NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Solid-State Synthesis
2.1.2. Sol-Gel Method
2.2. Characterization Techniques
3. Results and Discussion
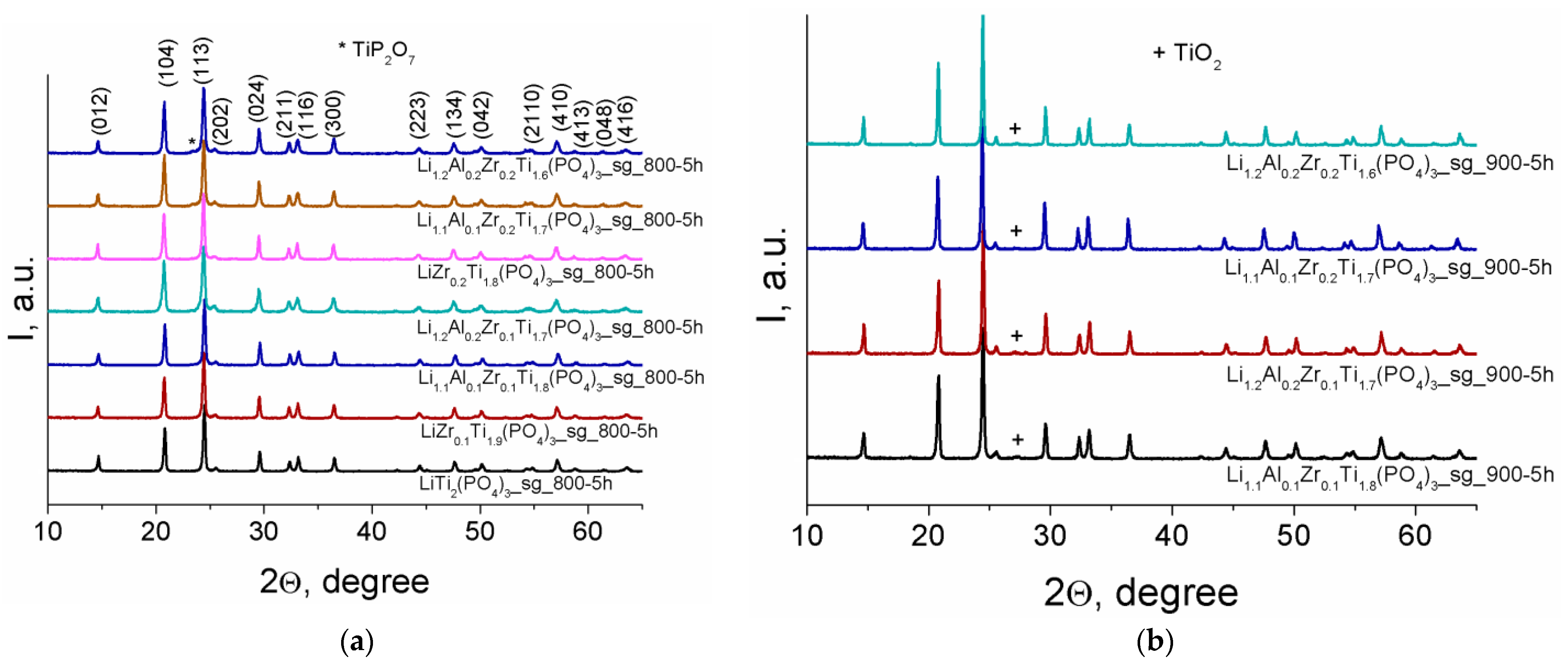
3.1. X-ray Diffraction Analysis
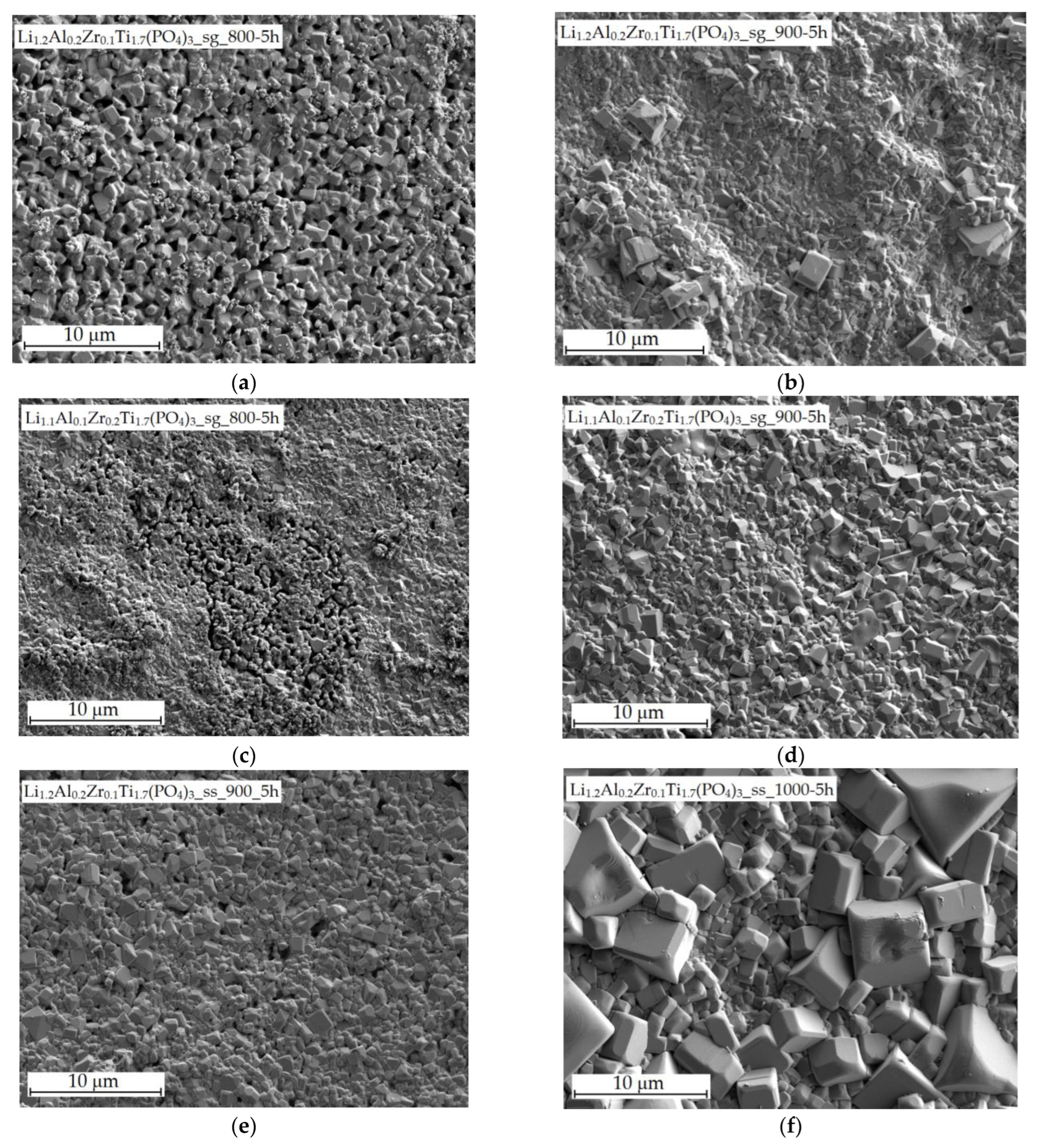
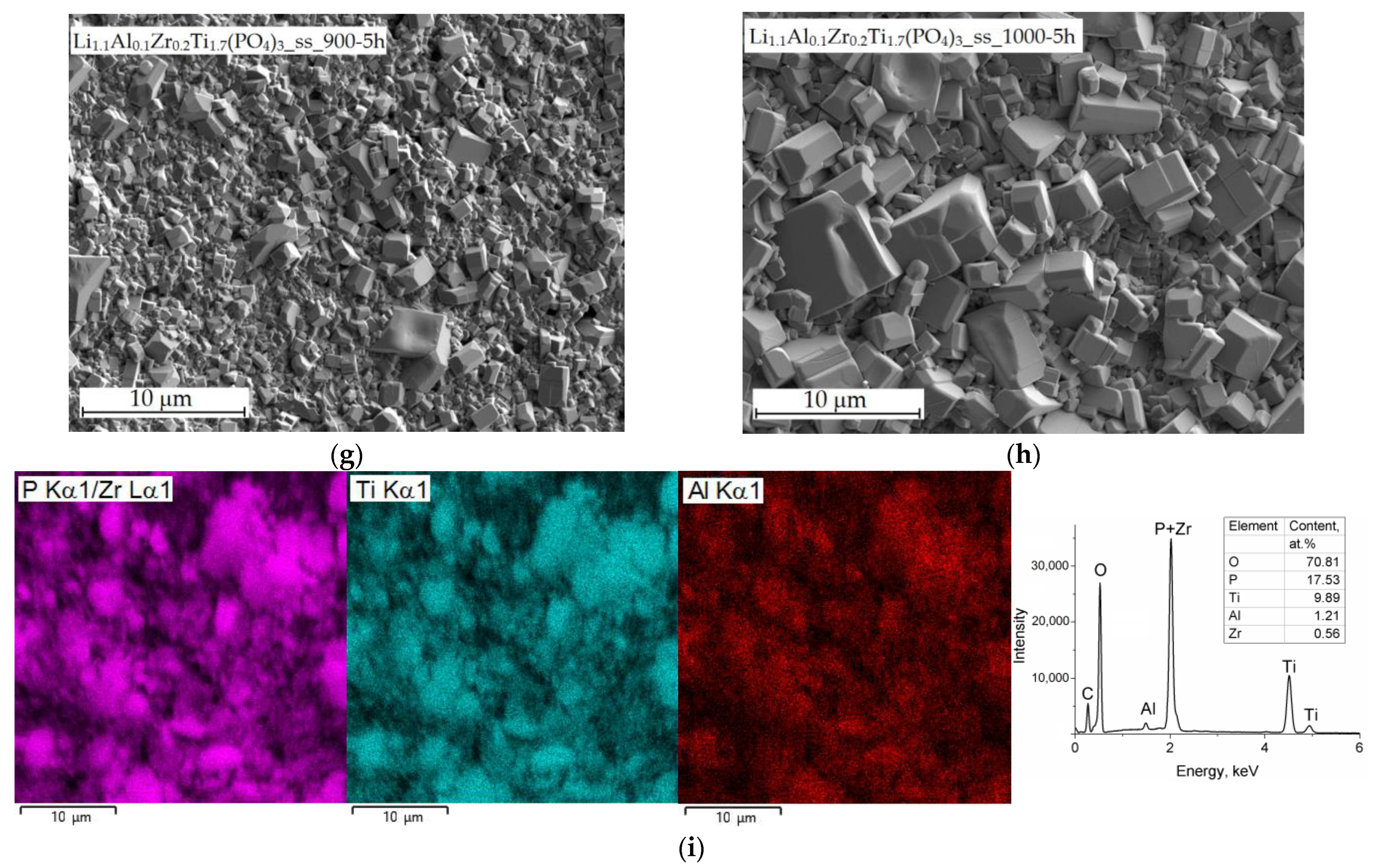
3.2. SEM
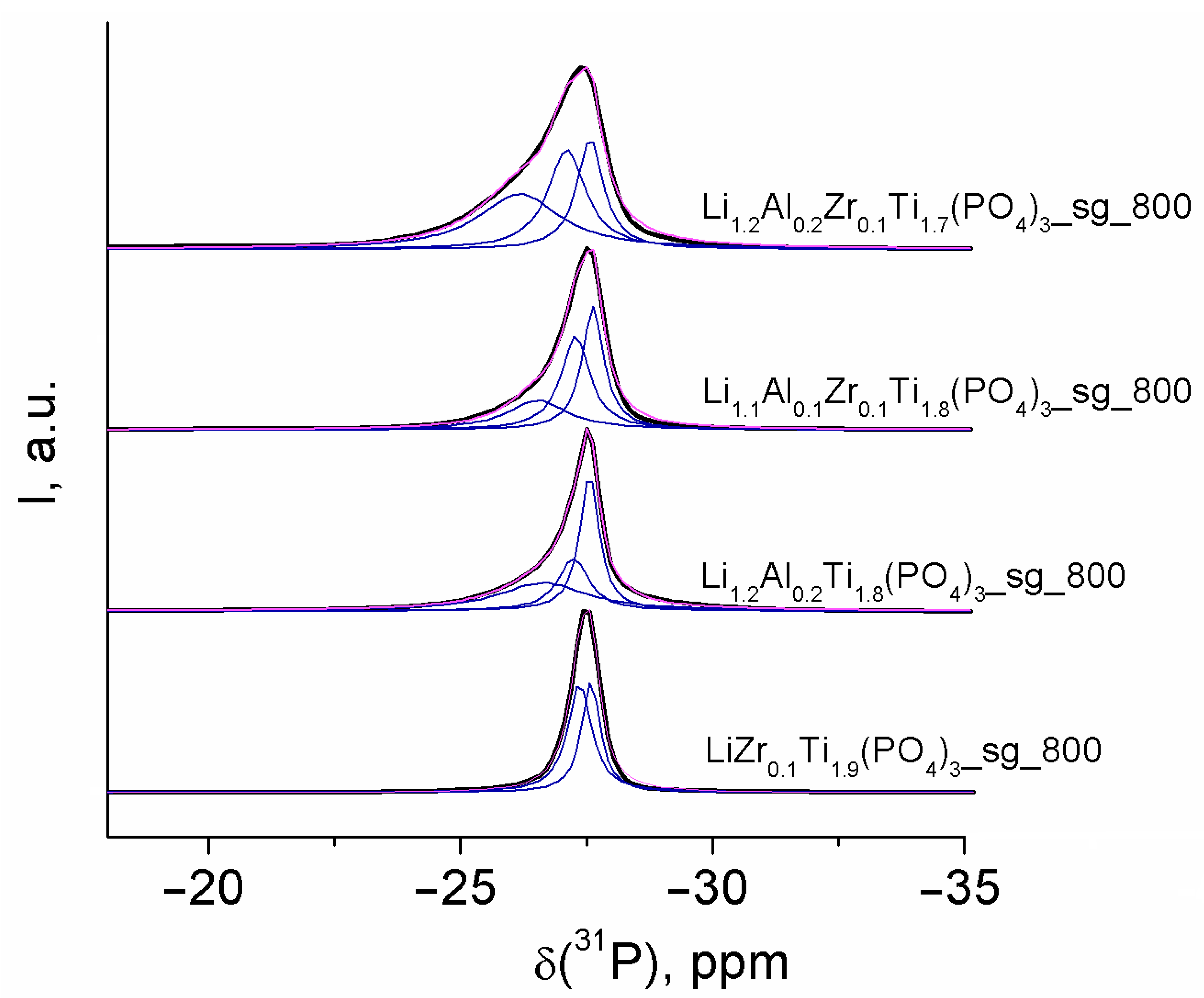
3.3. NMR Spectroscopy
3.4. Ionic Conductivity
3.5. Transfer Number and Electrolyte Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurasz, J.; Canales, F.A.; Kies, A.; Guezgouz, M.; Beluco, A. A review on the complementarity of renewable energy sources: Concept, metrics, application and future research directions. Solar Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- Gareiou, Z.; Drimili, E.; Zervas, E. Public acceptance of renewable energy sources. In Low Carbon Energy Technologies in Sustainable Energy Systems; Kyriakopoulos, G.L., Ed.; Academic Press: London, UK, 2021; pp. 309–327. [Google Scholar]
- Stenina, I.A.; Yaroslavtsev, A.B. Current progress in membranes for fuel cells and reverse electrodialysis. Mendeleev Commun. 2021, 31, 423–432. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane materials for energy production and storage. Pure Appl. Chem. 2020, 92, 1147–1157. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Bushkova, O.V.; Yaroslavtseva, T.V.; Dobrovolsky, Y.A. New lithium salts in electrolytes for lithium-ion batteries (Review). Russ. J. Electrochem. 2017, 53, 677–699. [Google Scholar] [CrossRef]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Lu, J. Solid electrolytes and interfaces in all-solid-state sodium batteries: Progress and perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of Electrolytes for Solid-State Batteries: Challenges and Perspectives. Front. Mater. 2020, 7, A111–A1115. [Google Scholar] [CrossRef]
- Braga, M.H.; Subramaniyam, C.; Murchison, A.J.; Goodenough, J.B. Extraordinary Dielectric Properties at Heterojunctions of Amorphous Ferroelectrics. J. Am. Chem. Soc. 2018, 140, 6343–6352. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashic, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Wang, C.; Liang, J.; Zhao, Y.; Zheng, M.; Li, X.; Sun, X. All-solid-state lithium batteries enabled by sulfide electrolytes: From fundamental research to practical engineering design. Energy Environ. Sci. 2021, 14, 2577–2619. [Google Scholar] [CrossRef]
- Sastre, J.; Priebe, A.; Döbeli, M.; Michler, J.; Tiwari, A.N.; Romanyuk, Y.E. Lithium Garnet Li7La3Zr2O12 Electrolyte for All-Solid-State Batteries: Closing the Gap between Bulk and Thin Film Li-Ion Conductivities. Adv. Mater. Interfaces 2020, 7, 2000425. [Google Scholar] [CrossRef]
- Huang, J.; Liang, F.; Hou, M.; Zhang, Y.; Chen, K.; Xue, D. Garnet-type solid-state electrolytes and interfaces in all-solid-state lithium batteries: Progress and perspective. Appl. Mater. Today 2020, 20, 100750. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, Q.; Niu, Z.; Chen, J. Electrolyte and Interface Engineering for Solid-State Sodium Batteries. Joule 2018, 2, 1747–1770. [Google Scholar] [CrossRef]
- Vizgalov, V.A.; Yashina, L.V.; Itkis, D.M.; Nestler, T.; Vyalikh, A.; Bobrikov, I.A.; Ivankov, O.I.; Petrenko, V.; Avdeev, M.V. The role of glass crystallization processes in preparation of high Li-conductive NASICON-type ceramics. CrystEngComm 2019, 21, 3106–3115. [Google Scholar] [CrossRef]
- Rossbach, A.; Tietz, F.; Grieshammer, S. Structural and transport properties of lithium-conducting NASICON materials. J. Power Sources 2018, 391, 1–9. [Google Scholar] [CrossRef]
- Dias, J.A.; Santagneli, S.H.; Messaddeq, Y. Methods for Lithium Ion NASICON Preparation: From Solid-State Synthesis to Highly Conductive Glass-Ceramics. J. Phys. Chem. C 2020, 124, 26518. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, E.; Yousaf, M.; Ma, L.; Wang, J.; Wang, F.; Lund, P. NASICON-Type Lithium-Ion Conductor Materials with High Proton Conductivity Enabled by Lithium Vacancies. Energy Fuels 2022, 36, 15154–15164. [Google Scholar] [CrossRef]
- Tolganbek, N.; Serikkazyyeva, A.; Kalybekkyzy, S.; Sarsembina, M.; Kanamura, K.; Bakenov, Z.; Mentbayeva, A. Interface modification of NASICON-type Li-ion conducting ceramic electrolytes: A critical evaluation. Mater. Adv. 2022, 3, 3055–3069. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Wilson, H.; Zhao, F.; Manthiram, A. Y-Doped NASICON-Type LiZr2(PO4)3 Solid Electrolytes for Lithium-Metal Batteries. Chem. Mater. 2017, 29, 7206–7212. [Google Scholar] [CrossRef]
- Kahlaoui, R.; Arbi, K.; Jimenez, R.; Sobrados, I.; Sanz, J.; Ternane, R. Distribution and mobility of lithium in NASICON-type Li1-xTi2-xNbx(PO4)3 (0 ≤ x ≤ 0.5) compounds. Mater. Res. Bull. 2018, 101, 146–154. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K. Inter-grain Li+ conduction in Sc and Y doped LATP compounds. Phys. B: Condens. Matter. 2022, 627, 413599. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; et al. Electrolytes for Li-ion transport—Review. Solid State Ionics 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Si, Q.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. High lithium ion conductivity solid electrolyte of chromium and aluminum co-doped NASICON-type LiTi2(PO4)3. Solid State Ionics 2015, 272, 101–106. [Google Scholar] [CrossRef]
- Yi, E.-J.; Yoon, K.-Y.; Jung, H.-A.; Nakayama, T.; Ji, M.-J.; Hwang, H. Fabrication and electrochemical properties of Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes by sol-gel method. Appl. Surf. Sci. 2019, 473, 622–626. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L.; Zhang, J.; Li, X. Recent advances in Li1+xAlxTi2−x(PO4)3 solid-state electrolyte for safe lithium batteries. Energy Stor. Mater. 2019, 19, 379–400. [Google Scholar] [CrossRef]
- Meesala, Y.; Jena, A.; Chang, H.; Liu, R.-S. Recent Advancements in Li-Ion Conductors for All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2017, 2, 2734–2751. [Google Scholar] [CrossRef]
- Arbi, K.; Bucheli, W.; Jiménez, R.; Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li1+xAlxM2−x(PO4)3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Das, A.; Sahu, S.; Mohapatra, M.; Verma, S.; Bhattacharyya, A.J.; Basu, S. Lithium-ion conductive glass-ceramic electrolytes enable safe and practical Li batteries. Mater. Today Energy 2022, 29, 101118. [Google Scholar] [CrossRef]
- Arbi, K.; Paris, M.A.; Sanz, J. Lithium exchange processes in the conduction network of the Nasicon LiTi2−xZrx(PO4)3 series (0 ≤ x ≤ 2). J. Phys. Chem. B 2006, 110, 6454–6457. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Veeraiah, V.; Prasada Rao, A.V.; Kishore Babu, B.; Vijaya Kumar, K. Influence of Zr4+ doping on structural, spectroscopic and conductivity studies of lithium titanium phosphate. Ceram. Int. 2014, 40, 13911–13916. [Google Scholar] [CrossRef]
- Borik, M.A.; Bredikhin, S.I.; Bublik, V.T.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, P.O.; Myzina, V.A.; Osiko, V.V.; Ryabochkina, P.A.; et al. Structure and conductivity of yttria and scandia-doped zirconia crystals grown by skull melting. J. Am. Ceram. Soc. 2017, 100, 5536–5547. [Google Scholar] [CrossRef]
- Xing, G.; Zhu, H.; Zhuang, A.; Meng, F.; Jiang, R.; Chen, S.; Chen, G.; Tang, Y. Doped superior garnet electrolyte toward all-solid-state Li metal batteries. Physics Open 2022, 13, 100119. [Google Scholar] [CrossRef]
- Kyono, N.; Bai, F.; Nemori, H.; Minami, H.; Mori, D.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Lithium-ion conducting solid electrolytes of Li1.4Al0.4Ge0.2Ti1.4(PO4)3 and MOx (M = Al, Ti, and Zr) composites. Solid State Ionics 2018, 324, 114–127. [Google Scholar] [CrossRef]
- Saffirio, S.; Falco, M.; Appetecchi, G.B.; Smeacetto, F.; Gerbaldi, C. Li1.4Al0.4Ge0.4Ti1.4(PO4)3 promising NASICON-structured glass-ceramic electrolyte for all-solid-state Li-based batteries: Unravelling the effect of diboron trioxide. J. Eur. Ceram. Soc. 2022, 42, 1023–1032. [Google Scholar] [CrossRef]
- Šalkus, T.; Dindune, A.; Kanepe, Z.; Ronis, J.; Určinskas, A.; Kežionis, A.; Orliukas, A.F. Lithium ion conductors in the system Li1+yGe2−x−yTixAly(PO4)3 (x = 0.1÷0.3, y = 0.07÷0.21). Solid State Ionics 2007, 178, 1282–1287. [Google Scholar] [CrossRef]
- Zhang, P.; Matsui, M.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Water-stable lithium ion conducting solid electrolyte of the Li1.4Al0.4Ti1.6−xGex(PO4)3 system (x = 0–1.0) with NASICON-type structure. Solid State Ionics 2013, 253, 175–180. [Google Scholar] [CrossRef]
- Maldonado-Manso, P.; Losilla, E.R.; Martinez-Lara, M.; Aranda, M.A.G.; Bruque, S.; Mouahid, F.E.; Zahir, M. High Lithium ionic conductivity in the Li1+xAlxGeyTi2-x-y(PO4)3 NASICON series. Chem. Mater. 2003, 15, 1879–1885. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K. Study of Study of electrical properties of gallium-doped lithium titanium aluminum phosphate compounds. Ionics 2015, 21, 1253–1259. [Google Scholar] [CrossRef]
- Shang, X.; Nemori, H.; Mitsuoka, S.; Mastuda, Y.; Takeda, Y.; Yamamoto, O.; Imanishi, N. High lithium-ion conducting NASICON-type Li1+x−yAlxNbyTi2–x−y(PO4)3 solid electrolytes. Solid State Ionics 2016, 297, 43–48. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Zhao, B.; Zhang, L.; Xiao, X.; Yan, H.; Xu, G.; Ma, L.; Liu, Y. Transport and interface characteristics of Te-doped NASICON solid electrolyte Li1.3Al0.3Ti1.7(PO4)3. Electrochim. Acta 2021, 399, 139367. [Google Scholar] [CrossRef]
- Rai, K.; Kundu, S. Fabrication and performances of high lithium-ion conducting solid electrolytes based on NASICON Li1.3Al0.3Ti1.7-xZrx(PO4)3 (0 ≤ x ≤ 0.2). Ceram. Int. 2020, 46, 23695–23705. [Google Scholar] [CrossRef]
- Rettenwander, D.; Welzl, A.; Pristat, S.; Tietz, F.; Taibl, S.; Redhammer, G.J.; Fleig, J. A microcontact impedance study on NASICON-type Li1+xAlxTi2-x(PO4)3, 0 ≤ x≤ 0.5 single crystals. J. Mater. Chem. 2016, 4, 1506–1513. [Google Scholar] [CrossRef]
- Yan, G.; Yu, S.; Nonemacher, J.F.; Tempel, H.; Kungl, H.; Malzbender, J.; Eichel, R.-A.; Krüger, M. Influence of sintering temperature on conductivity and mechanical behavior of the solid electrolyte LATP. Ceram. Int. 2019, 45, 14697–14703. [Google Scholar] [CrossRef]
- West, A.R. Basic Solid State Chemistry, Rev. ed.; John Wiley & Sons Ltd.: Chichester, UK, 1988; pp. 216–221. [Google Scholar]
- Stenina, I.A.; Pinus, I.Y.; Rebrov, A.I.; Yaroslavtsev, A.B. Lithium and hydrogen ions transport in materials with NASICON structure. Solid State Ionics 2004, 175, 445–449. [Google Scholar] [CrossRef]
- Stenina, I.A.; Velikodnyi, Y.A.; Ketsko, V.A.; Yaroslavtsev, A.B. Synthesis of NASICON-Type Lithium Zirconium Phosphate. Inorg. Mater. 2004, 40, 967–970. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Kwatek, K.; Ślubowska, W.; Trébosc, J.; Lafon, O.; Nowiński, J.L. Structural and electrical properties of ceramic Li-ion conductors based on Li1.3Al0.3Ti1.7(PO4)3-LiF. J. Eur. Ceram. Soc. 2020, 40, 85–93. [Google Scholar] [CrossRef]
- Arbi, K.; Hoelzel, M.; Kuhn, A.; García-Alvaradoc, F.; Sanza, J. Local structure and lithium mobility in intercalated Li3AlxTi2−x(PO4)3 NASICON type materials: A combined neutron diffraction and NMR study. Phys. Chem. Chem. Phys. 2014, 16, 18397–18405. [Google Scholar] [CrossRef]
- Diez-Gómez, V.; Arbi, K.; Sanz, J. Modeling Ti/Ge Distribution in LiTi2-xGex(PO4)3 NASICON Series by 31P MAS NMR and First-Principles DFT Calculations. J. Am. Chem. Soc. 2016, 138, 9479–9486. [Google Scholar] [CrossRef]
- Vinod Chandran, C.; Pristat, S.; Witt, E.; Tietz, F.; Heitjans, P. Solid-State NMR Investigations on the Structure and Dynamics of the Ionic Conductor Li1+xAlxTi2-x(PO4)3 (0 ≤ x ≤ 1.0). J. Phys. Chem. C. 2016, 120, 8436–8442. [Google Scholar] [CrossRef]
- Lavrova, G.V.; Shutova, E.S.; Ponomareva, V.G.; Dunyushkina, L.A. Proton conductivity and interphase interaction in CsH2PO4 –SrZrO3 composites. Russ. J. Electrochem. 2013, 49, 718–724. [Google Scholar] [CrossRef]
- Uvarov, N.F. Ionics of nanoheterogeneous materials. Russ. Chem. Rev. 2007, 76, 415–433. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Ion transport in heterogeneous solid systems. Russ. J. Inorg. Chem. 2000, 45, 249–267. [Google Scholar]
- Maier, J. Pushing Nanoionics to the Limits: Charge Carrier Chemistry in Extremely Small Systems. Chem. Mater. 2014, 26, 348–360. [Google Scholar] [CrossRef]
- Han, F.; Zhu, Y.; He, X.; Mo, Y.; Wang, C. Electrochemical Stability of Li10GeP2S12 and Li7La3Zr2O12 Solid Electrolytes. Adv. Energy Mater. 2016, 6, 1501590. [Google Scholar] [CrossRef]

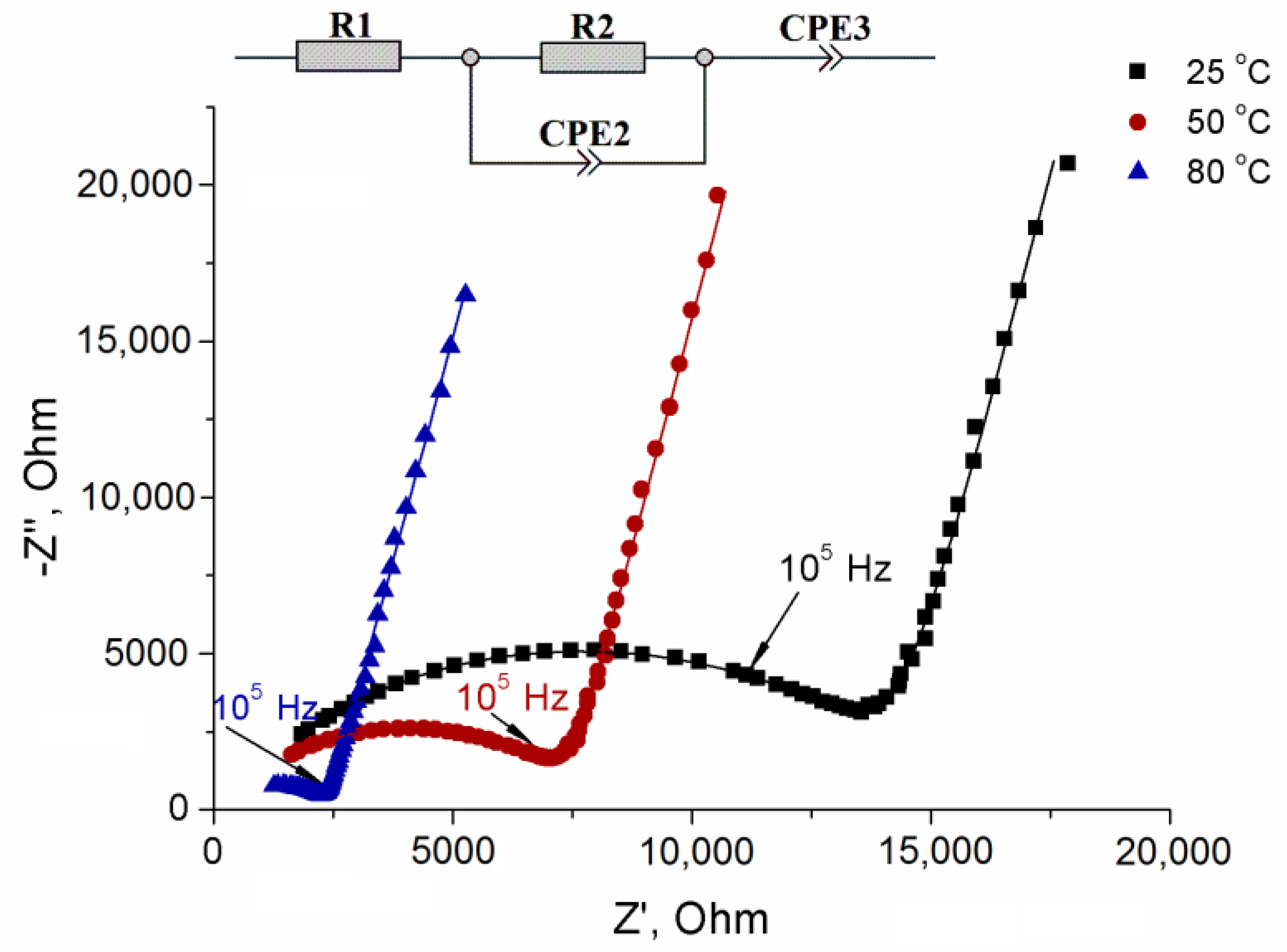
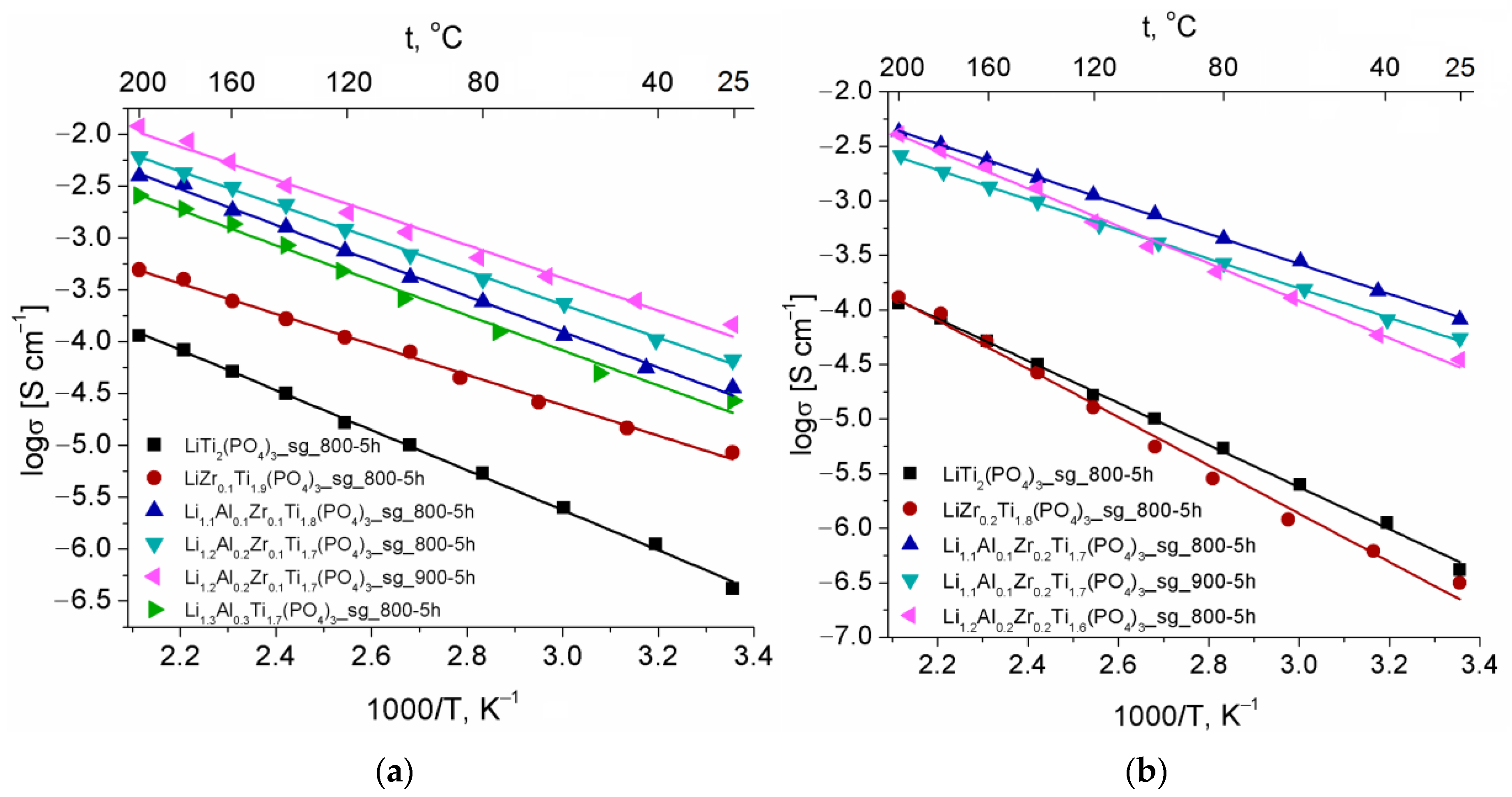
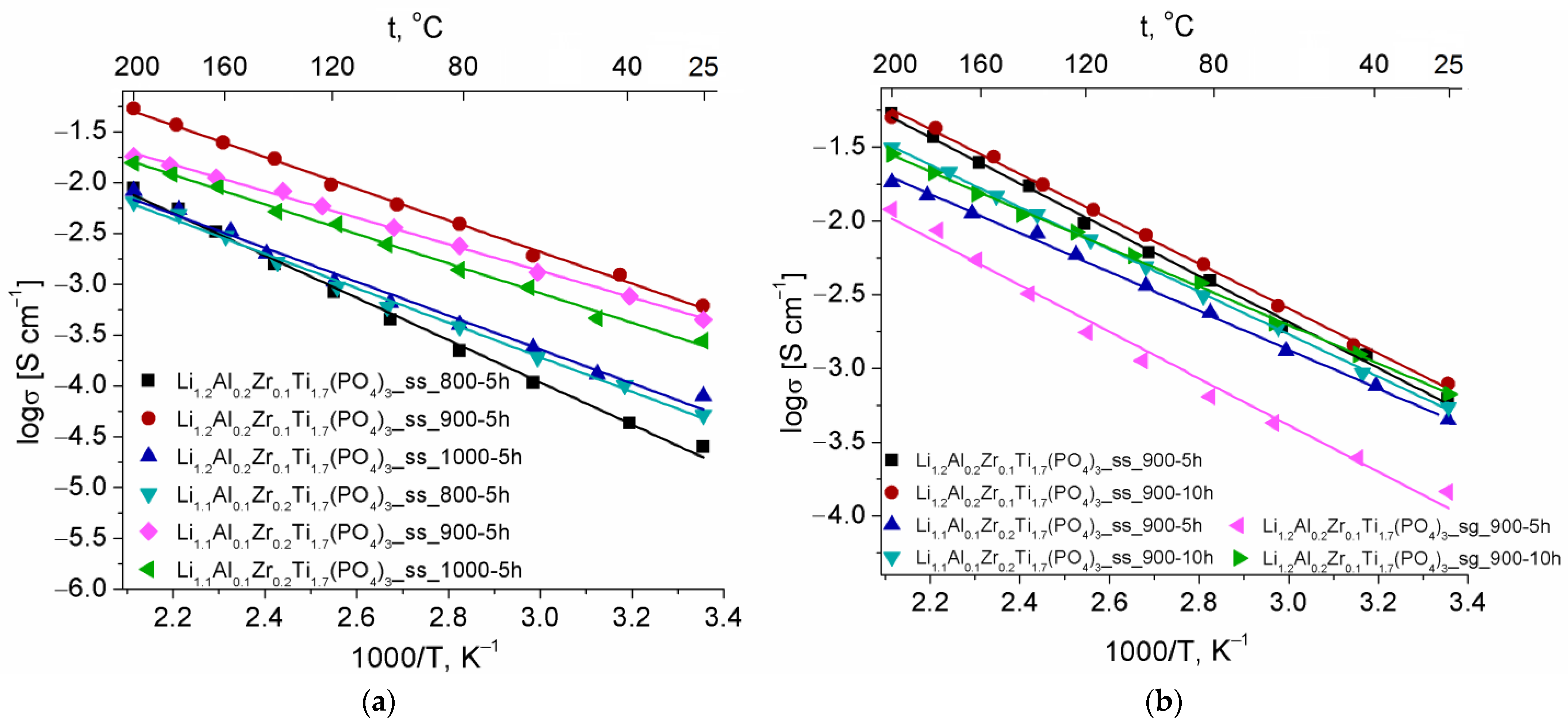
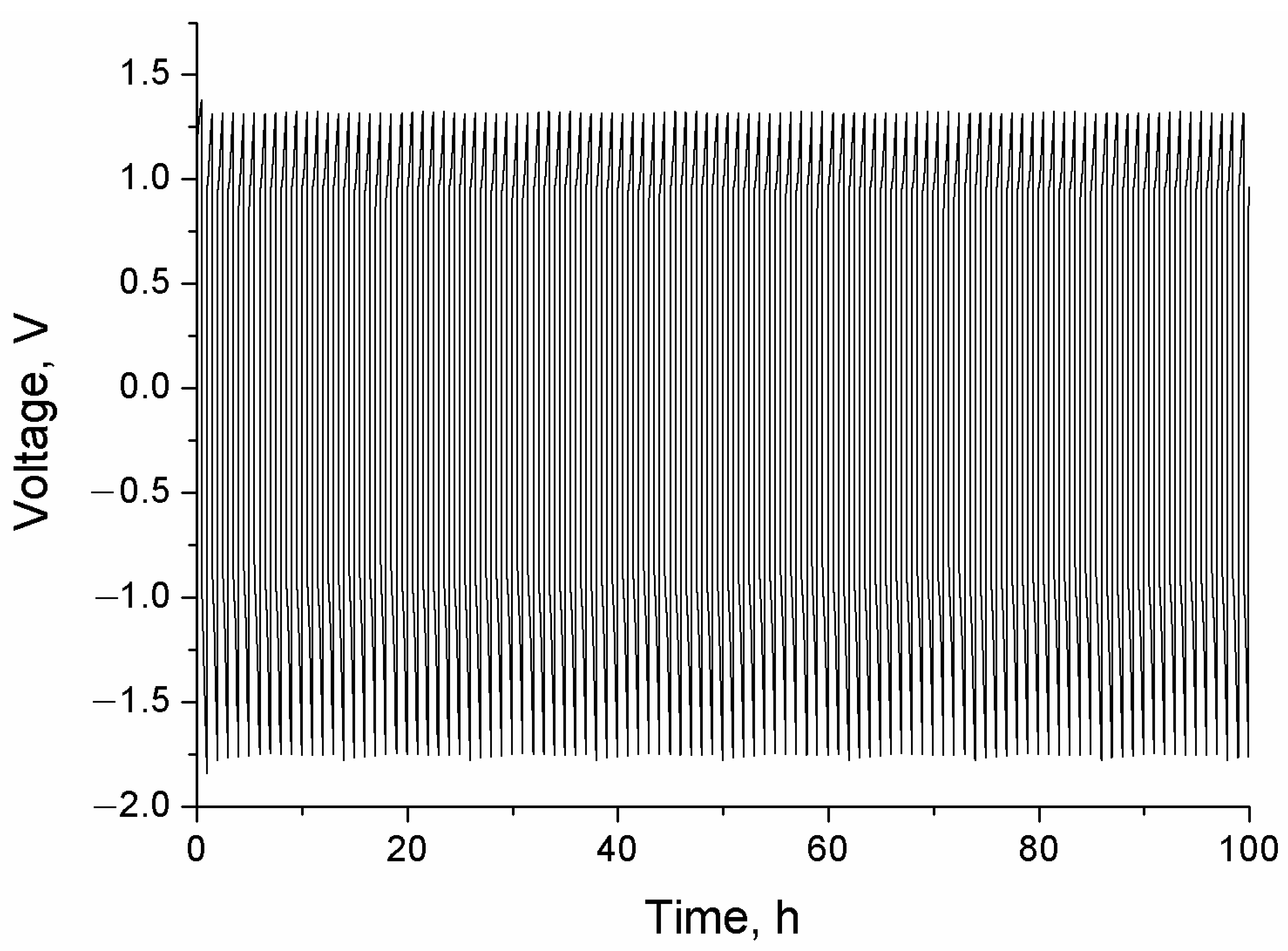
| Composition | a 1, Å | c, Å |
|---|---|---|
| LiTi2(PO4)3 | 8.5129 ± 0.0008 | 20.878± 0.004 |
| LiZr0.1Ti1.9(PO4)3 | 8.5238 ± 0.0003 | 20.982 ± 0.002 |
| Li1.1Zr0.1Al0.1Ti1.8(PO4)3 | 8.5109 ± 0.0006 | 20.956 ± 0.003 |
| Li1.2Zr0.1Al0.2Ti1.7(PO4)3 | 8.5158 ± 0.0005 | 20.942 ± 0.002 |
| LiZr0.2Ti1.8(PO4)3 | 8.5727 ± 0.0006 | 21.036 ± 0.002 |
| Li1.1Zr0.2Al0.1Ti1.7(PO4)3 | 8.5355 ± 0.0005 | 21.000 ± 0.003 |
| Li1.2Zr0.2Al0.2Ti1.6(PO4)3 | 8.5203 ± 0.0006 | 20.917 ± 0.002 |
| Sample | δ(31P), ppm | Assignment |
|---|---|---|
| LiZr0.1Ti1.9(PO4)3_sg_800 | −27.58 | P(O4Ti4) |
| −27.36 | P(O4Ti3Zr) | |
| Li1.2Al0.2Ti1.8(PO4)3_sg_800 | −27.56 | P(O4Ti4) |
| −27.24 | P(O4Ti3Al) | |
| −26.65 | P(O4Ti2Al2) | |
| Li1.1Al0.1Zr0.1Ti1.7(PO4)3_sg_800 | −27.59 | P(O4Ti4) |
| −27.28 | P(O4Ti3Zr) + P(O4Ti3Al) | |
| −26.52 | P(O4Ti2Al2) + P(O4Ti2ZrAl) | |
| Li1.2Al0.2Zr0.1Ti1.7(PO4)3_sg_800 | −27.57 | P(O4Ti4) |
| −27.11 | P(O4Ti3Zr) + P(O4Ti3Al) | |
| −26.17 | P(O4Ti2Al2) + P(O4Ti2ZrAl) |
| Sol-Gel Route | Solid-State Reaction | ||||
|---|---|---|---|---|---|
| Sample | σ25 °C, S cm−1 | Ea ± 1, kJ mol−1 | Sample | σ25 °C, S cm−1 | Ea ± 1, kJ mol−1 |
| LiTi2(PO4)3_800-5h | 4.1 × 10−7 | 37 | Li1.2Al0.2Zr0.1Ti1.7(PO4)3_800-5h | 2.5 × 10−5 | 41 |
| LiZr0.1Ti1.9(PO4)3_800-5h | 4.9 × 10−6 | 28 | Li1.2Al0.2Zr0.1Ti1.7(PO4)3_900-5h | 6.2 × 10−4 | 30 |
| Li1.1Al0.1Zr0.1Ti1.8(PO4)3_800-5h | 3.7 × 10−5 | 32 | Li1.2Al0.2Zr0.1Ti1.7(PO4)3_900-10h | 7.9 × 10−4 | 30 |
| Li1.2Al0.2Zr0.1Ti1.7(PO4)3_800-5h | 7.2 × 10−5 | 30 | Li1.2Al0.2Zr0.1Ti1.7(PO4)3_1000-5h | 7.8 × 10−5 | 33 |
| Li1.2Al0.2Zr0.1Ti1.7(PO4)3_900-5h | 1.5 × 10−4 | 31 | Li1.1Al0.1Zr0.2Ti1.7(PO4)3_800-5h | 5.1 × 10−5 | 32 |
| Li1.2Al0.2Zr0.1Ti1.7(PO4)3_900-10h 1 | 6.8 × 10−4 | 25 | Li1.1Al0.1Zr0.2Ti1.7(PO4)3_900-5h | 4.5 × 10−4 | 26 |
| LiZr0.2Ti1.8(PO4)3_800-5h | 3.2 × 10−7 | 42 | Li1.1Al0.1Zr0.2Ti1.7(PO4)3_900-10h | 5.4 × 10−4 | 28 |
| Li1.1Al0.1Zr0.2Ti1.7(PO4)3_800-5h | 6.7 × 10−5 | 26 | Li1.1Al0.1Zr0.2Ti1.7(PO4)3_1000-5h | 2.8 × 10−4 | 28 |
| Li1.1Al0.1Zr0.2Ti1.7(PO4)3_900-5h | 5.5 × 10−5 | 26 | |||
| Li1.2Al0.2Zr0.2Ti1.6(PO4)3_800-5h | 3.5 × 10−5 | 33 | |||
| Li1.3Al0.3Ti1.7(PO4)3_800-5h | 8.0 × 10−6 | 36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenina, I.; Pyrkova, A.; Yaroslavtsev, A. NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries 2023, 9, 59. https://doi.org/10.3390/batteries9010059
Stenina I, Pyrkova A, Yaroslavtsev A. NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries. 2023; 9(1):59. https://doi.org/10.3390/batteries9010059
Chicago/Turabian StyleStenina, Irina, Anastasia Pyrkova, and Andrey Yaroslavtsev. 2023. "NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method" Batteries 9, no. 1: 59. https://doi.org/10.3390/batteries9010059
APA StyleStenina, I., Pyrkova, A., & Yaroslavtsev, A. (2023). NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries, 9(1), 59. https://doi.org/10.3390/batteries9010059







