In-Situ Photoelectron Spectroscopy Investigation of Sulfurization-Induced Sodiophilic Sites with Model Systems of α-sexithiophene and p-sexiphenyl
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
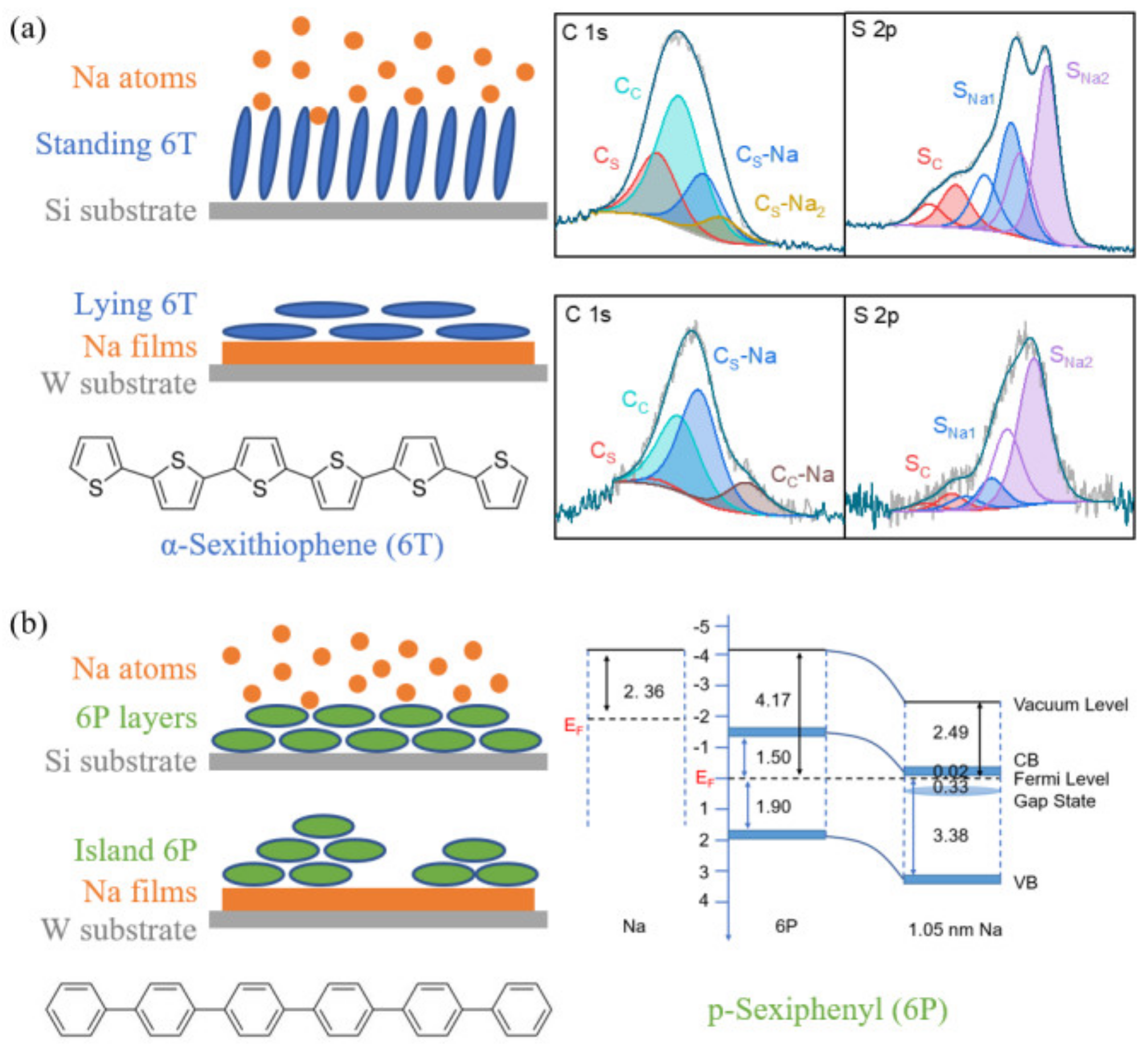
3.1. Na-6T
3.1.1. Na on 6T
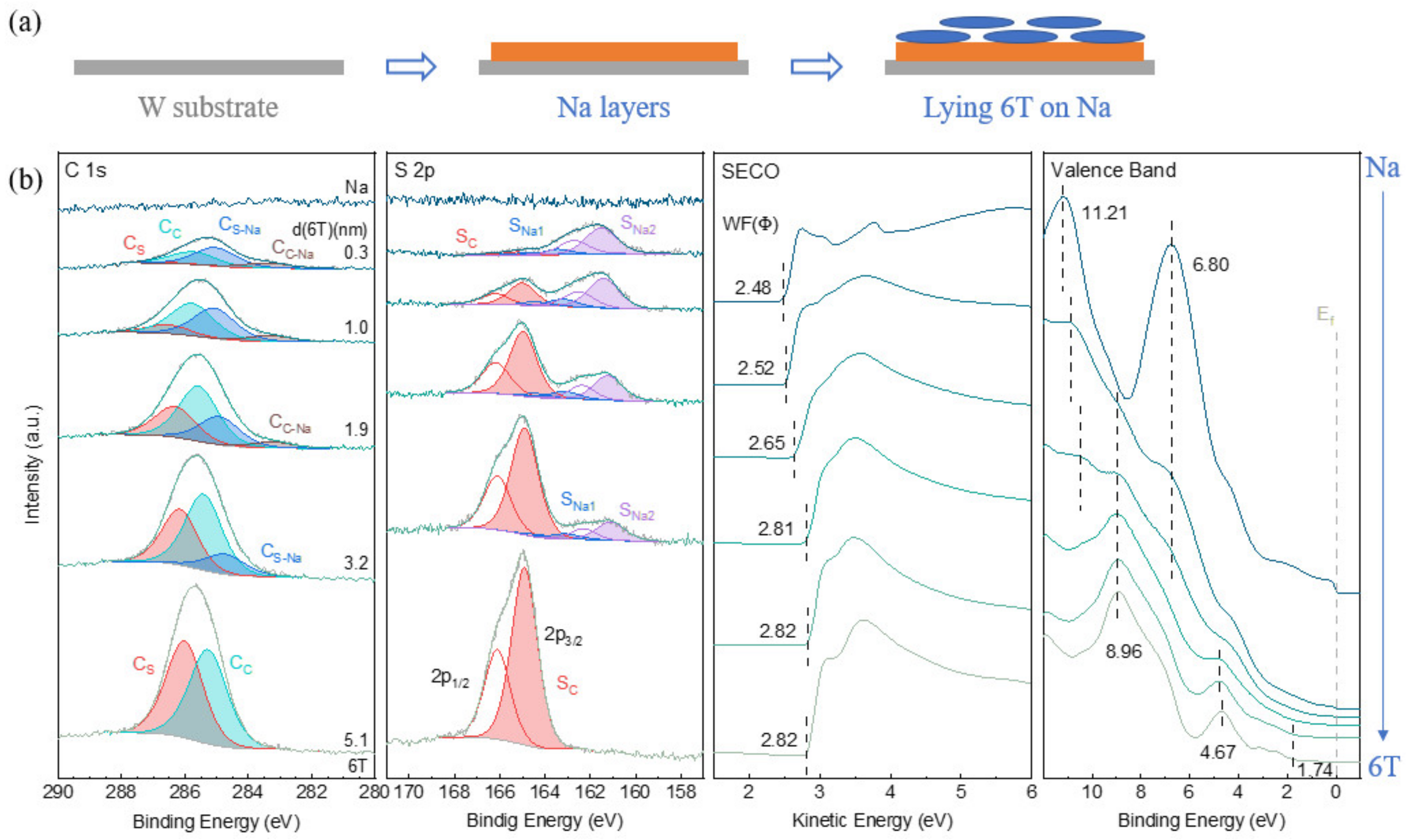
3.1.2. 6T on Na
3.2. Na-6P
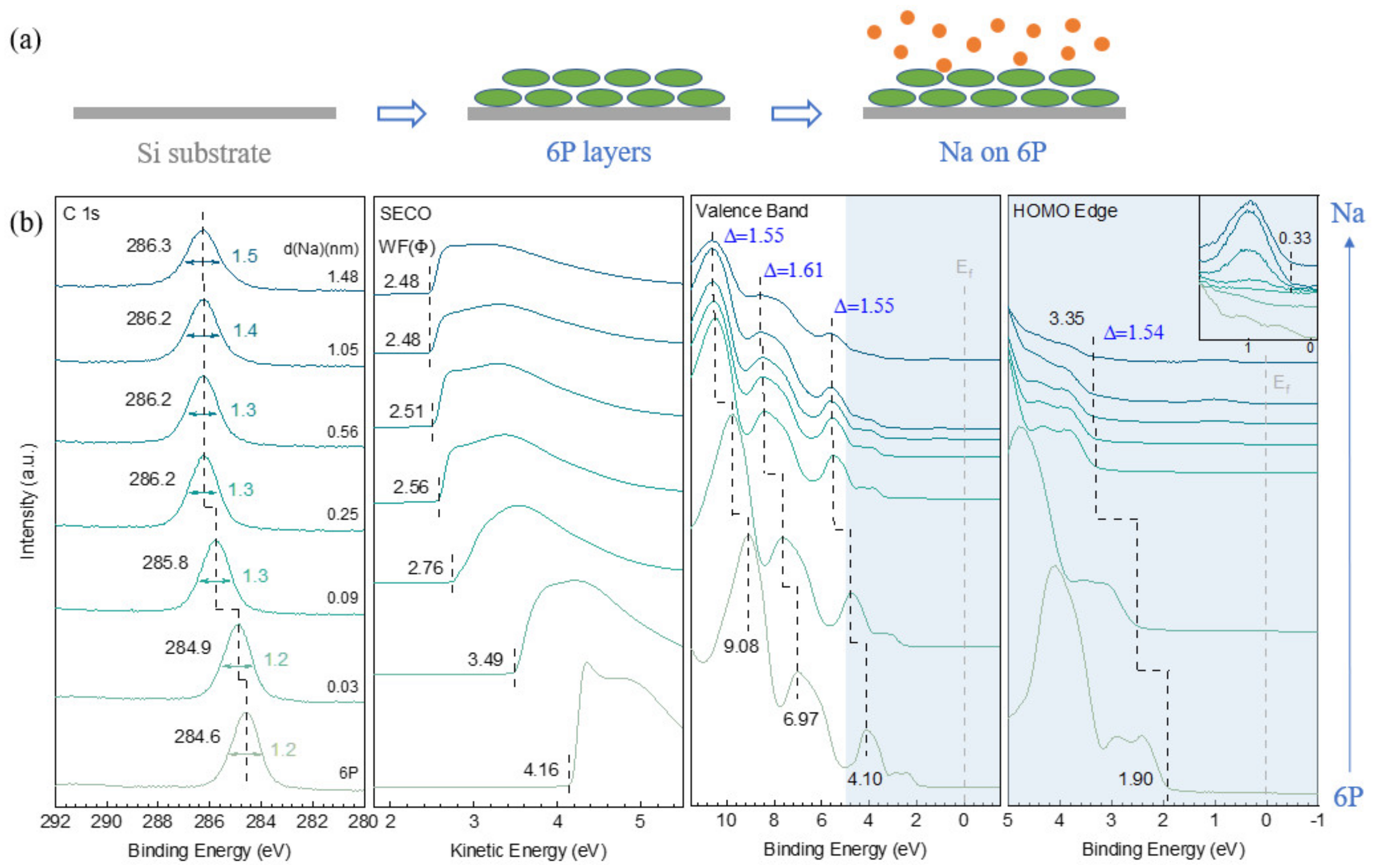
3.2.1. Na on 6P
3.2.2. 6P on Na
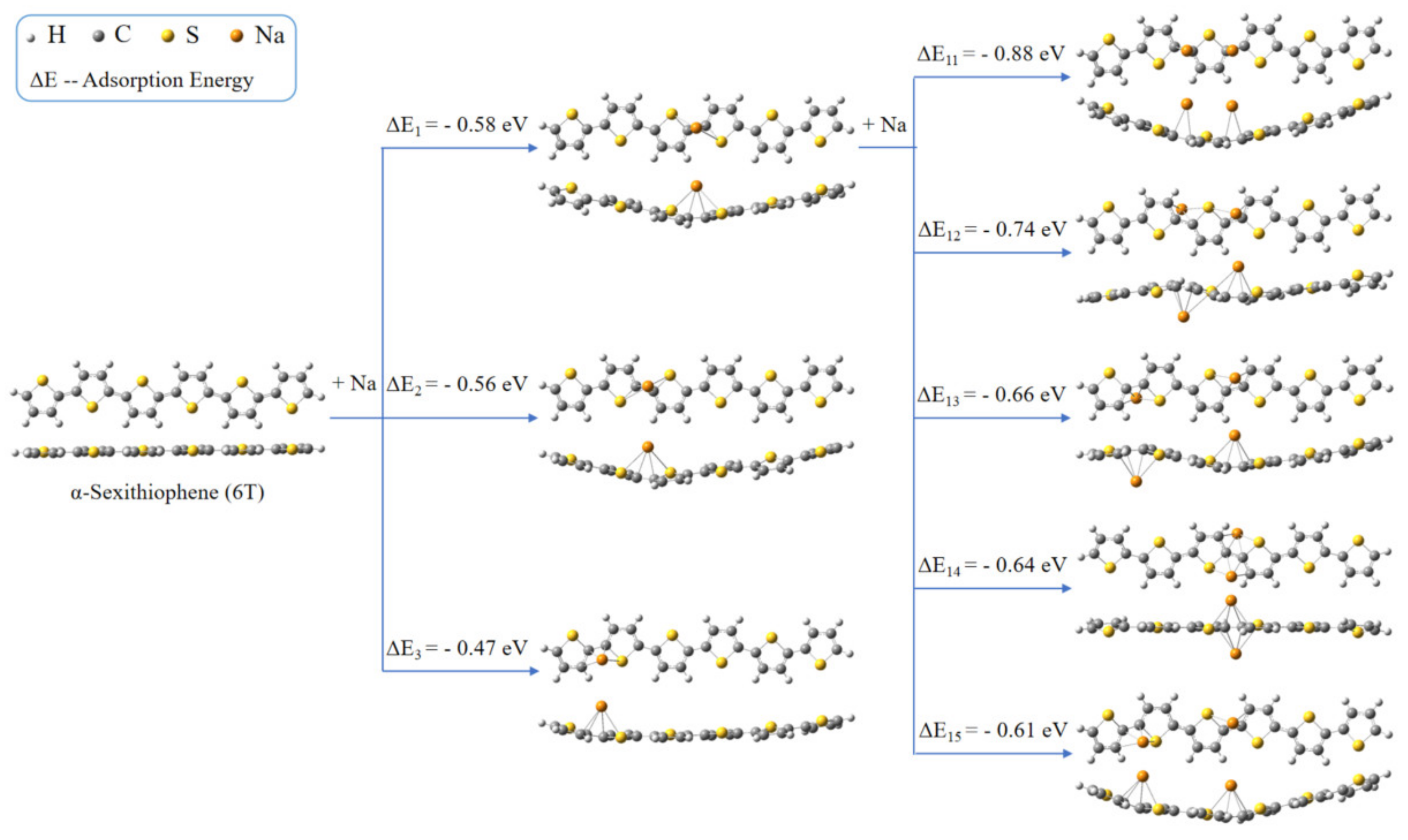
3.3. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Hua, Y.; Xu, Z.; Yu, J.S. Recent Advanced Development of Artificial Interphase Engineering for Stable Sodium Metal Anodes. Small 2022, 18, 2102250. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Matios, E.; Luo, J.; Li, W. Combining theories and experiments to understand the sodium nucleation behavior towards safe sodium metal batteries. Chem. Soc. Rev. 2020, 49, 3783–3805. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Kim, S.; Jo, C.; Lee, J. A review on recent approaches for designing the SEI layer on sodium metal anodes. Mater. Adv. 2020, 1, 3143–3166. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Y.; Yue, J.; Pan, D.; Qi, Y.; Hu, Y.-S.; Chen, L. Advanced Na metal anodes. J. Energy Chem. 2018, 27, 1584–1596. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, K.; Liu, P.; Jiao, L. 3D Confinement Strategy for Dendrite-Free Sodium Metal Batteries. Adv. Energy Mater. 2022, 12, 2100359. [Google Scholar] [CrossRef]
- Chu, C.; Li, R.; Cai, F.; Bai, Z.; Wang, Y.; Xu, X.; Wang, N.; Yang, J.; Dou, S. Recent advanced skeletons in sodium metal anodes. Energy Environ. Sci. 2021, 14, 4318–4340. [Google Scholar] [CrossRef]
- Cui, X.Y.; Wang, Y.J.; Wu, H.D.; Lin, X.D.; Tang, S.; Xu, P.; Liao, H.G.; Zheng, M.S.; Dong, Q.F. A Carbon Foam with Sodiophilic Surface for Highly Reversible, Ultra-Long Cycle Sodium Metal Anode. Adv. Sci. 2021, 8, 2003178. [Google Scholar] [CrossRef]
- Sun, B.; Li, P.; Zhang, J.; Wang, D.; Munroe, P.; Wang, C.; Notten, P.H.; Wang, G. Dendrite-free sodium-metal anodes for high-energy sodium-metal batteries. Adv. Mater. 2018, 30, 1801334. [Google Scholar] [CrossRef]
- He, X.; Jin, S.; Miao, L.; Cai, Y.; Hou, Y.; Li, H.; Zhang, K.; Yan, Z.; Chen, J. A 3D hydroxylated MXene/carbon nanotubes composite as a scaffold for dendrite-free sodium-metal electrodes. Angew. Chem. Int. Ed. 2020, 59, 16705–16711. [Google Scholar] [CrossRef]
- Wang, H.; Matios, E.; Wang, C.; Luo, J.; Lu, X.; Hu, X.; Zhang, Y.; Li, W. Tin nanoparticles embedded in a carbon buffer layer as preferential nucleation sites for stable sodium metal anodes. J. Mater. Chem. A 2019, 7, 23747–23755. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; Mou, J.; Xue, S.; Huang, S.; Hu, J.; Dong, Q.; Yang, C.; Liu, M. Single Cobalt Atoms Decorated N-doped Carbon Polyhedron Enabled Dendrite-Free Sodium Metal Anode. Small Methods 2021, 5, 2100833. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Du, C.F.; Zhong, S.; Jiang, Y.; Yu, H.; Sun, W.; Pan, H.; Rui, X.; Yu, Y. Homogeneous Na Deposition Enabling High-Energy Na-Metal Batteries. Adv. Funct. Mater. 2022, 32, 2110280. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, J.; Sun, Y.; Cui, Y. A highly reversible room-temperature sodium metal anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Jie, Y.; Lang, S.; Song, J.; Lei, Z.; Wang, S.; Ren, X.; Wang, D.; Li, X. Stable sodium metal batteries via manipulation of electrolyte solvation structure. Small Methods 2020, 4, 1900856. [Google Scholar]
- Cao, R.; Mishra, K.; Li, X.; Qian, J.; Engelhard, M.H.; Bowden, M.E.; Han, K.S.; Mueller, K.T.; Henderson, W.A.; Zhang, J.-G. Enabling room temperature sodium metal batteries. Nano Energy 2016, 30, 825–830. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Zhao, W.; Song, J.; Engelhard, M.H.; Zhang, J.-G. Extremely stable sodium metal batteries enabled by localized high-concentration electrolytes. ACS Energy Lett. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.; Xu, X.; Liao, M.; Li, Y.-Y.; Angell, M.; Gu, M.; Zhu, Y.; Hung, W.H.; Li, J. A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte. Nat. Commun. 2019, 10, 3302. [Google Scholar] [CrossRef]
- Hu, X.; Matios, E.; Zhang, Y.; Wang, C.; Luo, J.; Li, W. Deeply cycled sodium metal anodes at low temperature and in lean electrolyte conditions. Angew. Chem. 2021, 133, 6043–6048. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Lu, Z.; Lin, K.; Lyu, Y.-Q.; Li, B.; Ciucci, F.; Kim, J.-K. Non-flammable electrolyte for dendrite-free sodium-sulfur battery. Energy Storage Mater. 2019, 23, 8–16. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Z.; Huang, X.; Lu, X.; Xie, J.; Cheng, S. Nitrofullerene as an electrolyte-compatible additive for high-performance sodium metal batteries. Nano Energy 2021, 89, 106396. [Google Scholar] [CrossRef]
- Shi, Q.; Zhong, Y.; Wu, M.; Wang, H.; Wang, H. High-performance sodium metal anodes enabled by a bifunctional potassium salt. Angew. Chem. 2018, 130, 9207–9210. [Google Scholar] [CrossRef]
- Teng, W.; Wu, J.; Liang, Q.; Deng, J.; Xu, Y.; Liu, Q.; Wang, B.; Ma, T.; Nan, D.; Liu, J. Designing Advanced Liquid Electrolytes for Alkali Metal Batteries: Principles, Progress, and Perspectives. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Bao, C.; Wang, B.; Liu, P.; Wu, H.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Solid electrolyte interphases on sodium metal anodes. Adv. Funct. Mater. 2020, 30, 2004891. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Yu, Y.; Zhao, X.; Chen, Q.; Zhao, L.; Di, Q.; Ju, H.; Quan, Z. Poly (vinylidene difluoride) coating on Cu current collector for high-performance Na metal anode. Energy Storage Mater. 2020, 24, 588–593. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, G.; Liu, X.; Guo, B.; Xu, G.; Huang, Z.; Wu, M.; Liu, H.K.; Dou, S.X.; Wu, C. Dendrite-free sodium metal anodes enabled by a sodium benzenedithiolate-rich protection layer. Angew. Chem. 2020, 132, 6658–6662. [Google Scholar] [CrossRef]
- Tian, H.; Shao, H.; Chen, Y.; Fang, X.; Xiong, P.; Sun, B.; Notten, P.H.; Wang, G. Ultra-stable sodium metal-iodine batteries enabled by an in-situ solid electrolyte interphase. Nano Energy 2019, 57, 692–702. [Google Scholar] [CrossRef]
- Choudhury, S.; Wei, S.; Ozhabes, Y.; Gunceler, D.; Zachman, M.J.; Tu, Z.; Shin, J.H.; Nath, P.; Agrawal, A.; Kourkoutis, L.F. Designing solid-liquid interphases for sodium batteries. Nat. Commun. 2017, 8, 898. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, S.; Lu, G.; Wang, L.; Jiang, Y.; Liu, F.; Yao, Y.; Yang, H.; Ma, M.; Ye, S. Red Phosphorous-Derived Protective Layers with High Ionic Conductivity and Mechanical Strength on Dendrite-Free Sodium and Potassium Metal Anodes. Adv. Energy Mater. 2021, 11, 2003381. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Lushington, A.; Sun, Q.; Yadegari, H.; Wang, B.; Xiao, W.; Li, R.; Sun, X. Superior stable and long life sodium metal anodes achieved by atomic layer deposition. Adv. Mater. 2017, 29, 1606663. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, W.; Chen, Q.; Yu, Y.; Zhao, X.; Tang, M.; Zheng, Y.; Quan, Z. Hybrid protective layer for stable sodium metal anodes at high utilization. ACS Appl. Mater. Interfaces 2019, 11, 37693–37700. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, H.; Noh, H.; Lee, J.; Kim, S.; Ryou, M.-H.; Lee, Y.M.; Kim, H.-T. Enhancing the cycling stability of sodium metal electrodes by building an inorganic–organic composite protective layer. ACS Appl. Mater. Interfaces 2017, 9, 6000–6006. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Matios, E.; Li, W. Facile stabilization of the sodium metal anode with additives: Unexpected key role of sodium polysulfide and adverse effect of sodium nitrate. Angew. Chem. 2018, 130, 7860–7863. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.-M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar]
- Wang, K.; Li, X.; Gao, J.; Sun, Q.; Yang, Z.; He, J.; Cui, S.; Huang, C. Self-Regulation Seaweed-Like Lithium Metal Anode Enables Stable Cycle Life of Lithium Battery. Adv. Funct. Mater. 2021, 31, 2009917. [Google Scholar] [CrossRef]
- Yao, W.; Xu, M.; Qiu, W.; Wang, J.; Sun, Y.; Xu, J.; Zhang, Q. Ultralight PEDOT Functionalized Separators toward High-Performance Lithium Metal Anodes. ChemElectroChem 2021, 8, 2836–2845. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, W.; Zheng, H.; Su, Y.; Liang, X.; Chen, C.; Xiang, H. A multifunctional thiophene-based electrolyte additive for lithium metal batteries using high-voltage LiCoO2 cathode. J. Electrochem. Soc. 2019, 166, A3222. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Li, J. Carbon nanotube enables high-performance thiophene-containing organic anodes for lithium ion batteries. Electrochim. Acta 2022, 408, 139947. [Google Scholar]
- Hou, C.-C.; Ma, C.; Zhang, S.-N.; Wang, L.-Y.; Wang, K.-X.; Chen, J.-S. Polymeric Schiff Base with Thiophene Rings for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 13802–13807. [Google Scholar] [CrossRef]
- Liu, Y.; Lian, X.; Xie, Z.; Yang, J.; Ding, Y.; Chen, W. Probing fluorination promoted sodiophilic sites with model systems of F16CuPc and CuPc. Front. Optoelectron. 2022, 15, 19. [Google Scholar]
- Powell, C. The quest for universal curves to describe the surface sensitivity of electron spectroscopies. J. Electron. Spectrosc. Relat. Phenom. 1988, 47, 197–214. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Ivanco, J.; Haber, T.; Resel, R.; Netzer, F.P.; Ramsey, M.G. Electronic and geometric structure of electro-optically active organic films and associated interfaces. Thin Solid Film. 2006, 514, 156–164. [Google Scholar] [CrossRef]
- Toyoshima, H.; Inoue, K.; Hiraga, K.; Ohno, S.; Tanaka, M. Determination of molecular orientation of α-sexithiophene on passivated Si (001) by means of optical reflectance spectroscopic methods. Surf. Sci. 2013, 616, 36–43. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Wee, A.T.S. One-dimensional molecular chains with dispersive electronic states. Nano Lett. 2009, 9, 4292–4296. [Google Scholar] [CrossRef] [PubMed]
- Duhm, S.; Heimel, G.; Salzmann, I.; Glowatzki, H.; Johnson, R.L.; Vollmer, A.; Rabe, J.P.; Koch, N. Orientation-dependent ionization energies and interface dipoles in ordered molecular assemblies. Nat. Mater. 2008, 7, 326–332. [Google Scholar] [CrossRef]
- Wagner, T.; Fritz, D.R.; Zeppenfeld, P. α-6T on Ag(110): The formation of the wetting layer. Synth. Met. 2011, 161, 2006–2010. [Google Scholar] [CrossRef]
- Glowatzki, H.; Duhm, S.; Braun, K.F.; Rabe, J.P.; Koch, N. Molecular chains and carpets of sexithiophenes on Au(111). Phys. Rev. B 2007, 76, 125425. [Google Scholar] [CrossRef]
- Grobosch, M.; Knupfer, M. Electronic properties of the interface between the organic semiconductor α-sexithiophene and polycrystalline palladium. Org. Electron. 2008, 9, 767–774. [Google Scholar] [CrossRef]
- Schwieger, T.; Liu, X.; Peisert, H.; Adolphi, B.; Kiriy, N.; Knupfer, M. Electronic properties of interfaces between different sexithiophenes and gold. J. Appl. Phys. 2005, 97, 123712. [Google Scholar] [CrossRef]
- Grobosch, M.; Knupfer, M. Energy level alignment and interface states at α-sexithiophene/Ag interfaces. Org. Electron. 2007, 8, 625–630. [Google Scholar] [CrossRef]
- Blumstengel, S.; Koch, N.; Sadofev, S.; Schäfer, P.; Glowatzki, H.; Johnson, R.L.; Rabe, J.P.; Henneberger, F. Interface formation and electronic structure of α-sexithiophene on ZnO. Appl. Phys. Lett. 2008, 92, 193303. [Google Scholar] [CrossRef]
- Endres, J.; Pelczer, I.; Rand, B.P.; Kahn, A. Determination of Energy Level Alignment within an Energy Cascade Organic Solar Cell. Chem. Mater. 2016, 28, 794–801. [Google Scholar] [CrossRef]
- Ivanco, J.; Netzer, F.P.; Ramsey, M.G. Dissociation of sexithiophene on Al(111) surface. Org. Electron. 2007, 8, 545–551. [Google Scholar] [CrossRef]
- Koch, N.; Chan, C.; Kahn, A.; Schwartz, J. Lack of thermodynamic equilibrium in conjugated organic molecular thin films. Phys. Rev. B 2003, 67, 195330. [Google Scholar] [CrossRef]
- Koch, N.; Yu, L.M.; Parenté, V.; Lazzaroni, R.; Johnson, R.L.; Leising, G.; Pireaux, J.J.; Brédas, J.L. Evidence for physisorption of aluminum on the surface of electroluminescent sexiphenyl. Adv. Mater. 1998, 10, 1038–1043. [Google Scholar] [CrossRef]
- Schroeder, P.G.; France, C.B.; Parkinson, B.A.; Schlaf, R. Orbital alignment atp-sexiphenyl and coronene/layered materials interfaces measured with photoemission spectroscopy. J. Appl. Phys. 2002, 91, 9095–9107. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Koch, N.; Jäckel, F.; Ghijsen, J.; Rojas, M.C.; Grioni, M.; Rabe, J.P.; Johnson, R.L.; Kahn, A.; Pireaux, J.J. Observation of filled states at the Fermi-level in alkali-metal intercalated organic films: Dependence on substrate work function. J. Electron. Spectrosc. Relat. Phenom. 2005, 144–147, 495–498. [Google Scholar] [CrossRef]
- Koch, N.; Rajagopal, A.; Ghijsen, J.; Johnson, R.; Leising, G.; Pireaux, J.-J. Bipolaron: The stable charged species in n-doped p-sexiphenyl. J. Phys. Chem. B 2000, 104, 1434–1438. [Google Scholar] [CrossRef]
- Andreev, A. Morphology and growth kinetics of organic thin films deposited by hot wall epitaxy. Org. Electron. 2004, 5, 23–27. [Google Scholar] [CrossRef]
- Teichert, C.; Hlawacek, G.; Andreev, A.Y.; Sitter, H.; Frank, P.; Winkler, A.; Sariciftci, N.S. Spontaneous rearrangement of para-sexiphenyl crystallites into nano-fibers. Appl. Phys. A 2005, 82, 665–669. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lian, X.; Jiang, C.; Sun, Z.; Yang, J.; Ding, Y.; Chen, W. In-Situ Photoelectron Spectroscopy Investigation of Sulfurization-Induced Sodiophilic Sites with Model Systems of α-sexithiophene and p-sexiphenyl. Batteries 2023, 9, 21. https://doi.org/10.3390/batteries9010021
Liu Y, Lian X, Jiang C, Sun Z, Yang J, Ding Y, Chen W. In-Situ Photoelectron Spectroscopy Investigation of Sulfurization-Induced Sodiophilic Sites with Model Systems of α-sexithiophene and p-sexiphenyl. Batteries. 2023; 9(1):21. https://doi.org/10.3390/batteries9010021
Chicago/Turabian StyleLiu, Yuan, Xu Lian, Chonglai Jiang, Zejun Sun, Jinlin Yang, Yishui Ding, and Wei Chen. 2023. "In-Situ Photoelectron Spectroscopy Investigation of Sulfurization-Induced Sodiophilic Sites with Model Systems of α-sexithiophene and p-sexiphenyl" Batteries 9, no. 1: 21. https://doi.org/10.3390/batteries9010021
APA StyleLiu, Y., Lian, X., Jiang, C., Sun, Z., Yang, J., Ding, Y., & Chen, W. (2023). In-Situ Photoelectron Spectroscopy Investigation of Sulfurization-Induced Sodiophilic Sites with Model Systems of α-sexithiophene and p-sexiphenyl. Batteries, 9(1), 21. https://doi.org/10.3390/batteries9010021









