Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition
Abstract
:1. Introduction
2. Results
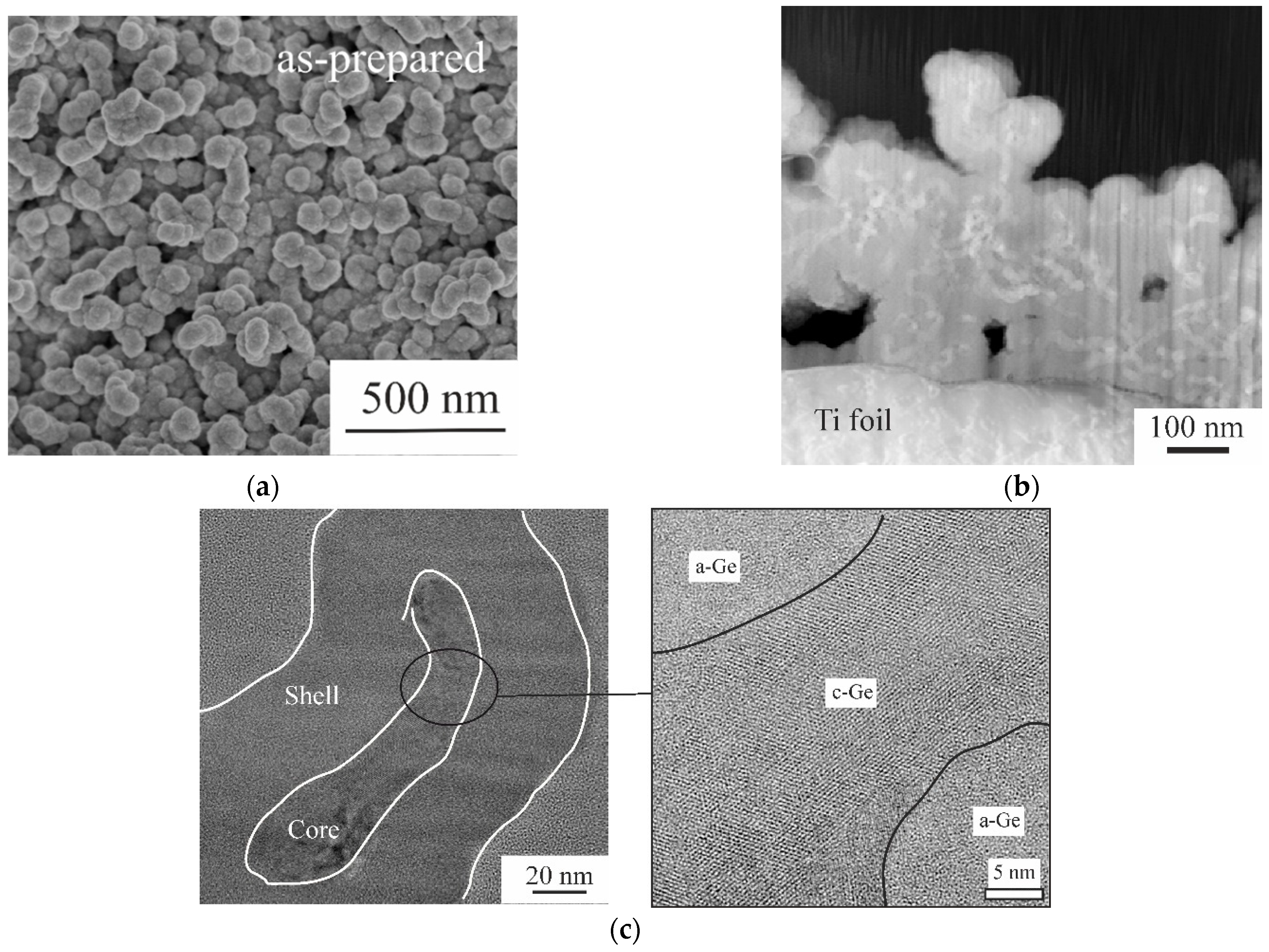
2.1. Morphological and Physical Studies of Ge Samples before Sodium Insertion/Extraction
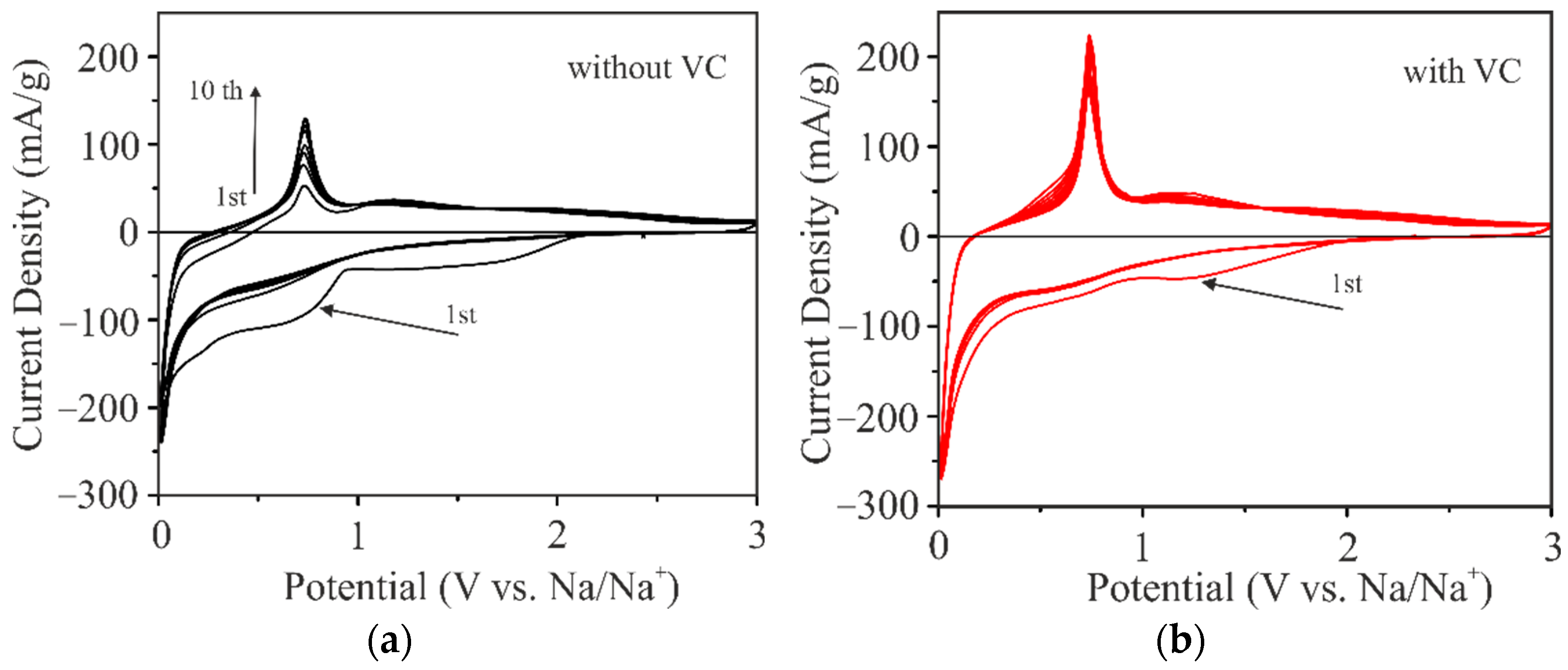
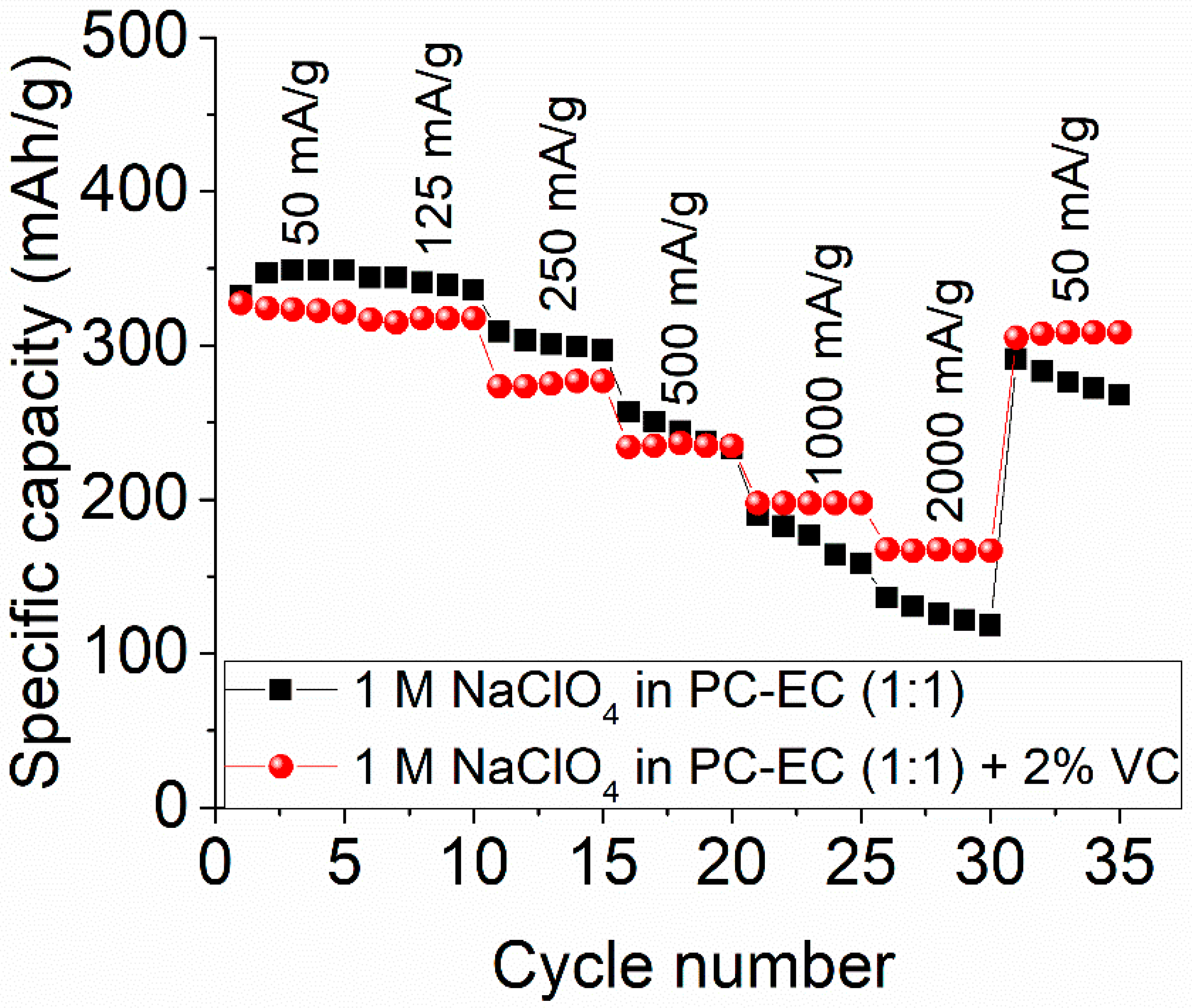
2.2. Electrochemical Studies of Sodium Insertion/Extraction
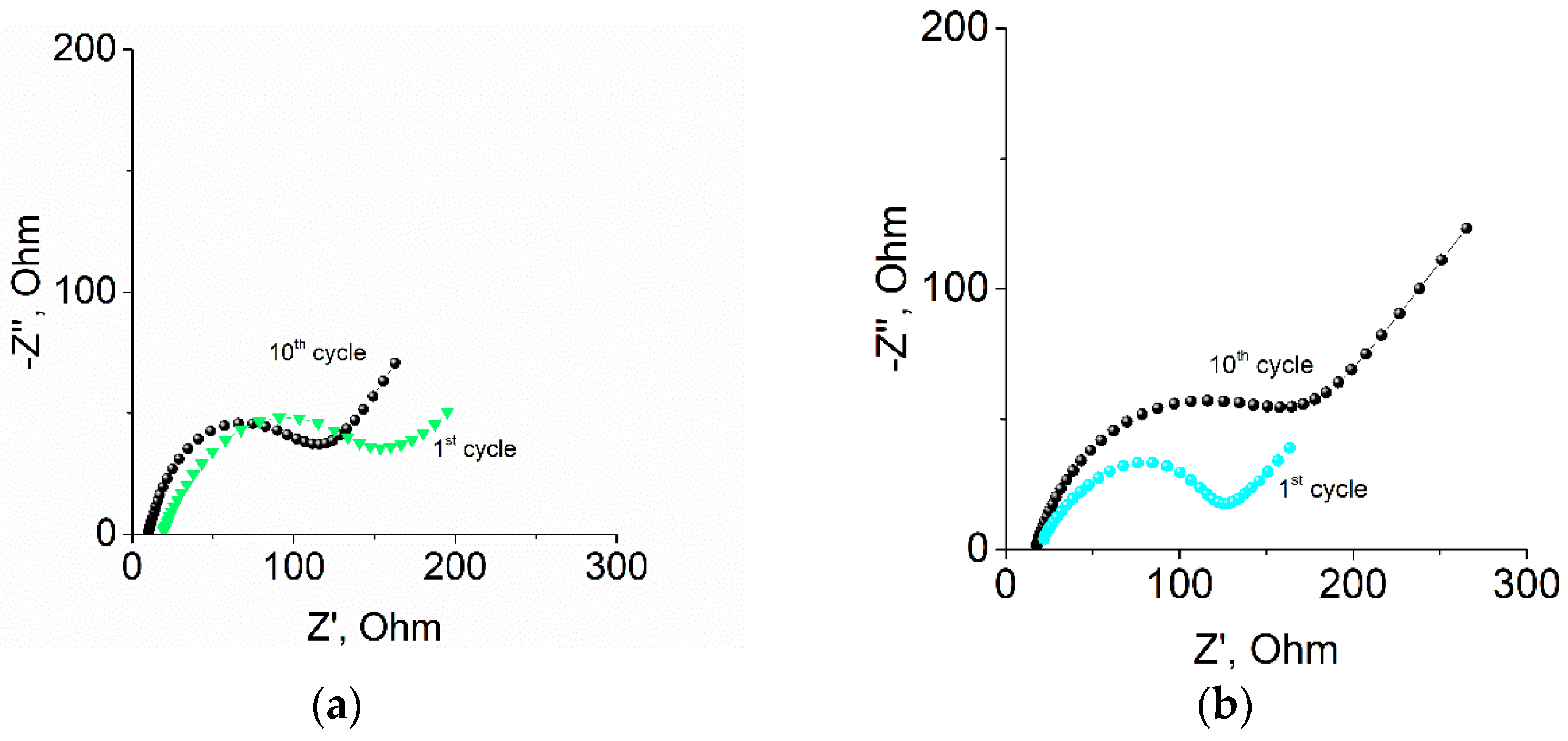
2.3. Impedance Measurements of Samples
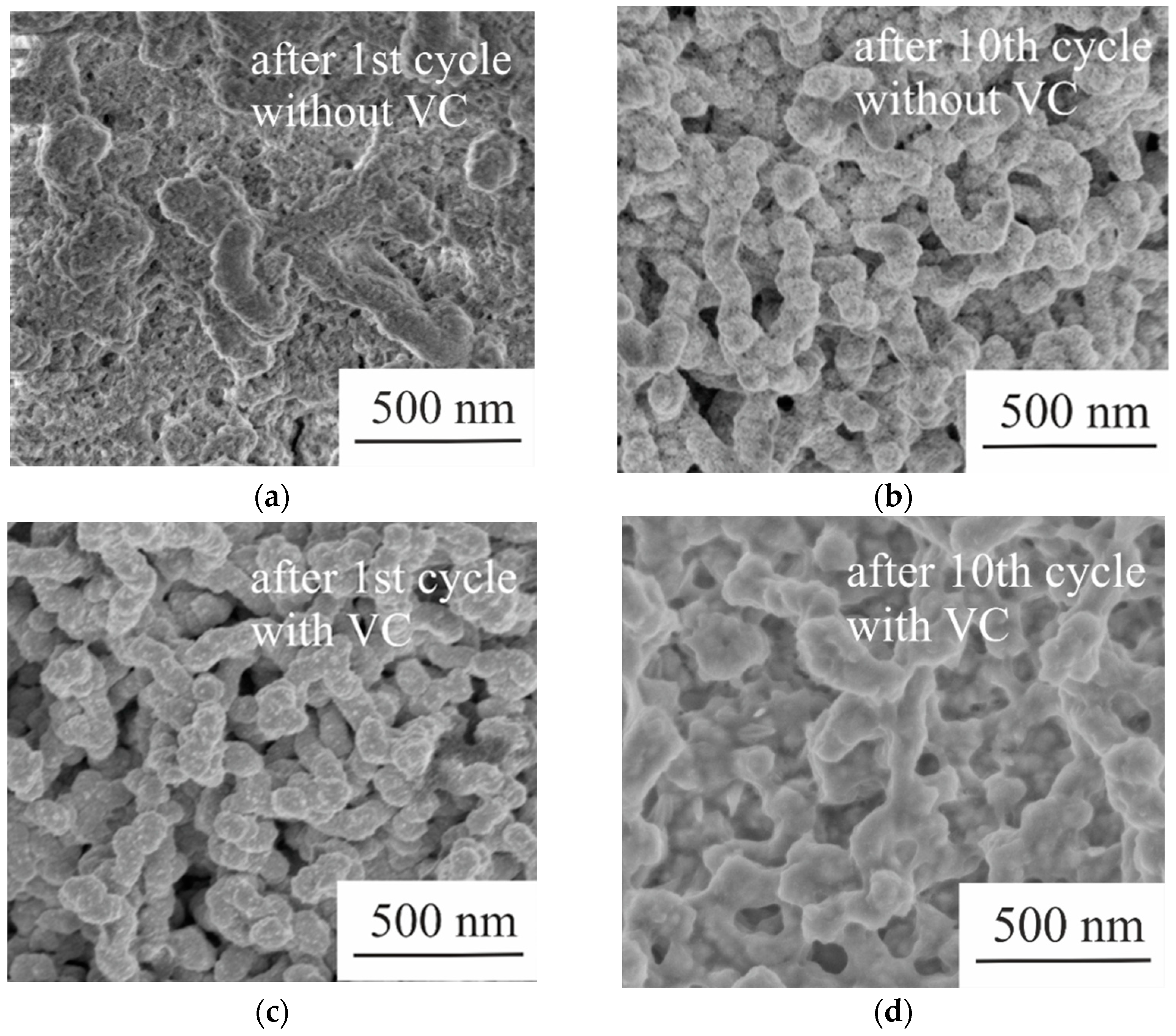
2.4. Morphological and Physical Studies of Samples after Sodium Insertion/Extraction
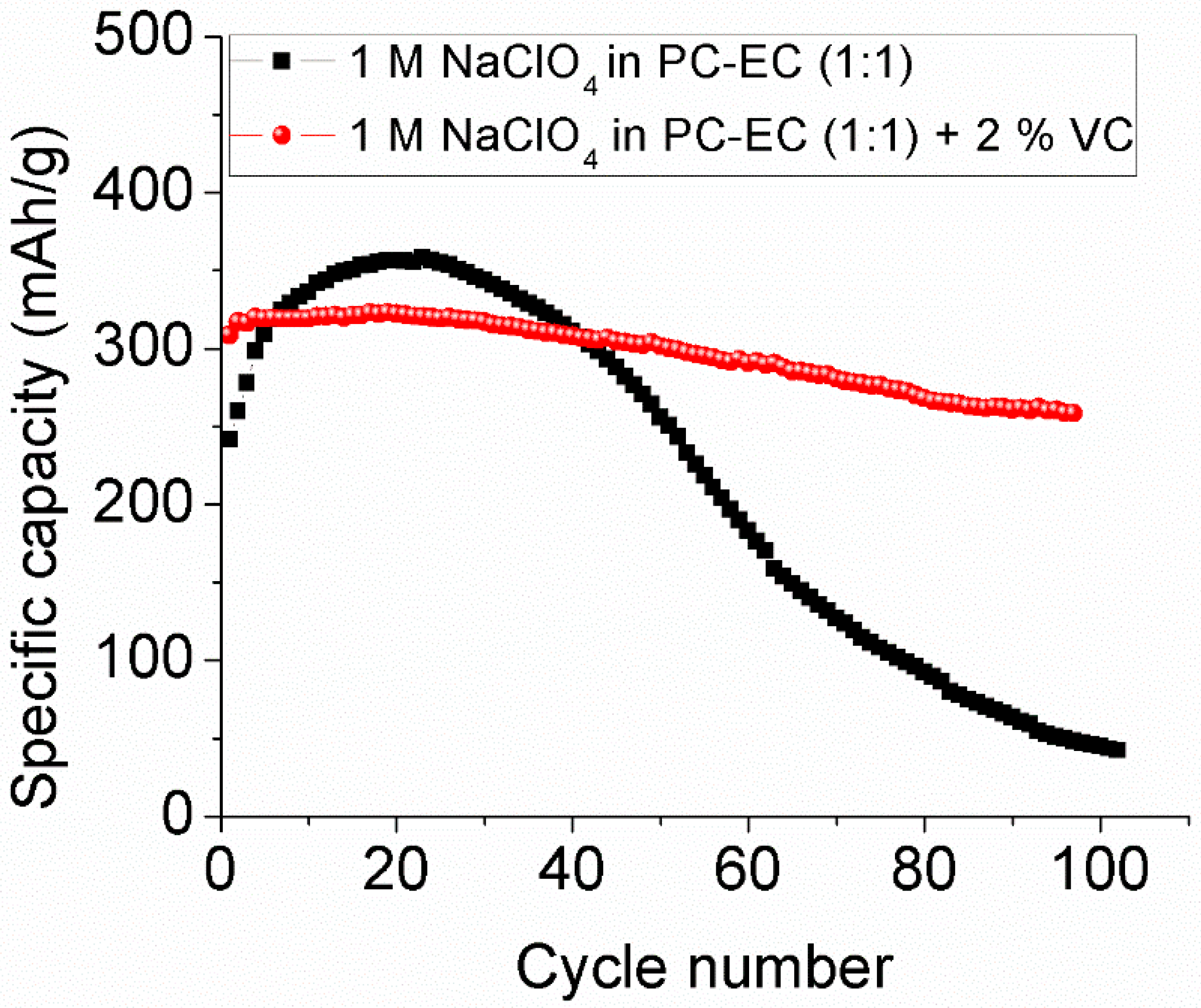
2.5. Long-Term Cycling of Samples
3. Discussion
4. Materials and Methods
4.1. Samples Preparation
4.2. Material Characterization
4.3. Electrochemical Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Hu, Y.-S.; Lu, Y. Nobel prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries. ACS Energy Lett. 2019, 4, 2689–2690. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Talaie, E.; Duffort, V.; Nazar, L.F. The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 3431–3448. [Google Scholar] [CrossRef]
- Skundin, A.M.; Kulova, T.L.; Yaroslavtsev, A.B. Sodium-Ion Batteries (a Review). Russ. J. Electrochem. 2018, 54, 113–152. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Sb–Si Alloys and Multilayers for Sodium-Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 2, 2205–2213. [Google Scholar] [CrossRef]
- Farbod, B.; Cui, K.; Kalisvaart, W.P.; Kupsta, M.; Zahiri, B.; Kohandehghan, A.; Lotfabad, E.M.; Li, Z.; Luber, E.J.; Mitlin, D. Anodes for Sodium Ion Batteries Based on Tin–Germanium–Antimony Alloys. ACS Nano 2014, 8, 4415–4429. [Google Scholar] [CrossRef]
- Niu, L.; Guo, S.; Liang, W.; Song, L.; Song, B.; Zhang, Q.; Wu, L. In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics. Crystals 2022, 12, 575. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Chen, C.; Guo, H.; Liu, X.; Xu, G.; Zhong, H.; Cheng, S.; Wu, P.; Meng, J.; et al. In operando mechanism analysis on nanocrystalline silicon anode material for reversible and ultrafast sodium storage. Adv. Mater. 2017, 29, 1604708. [Google Scholar] [CrossRef]
- Gibertini, E.; Liberale, F.; Dossi, C.; Binda, G.; Mattioli, B.; Bettinetti, R.; Maspero, A.; Fiore, M.; Ruffo, R.; Magagnin, L. Algae-derived hard carbon anodes for Na-ion batteries. J. Appl. Electrochem. 2021, 51, 1665–1673. [Google Scholar] [CrossRef]
- Simone, V.; Boulineau, A.; de Geyer, A.; Rouchon, D.; Simonin, L.; Martinet, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Jiao, X.; Zhang, C.; Dai, X.; Song, J. Stable Cycling of Phosphorus Anode for Sodium-Ion Batteries through Chemical Bonding with Sulfurized Polyacrylonitrile. Adv. Funct. Mater. 2018, 28, 1801010. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Jian, C.; Cui, D.; Chen, M.; Li, Z.; Li, T.; Nilges, T.; He, K.; Jia, Z.; et al. Red-phosphorus-impregnated carbon nanofibers for sodium-ion batteries and liquefaction of red phosphorus. Nat. Commun. 2020, 11, 2520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, A.; Shen, C.; Liu, Q.; Cao, X.; Ma, Y.; Chen, L.; Lau, C.; Chen, T.C.; Wei, F.; et al. Red phosphorus nanodots on reduced graphene oxide as a flexible and ultra-fast anode for sodium-ion batteries. ACS Nano 2017, 11, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Yaru, W.; Wang, P.; Zhao, D.; Hu, B.; Du, Y.; Xu, H.; Chang, K. Thermodynamic description of the Ge–Na and Ge–K systems using the CALPHAD approach supported by first-principles calculations. Calphad 2012, 37, 72–76. [Google Scholar] [CrossRef]
- Legrain, F.; Malyi, O.; Manzhos, S. Comparative computational study of the diffusion of Li, Na, and Mg in silicon including the effect of vibrations. Solid State Ion. 2013, 253, 157–163. [Google Scholar] [CrossRef]
- Kohandehghan, A.; Cui, K.; Kupsta, M.; Ding, J.; Lotfabad, E.M.; Kalisvaart, W.P.; Mitlin, D. Activation with Li enables facile sodium storage in germanium. Nano Lett. 2014, 14, 5873–5882. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Smolyaninov, V.A.; Dronov, A.A.; Gavrilov, S.A.; Trifonov, A.Y.; Kulova, T.L.; Kuz’mina, A.A.; Skundin, A.M. Electrochemical insertion of sodium into nanostructured materials based on germanium. Mendeleev Commun. 2018, 28, 659–660. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kulova, T.L.; Skundin, A.M.; Gavrilov, S.A. The effect of growth temperature on the process of insertion/extraction of sodium into germanium nanowires formed by electrodeposition using indium nanoparticles. Mater. Lett. 2021, 287, 129303. [Google Scholar] [CrossRef]
- Iermakov, I.; Dugas, R.; Palacín, M.R.; Ponrouch, A. On the Comparative Stability of Li and Na Metal Anode Interfaces in Conventional Alkyl Carbonate Electrolytes. J. Electrochem. Soc. 2015, 162, A7060. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Chen, Q.; Kong, X. The chemical evolution of solid electrolyte interface in sodium metal batteries. Sci. Adv. 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Mogensen, R.; Brandell, D.; Younesi, R. Solubility of the Solid Electrolyte Interphase (SEI) in Sodium Ion Batteries. ACS Energy Lett. 2016, 1, 1173–1178. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Lin, D. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.-M.; Palacín, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572. [Google Scholar] [CrossRef]
- Ushirogata, K.; Sodeyama, K.; Tateyama, Y.; Okuno, Y.; Tateyama, Y. Additive Effect on Reductive Decomposition and Binding of Carbonate-Based Solvent toward Solid Electrolyte Interphase Formation in Lithium-Ion Battery. J. Am. Chem. Soc. 2013, 135, 11967–11974. [Google Scholar] [CrossRef]
- Jaumann, T.; Balach, J.; Langklotz, U.; Sauchuk, V.; Fritsch, M.; Michaelis, A.; Teltevskij, V.; Mikhailova, D.; Oswald, S.; Klose, M. Lifetime vs. rate capability: Understanding the role of FEC and VC in high-energy Li-ion batteries with nano-silicon anodes. Energy Storage Mater. 2017, 6, 26–35. [Google Scholar] [CrossRef]
- Nie, M.; Demeaux, J.; Young, B.T.; Heskett, D.R.; Chen, Y.; Bose, A.; Woicik, J.C.; Lucht, B.L. Effect of Vinylene Carbonate and Fluoroethylene Carbonate on SEI Formation on Graphitic Anodes in Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A7008–A7014. [Google Scholar] [CrossRef]
- Ahn, S.; Fukushima, M.; Nara, H.; Momma, T.; Sugimoto, W.; Osaka, T. Effect of fluoroethylene carbonate and vinylene carbonate additives on full-cell optimization of Li-ion capacitors. Electrochem. Commun. 2021, 122, 106905. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.P.; Lucht, B.L. Fluoroethylene Carbonate and Vinylene Carbonate Reduction: Understanding Lithium-Ion Battery Electrolyte Additives and Solid Electrolyte Interphase Formation. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef] [Green Version]
- Kitz, P.G.; Lacey, M.J.; Novák, P.; Berg, E.J. Operando investigation of the solid electrolyte interphase mechanical and transport properties formed from vinylene carbonate and fluoroethylene carbonate. J. Power Sources 2020, 477, 228567. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kuz’mina, A.A.; Kulova, T.L.; Skundin, A.M.; Emets, V.V.; Volkov, R.L.; Dronov, A.A.; Borgardt, N.I.; Gavrilov, S.A. High-rate and low-temperature performance of germanium nanowires anode for lithium-ion batteries. J. Electroanal. Chem. 2021, 888, 115209. [Google Scholar] [CrossRef]
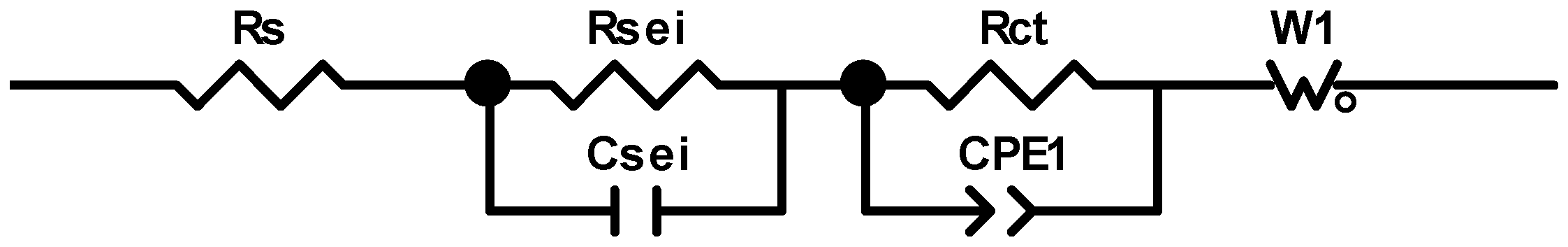

| Ge Microrods | Rs, Ohm | Rsei, Ohm/cm2 | Csei, F/cm2 | Rct, Ohm/cm2 | Cdl, F/cm2 | W, Ohm/s0.5 |
|---|---|---|---|---|---|---|
| 1M NaClO4 in PC-EC after 1 cycle | 13 | 12.55 | 4.47 × 10−7 | 295.5 | 4.3 × 10−5 | 133 |
| 1M NaClO4 in PC-EC after 10 cycles | 9 | 1.2 | 4.25 × 10−7 | 175 | 1.4 × 10−5 | 215 |
| 1M NaClO4 in PC-EC + 2%VC after 1 cycle | 13 | 1.05 | 4.7 × 10−7 | 219.7 | 6 × 10−6 | 342 |
| 1M NaClO4 in PC-EC + 2%VC after 10 cycles | 15 | 4 | 1.0 × 10−7 | 270 | 5.5 × 10−6 | 202 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, E.A.; Gavrilin, I.M.; Kudryashova, Y.O.; Martynova, I.K.; Volkov, R.L.; Kulova, T.L.; Skundin, A.M.; Borgardt, N.I.; Gavrilov, S.A. Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition. Batteries 2022, 8, 109. https://doi.org/10.3390/batteries8090109
Lebedev EA, Gavrilin IM, Kudryashova YO, Martynova IK, Volkov RL, Kulova TL, Skundin AM, Borgardt NI, Gavrilov SA. Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition. Batteries. 2022; 8(9):109. https://doi.org/10.3390/batteries8090109
Chicago/Turabian StyleLebedev, Egor A., Ilya M. Gavrilin, Yulia O. Kudryashova, Irina K. Martynova, Roman L. Volkov, Tatiana L. Kulova, Alexander M. Skundin, Nikolay I. Borgardt, and Sergey A. Gavrilov. 2022. "Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition" Batteries 8, no. 9: 109. https://doi.org/10.3390/batteries8090109
APA StyleLebedev, E. A., Gavrilin, I. M., Kudryashova, Y. O., Martynova, I. K., Volkov, R. L., Kulova, T. L., Skundin, A. M., Borgardt, N. I., & Gavrilov, S. A. (2022). Effect of Vinylene Carbonate Electrolyte Additive on the Process of Insertion/Extraction of Na into Ge Microrods Formed by Electrodeposition. Batteries, 8(9), 109. https://doi.org/10.3390/batteries8090109








