Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Na3Zr2Si2PO12
2.2. Structural Characterization
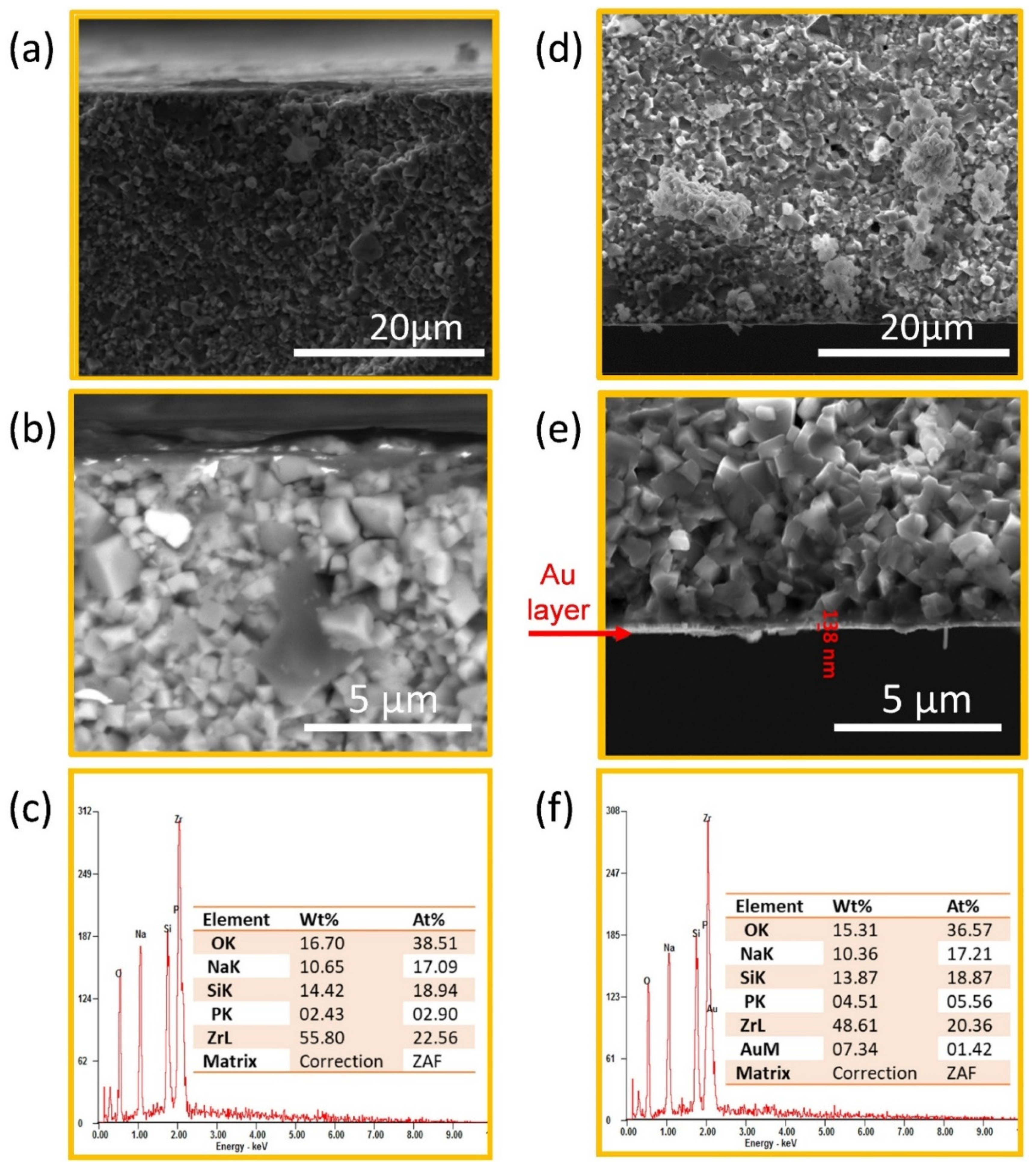
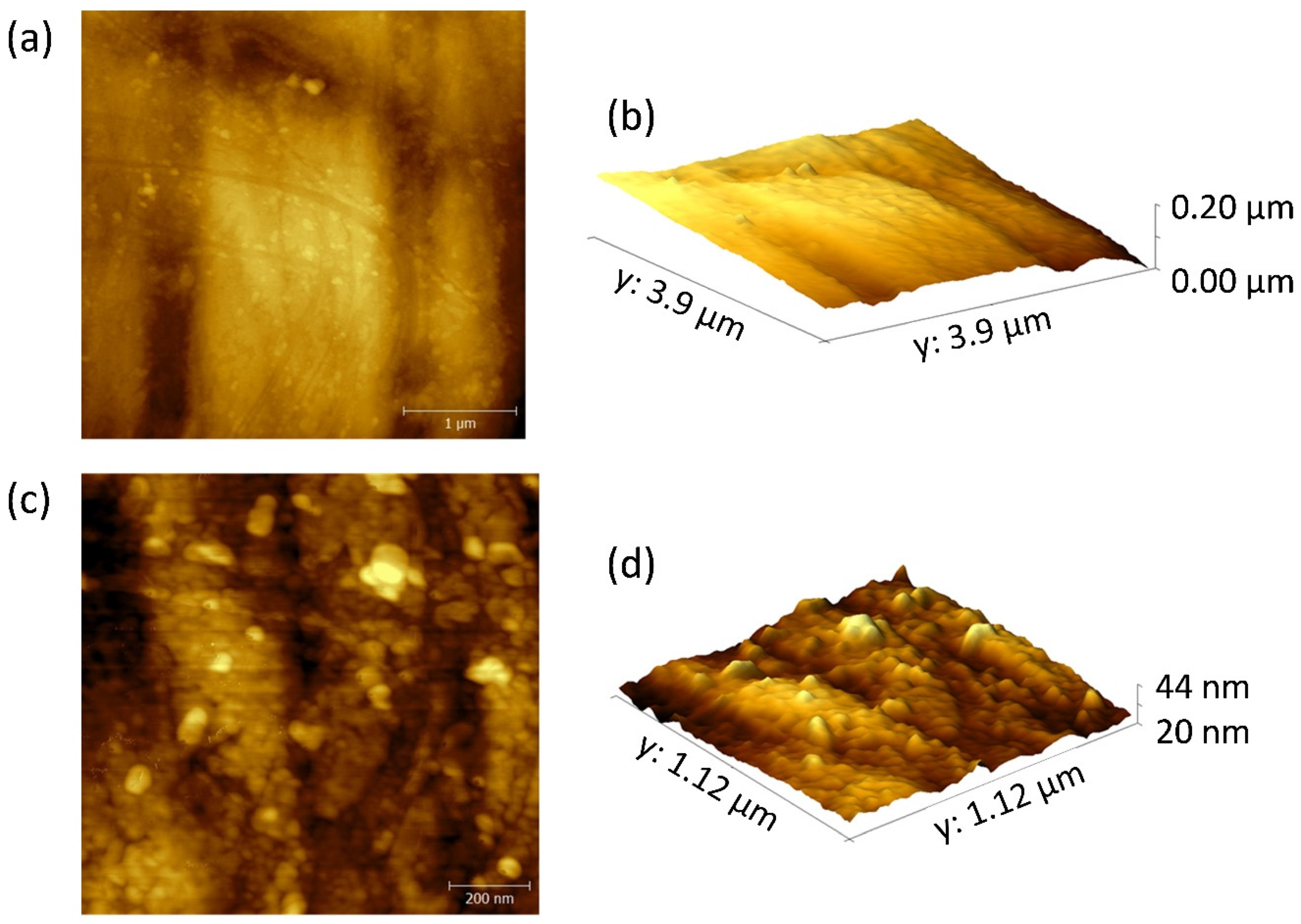
2.3. Microstructural Characterization
2.4. Fabrication of the Symmetric Cells
- (i)
- Na||NZSP||Na: A Swagelok-type cell was assembled by placing the sodium foil on both surfaces of the pellet by applying a gentle pressure. The cell was then heated to 80 °C overnight under an argon atmosphere to soften the sodium and thus enhance the contact.
- (ii)
- Na||Au-NZSP-Au||Na: A thin layer of gold was sputtered for 1 min on each side of the NZSP at a current of 50 µA using the DC-sputtering technique. The coated sample was then moved to the glove box. Subsequently, sodium discs were placed above the Au-sputtered NSZP, and a Swagelok-type cell was fabricated. The Swagelok cell was then heated to 80 °C overnight to form a sodium–gold alloy.
2.5. Electrical Conductivity Studies
2.6. Electrode Preparation
2.7. Fabrication of the Quasi-Solid-State Sodium Metal Battery
2.8. Electrochemical Characterization
3. Results and Discussions
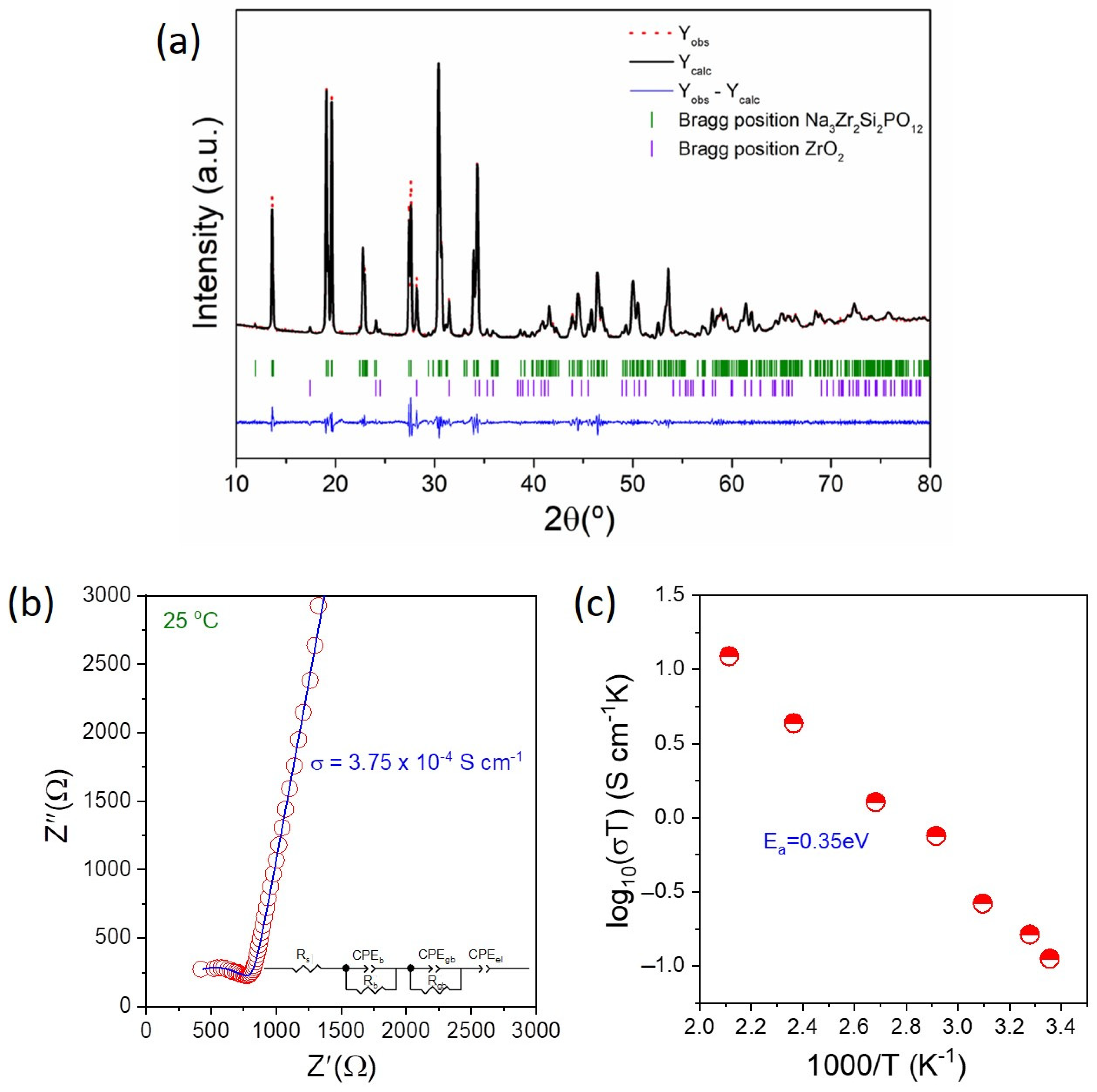
3.1. Characterization of Na3Zr2Si2PO12
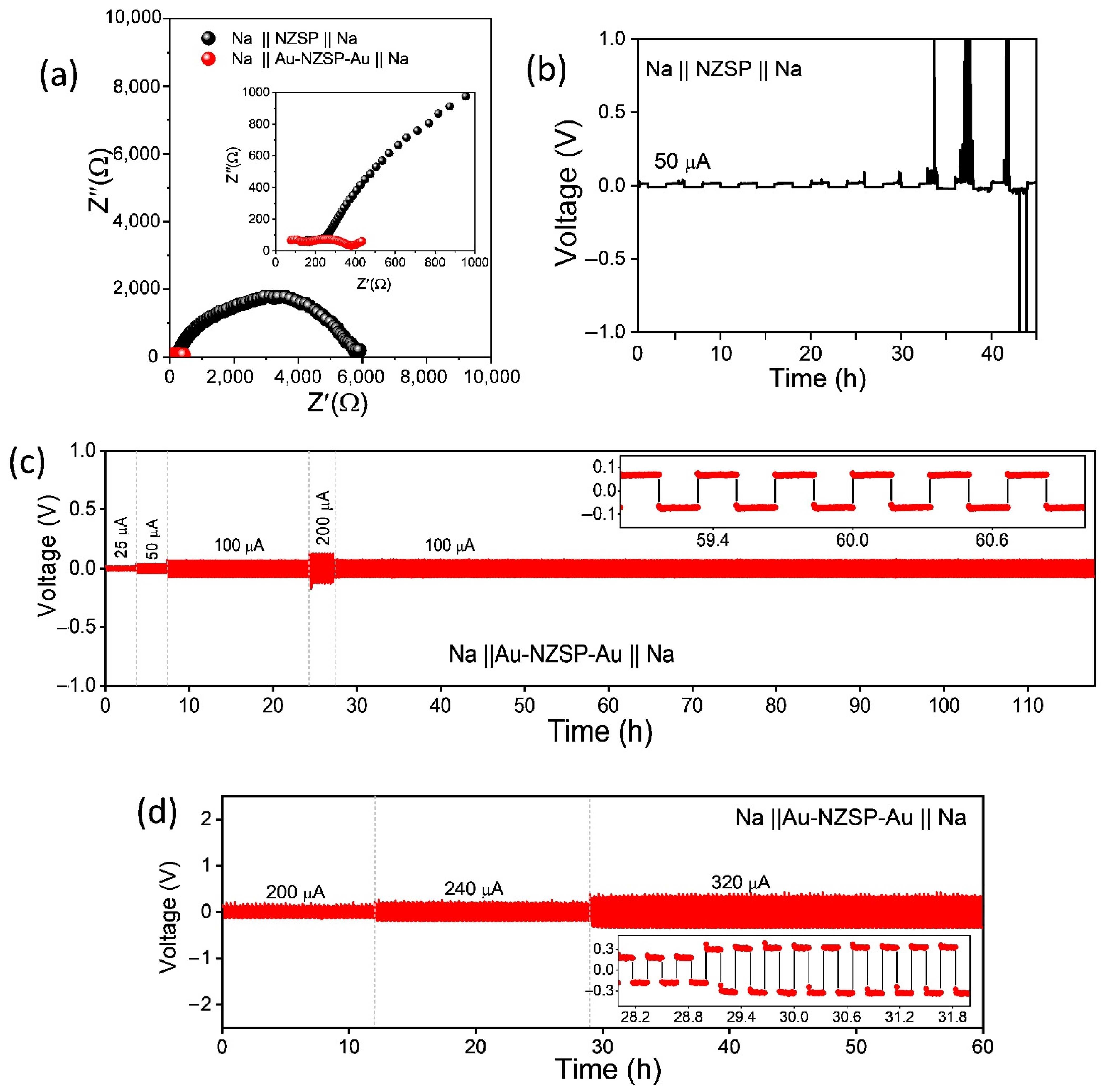
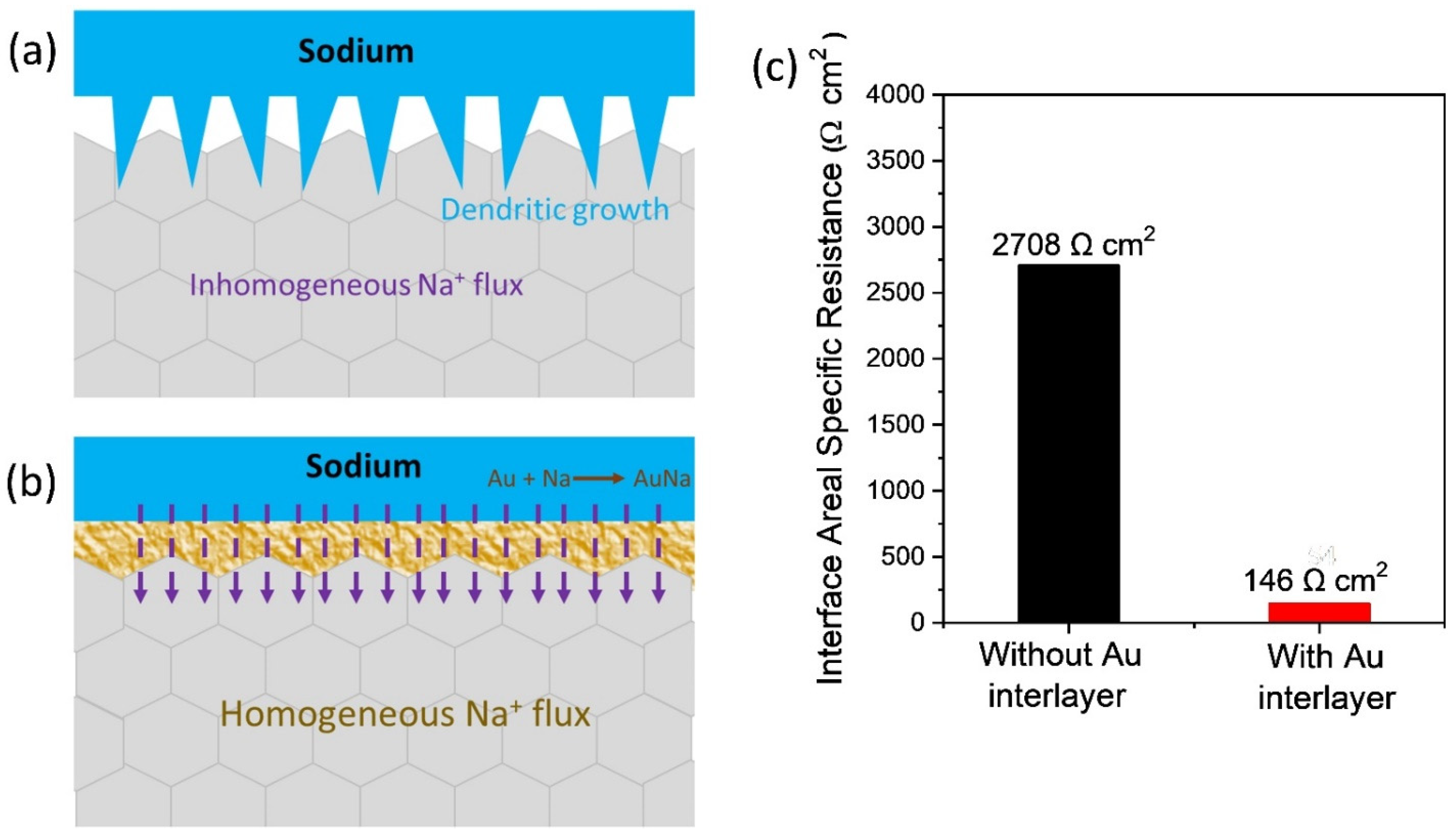
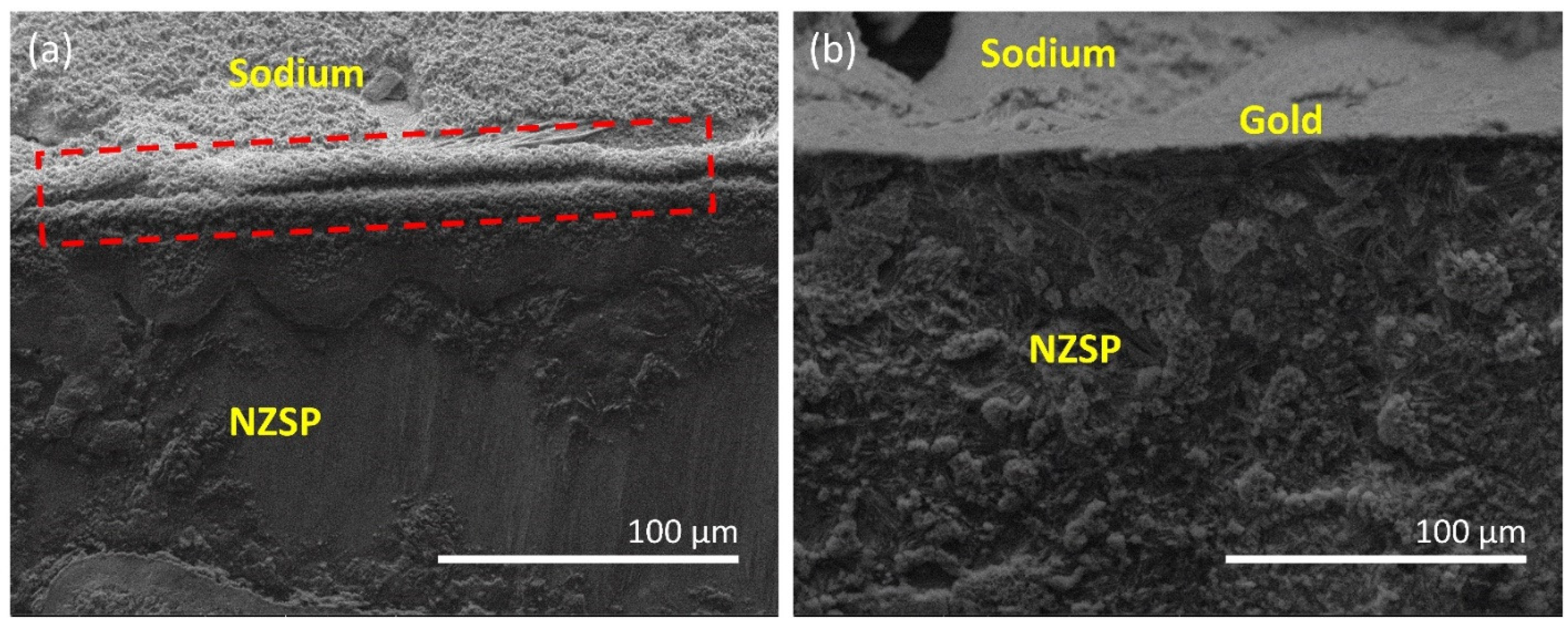
3.2. Symmetric Cell Evaluation
3.3. Fabrication and Electrochemical Performance Evaluation of Na-Au||NZSP||PP-NFP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawicki, M.; Shaw, L. Advances and challenges of sodium ion batteries as post lithium ion batteries. RSC Adv. 2015, 5, 53129. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low-cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529. [Google Scholar] [CrossRef]
- Barpanda, P.; Chotard, J.N.; Recham, N.; Delacourt, C.; Ati, M.; Dupont, L.; Armand, M.; Tarascon, J.M. Structural, transport, and electrochemical investigation of novel AMSO4F (A = Na, Li; M = Fe, Co, Ni, Mn) metal fluorosulphates prepared using low temperature synthesis routes. Inorg. Chem. 2010, 49, 7401. [Google Scholar] [CrossRef]
- Plashnitsa, L.S.; Kobayashi, E.; Noguchi, Y.; Okada, S.; Yamaki, J.I. Performance of NASICON Symmetric Cell with Ionic Liquid Electrolyte. J. Electrochem. Soc. 2010, 157, A536. [Google Scholar] [CrossRef]
- Kim, H.; Park, I.; Seo, D.H.; Lee, S.; Kim, S.W.; Kwon, W.J.; Park, Y.U.; Kim, C.S.; Jeon, S.; Kang, K. New iron-based mixed-polyanion cathodes for lithium and sodium rechargeable batteries: Combined first principles calculations and experimental study. J. Am. Chem. Soc. 2012, 134, 10369. [Google Scholar] [CrossRef]
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. Recent progress of emerging cathode materials for sodium ion batteries. Mater. Chem. Front. 2021, 5, 3735. [Google Scholar] [CrossRef]
- Wang, H.; Liao, X.Z.; Yang, Y.; Yan, X.; He, Y.S.; Ma, Z.F. Large-Scale Synthesis of NaNi1/3Fe1/3Mn1/3O2 as High Performance Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2016, 3, A565. [Google Scholar] [CrossRef]
- Jian, Z.L.; Han, W.Z.; Lu, X.; Yang, H.X.; Hu, Y.S.; Zhou, J.; Zhou, Z.B.; Li, J.Q.; Chen, W.; Chen, D.; et al. Superior Electrochemical Performance and Storage Mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2013, 3, 156. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.M.; Palacin, M.R. Na2Ti3O7: Lowest Voltage Ever Reported Oxide Insertion Electrode for Sodium Ion Batteries. Chem. Mater. 2011, 23, 4109. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, S.Y.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L. A self-forming composite electrolyte for solid-state sodium battery with ultralong cycle life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Kim, J.J.; Yoon, K.; Park, I.; Kang, K. Progress in the Development of Sodium-Ion Solid Electrolytes. Small Methods 2017, 1, 1700219. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Hou, H.; Xu, Q.; Pang, Y.; Li, L.; Wang, J.; Zhang, C.; Sun, C. Efficient Storing Energy Harvested by Triboelectric Nanogenerators Using a Safe and Durable All-Solid-State Sodium-Ion Battery. Adv. Sci. 2017, 4, 1700072. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Liu, C.; Zou, G.; Hou, H.; Ji, X. Research Progress in Inorganic Solid-State Electrolytes for Sodium-Ion Batteries. Acta Phys.-Chim. Sin. 2020, 36, 1905013. [Google Scholar]
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable Sodium All-Solid-State Battery. ACS Cent. Sci. 2017, 3, 52. [Google Scholar] [CrossRef]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, Q.; Niu, Z.; Chen, J. Electrolyte and Interface Engineering for Solid-State Sodium Batteries. Joule 2018, 2, 1747. [Google Scholar] [CrossRef]
- Hong, H. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203. [Google Scholar] [CrossRef]
- Von Alpen, U.; Bell, M.F.; Wichelhaus, W. Phase transition in nasicon (Na3Zr2Si2PO12). Mater. Res. Bull. 1979, 14, 1317. [Google Scholar] [CrossRef]
- Perthuis, H.; Colomban, P. Sol-gel Routes Leading to NASICON Ceramics. Ceram. Int. 1986, 12, 39. [Google Scholar] [CrossRef]
- Bohnke, O.; Ronchetti, S.; Mazza, D. Conductivity measurements on nasicon and nasicon-modified materials. Solid State Ion. 1999, 122, 127. [Google Scholar] [CrossRef]
- Niu, Y.; Yin, Y.X.; Guo, Y. Nonaqueous Sodium-Ion Full Cells: Status, Strategies, and Prospects. Small 2019, 15, 1900233. [Google Scholar] [CrossRef]
- Ruan, Y.; Guo, F.; Liu, J.; Song, S.; Jiang, N.; Cheng, B. Optimization of Na3Zr2Si2PO12 ceramic electrolyte and interface for high performance solid-state sodium battery. Ceram Int. 2019, 45, 1770. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective. Nano Energy 2018, 52, 279. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168. [Google Scholar] [CrossRef]
- Sumaletha, N.; Samuel, R.; Shantel, B.; Thangadurai, V. Sintering temperature, excess sodium, and phosphorous dependencies on morphology and ionic conductivity of NASICON Na3Zr2Si2PO12. Solid State Ion. 2019, 331, 22. [Google Scholar]
- Goodenough, J.B.; Singh, P.J. Review-Solid Electrolytes in Rechargeable Electrochemical Cells. Electrochem. Mater. 2015, 162, A2387. [Google Scholar] [CrossRef]
- Luntz, A.C.; Voss, J.; Reuter, K.J. Interfacial Challenges in Solid-State Li Ion Batteries. Phys. Chem. Lett. 2015, 6, 4599. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yang, J.; Yuan, H.; Fu, H.; Li, Y.; Li, Y.; Ferber, T.; Guhl, C.; Sun, H.; Jaegermann, W.; et al. Stabilizing Na3Zr2Si2PO12/Na Interfacial Performance by Introducing a Clean and Na-Deficient Surface. Chem. Mater. 2020, 32, 3970. [Google Scholar] [CrossRef]
- Miao, X.; Di, H.; Ge, X.; Zhao, D.; Wang, P.; Wang, R.; Wang, C.; Yin, L. AlF3-modified anode-electrolyte interface for effective Na dendrites restriction in nasicon-based solid-state electrolyte. Energy Storage Mater. 2020, 30, 170. [Google Scholar] [CrossRef]
- Uchida, Y.; Hasegawa, G.; Shima, K.; Inada, M.; Enomoto, N.; Akamatsu, H.; Hayashi, K. Insights into Sodium Ion Transfer at the Na/NASICON Interface Improved by Uniaxial Compression. ACS Appl. Energy Mater. 2019, 2, 2913. [Google Scholar] [CrossRef]
- Yang, J.Y.; Xu, H.; Wu, J.; Gao, Z.; Hu, F.; Wei, Y.; Li, Y.; Liu, D.; Huang, Y.H. Improving Na/Na3Zr2Si2PO12 Interface via SnOx/Sn Film for High-Performance Solid-State Sodium Metal Batteries. Small Methods 2021, 5, 2100339. [Google Scholar] [CrossRef]
- He, M.; Cui, Z.; Chen, C.; Li, Y.; Guo, X. Formation of self-limited, stable and conductive interfaces between garnet electrolytes and lithium anodes for reversible lithium cycling in solid-state batteries. J. Mater. Chem. A 2018, 6, 11463. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Xu, S.; McOwen, D.; Gong, Y.; Yang, C.; Pastel, G.; Xie, H.; Fu, K.; Dai, J.; et al. 3D lithium metal anodes hosted in asymmetric garnet frameworks toward high energy density batteries. Energy Storage Mater. 2018, 14, 376. [Google Scholar] [CrossRef]
- Fu, K.K.; Gong, Y.; Liu, B.; Zhu, Y.; Xu, S.; Yao, Y.; Luo, W.; Wang, C.; Lacey, S.D.; Dai, J.; et al. Toward garnet electrolyte–based Li metal batteries: An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv. 2017, 3, e1601659. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, Y.; Liu, B.; Fu, K.; Yao, Y.; Hitz, E.; Li, Y.; Dai, J.; Xu, S.; Luo, W.; et al. Conformal, Nanoscale ZnO Surface Modification of Garnet-Based Solid-State Electrolyte for Lithium Metal Anodes. Nano Lett. 2017, 17, 565. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2017, 16, 572. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gong, Y.; Zhu, Y.; Li, Y.; Yao, Y.; Zhang, Y.; Fu, K.; Pastel, G.; Lin, C.F.; Mo, Y.; et al. Reducing Interfacial Resistance between Garnet-Structured Solid-State Electrolyte and Li-Metal Anode by a Germanium Layer. Adv. Mater. 2017, 29, 1606042. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gong, Y.; Zhu, Y.; Fu, K.K.; Dai, J.; Lacey, S.D.; Wang, C.; Liu, B.; Han, X.; Mo, Y.; et al. In Situ Neutron Depth Profiling of Lithium Metal–Garnet Interfaces for Solid State Batteries. J. Am. Chem. Soc. 2016, 138, 12258. [Google Scholar] [CrossRef]
- Tsai, C.L.; Roddatis, V.; Chandran, C.; Ma, Q.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617. [Google Scholar] [CrossRef]
- Alexander, G.V.; Patra, S.; Raj, S.V.S.; Sugumar, M.; Din, M.; Muru-gan, R.J. Electrodes-electrolyte interfacial engineering for realizing room temperature lithium metal battery based on garnet structured solid fast Li+ conductors. Power Sources 2018, 396, 764. [Google Scholar] [CrossRef]
- Park, K.; Yu, B.-C.; Jung, J.-W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical Nature of the Cathode Interface for a Solid-State Lithium-Ion Battery: Interface between LiCoO2 and Garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051. [Google Scholar] [CrossRef]
- Alexander, G.V.; Indu, M.S.; Kamakshy, S.; Murugan, R. Development of stable and conductive interface between garnet structured solid electrolyte and lithium metal anode for high performance solid-state battery. Electrochim. Acta 2020, 332, 135511. [Google Scholar] [CrossRef]
- Indu, M.S.; Alexander, G.V.; Deviannapoorani, C.; Murugan, R. Realization of room temperature lithium metal battery with high Li+ conductive lithium garnet solid electrolyte. Ceram. Int. 2019, 45, 22610. [Google Scholar] [CrossRef]
- Berlanga, C.; Monterrubio, I.; Armand, M.; Rojo, T.; Galceran, M.; Casas-Cabanas, M. Cost-effective synthesis of triphylite-NaFePO4 cathode: A zero-waste process. ACS Sustain. Chem. Eng. 2020, 8, 725–730. [Google Scholar] [CrossRef]
- Boilot, J.P.; Collin, G.; Colomban, P. Crystal structure of the true nasicon: Na3Zr2Si2PO12. Mater. Res. Bull. 1987, 5, 669. [Google Scholar] [CrossRef]
- Krok, F. Influence of sintering conditions on chemical composition of NASICON. Solid State Ionics. 1987, 24, 21. [Google Scholar] [CrossRef]
- Gordon, R.; Miller, G.; McEntire, B.; Beck, E.; Rasmussen, J. Fabrication and characterization of NASICON electrolytes. Solid State Ion. 1981, 3−4, 243. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Figueiredo, F.M.; Marques, F.M.B.; Franco, J.I. Submicrometric NASICON Ceramics with Improved Electrical Conductivity Obtained from Mechanically Activated Precursors. J. Eur. Ceram. Soc. 2001, 21, 737. [Google Scholar] [CrossRef]
- Wakasugi, J.; Munakata, H.; Kanamura, K. Effect of Gold Layer on Interface Resistance between Lithium Metal Anode and Li6.25Al0.25La3Zr2O12 Solid Electrolyte. J. Electrochem. Soc. 2017, 164, A1022. [Google Scholar] [CrossRef]
- Galceran, M.; Saurel, D.; Acebedo, B.; Roddatis, V.; Martin, E.; Rojo, T.; Casas-Cabanas, M. The mechanism of NaFePO4 (de)sodiation determined by in situ X-ray diffraction. Phys. Chem. Chem. Phys. 2014, 16, 8837. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampathkumar, R.; Echeverría, M.; Zhang, Y.; Armand, M.; Galceran, M. Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery. Batteries 2023, 9, 8. https://doi.org/10.3390/batteries9010008
Sampathkumar R, Echeverría M, Zhang Y, Armand M, Galceran M. Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery. Batteries. 2023; 9(1):8. https://doi.org/10.3390/batteries9010008
Chicago/Turabian StyleSampathkumar, Ramakumar, María Echeverría, Yan Zhang, Michel Armand, and Montserrat Galceran. 2023. "Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery" Batteries 9, no. 1: 8. https://doi.org/10.3390/batteries9010008
APA StyleSampathkumar, R., Echeverría, M., Zhang, Y., Armand, M., & Galceran, M. (2023). Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery. Batteries, 9(1), 8. https://doi.org/10.3390/batteries9010008





