1. Introduction
The continuous increase in electricity consumption in a world of finite fuel sources and a changing climate, urgently requires the impulse of renewable energy sources [
1]. The fluctuating nature of renewable energy generation [
2] entails the development of reliable large-scale energy storage systems (ESSs) that improve the stability, efficiency and sustainability of the power grid [
3].
Among the different electrochemical ESSs, redox flow batteries (RFBs) are considered an excellent technology for cost-effective large-scale stationary applications [
4] due to their high energy density, good cyclability, flexible architecture and distinctive decoupling between power and energy [
5]. The core of RFBs lies in the electroactive species in which the energy is stored [
6], and several metal-based redox couples have been investigated over the past few decades [
7]. Among them, “all-vanadium” RFBs (VRFBs) are the most studied and are among the few RFBs that have been tested and demonstrated at utility scale [
8,
9].
The VRFB technology was developed in 1985 by Skyllas-Kazakos [
10] and consists of an electrochemical cell where the energy conversions take place and two external tanks where the positive (catholyte, containing VO
2+/VO
2+ ions) and negative (anolyte, containing V
2+/V
3+ ions) electrolytes are stored. The electrolyte flows through each of the two half-cells, which are separated by a membrane that allows selective ion exchange to maintain charge balance, to return to the storage tank.
Considering that they provide the reaction sites, electrodes are an essential component of RFBs that directly affect battery performance [
11]. The main requirements of the electrodes are (1) good electrocatalytic activity, (2) chemical stability, (3) electrochemical stability in the operating potential window, (4) excellent electrical conductivity and (5) mechanical durability under flow conditions. Carbonaceous materials have been identified as the best candidates for both the negative and positive half-cells in vanadium flow batteries (VRFB) [
12,
13,
14] as they exhibit excellent catalytic activity, good conductivity, good chemical and mechanical stabilities, and are easy to manufacture at a low price in different forms [
15]. The most common carbonaceous electrode formats are graphite felt (GF) and carbon paper (CP), based on rayon and polyacrylonitrile (PAN) precursors [
16], [
17]. Despite their overall favorable properties, they also present disadvantages such as their inherent hydrophobicity [
18] attributed to the C-C structure [
19] and their low surface area, which can lead to low electrode wettability with associated pumping losses and slower reaction kinetics.
To improve the electrical, electrochemical, and surface properties and extend the lifetime of carbon electrodes, different surface treatments have been studied. The most common strategies described in the literature can be mainly classified into thermal [
20,
21,
22], chemical [
23,
24], and plasma [
25,
26] treatments. Most studies focus on (i) morphological modifications that increase the surface area, which creates more active sites, or on (ii) chemical modifications of the surface, often introducing oxygen groups such as hydroxyl (-OH) or carboxyl (-COOH) to improve the wettability of the electrode. However, there is a lack of understanding regarding the influence of the chemical and morphological modifications in the electrode surface on the final electrochemical performance of the redox flow batteries. In addition, other approaches, including heteroatom doping [
19,
27,
28] in order to increase the donor or acceptor levels to accelerate the charge transfer, and the decoration of GF with different materials, such as carbon-based catalysts [
29] (e.g., carbon nanotubes [
30], nanoparticles [
31], graphene [
32], nanofibers [
33], …) or metal and metal-oxides (e.g., bismuth [
34], zirconium oxide [
35], niobium [
36], manganese oxide [
37], …), have also been recurrent strategies to increase the electrocatalytic properties of the electrode over a specific surface area. Nevertheless, these treatments are more complex, costly, and time consuming, which may impede their further exploitation in cells. This is the main reason why, during the present work, only surface treatments (thermal, chemical, and plasma) were selected as the most promising to overcome the main drawbacks inferred by graphite felt properties. However, they must be carefully evaluated to further understand their influence in the electrochemical performance of graphite felt as electrode in redox flow batteries.
The thermal treatment stands out as one of the most attractive common methods for improving the electrode performance of RFBs. There is consensus that the functionalization of electrodes by heat treatment is carried out at temperatures above 400 °C and in oxidizing atmospheres. The exposure time is an important parameter since it has been shown that an excessive time at high temperatures generates a deterioration of the fibers and produces a decrease in surface oxygen. In general, optimum conditions for good surface functionalization are obtained at temperatures of 500–550 °C and a minimum of 5 h of treatment [
22]. To reduce the time of this type of treatment, different works have studied the modification of the treatment atmosphere using oxygen or nitrogen-enriched atmospheres [
21]. Generally, this type of treatment results in an improvement in cell performance associated with the generation of oxygen-based functional groups (also related to wettability) and the increase in surface area produced by this type of treatment. However, thermal treatment is presented as a method of low reproducibility and selectivity and their specific effects are under discussion [
38], being not clear whether the enhanced kinetics on the treated electrode surfaces are related to the oxygen functional groups and its associated wettability improvement or to the increased surface area [
21]. All studies agree that there is an improvement in electrochemical activity as a result of increased wettability attributed to oxygen functional groups. Nevertheless, the connection between increased surface area and electrode activity is not so clear and has not been conclusively verified.
In the chemical treatments, the different surface modification is performed by an oxidizing agent. The degree of functionalization is controllable [
24], and this type of treatment involves simple reaction steps but with a very thorough final washing step. The treatments are carried out at room or moderate temperature by refluxing or boiling in the oxidizing agent generating a large amount of chemisorbed oxygen functional groups that improve the wettability of the electrode. Several studies demonstrate the increase of oxygen groups without involving any morphological difference in the treated felt implying substantial improvements in cell performance [
23,
39]. Regarding the possibility of using acid mixtures, in a more recent work [
24,
40,
41], it was shown that the combination of acids (nitric acid with phosphoric acid or with sulfuric acid) induced more oxygen groups compared to a simple treatment with nitric acid.
Plasma treatments are a versatile, controllable, fast, and simple method to modify the surface properties of the graphite felt in a uniform manner by physically bombarding molecules of different gases (O
2, N
2, NH
3, CH
4 or Ar), accelerated through various discharge methods (glow, arc, radiofrequency, microwave, corona...) [
42]. This process is an environmentally friendly route to improve the physicochemical properties of the graphite felt, as it is solvent-free, produces low or no waste, and involves shorter treatment times [
43]. In addition, plasma treatments are used to implant functional groups without changing the bulk structure compared to other techniques because bond breaking by high-energy electrons and radicals introduces chemically active species more easily and with less damage [
44]. Some nitrogen plasma treatments have been shown to improve the electrochemical reactivity of graphite felts by creating N-doped heteroatom defects, which generate an increase in the number of active sites and improve the wettability of the electrode [
25]. Using oxygen plasma, increased redox activity has also been reported without an associated significant improvement in surface area, thus attributing the improved properties to the surface functional groups [
26,
45].
In this context, it is difficult to distinguish between the benefit obtained by increasing surface area and functional groups in the evaluation of cell performance. The increase of the number of oxygen groups in the electrode surface is consistently related with an improvement of the kinetics of the V
+2/V
+3 redox reaction; however, it is unclear how it affects the VO
2+/VO
2+ half-reaction [
46]. Therefore, it is necessary to evaluate the effect of GF modification, especially on the positive electrode.
The aim of this paper is, firstly, to understand which process parameters in the thermal, chemical, and plasma treatment have more influence on the functionalization of GFs and, secondly, how this functionalization (related to surface area modification and incorporation of new functional groups) is affecting the electrochemistry of the VO2+/VO2+ half-reaction. Therefore, in this study, a commercial GF has been modified by thermal, chemical, and plasma treatments to create different morphologies and different oxygen group contents. For each treatment, key variables influencing acidic oxygen group content, surface area, and wettability have been selected based on a deep physicochemical characterization of the treated felts. Furthermore, the electrochemical activity of the electrodes is evaluated to discern among the influence of the specific variables under study on the cell performance.
2. Materials and Methods
2.1. Materials and Reagents
The GF used in this study was a polyacrylonitrile (PAN)-based precursor from SGL Carbon (GFD 4.6 EA PAN). The sample dimension used for the application of the different treatments was 10 × 10 cm2.
The reagents used in the chemical treatment were purchased from Sigma Aldrich. Analytical grade HNO3 (60% vol/vol) and H3PO4 (85% vol/vol) were used without further purification. The reagents used in the titration were NaOH and HCl, 0.1 N and phenolphthalein as indicator, all from Sigma Aldrich. Each reagent was diluted with deionized H2O to the required concentration.
2.2. Graphite Felt Treatments
Prior to the application of the different treatments, the samples were rinsed with water and isopropanol to remove any residual impurities and were dried at 120 °C for one hour.
For the thermal treatments, samples were introduced in a furnace (Nabertherm P 330) and heated from room temperature to the set-point temperature in an hour. After the defined exposure time, the samples were let to cool down to room temperature. The studied variables were oven atmosphere (air or nitrogen), temperature (400–650 °C), and exposure time (3–27 h).
Chemical treatments were performed by immersion. Due to the hydrophobic nature of GF, it tends to float in the liquid, so specific tooling was manufactured to ensure the complete immersion of the sample. Homogeneous contact with the mixture of acids was achieved by constant magnetic stirring of the solution on a temperature-controlled hot plate. After applying the different chemical treatments, the treated samples were rinsed twice in a deionized water bath to remove acid residues. Finally, the samples were dried in an oven for 2 h at 150 °C. The controlled variables in the chemical treatment were the ratio between both acids in weight H3PO4: HNO3 (1:3 (25%), 1:1 (50%), 3:1 (75%)), the temperature (30 °C, 55 °C, 80 °C), and the exposure time (2, 5, 8 h).
For the plasma treatments, a Diener Zepto Plasma equipment was used at 40 mHZ. The evaluated variables were the power rating (50, 75, 100 W), pressure (0.1, 0.75, 1 bar), and exposure time (from 1 to 10 min) with O2 gas.
2.3. Physicochemical Characterization
The physicochemical characterization of the treated GFs comprised a specific surface area measurement by BET (Brunauer–Emmett–Teller), oxygen acid groups concentration determination through the Boehm method and wettability measurements to determine the hydrophilicity or hydrophobicity character of the material. In addition, resistance measurements have been carried out with the 4-point probe method and the identification of surface functional groups by XPS has been performed.
2.3.1. BET Surface Area Analysis
Multi-point BET measurement was carried out by N2 (77 K) adsorption with a Quantachrome Autosorb-iQ-MP (Quantachrome Instruments, Boynton Beach, FL, USA). The process of isothermal absorption started after the vacuum degasification and was maintained at 250 °C for 10 h.
2.3.2. Boehm Method for Oxygen Groups Determination
The titration in aqueous solution was performed by the method proposed by Boehm [
47,
48] which was subsequent adapted by Sara Goertzen [
49]. By this method, it is possible to determine the concentration of acidic oxygen groups (hydroxyl, carbonyl, and lactone groups) at GF surface. For this purpose, 1.5 g of GF was added to 50 mL of 0.05 M NaOH (Solution B). This mixture was maintained under magnetic stirring for 24 h and subsequently filtered with Whatman paper grade 1 to separate the GF.
The resulting solution was back-titrated, taking 3 aliquots (10 mL of Solution B each) to ensure the repeatability of the measurements. The process consisted in the addition of 20 mL of 0.05 M HCl to each aliquot for the back-titration using phenolphthalein as an indicator.
The functionalization grade of the surface of the GF is calculated by the following equations:
where
nCSF represents the moles of surface functionalized [
B] and
VB are the concentration and the volume of the Solution B.
Va is the volume of the aliquot taken from the Solution B, [
HCl] and
VHCl are the concentration and volume of the acid added to the aliquot and [
NaOH] and
VNaOH are the concentration and the volume of the amount of titrant.
2.3.3. Wettability Measurements
Static water contact angle (WCA) measurements are often used to characterize the intrinsic wettability of surfaces. For this purpose, the GFs were measured with a SURFTENS universal goniometer by depositing a 5 µL drop of distilled water on the different treated surfaces. However, it was only possible to discern between the hydrophilic (0°) or hydrophobic (122°) character of the samples, since when the electrode has a hydrophilic character, the droplets are completely adsorbed on contact with the surface giving rise to a zero-contact angle.
2.3.4. Scanning Electron Microscopy (SEM)
SEM was employed to analyze the morphology and topography of the CF electrodes. A Carl Zeiss SMT Ultra Gemini-II microscope (Carl Zeiss, Thornwood, NY, USA) was employed. Samples were analyzed without being coated.
2.3.5. Resistance Test
Through-plane area specific resistance (ASR) of the graphite felts was measured by Keithley 2400 SourceMeter with the S302-4 resistivity measurement stand and SP4-500855TRY 4-point probe. The samples were held between two copper sheets and compressed to 23% of their initial thickness.
2.3.6. X-ray Photoelectron Spectroscopy (XPS)
X-ray Photoelectron Spectra were recorded using a Phoibos 150 XPS spectrometer (SPECS Surface Nano Analysis) in Fixed Analyzer Transmission mode with 2 mm lateral view on the sample. The chamber base pressure was 1 × 10
−10 mbar and a non-monochromatic Mg source (Mg Kα with hν = 1253.6 eV) was employed. Pass energies of 90 eV and 30 eV were used respectively for acquiring the survey spectra and the high-resolution regions (C 1s, O 1s, and N 1s). Quantifications of atomic surface species were done by applying tabulated Scofield cross sections for each element and core level to the corresponding integrated intensity [
50] after correcting the energy dependent analyzer transmission function and differences in effective attenuation length of the collected photoelectrons depending on their kinetic energies [
51,
52]. The background of inelastically scattered photoelectrons was simulated by a Shirley function, and a Voigt profile (30% Gaussian, 70% Lorentzian) was employed as a line shape for all components except the semi-metallic sp
2 C=C. Aromatic carbon asymmetry was defined in the Doniach–Sunjic (DS) model [
53] as using an asymmetric pseudo-Voigt (APV) function instead to overcome the integral divergence of the DS function. The fitting model was constrained to be quantitatively consistent so that the O % obtained directly by integration of the O 1s peak agrees with the amount indirectly calculated through deconvolution of the C 1s peak. Peak positions of the different carbon species were set to: 284.4 eV for aromatic carbon (C=C), 284.8 eV for aliphatic carbon (C-C) and/or defects, 286.5 ± 0.3 eV for hydroxyl (C-OH) and epoxy (C-O-C), 287.8 ± 0.2 eV for carbonyl (C=O), and 289.0 ± 0.2 eV for carboxyl (O-C=O) groups, with secondary peaks of plasmon/shake-up contributions at +6.4 eV and +10.1 eV with respect to the main C=C peak [
54].
2.4. Electrochemical Characterization
For the electrochemical measurements, a three-electrode configuration was employed, with the modified graphite felt under study as working electrode (WE), an Ag/AgCl reference electrode (RE), and a Pt spiral wire as counter electrode (CE). To ensure the comparability, the orientation and distance of all three electrodes were kept the same by means of a Teflon cap. For the preparation of the WE, the different graphite felt were cut into 1 × 1 cm2 samples, which were soaked in ethanol and then rinsed with DI water before immersing them in the electrolyte by means of a Pt wire holder. 10 mL of the electrolyte (160 mM VOSO4 dissolved in 4 M H2SO4) were used for all the measurements. Cyclic voltammetry (CV) measurements were conducted with a Biologic SP-300-bi-potentiostat between 0 V and 1.6 V (vs. Ag/AgCl) at different scan rates (1, 3, 5, 7, 10 mV/s) to study the performance of each electrode to catalyze the VO2+/VO2+ half-reaction.
3. Results
A commercial GF was treated by a thermal, chemical, and plasma treatment. The main parameters of these electrode treatments were varied to study their influence on the surface area, the formation of oxygen groups, and the wettability of the electrode. Based on the obtained results, it is discussed which of the process parameters have a greater influence on the physicochemical properties of the electrodes.
Furthermore, some treated GF electrodes were characterized electrochemically to relate the surface area, oxygen cluster formation, and wettability of the electrodes with their electrochemical performance.
3.1. Thermal Treatment
The electrode samples were submitted to several thermal processes under two different atmospheres (air and nitrogen [
21,
55]). Temperatures between 450 and 650 °C [
38,
56] and exposure times from 3 to 27 h were applied.
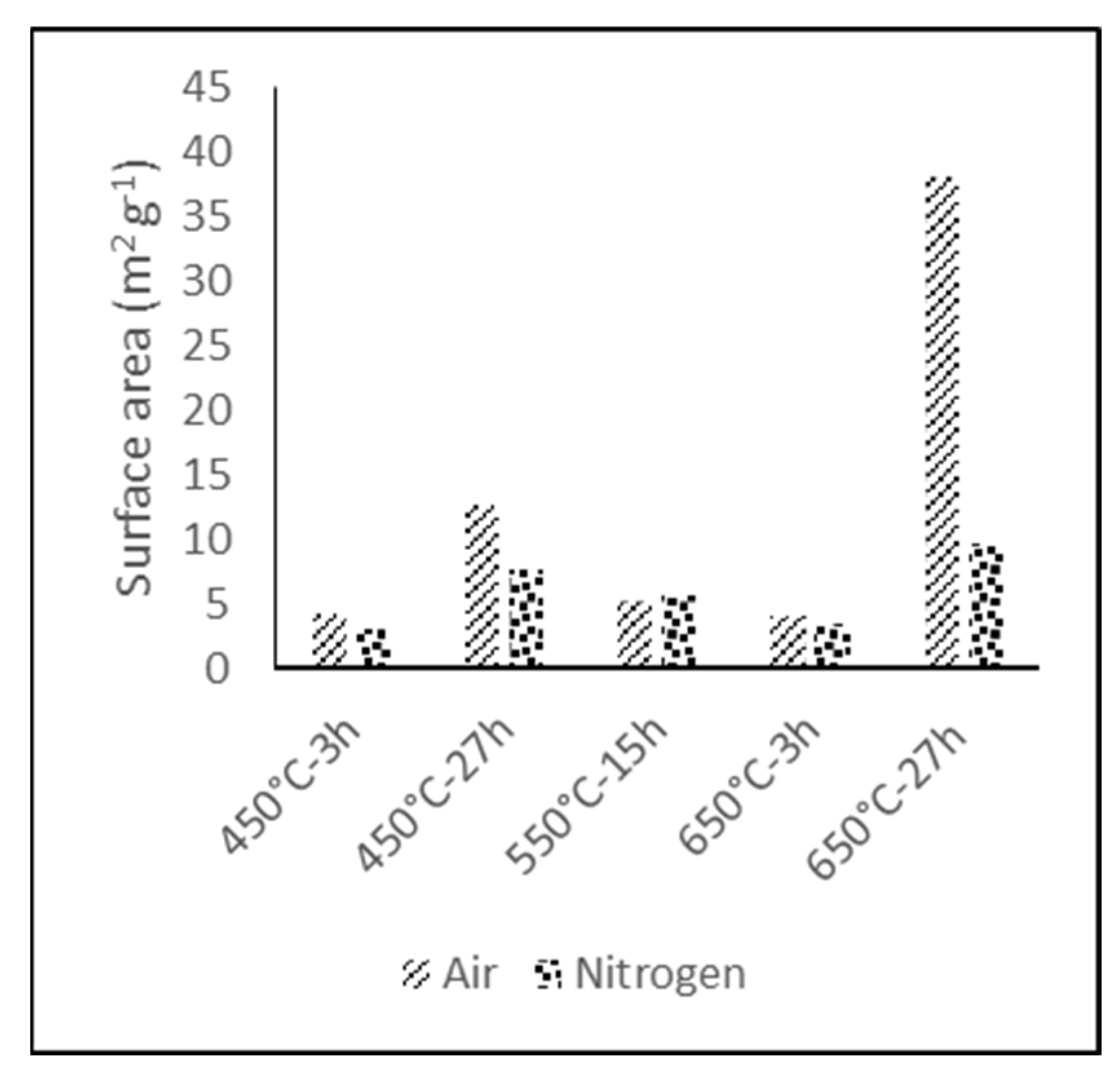
The objective of the developed thermal processes was to introduce functional groups on the surface of the electrode, enhancing its wettability but simultaneously increasing the surface area of the electrode. The specific combination of variables and associated measurements are shown in
Table 1. Overall, the modification of the variables of the thermal treatments allowed to obtain a wide range of hydrophilic samples, with contents of oxygen groups from 0.57 to 1.12 mM and specific surface areas from 4 to 38 m
2 g
−1.
The thermal treatment variable with major influence in the BET specific surface area is the type of atmosphere, although it is also dependent on temperature and exposure time, as it is shown in
Figure 1.
BET specific surface area of the electrodes treated for prolonged times (27 h) under air atmosphere underwent a significant gain with respect to the reference reaching 12 m2 g−1 and 38 m2 g−1 for the electrode treated at 450 °C and 650 °C, respectively. In the case of the electrodes treated under nitrogen atmosphere or with shorter treatment times, the increase was not significant. This drastic increase in the specific surface area under air atmosphere could be due to the reduction of the fiber diameter.
Figure 2 shows the morphology of the original and the treated GF fibers at 650 °C during 27 h in air and nitrogen, the thermal treatments with higher effects in the specific surface area. It is observed that these treatments caused a fiber degradation, reaching around a 50% decrease of the original diameter in the treated fibers.
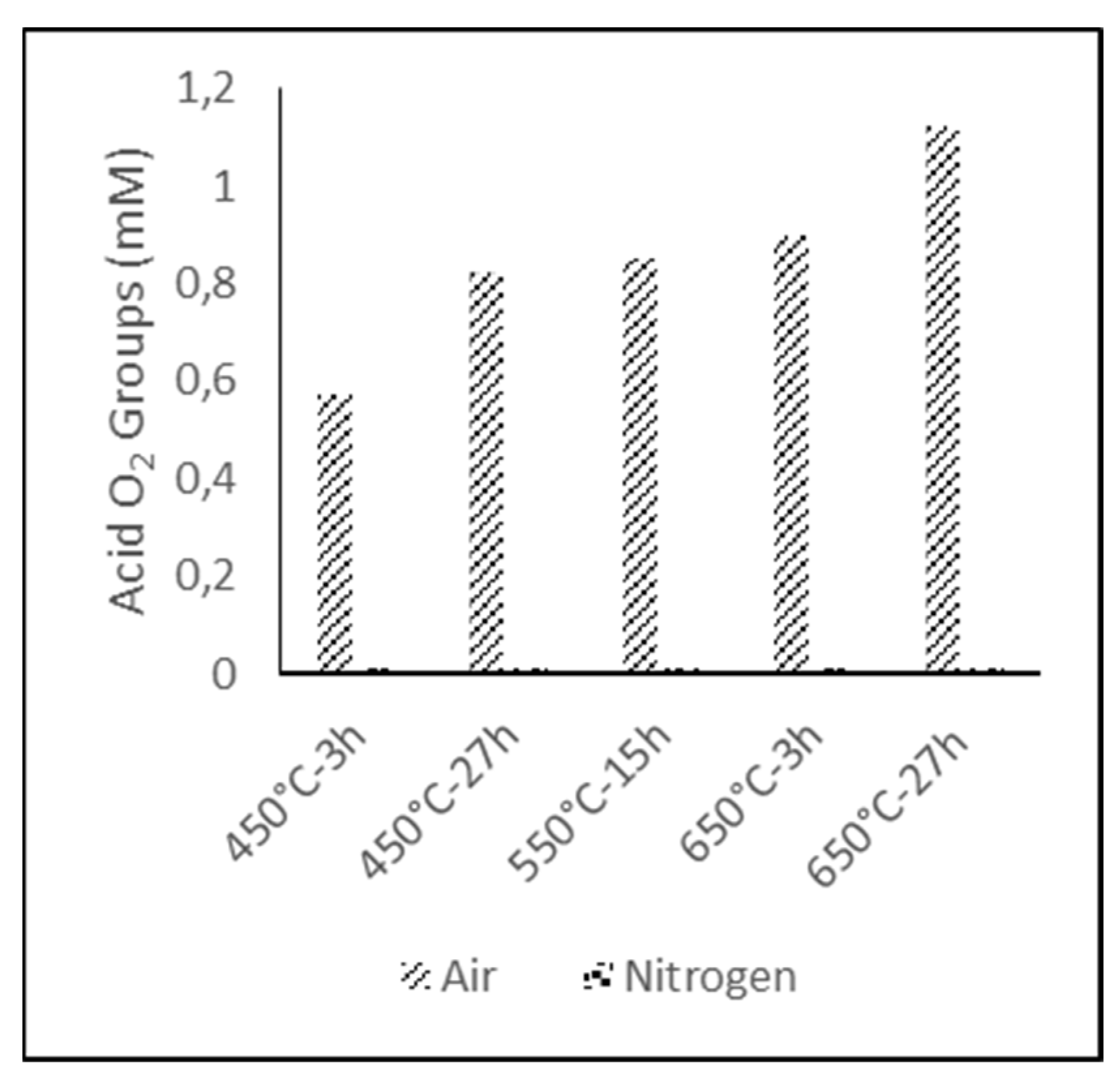
The amount of acidic oxygen groups on the surface, represented in
Figure 3, revealed that the samples treated under nitrogen atmosphere could not be performed by Boehm titration, due to their hydrophobic character, so this value was assumed to be zero. For the electrodes treated under air atmosphere, the results showed that higher functionalization degrees could be obtained either increasing the temperature during the treatment or its duration, being possible to achieve the same level of functionalization by adjusting the time and temperature in an operational window [
57]. For example, a surface acid oxygen group concentration of 0.8–0.9 mM (miliMoles) can be obtained at 450 °C for 27 h, 550 °C for 15 h, or 650 °C for 3 h; thus, the treatment conditions can be adjusted to be as energy efficient as possible.
The electrochemical performance of the redox flow batteries is also influenced by the wettability of the electrodes, that is, of the GF [
22]. Functionalization with oxygen functional groups improves the surface wettability. In this sense, it has been confirmed that the GF treated in an inert atmosphere, with an inappreciable content of oxygen groups, presents a hydrophobic character, so as the untreated GF.
The results indicated the prominent influence of the treatment atmosphere compared to the rest of the parameters. In the case of the thermally treated GF under a nitrogen atmosphere, the droplets remain and roll on the surface at a low inclination, leaving the felt dry (water contact angle of 122°). On the other hand, when GF is treated under an air atmosphere, the droplets are adsorbed by the felts as soon as they contact the surface. This is because of their superhydrophilic character.
Furthermore, there is a clear relationship between the content of oxygen groups on the surface and the hydrophilic character. Values higher than 0.5 mM of oxygen acid groups lead to superhydrophilic surfaces, which implies a total adsorption of water on the surface. A higher number of oxygen acid groups does not have a major impact on wettability, since at this level, the wettability obtained is total.
3.2. Chemical Treatment
The chemical treatments were performed in liquid phase using acids [
23], with the aim of increasing the concentration of surface oxygen groups. The treatment of carbonaceous materials with nitric acid, or with a mixture of acids, has been studied by several researchers, concluding that it generated the formation of functional oxygen groups as carboxylic, phenolic, and free radicals.
In this work, the modifications of the GFs were performed at different ratios of a mixture of H
3PO
4:HNO
3 [
58] by varying the treatment time and temperature [
23,
24,
59]. The combination of variables used in the different treatments and the associated measurements are shown in
Table 2. The modification of the defined variables of the chemical process allowed to obtain hydrophobic to hydrophilic surfaces, with contents of oxygen groups from 2.88 to 4.98 mM and specific surface areas from 0.82 to 6.49 m
2 g
−1.
The specific surface area of GF is not significantly affected by the variables of the chemical process in the analyzed range of values (varying from 0.82 to 6.49 m2 g−1). The largest increases were observed at the shortest treatment times (2 h).
Prolonged exposure times caused a strong decrease in BET specific surface area, as already described in previous work [
60,
61], attributed to both blockage of the pores, due to oxygenated groups formation on the surface of the activated carbon, and/or damages in the porous structure.
Regarding the amount of surface oxygen groups, all the samples showed a significant increase, though short treatment times and high ratios H3PO4:HNO3 (that is, higher amounts of phosphoric acid) led to a higher amount of oxygen groups. However, it was observed that the temperature of the treatment hardly affected the generation of oxygenated groups.
By chemical treatments, values higher than 3.55 mM of oxygen acid groups provide superhydrophilicity at the surface, which implies a total adsorption of water on the surface. A higher number of oxygen groups does not have a major impact on wettability, since at this level, the obtained wettability is total.
When comparing the thermal and chemical treatments, 0.57 mM of oxygen groups already provided this hydrophilic behavior in thermal treatment, so it is concluded that hydrophilicity does not only depend on the oxygen groups as other factors, such as surface area, also influence it. In addition, chemical treatments provided a higher amount of oxygen groups and lower surface areas, which is in good agreement with previously published results [
62].
3.3. Plasma Treatment
The oxygen plasma treatment is used to modify the physical and chemical properties of GF simultaneously with the final aim of generating hydrophilic groups, thus improving the wettability of the GF. For the oxygen plasma treatment, the parameters studied were time, power, and pressure [
26]. The specific combination of these variables and the main physicochemical properties of the GF obtained at those conditions are shown in
Table 3. This series of plasma treatments allowed to obtain hydrophilic samples with different contents of oxygen acid groups (from 2.13 to 4.05 mM) and different surface areas (from 2.82 to 8.43 m
2 g
−1).
The surface area showed a moderate increment (up to 8.43 m
2 g
−1), greater with longer treatment times and higher power, in good agreement with previous work [
12].
Regarding the amount of surface oxygen groups, all samples showed a moderate increase, although it is observed that higher exposure times generated a higher concentration of oxygen on the surface. Therefore, the treatment time is the variable that has the major influence over the oxygen groups concentration. This phenomenon has been previously described [
42]. It must also be mentioned that values higher than 2 mM of oxygen groups provide superhydrophilicity at the surface.
As for the rest of the variables, the influence of pressure on the generation of oxygen groups on the electrode surface is noteworthy, with lower pressures leading to higher levels of functionalization.
However, oxygen plasma has a limited penetration ability [
42] to the graphite felt surface, and it is difficult to observe drastic changes inside the graphite felt even after long-term treatments.
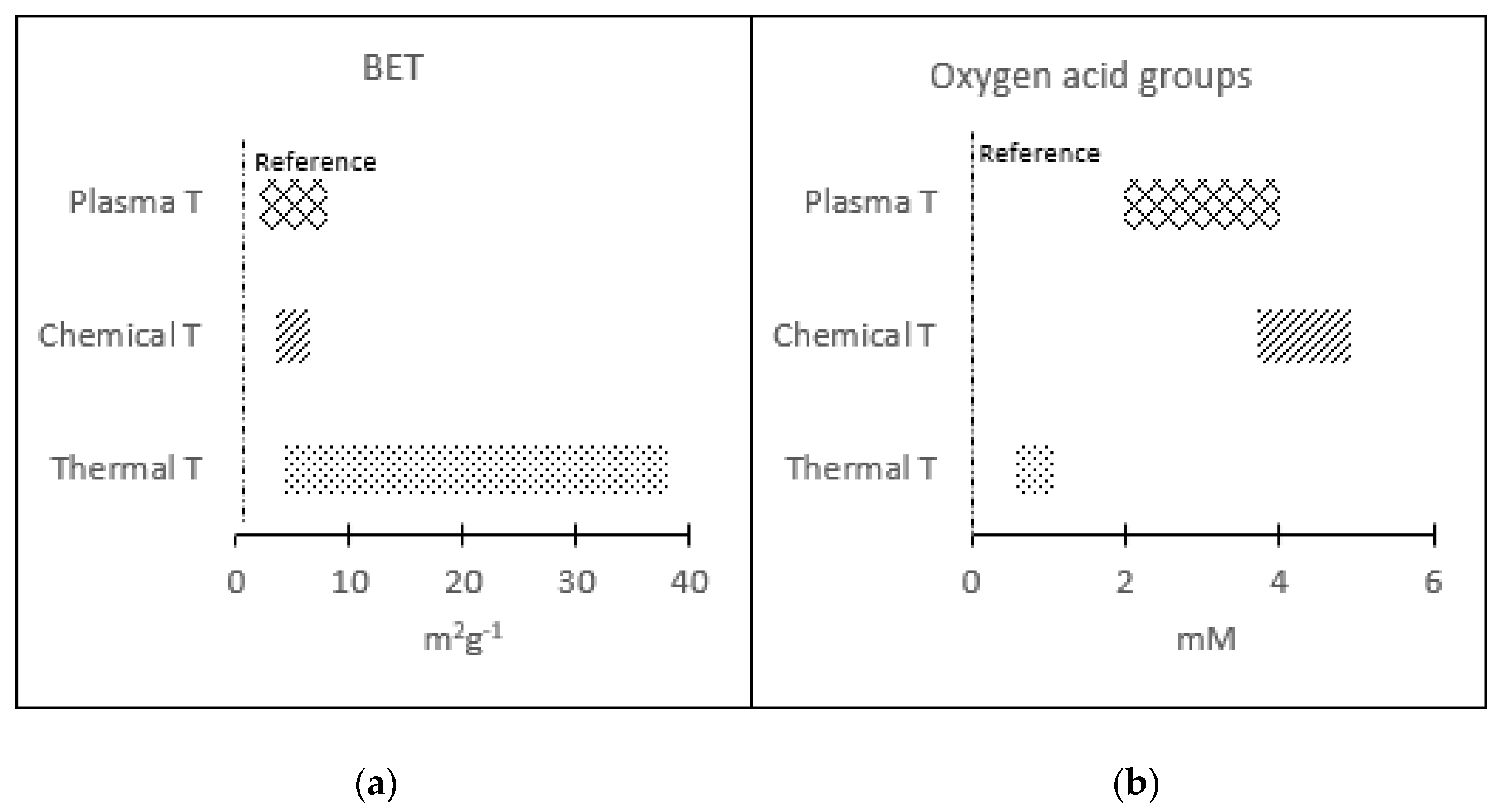
Comparing the results obtained by applying the three types of treatments, it can be concluded that it is possible to obtain electrodes with hydrophilic character, but with different physicochemical properties. A comparative analysis of the BET and Oxygen acid groups of the different hydrophilic samples obtained during the different treatments is shown in
Table 4 and
Figure 4.
The results indicated that thermal treatments generate larger changes in the specific surface area when the generation of oxygenated groups is modest. Plasma or chemical treatments achieved the opposite effect: a significant generation of oxygenated groups with no significant changes in surface area. The chemical treatments were the ones that caused the greatest generation of oxygenated groups.
In order to be able to distinguish between the positive benefit obtained from an increase in the specific surface area and functional groups, it was decided to electrochemically evaluate different hydrophilic samples obtained by the different studied treatments. In this context, it was decided to focus the study on comparing samples with similar surface area levels but different oxygenated group concentrations, and conversely, samples with similar concentrations of oxygenated groups but different surface area values (see
Table 5).
Futhermore, the electrical resistance was measured in the selected treated GFs to further understand the influence of the surface treatments on the properties of the electrodes. As it can be perceived, all the treatments significantly decreased the resistance in comparison to the reference GF. This fact is noteworthy because it translates in a reduction of the ohmic losses, thus leading to an increase in the energy efficiency of the cell.
3.4. Electrochemical Performance
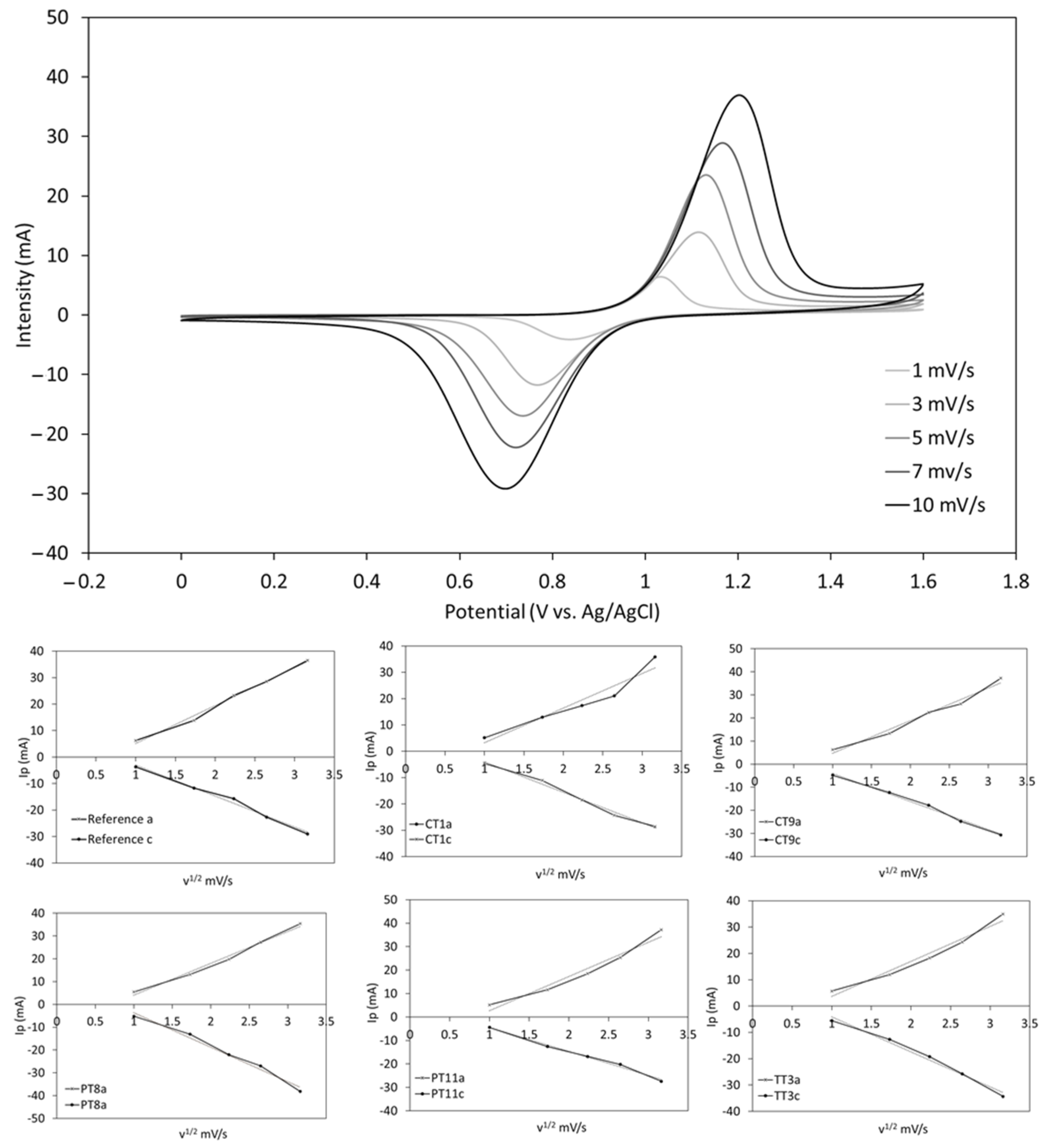
Samples listed in
Table 5 (Reference electrode, CT1, CT9, PT8, PT11 and TT3) were electrochemically characterized by comparing the onset potentials, peak potential separation (ΔEp) and peak current ratios (Ipa/Ipc) at different scan rates. The CV measurements at different scan rates revealed the chemical reversibility of the system for all the treated electrodes, as they all show a linear relationship between Ip and ν1/2, following the Randles–Sevcik equation [
63], as shown in
Figure 5.
Regarding the electrochemical reversibility of the VO
2+/VO
2+ reaction, the variation of ΔEp with the scan rate indicates that the process is quasi-reversible. However, the comparison of the ΔEp values at 10 mV/s shows that all the treatments significantly improve the electron transfer kinetics as compared to the untreated (Reference) graphite, as shown in
Figure 6 and
Table 6. While both the plasma (PT8, PT11) and chemical (CT1, CT9) treatments show a similar performance enhancement, the thermal treatment (TT3) provides a remarkable increase in the electrochemical reversibility of the system with an over 200 mV reduction of ΔEp. The near to 1 Ipa/Ipc ratio also indicates the excellent chemical reversibility of the process using the thermically treated felts. Moreover, a 110 mV reduction in the onset potential (vs. untreated electrode) was achieved by this method, which eventually translates into a better energy storage efficiency as it requires a lower charge voltage for the battery [
64]. This response is in accordance with the smaller resistance obtained in the resistance test.
A low oxygen surface group concentration generally leads to lower peak to peak voltage separations, indicating an improved electrochemical reversibility of the VO2+/VO2+ reaction, as deduced from the comparison between treatments that generate different surface chemical compositions but similar BET results (TT3, CT9 and PT11). Interestingly, this effect has been found to be independent of the measured surface area, as inferred from the evaluation of two electrode treatments that produce similar oxygen groups but different surface areas (CT1 and PT8). However, this outcome is only achieved when a minimum surface area threshold is surpassed and, therefore, remarkably low BET measurements tend to put that reversibility at stake (treatment CT1). Moreover, high surface areas generally lead to an improved performance as they also favor the chemical reversibility of the VO2+/VO2+ reaction, as shown by the closer to a unit Ipa/Ipc ratio of treatments with alike surface compositions and different BET results (PT8 vs. CT1).
To sum up, according to the evaluation of the electrochemical activity of the VO2+/VO2+ redox pair with the different treated GF, it can be stated that TT3 treatment revealed promising electrochemical performance to take part as an electrode in a RFB.
3.5. XPS Analysis
XPS measurements were performed in the reference GF and most promising treated electrode (TT3) to further understand the changes in the surface composition caused by the thermal treatment. The results (
Table 7) revealed an increase in %O content from 1.5% in the reference sample to 7.9% in the thermally treated TT3, in good agreement with previously published results for thermal treatments [
65]. Although a significant increase in the amount of surface O is appreciated after the thermal treatment, the reported results for plasma or chemical treatments show a O percentage around 20 [
66] or 40% [
67], respectively. This trend perfectly correlates with the obtained results for the Boehm method.
The relative concentration of different functional groups was calculated from the C 1s peak fitting, as shown in the results presented in
Table 8 (corresponding to
Figure 7). There is a significant decrease in the sp
2 C proportion from the reference (88.4%) to the TT3 thermally treated (61.6%) sample. Consequently, an increase in the second peak (284.8 eV) corresponding to sp
3 C and defects is appreciated, changing from 3.1% in the reference to 24.6% in TT3. Moreover, the rest of the peaks corresponding to different oxygen functionalities increase in TT3 compared to the reference. XPS analysis revealed that the more abundant oxygenated groups in the surface of the thermally treated felt are hydroxyl (C-OH) and epoxy (C-O-C), resulting in the contribution at 286.5 eV, followed by carbonyl C=O groups (at 287.8 eV) and finally carboxyl O-C=O groups (at 289.0 eV).
From the C 1s peak deconvolution, the relative concentration of different functional groups were calculated, as shown in the results presented in
Table 8 (obtained from
Figure 7). There is a significant decrease from the reference to the TT3 thermally treated in sp
2 (284.4 eV) proportion from 87.85% to 54.85%. Consequently, an increased in sp
3 (285.6 eV) is appreciated from 3.58% in the reference to 29.19% in TT3. Moreover, XPS analysis revealed that the main contribution of oxygenated groups in the surface of the thermally treated felt is C-O-(C/H) (286.1 eV), followed by the presence of C=O groups (287.4 eV).
4. Conclusions
The present work reports the modification of a commercial GF by thermal, chemical, and plasma treatments. As a result, it reveals which process parameters have significant influence on the GF functionalization depending on the treatment, so as the effect on their electrochemical activity as electrodes for a vanadium RFB.
During the thermal treatment, the control of the atmosphere is the main feature to successfully modify the GF surface. It is required air atmosphere to ensure hydrophilic behavior. In the chemical treatment, the ratio between acids and time must be carefully selected. It has been demonstrated that large periods of time may cause the blockage of the pores because of the great number of functional groups. For the plasma treatment, time and power are the main variables to control during the GF functionalization.
Comparing the treatments’ performance, it can be stated that carefully selecting the main parameters during the functionalization, a wide range of the GF properties can be designed regarding specific surface area, oxygen groups content, electrical resistance, or wettability. Thermal treatments generate larger changes in surface area, while a modest generation of oxygenated groups. Contrary, chemical, or plasma treatments allowed to reach significant number of oxygenated groups without changes in the surface area.
The study of the electrochemical activity of the treated GFs revealed an increased performance for all the treatments in comparison to the untreated one. Moreover, this study brought the importance of having and adequate relation between surface area and surface oxygenated groups, showed enhanced performance for the VO2+/VO2+ redox pair. It was demonstrated that GF with surface area in the ranges 4.5–5.6 m2 g−1 with a minimum presence of surface oxygenated groups, 0.85 mM, are more favourable to VO2+/VO2+ reactions than GF with higher oxygenated groups regardless of the surface area. This implies an excess of oxygenated groups, may interfere in the reaction VO2+/VO2+ creating a polarization in the cell. This is demonstrated by the enhanced activity for TT3 in the electrochemical measurements, being decreased over 200 mV the peak-to-peak potential and 110 mV the onset potential, which reveals an improved reversibility of the VO2+/VO2+ reaction. Consequently, enhanced energy efficiency in the cell is expected, thanks to the enhanced electrochemical activity, so as the reduced ohmic resistance losses of the electrode, which have been shown during the resistance test.
To conclude, this study discerns among the main variables that affect the GF electrode functionalization and what it is more, it assesses some of the most important properties of the developed electrodes, so as their electrochemical activity depending on their chemical composition and morphology.