater. 2022, 37, 371–379. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bin Mujib, S.; Soares, D.; Singh, G. Electrode materials for high-performance sodium-ion batteries. Materials 2019, 12, 1952. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Shan, H.; Zhao, Y.; Dong, L.; Xu, H.; Zhang, X.; Li, D.; Sun, X. Oxygen-containing Functional Groups Enhancing Electrochemical Performance of Porous Reduced Graphene Oxide Cathode in Lithium Ion Batteries. Electrochim. Acta 2015, 174, 762–769. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, X.; Fan, L.; Yan, L.; Wang, Z.; Lei, W.; Ruan, D.; Shi, Z. Boosting the High Capacitance-Controlled Capacity of Hard Carbon by Using Surface Oxygen Functional Groups for Fast and Stable Sodium Storage. ACS Appl. Energy Mater. 2021, 4, 11436–11446. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jung, S.C.; Choi, J.W.; Han, Y.-K. Important Role of Functional Groups for Sodium Ion Intercalation in Expanded graphite. Chem. Mater. 2015, 27, 5402–5406. [Google Scholar] [CrossRef]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.; Wang, C.; Koratkar, N. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Shen, D.H.; Surampudi, S.; Attia, A.I.; Halpert, G. Electrochemical Impedance Spectroscopy of Lithium-Titanium Disulfide Rechargeable Cells. J. Electrochem. Soc. 1993, 140, 1854. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Antonacci, P. X-ray and Electrochemical Impedance Spectroscopy Diagnostic Investigations of Liquid Water in Polymer Electrolyte Membrane Fuel Cell Gas Diffusion Layers; University of Toronto (Canada): Toronto, ON, Canada, 2016. [Google Scholar]
- Setiadji, S.; Nuryadin, B.W.; Ramadhan, H.; Sundari, C.D.D.; Sudiarti, T.; Supriadin, A.; Ivansyah, A.L. Preparation of reduced Graphene Oxide (rGO) assisted by microwave irradiation and hydrothermal for reduction methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012079. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Yu, L.; Alam, F.E.; Dai, W.; Li, C.; Jiang, N. Enhancing the thermal, electrical, and mechanical properties of silicone rubber by addition of graphene nanoplatelets. Mater. Des. 2015, 88, 950–957. [Google Scholar] [CrossRef]
- Inagaki, M.; Fujita, K.; Takeuchi, Y.; Oshida, K.; Iwata, H.; Konno, H. Formation of graphite crystals at 1000–1200 °C from mixtures of vinyl polymers with metal oxides. Carbon 2001, 39, 921–929. [Google Scholar] [CrossRef]
- Theuwissen, K.; Lacaze, J.; Laffont, L. Structure of graphite precipitates in cast iron. Carbon 2016, 96, 1120–1128. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K.; Kumar, S.S. Reduced graphene oxide derived from used cell graphite and its green fabrication as an eco-friendly supercapacitor. RSC Adv. 2014, 4, 60039–60051. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, J.S.; Lee, K.Y.; Park, K.H.; Bae, J.-S.; Mubarak, M.; Lee, H. Elucidation of an intrinsic parameter for evaluating the electrical quality of graphene flakes. Sci. Rep. 2019, 9, 557. [Google Scholar] [CrossRef]
- Shalaby, A.; Nihtianova, D.; Markov, P.; Staneva, A.D.; Iordanova, R.S.; Dimitriev, Y.B. Structural analysis of reduced graphene oxide by transmission electron microscopy. Bulg. Chem. Commun. 2015, 47, 291–295. [Google Scholar]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Obergfell, D.; Roth, S.; Girit, C.; Zettl, A. On the roughness of single- and bi-layer graphene membranes. Solid State Commun. 2007, 143, 101–109. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; Degirmenci, V.; Sloan, J.; Presvytis, D.; Watson, M.; McNally, T. Characterisation of graphite nanoplatelets (GNP) prepared at scale by high-pressure homogenisation. J. Mater. Chem. C 2019, 7, 6383–6390. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Ozga, P.; Maziarz, W.; Pstruś, J.; Kania, B.; Bobrowski, P.; Stolarska, J. Microstructure and properties of bulk copper matrix composites strengthened with various kinds of graphene nanoplatelets. Mater. Sci. Eng. A 2015, 628, 124–134. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Bolaños-Morales, D.; Bonilla-Martínez, J.; Estrada-Guel, I.; Martínez-Sánchez, R. Microstructural and hardness behavior of graphene-nanoplatelets/aluminum composites synthesized by mechanical alloying. J. Alloys Compd. 2014, 615, S578–S582. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Jerng, S.-K.; Seong Yu, D.; Hong Lee, J.; Kim, C.; Yoon, S.; Chun, S.-H. Graphitic carbon growth on crystalline and amorphous oxide substrates using molecular beam epitaxy. Nanoscale Res. Lett. 2011, 6, 565. [Google Scholar] [CrossRef]
- Mera, G.; Navrotsky, A.; Sen, S.; Kleebe, H.-J.; Riedel, R. Polymer-derived SiCN and SiOC ceramics—Structure and energetics at the nanoscale. J. Mater. Chem. A 2013, 1, 3826–3836. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Priya Rose, T.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Zhang, X.; Ma, S. Achieving concentrated graphene dispersions in water/acetone mixtures by the strategy of tailoring Hansen solubility parameters. J. Phys. D Appl. Phys. 2013, 46, 025301. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, T. Study of Ultrasonic Dispersion of Graphene Nanoplatelets. Materials 2019, 12, 1757. [Google Scholar] [CrossRef]
- Dash, P.; Dash, T.; Rout, T.K.; Sahu, A.K.; Biswal, S.K.; Mishra, B.K. Preparation of graphene oxide by dry planetary ball milling process from natural graphite. RSC Adv. 2016, 6, 12657–12668. [Google Scholar] [CrossRef]
- Rui, B.; Yang, M.; Zhang, L.; Jia, Y.; Shi, Y.; Histed, R.; Liao, Y.; Xie, J.; Lei, F.; Fan, L. Reduced graphene oxide-modified biochar electrodes via electrophoretic deposition with high rate capability for supercapacitors. J. Appl. Electrochem. 2020, 50, 407–420. [Google Scholar] [CrossRef]
- Alam, K.; Sim, Y.; Yu, J.-H.; Gnanaprakasam, J.; Choi, H.; Chae, Y.; Sim, U.; Cho, H. In-situ Deposition of Graphene Oxide Catalyst for Efficient Photoelectrochemical Hydrogen Evolution Reaction Using Atmospheric Plasma. Materials 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Mehdizadeh, A.; Hosseini, M.A.; Jafari, S.A.; Ashtari, P. Reduced graphene oxide/polymethyl methacrylate (rGO/PMMA) nanocomposite for real time gamma radiation detection. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 940, 72–77. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Alam, S.N.; Kumar, L. Mechanical properties of aluminium based metal matrix composites reinforced with graphite nanoplatelets. Mater. Sci. Eng. A 2016, 667, 16–32. [Google Scholar] [CrossRef]
- Sarkar, A.; Manohar, C.V.; Mitra, S. A simple approach to minimize the first cycle irreversible loss of sodium titanate anode towards the development of sodium-ion battery. Nano Energy 2020, 70, 104520. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Reduced graphene oxide with superior cycling stability and rate capability for sodium storage. Carbon 2013, 57, 202–208. [Google Scholar] [CrossRef]
- Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Environ. Sci. 2011, 4, 3342–3345. [Google Scholar] [CrossRef]
- Fu, H.; Xu, Z.; Guan, W.; Shen, X.; Cao, L.; Huang, J. Adsorption contributions of graphene to sodium ion storage performance. J. Phys. D Appl. Phys. 2018, 51, 205501. [Google Scholar] [CrossRef]
- Mochida, I.; Ku, C.-H.; Korai, Y. Anodic performance and insertion mechanism of hard carbons prepared from synthetic isotropic pitches. Carbon 2001, 39, 399–410. [Google Scholar] [CrossRef]
- Kumar, N.A.; Gaddam, R.R.; Varanasi, S.R.; Yang, D.; Bhatia, S.K.; Zhao, X.S. Sodium ion storage in reduced graphene oxide. Electrochim. Acta 2016, 214, 319–325. [Google Scholar] [CrossRef]
- Schürholz, D.; Schweighofer, B.; Neumayer, M.; Klug, A.; Teichmann, R.; Wegleiter, H. Investigation of Accuracy Requirements for Delta Differential Capacity and Voltage Measurements of Li-Ion Batteries. In Proceedings of the 2022 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Ottawa, ON, Canada, 16–19 May 2022; pp. 1–6. [Google Scholar]
- Kurzweil, P.; Frenzel, B.; Scheuerpflug, W. A Novel Evaluation Criterion for the Rapid Estimation of the Overcharge and Deep Discharge of Lithium-Ion Batteries Using Differential Capacity. Batteries 2022, 8, 86. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Source 2020, 480, 228742. [Google Scholar] [CrossRef]
- Steinhauer, M.; Risse, S.; Wagner, N.; Friedrich, K.A. Investigation of the Solid Electrolyte Interphase Formation at graphite Anodes in Lithium-Ion Batteries with Electrochemical Impedance Spectroscopy. Electrochim. Acta 2017, 228, 652–658. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Teller, H.; Markovsky, B.; Salitra, G.; Heider, U.; Heider, L. Common Electroanalytical Behavior of Li Intercalation Processes into graphite and Transition Metal Oxides. J. Electrochem. Soc. 1998, 145, 3024. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Song, J.; Bazant, M.Z. Electrochemical Impedance of a Battery Electrode with Anisotropic Active Particles. Electrochim. Acta 2014, 131, 214–227. [Google Scholar] [CrossRef]
- Song, J.; Bazant, M.Z. Effects of Nanoparticle Geometry and Size Distribution on Diffusion Impedance of Battery Electrodes. J. Electrochem. Soc. 2013, 160, A15. [Google Scholar] [CrossRef]
- Yazami, R.; Reynier, Y.F. Mechanism of self-discharge in graphite—Lithium anode. Electrochim. Acta 2002, 47, 1217–1223. [Google Scholar] [CrossRef]
- Jiang, C.; Hosono, E.; Zhou, H. Nanomaterials for lithium ion batteries. Nano Today 2006, 1, 28–33. [Google Scholar] [CrossRef]
- Estaller, J.; Kersten, A.; Kuder, M.; Thiringer, T.; Eckerle, R.; Weyh, T. Overview of Battery Impedance Modeling Including Detailed State-of-the-Art Cylindrical 18650 Lithium-Ion Battery Cell Comparisons. Energies 2022, 15, 3822. [Google Scholar] [CrossRef]
- Zhang, J.A.; Wang, P.; Liu, Y.; Cheng, Z. Variable-order equivalent circuit modeling and state of charge estimation of lithium-ion battery based on electrochemical impedance spectroscopy. Energies 2021, 14, 769. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef]
- Darwiche, A.; Bodenes, L.; Madec, L.; Monconduit, L.; Martinez, H. Impact of the salts and solvents on the SEI formation in Sb/Na batteries: An XPS analysis. Electrochim. Acta 2016, 207, 284–292. [Google Scholar] [CrossRef]
- Philippe, B.; Valvo, M.; Lindgren, F.; Rensmo, H.; Edström, K. Investigation of the Electrode/Electrolyte Interface of Fe2O3 Composite Electrodes: Li vs. Na Batteries. Chem. Mater. 2014, 26, 5028–5041. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Source 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Park, J.; Yoon, G.; Kim, H.; Kang, K. Graphitic Carbon Materials for Advanced Sodium-Ion Batteries. Small Methods 2019, 3, 1800227. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Widanage, W.D.; Marco, J.; Gama-Valdez, M.Á.; Chouchelamane, G.H. Identification and Quantification of Ageing Mechanisms in Lithium-Ion Batteries Using the EIS Technique. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 27–29 June 2016; pp. 1–6. [Google Scholar]
- McNealy, B.E.; Hertz, J.L. On the use of the constant phase element to understand variation in grain boundary properties. Solid State Ion. 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Bagheri, S.; Monajjemi, M.; Ziglari, A.; Manesh, A.T. Lithium Including Mixed Sodium Inside Graphene Oxide (GO) as Anodic Electrodes for ion Batteries. Orient. J. Chem. 2018, 34, 981. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-principles study of alkali metal-graphite intercalation compounds. J. Power Source 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Messing, M.; Shoa, T.; Habibi, S. EIS from Accelerated and Realistic Battery Aging. In Proceedings of the 2021 IEEE Transportation Electrification Conference & Expo (ITEC), Chicago, IL, USA, 21–25 June 2021; pp. 720–725. [Google Scholar]
- Levi, M.D.; Aurbach, D. Diffusion Coefficients of Lithium Ions during Intercalation into graphite Derived from the Simultaneous Measurements and Modeling of Electrochemical Impedance and Potentiostatic Intermittent Titration Characteristics of Thin graphite Electrodes. J. Phys. Chem. B 1997, 101, 4641–4647. [Google Scholar] [CrossRef]
- Solchenbach, S.; Huang, X.; Pritzl, D.; Landesfeind, J.; Gasteiger, H.A. Monitoring SEI Formation on graphite Electrodes in Lithium-Ion Cells by Impedance Spectroscopy. J. Electrochem. Soc. 2021, 168, 110503. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J. Theory of Impedance Response of Porous Electrodes: Simplifications, Inhomogeneities, Non-Stationarities and Applications. J. Electrochem. Soc. 2016, 163, A1983. [Google Scholar] [CrossRef]
- Sikha, G.; White, R.E. Analytical Expression for the Impedance Response of an Insertion Electrode Cell. J. Electrochem. Soc. 2007, 154, A43. [Google Scholar] [CrossRef]
- Wang, J.-W.; Chen, Y.; Chen, B.-Z. A Synthesis Method of MnO2/Activated Carbon Composite for Electrochemical Supercapacitors. J. Electrochem. Soc. 2015, 162, A1654. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, S.; Singh, G. Differentiating Cyclability and Kinetics of Na+ Ions in Surface-Functionalized and Nanostructured Graphite Using Electrochemical Impedance Spectroscopy. Batteries 2023, 9, 534. https://doi.org/10.3390/batteries9110534
Dey S, Singh G. Differentiating Cyclability and Kinetics of Na+ Ions in Surface-Functionalized and Nanostructured Graphite Using Electrochemical Impedance Spectroscopy. Batteries. 2023; 9(11):534. https://doi.org/10.3390/batteries9110534
Chicago/Turabian StyleDey, Sonjoy, and Gurpreet Singh. 2023. "Differentiating Cyclability and Kinetics of Na+ Ions in Surface-Functionalized and Nanostructured Graphite Using Electrochemical Impedance Spectroscopy" Batteries 9, no. 11: 534. https://doi.org/10.3390/batteries9110534
APA StyleDey, S., & Singh, G. (2023). Differentiating Cyclability and Kinetics of Na+ Ions in Surface-Functionalized and Nanostructured Graphite Using Electrochemical Impedance Spectroscopy. Batteries, 9(11), 534. https://doi.org/10.3390/batteries9110534





