Abstract
Due to their unique spatial structures, layered double hydroxides (LDHs) have been considered as prospective electrode materials for supercapacitors. In this work, several NiCo-LDH materials are obtained via a facile selenization process. This can improve the conductivity and reduce the electrochemical impedance of the samples. The 0.4Se-NiCo-LDH materials deliver a specific capacitance of 1396 F/g at 1 A/g. The capacity retention rate can reach 91.38% after 10,000 cycles. In addition, using the prepared materials as a positive electrode, an asymmetric supercapacitor is constructed. It offers an energy density of 60 Wh/kg at a power density of 2700 W/kg, demonstrating that the synthesized samples possess promising applications in future flexible energy-storage systems.
1. Introduction
Due to the increasing energy crisis and natural disasters in recent years, people are committed to designing and developing some emerging energy conversion and storage systems [1,2,3]. The quick development of science and technology has led to a vast demand for energy-storage devices [4,5,6]. Among them, the supercapacitor has been a research focus as a kind of efficient and green energy storage equipment [7,8,9]. One of the most critical elements of a supercapacitor is its active electrode material, which undoubtedly determines its performances, such as its specific capacitance and the structural stability [10,11,12]. Transition metal layered hydroxide has also been widely investigated because of its outstanding electrochemical performance [13,14,15,16,17].
NiCo-LDH is an excellent electrode material due to its distinctive layered structure and the interaction of the Ni and Co elements [18,19]. NiCo-LDH material can provide many active sites and reaction spaces. Simultaneously, the synergistic effect between Ni and Co ions can effectively improve the electrochemical performance. All these characteristics make NiCo-LDH products suitable electrode materials for supercapacitors. For example, Li et al., prepared a silver-plated NiCo-LDH material with a capacitance of 1338 F/g at 10 A/g [20]. In addition, a V-doped NiCo-LDH electrode was also synthesized by Hong’s group, delivering a specific capacitance of 2960 F/g at 1 A/g [21]. However, its poor structural stability resulted in fading cyclability during successive cycling, and its low electrical conductivity also limits its wide application for supercapacitors. To address these issues, we turn our attention to some emerging selenides materials. The characteristic 4s24p4 electronic structure of Se makes it easy to combine with transition metal atoms to form covalent bonds [22]. Because of this feature, transition metal selenides possess many metallic properties, so they are conducive to the electrochemical reaction. In this regard, the NiCo2Se4/MXene composite electrode achieved a capacitance of 953.8 F/g at 1 A/g [23]. Yang et al., prepared a (Ni, Co)Se2 nanorod array created on NiCo-LDH, which is generated from conical ZIF-L. The specific capacity of the material is 188.8 mAh/g when the current density is 1 A/g [24]. These outstanding electrochemical characteristics result from the synergistic interaction between selenium and transition metals. Consequently, surface selenization of transition metal hydroxide will theoretically obtain outstanding electrochemical performance.
Herein, we prepared 0.4Se-NiCo-LDH sample on a nickel foam substrate using a hydrothermal method. The prepared sample delivers a specific capacitance of 1396 F/g at 1 A/g and can still maintain 91.38% of its initial specific capacitance at 10,000 cycles. Then, a hybrid capacitor is constructed with the composite samples as the cathode and activated carbon as the anode, respectively. The device can keep 84.45% of its original capacity after 8000 tests. In addition, when the power density of the equipment is 2700 W/kg, the energy density can reach 60 Wh/Kg.
2. Materials and Methods
In this work, all purchased chemicals were used directly. In the pretreatment stage of the experiment, a piece of nickel foam (NF) was washed with 0.5 mol/L HCl solution and deionized water under sonication, respectively. First, the NiCo-LDH nanosheets were prepared using a hydrothermal method [19]. Then, 5 g NaOH and 0.4 g Se were dissolved into 60 mL deionized water and stirred for 30 min. After that, the solution was heated to 180 °C and stored for 12 h. Subsequently, the prepared NiCo-LDH precursor was transferred to the above solution, heated to 140 °C, and stored for 4 h. The sample was named 0.4Se-NiCo-LDH, and it had an average loading mass of 1.5 mg cm−2. For comparison, we also prepared two samples with 0.2 g and 0.6 g Se raw material. Their average loading masses are 1.2 and 1.7 mg cm−2; the as-prepared products were named as 0.2Se-NiCo-LDH and 0.6Se-NiCo-LDH, respectively.
3. Results and Discussion
XRD was first used to investigate the crystal structure and phase purity of the samples. From Figure 1a, the well-defined diffraction peaks are associated with the (003), (006), (012), (015), and (110) planes of the hydrotalcite-like phas at 11.21°, 22.44°, 34.25°, and 39.10°, respectively (PDF No. 40-0216). Meanwhile, the 0.4Se-NiCo-LDH peaks are located at 28.28° (100), 32.9° (101), 50.07° (110), 58.1° (103), 63.29° (201), and 72.24° (202). They can be assigned to NiCoSe2 phases (PDF No. 70-2851) [25].
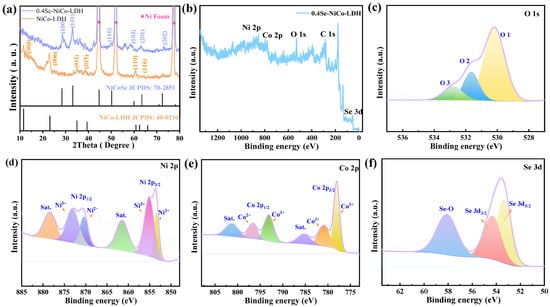
Figure 1.
Structural characterization (a) XRD patterns of the as-prepared samples, (b) the XPS full spectra of 0.4Se-NiCo-LDH sample, (c) O1s, (d) Ni 2p, (e) Co 2p, (f) Se 3d.
We further tested the element composition and valence information of the sample using XPS. The full spectrum indicates the existence of Ni, Co, Se, and O elements in the 0.4Se-NiCo-LDH sample (as clearly seen in Figure 1b). As illustrated in Figure 1c, the O 1s spectra consist of three primary peaks. O1 presents the metal–oxygen bond, which is located at 530.2 eV. O2 delegates the oxygen ion and O3 is the physicochemical water on the surface of the active material, which is at 531.7 eV and 532.9 eV, respectively [26]. In Figure 1d, the binding energies of Ni3+ are located at the fitting peaks 855.3 and 873.1 eV, while the other two peaks at 853.5 and 870.5 eV belong to Ni2+. Additionally, the two satellite peaks in the Ni 2p spectrum can be found at 861.4 and 878.5 eV, respectively [27]. Two spin-orbit double peaks (Co 2p3/2 and Co 2p1/2) and two satellites make up the Co 2p XPS spectra (Figure 1e) due to the co-existence of Co2+ and Co3+. The fitted peaks at 781.1 and 796.8 eV are Co2+; the two peaks at 778.1 and 793.2 eV can be indexed to Co3+ [28]. The characteristic peaks at 53.3 and 54.4 eV are typical characteristics of metal–selenium, and the characteristic peaks at 58.1 eV in Se 3d spectrum may come from Se-O [29,30].
The scanning electron microscope (SEM) was used to study the morphology of the samples. NiCo-LDH samples are depicted in Figure 2a, where the surface of Ni foam is covered by uniformly overlapping nanosheets, which indicates that the samples show good homogeneity. High-magnification SEM images demonstrate that the nanosheets possess an average thickness of 40 nm (Figure 2e). As seen in Figure 2b,f, the smooth nanosheets become rough after Se doping, and a layer of ‘small balls’ covers the surface [31]. The porous surface can promote electrochemical kinetics and provide many electrode/electrolyte contact surfaces.

Figure 2.
Morphology characterization. (a–d) Low-magnification SEM images; (e–h) high-magnification SEM images.
These ‘small balls’ gradually appear with the increase in Se content, and the surface of Ni foam forms a 3D honeycomb structure based on the cross-linked nanosheet array (Figure 2c,g). Notably, this interconnected honeycomb structure possesses high porosity and can provide many internal spaces as active sites. Nevertheless, when the Se content continuously increases, the small balls on the 0.6Se-NiCo-LDH sample surface gradually connect (Figure 2d,h), resulting in the small pore diameter of the 3D honeycomb structure. The material surface shows a serious agglomeration phenomenon.
Figure 3a depicts the CV curves of the samples. It can be found that the 0.4Se-NiCo-LDH sample presents high current density and a large curve integral area. This means that surface selenization can lead to an improvement in the electrochemical activity of the NiCo-LDH electrode. Moreover, the CV curves possess symmetric redox peaks, which reflect a typical pseudocapacitive characteristic different from electrochemical double-layer capacitance [31,32]. This can also be verified in the GCD curves of the electrode materials at 1 A/g (Figure 3b). A pair of charge–discharge voltage platforms are clearly observed in each GCD curve. The platforms are derived from the redox reaction between the active substance in the electrode and OH− between the electrode and the electrolyte/near surface. At the current density of 1 A/g, the 0.4Se-NiCo-LDH electrode shows an obvious charging platform at the potential window of ~0.35 V. The relevant redox reactions can be described by the following equation [32]
NiCoSe2 + 2H2O + O2 = Co(OH)2 + Ni(OH)2 + 2Se
3Se + 6OH− → 2Se2− + SeO32− + 3H2O
Co(OH)2 + OH− ↔ CoOOH + H2O + e−
CoOOH + OH− ↔ CoO2 + H2O + e−
Ni(OH)2 + OH− ↔ NiOOH + H2O + e−
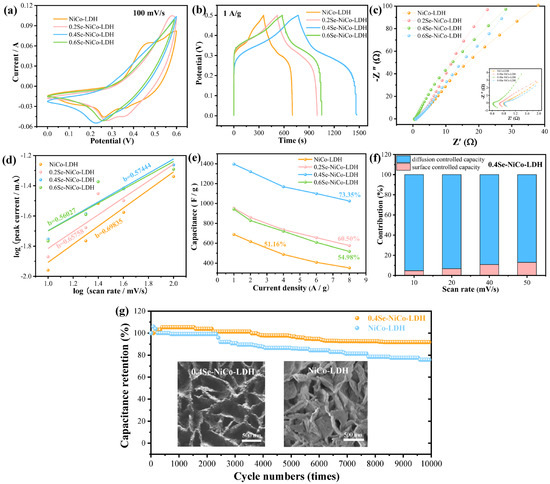
Figure 3.
Electrochemical characterizations of the electrode materials. (a) CV curves, (b) GCD curves, (c) Nyquist plots, (d) b values, (e) specific capacitance, (f) contribution ratio between capacitance and the diffusion-limited result (g) cycling performance and SEM images after cycling.
It is clear that there are the different energy-storage mechanisms for Ni and Co elements. Therefore, 0.4Se-NiCo-LDH materials have multiple potential oxidation reduction states.
Figure 3c presents the Nyquist curves of the prepared samples. The EISs of the samples show a similar shape. From the enlarged image, the semicircular intercept of the 0.4Se-NiCo-LDH sample is distinctly smaller than that of single NiCo-LDH, indicating that the introduction of the Se element can greatly reduce the charge-transfer resistance (Rct). In the low-frequency region, the slope of 0.4Se-NiCo-LDH material is significantly higher than that of other samples. This suggests that the sample possesses a fast charge-transfer rate and interface absorption/desorption rate.
To determine whether the electrode materials show pseudo capacitance behavior during charging and discharging, the following formula can be utilized for calculation [33]:
I = avb
In the formula, I refers to the peak current, v is the scan rate, and a and b are constants. Obviously, the b values of these samples are all between 0.5 and 1 (Figure 3d), which demonstrates the pseudo capacitance dominated behavior. Since both Ni and Co ions are in a low-valence state in the 0.4Se-NiCo-LDH samples, the two ions can directly contribute to the pseudo capacitance performance. Consequently, with their high specific capacity, great rate performance, and exceptional durability, 0.4Se-NiCo-LDH electrodes present optimal electrochemical performance [31].
The specific capacitance of the samples is estimated from GCD curves through Figure 3e. It is worth noting that the specific capacitance of 0.4Se-NiCo-LDH sample is 1398 F/g at 1 A/g and remains at 73.35% of the initial capacitance when the current density is elevated to 8 A/g. At the same current density, NiCo-LDH maintains 51.16% capacitive retention. The following equation provides a capacitance contribution to the system:
i = k1v + k2v1/2
By using CV curves, it is possible to determine the values of k1 and k2. According to Figure 3f, the 0.4Se-NiCo-LDH electrode’s diffusion capacitive contribution is 95.38% at 10 mV/s. It can be found that when the scan rate rises, the diffusion control contribution gradually decreases. The surface control contribution gradually replaces the diffusion control contribution as the scanning speed continues to increase. As illustrated in Figure 3g, the electrode materials maintain 91.8% capacitance retention at 10 A/g after 10,000 cycles, which is much higher than that of NiCo-LDH (75.9%). These cycling results confirm the superior capacitance stability of the 0.4Se-NiCo-LDH nanocomposite. SEM images after cycling further prove that the honeycomb structure provides a stable structure.
To evaluate the practical application of 0.4Se-NiCo-LDH samples, quasi-solid-state asymmetric devices are assembled. Figure 4a reveals that the voltage of the device can reach 1.6 V according to the CV curves. As shown in Figure 4b, the device owns the ability of quick charge and discharge; the discharge time of the device is 78 s at 1 A/g. The reaction kinetics can be investigated by the Nyquist plot (Figure 4c), and the measurement frequency range is 10−2 to 105 Hz. In the whole frequency range, the device is practically straight and does not represent a full semicircle.
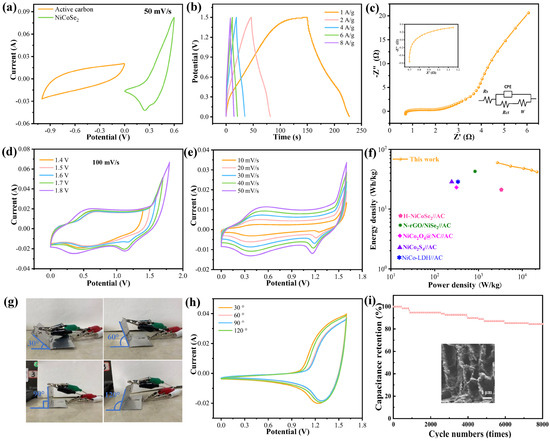
Figure 4.
Electrochemical characterization of ASC. (a) CV curves of 0.4Se-NiCo-LDH and active carbon, (b) GCD curves, (c) Nyquist plots, (d) CV curves with various voltage windows, (e) CV curves with various scan rates, (f) Ragone plots, (g) digital photographs of the folded device (h) CV curves of device at different bending angles, (i) cycling performance and SEM image after cycling.
The Warburg impedance (Zw) represents the ion diffusion. It is correlated with the slope of the line in the low-frequency zone. The asymmetric devices show low ion diffusion resistance, which is ascribed to the internal electrical characteristics and synergistic effects of surface selenization. Meanwhile, the three-dimensional porous structure allows many electrochemical active sites to be exposed, increasing the utilization of the entire electrode material [34,35]. We present the equivalent fitting circuit with a Rs of 0.76 for the device, indicating its excellent conductivity. The CV curves of the device under different voltage windows are shown in Figure 4d. It can be found that the shapes of the curves remain almost unchanged with the increase in the potential window from 1.4 to 1.8 V. The area of the curve increases with the enlarging of the scanning speed.
As can be seen from the Ragone plot, 0.4-Se-NiCo-LDH//AC can provide an energy density of 60 Wh/kg at a power density of 2700 W/kg. This result is apparently higher than that of the previously reported work (Figure 4f). Moreover, Table 1 further represents the electrochemical performances of several composite electrodes [25,36,37,38,39]. It demonstrates the large power density and energy density of our fabricated 0.4-Se-NiCo-LDH sample. The CV test is also performed at the scanning speed of 100 mV/s, as shown in Figure 4g,h. Different bending angles show no discernible effect on the shape of CV curves, revealing that the performance is almost affected by bending angles. The device presents exceptional long-cycle stability by maintaining 84.46% of the original capacitance after 8000 cycles (Figure 4i). The prepared 0.4Se-NiCo-LDH composite possesses an outstanding electrochemical performance for flexible energy storage devices.

Table 1.
Comparison of energy density and power density of 0.4Se-NiCo-LDH device with previous reports.
4. Conclusions
Overall, we have synthesized a 0.4Se-NiCo-LDH sample via a facile hydrothermal approach. The optimized 0.4Se-NiCo-LDH sample possesses high porosity and can provide many active sites during electrochemical reactions. Moreover, selenium itself shows a unique electronic structure, which provides the sample with high conductivity. Therefore, this growth route is simple and widely applicable to the design of some other hydroxide/oxide electrode materials. In our work, the unique nanostructure enables it to maintain excellent capacitance and mechanical stability after long cycling. The assembled device using the obtained samples as cathode materials demonstrates outstanding cycle stability and high energy density. The results suggest that the synthesized materials possess promising application prospects in future energy-storage devices.
Author Contributions
Conceptualization, M.W.; methodology, X.L.; software, X.L.; validation, M.W.; formal analysis, M.W.; investigation, M.W.; resources, X.W.; data curation, M.W.; writing—original draft preparation, M.W.; writing—review and editing, X.W.; visualization, X.L.; supervision, X.W.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
The work is supported by the National Natural Science Foundation of China (No. 52172218).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Liu, Y.; Wu, X.; Cho, Y.R. Enhanced electrochemical performance of Zn/VOx batteries by a carbon encapsulation strategy. ACS Appl. Mater. Interfaces 2022, 14, 11654–11662. [Google Scholar]
- Liu, Y.; Wu, X. Hydrogen and sodium ions cointercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity. Nano Energy 2021, 86, 106124. [Google Scholar] [CrossRef]
- Kamel, A.A.; Rezk, H.; Shehata, N.; Thomas, J. Energy management of a DC microgrid composed of photovoltaic/fuel cell/battery/supercapacitor systems. Batteries 2019, 5, 63. [Google Scholar] [CrossRef]
- Wang, M.D.; Liu, X.Y.; Sun, Y.C.; Wu, X. High-efficient NiCo layered double hydroxide electrocatalyst. New J. Chem. 2022, 46, 18535–18542. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.X.; Shi, X.Y.; Zhu, Y.Y.; Wu, Z.S. Recent status and future perspectives of ultracompact and customizable micro-supercapacitors. Nano Res. Energy 2022, 1, e9120018. [Google Scholar] [CrossRef]
- Rastabi, S.A.; Mamoory, R.S.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of a NiMoO4/3D-rGO nanocomposite via starch medium precipitation method for supercapacitor performance. Batteries 2020, 6, 5. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, M.D.; Wu, X. Tailoring Electrochemical Performance of Co3O4 Electrode Materials by Mn Doping. Molecules 2022, 27, 7344. [Google Scholar] [CrossRef]
- Gu, J.W.; Peng, Y.; Zhou, T.; Ma, J.; Pang, H.; Yamauchi, Y.S. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, e9120009. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.F.; Liu, H.Q.; Song, J.R.; Umar, A.; Wu, X. Toward high performance asymmetric hybrid capacitor by electrode optimization. Inorg. Chem. Front. 2019, 6, 2824–2831. [Google Scholar] [CrossRef]
- Sun, Y.C.; Wang, X.W.; Wu, X. High-performance flexible hybrid capacitors by regulating NiCoMoS@Mo0.75-LDH electrode structure. Mater. Res. Bull. 2023, 158, 112073. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Q.; Alshehri, A.A.; Sun, X.P. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy 2022, 1, e9120010. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, D.P.; Liu, H.Q.; Umar, A.; Xiang, W. High performance hybrid supercapacitor based on hierarchical MoS2/Ni3S2 metal chalcogenide. Chin. Chem. Lett. 2019, 30, 1105–1110. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.Q.; Li, X.M.; Du, X.; Ma, X.L.; Hao, X.G.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 36, 526–534. [Google Scholar] [CrossRef]
- Ni, G.; Cheng, J.; Dai, X.; Guo, Z.H.; Ling, X.; Yu, T.; Sun, Z.J. Integrating ultrathin polypyrrole framework on nickel-cobalt layered double hydroxide as an amperometric sensor for nonenzymatic glucose determination. Electroanalysis 2018, 30, 2366–2373. [Google Scholar] [CrossRef]
- Wang, W.C.; Zhang, N.; Shi, Z.Y.; Ye, Z.R.; Gao, Q.Y.; Zhi, M.J.; Hong, Z.L. Preparation of Ni-Al layered double hydroxide hollow microspheres for supercapacitor electrode. Chem. Eng. J. 2018, 338, 55–61. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.L.; Wu, F.; Hu, B.S.; Meng, F.; Niu, K.Y.; Lin, F.; Zheng, H.M. An investigation of ultrathin nickel-iron layered double hydroxide nanosheets grown on nickel foam for high-performance supercapacitor electrodes. J. Alloys Compd. 2017, 714, 63–70. [Google Scholar] [CrossRef]
- Khalafallah, D.; Li, X.Y.; Zhi, M.J.; Hong, Z.L. Three-dimensional hierarchical NiCo layered double hydroxide nanosheet arrays decorated with noble metals nanoparticles for enhanced urea electrocatalysis. ChemElectroChem 2019, 7, 163–174. [Google Scholar] [CrossRef]
- Wang, M.D.; Liu, X.Y.; Liu, H.Q.; Zhao, D.P.; Wu, X. NiCo layered double hydroxide nanosheets with enhanced electrochemical performance. J. Alloys Compd. 2022, 903, 163926. [Google Scholar] [CrossRef]
- Li, X.Y.; Khalafallah, D.; Wu, Z.X.; Zhi, M.J.; Hong, Z.L. Silver incorporated partially reduced NiCo-layered double hydroxide frameworks for asymmetric supercapacitors. J. Energy Storage 2020, 31, 101578. [Google Scholar] [CrossRef]
- Wu, Z.X.; Khalafallah, D.; Teng, C.Q.; Wang, X.Q.; Zou, Q.; Chen, J.H.; Zhi, M.J.; Hong, Z.L. Vanadium doped hierarchical porous nickel-cobalt layered double hydroxides nanosheet arrays for high-performance supercapacitor. J. Alloys Compd. 2020, 838, 155604. [Google Scholar] [CrossRef]
- Zhang, K.L.; Li, Y.H.; Deng, S.J.; Shen, S.H.; Zhang, Y.; Pan, G.X.; Xiong, Q.Q.; Liu, Q.; Xia, X.H.; Wang, X.L.; et al. Molybdenum selenide electrocatalysts for electrochemical hydrogen evolution reaction. ChemElectroChem 2019, 6, 3530–3548. [Google Scholar] [CrossRef]
- Liu, Y.X.; Gong, J.X.; Wang, J.H.; Hu, C.H.; Xie, M.Z.; Jin, X.L.; Wang, S.; Dai, Y.T. Facile fabrication of MXene supported nickel-cobalt selenide ternary composite via one-step hydrothermal for high-performance asymmetric supercapacitors. J. Alloys Compd. 2022, 899, 163354. [Google Scholar] [CrossRef]
- Yang, Q.J.; Liu, Y.; Deng, C.Y.; Sun, L.; Shi, W.D. In-situ construction of heterostructure (Ni, Co)Se2 nanoarrays derived from cone-like ZIF-L for high performance hybrid supercapacitors. J. Colloid Interface Sci. 2022, 608, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.R.; Shi, Y.Y.; Wu, C.; Zhang, Y.R.; Ma, Y.Z.; Sun, X.; Sun, J.F.; Zhang, X.G.; Yuan, C.Z. Monodisperse metallic NiCoSe2 hollow sub-microspheres: Formation process, intrinsic charge-storage mechanism, and appealing pseudocapacitance as highly conductive electrode for electrochemical supercapacitors. Adv. Funct. Mater. 2018, 28, 1705921. [Google Scholar] [CrossRef]
- Guo, K.L.; Cui, S.Z.; Hou, H.W.; Chen, W.H.; Mi, L.W. Hierarchical ternary Ni-Co-Se nanowires for high-performance supercapacitor device design. Dalton Trans. 2016, 45, 19458–19465. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.Y.; Ma, S.L.; Li, M.H.; He, G.X.; Xie, M.J.; Guo, X.F. Template ion-exchange synthesis of Co-Ni composite hydroxides nanosheets for supercapacitor with unprecedented rate capability. Chem. Eng. J. 2022, 432, 134319. [Google Scholar] [CrossRef]
- Jia, H.A.; Wang, Z.Y.; Zheng, X.H.; Lin, J.H.; Liang, H.Y.; Cai, Y.F.; Qi, J.L.; Cao, J.; Feng, J.C.; Fei, W.D. Interlaced Ni-Co LDH nanosheets wrapped Co9S8 nanotube with hierarchical structure toward high performance supercapacitors. Chem. Eng. J. 2018, 351, 348–355. [Google Scholar] [CrossRef]
- Xu, Z.F.; Pan, H.L.; Lin, Y.; Yang, Z.; Wang, J.L.; Gong, Y.Q. Constructing a hexagonal copper-coin-shaped NiCoSe2@NiO@CoNi2S4@CoS2 hybrid nanoarray on nickel foam as a robust oxygen evolution reaction electrocatalyst. J. Mater. Chem. A 2018, 6, 18641–18648. [Google Scholar] [CrossRef]
- Jia, H.; Zhu, X.Y.; Song, T.T.; Pan, J.P.; Peng, F.; Li, L.H.; Liu, Y. Hierarchical nanocomposite of carbon-fiber-supported Ni-Co-based layered double-hydroxide nanosheets decorated with (NiCo)Se2 nanoparticles for high performance energy storage. J. Colloid Interface Sci. 2021, 608, 175–185. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, S.; Fan, M.Q.; Li, C.; Chen, D.; Tian, G.L.; Shu, K.Y. Bimetallic nickel cobalt selenides: A new kind of electroactive material for high-power energy storage. J. Mater. Chem. A 2015, 3, 23653. [Google Scholar] [CrossRef]
- Li, S.; Ruan, Y.J.; Xie, Q. Morphological modulation of NiCo2Se4 nanotubes through hydrothermal selenization for asymmetric supercapacitor. Electrochim. Acta 2020, 356, 136837. [Google Scholar] [CrossRef]
- Dai, M.Z.; Liu, H.Q.; Zhao, D.P.; Zhu, X.F.; Umar, A.; Algarni, H.; Wu, X. Ni Foam substrates modifified with a ZnCo2O4 nanowire-coated Ni(OH)2 nanosheet electrode for hybrid capacitors and electrocatalysts. ACS Appl. Nano Mater. 2021, 4, 5461–5468. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, Y.F.; Peng, H.Q.; Zhang, Z.Y.; Sit, C.K.; Yuen, M.F.; Zhang, T.R.; Lee, C.S.; Zhang, W.J. Nickel-cobalt diselenide 3D mesoporous nanosheet networks supported on Ni Foam: An all-pH highly efficient integrated electrocatalyst for hydrogen evolution. Adv. Mater. 2017, 29, 1606521. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, J.Z.; Sun, K.J.; Ma, G.F.; Zhang, Z.G.; Feng, E.; Lei, Z.Q. High performance asymmetric supercapacitor designed with a novel NiSe@MoSe2 nanosheet array and nitrogen doped carbon nanosheet. ACS Sustain. Chem. Eng. 2017, 5, 5951–5963. [Google Scholar] [CrossRef]
- Kuai, Y.Q.; Wang, T.L.; Liu, M.T.; Ma, H.W.; Zhang, C.J. Flower-like Ni0.85Se nanosheets with enhanced performance toward hybrid supercapacitor. Electrochim. Acta 2019, 321, 134701. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Ren, J.C.; Meng, Q.; Zhang, X.H.; Du, C.C.; Chen, J.H. Facilely hierarchical growth of N-doped carbon-coated NiCo2O4 nanowire arrays on Ni foam for advanced supercapacitor electrodes. ACS Sustain. Chem. Eng. 2019, 7, 12447–12456. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Wu, Z.B.; Jing, M.J.; Yang, X.M.; Song, W.X.; Ji, X.B. Mesoporous NiCo2S4 nanoparticles as high-performance electrode materials for supercapacitors. J. Power Sources 2015, 273, 584–590. [Google Scholar] [CrossRef]
- Wang, P.Y.; Li, Y.N.; Li, S.D.; Liao, X.Q.; Sun, S.M. Water-promoted zeolitic imidazolate framework-67 transformation to Ni-Co layered double hydroxide hollow microsphere for supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2017, 28, 9221–9227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).