Binder-Free Ge-Co-P Anode Material for Lithium-Ion and Sodium-Ion Batteries
Abstract
:1. Introduction
2. Results
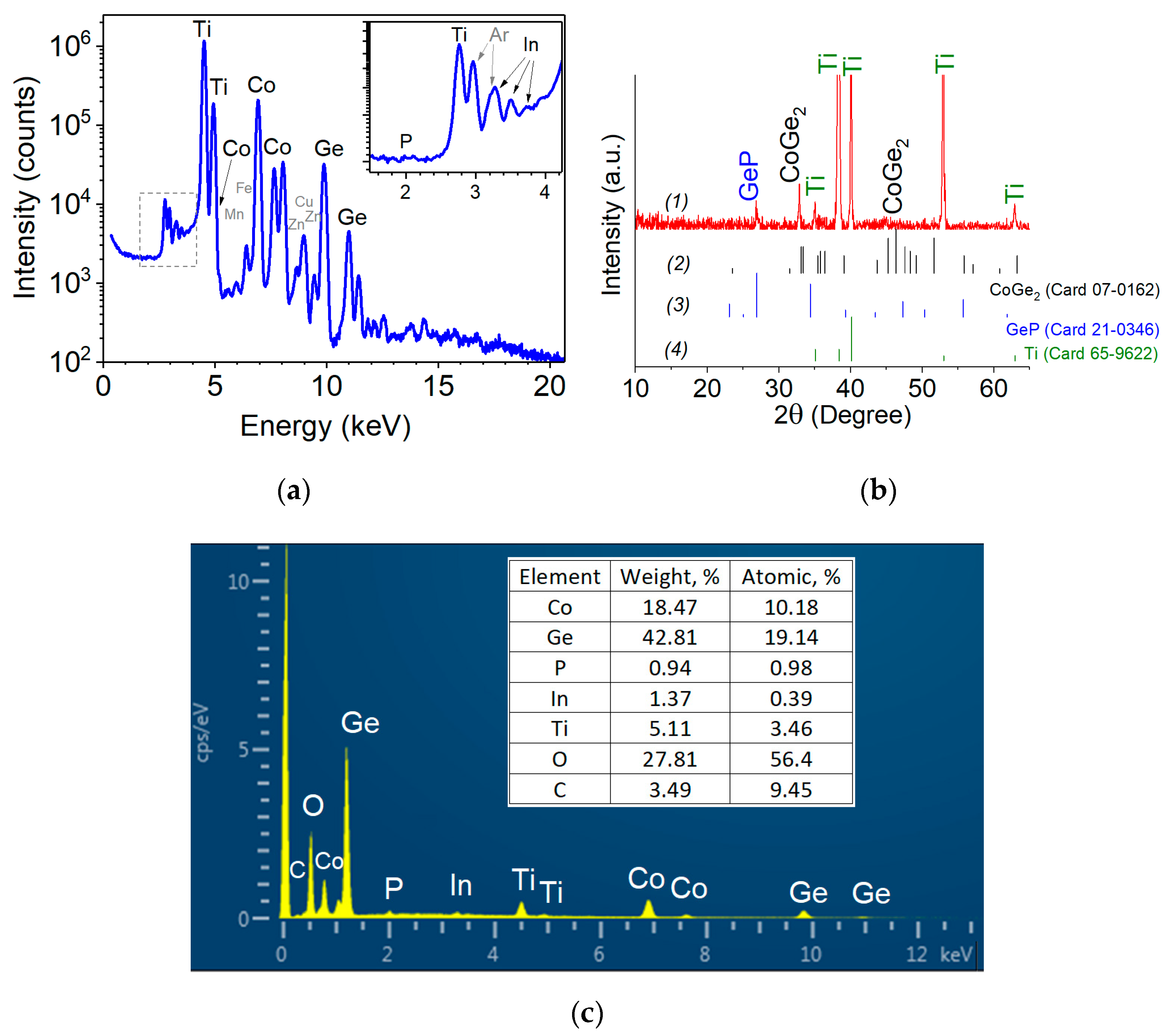
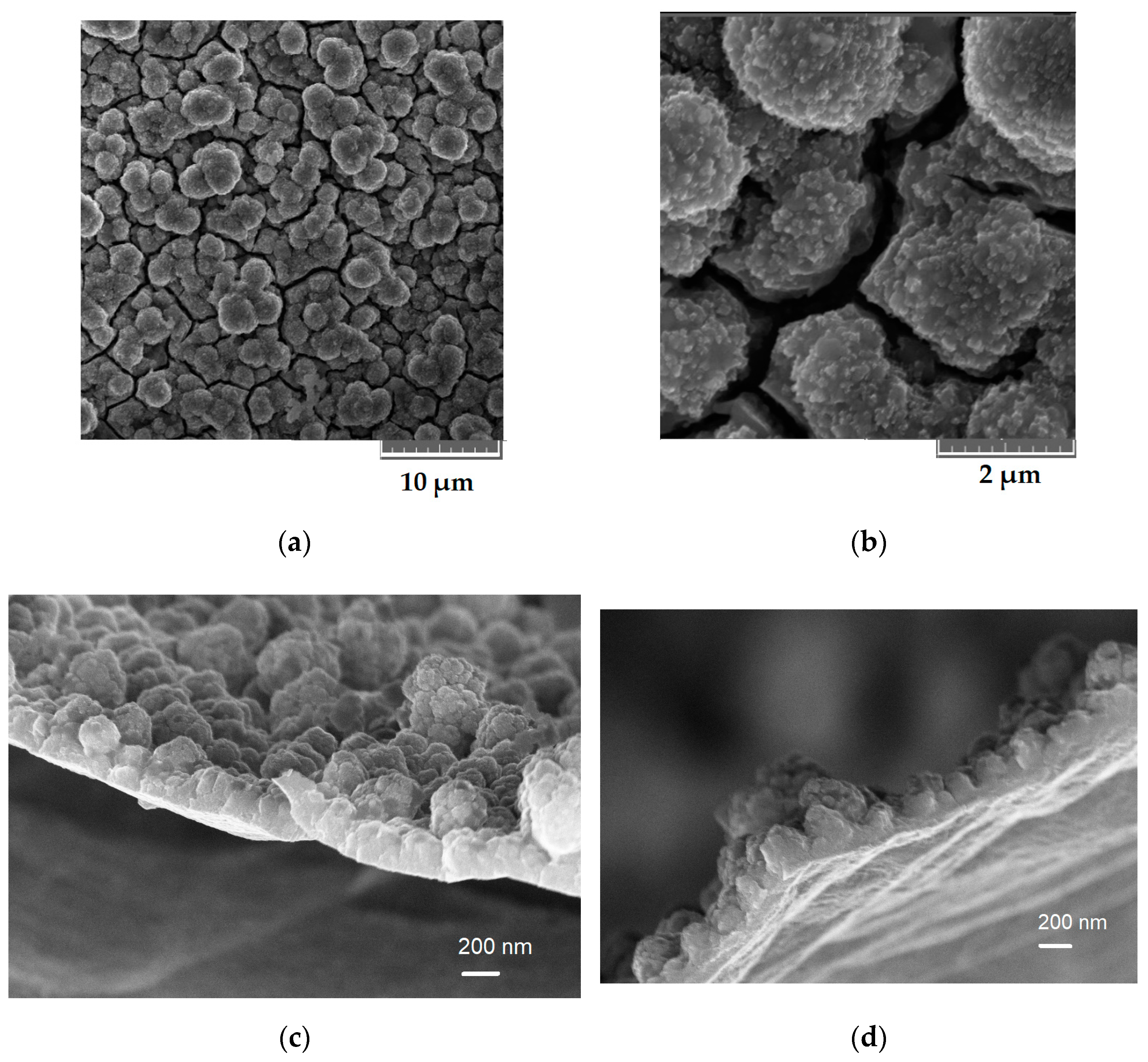
2.1. Morphological and Physical Studies
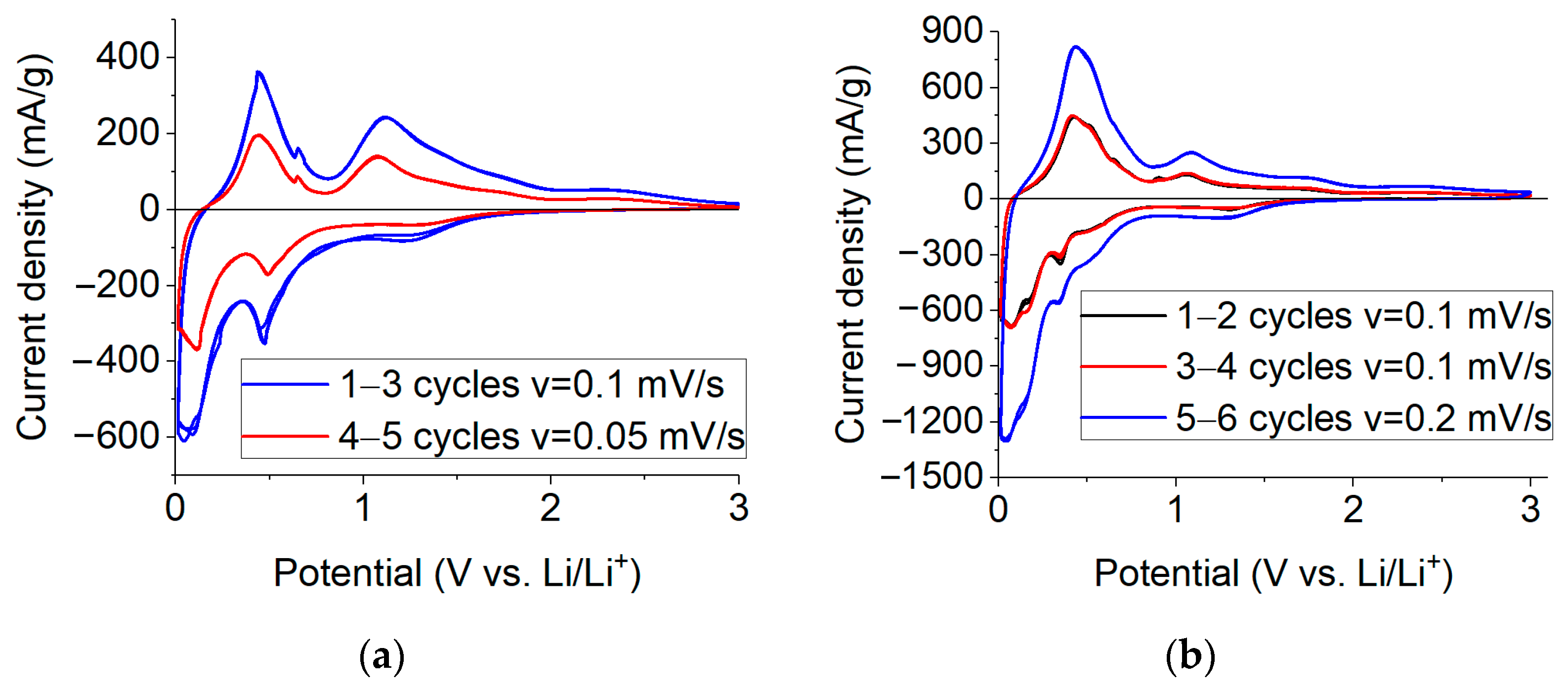
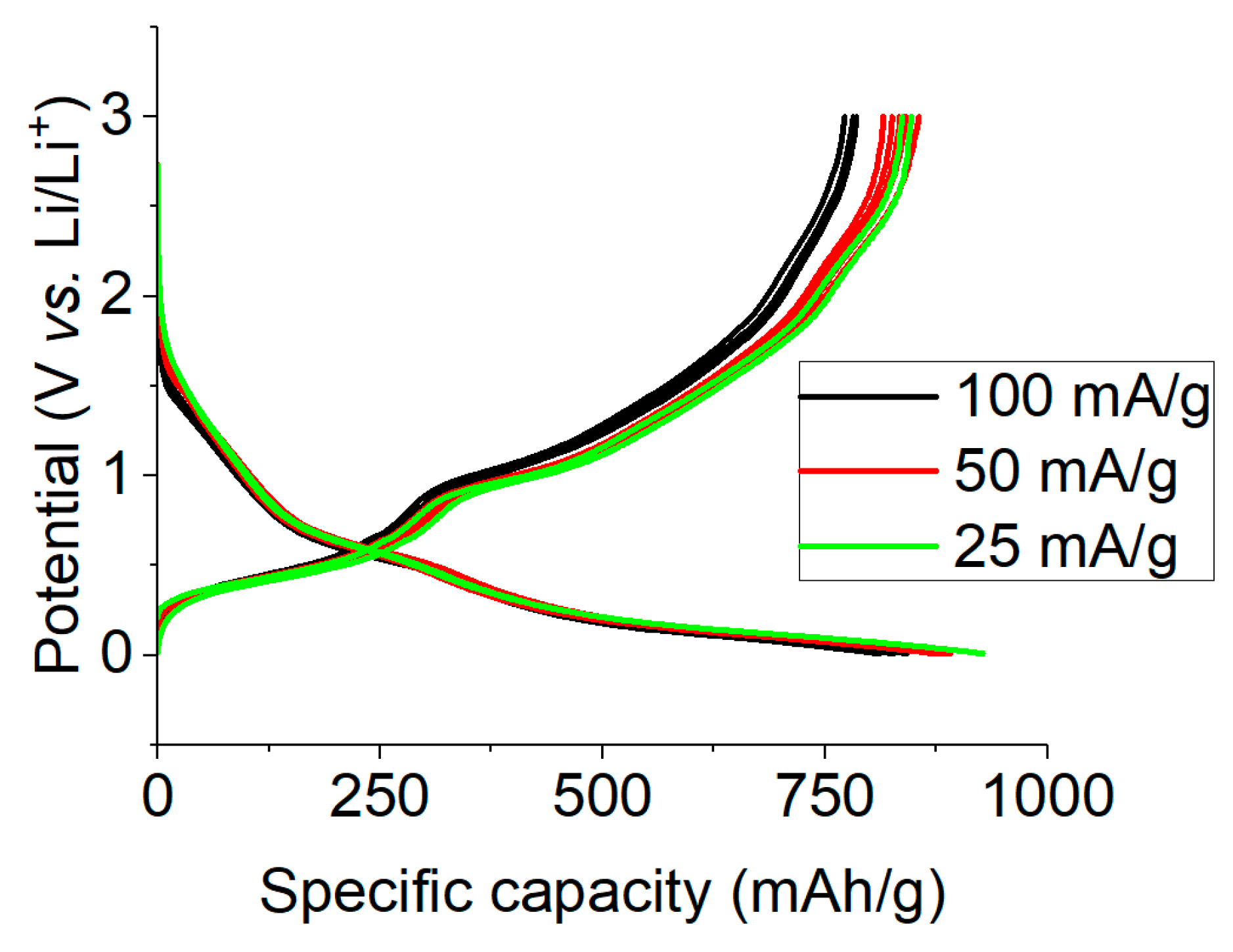
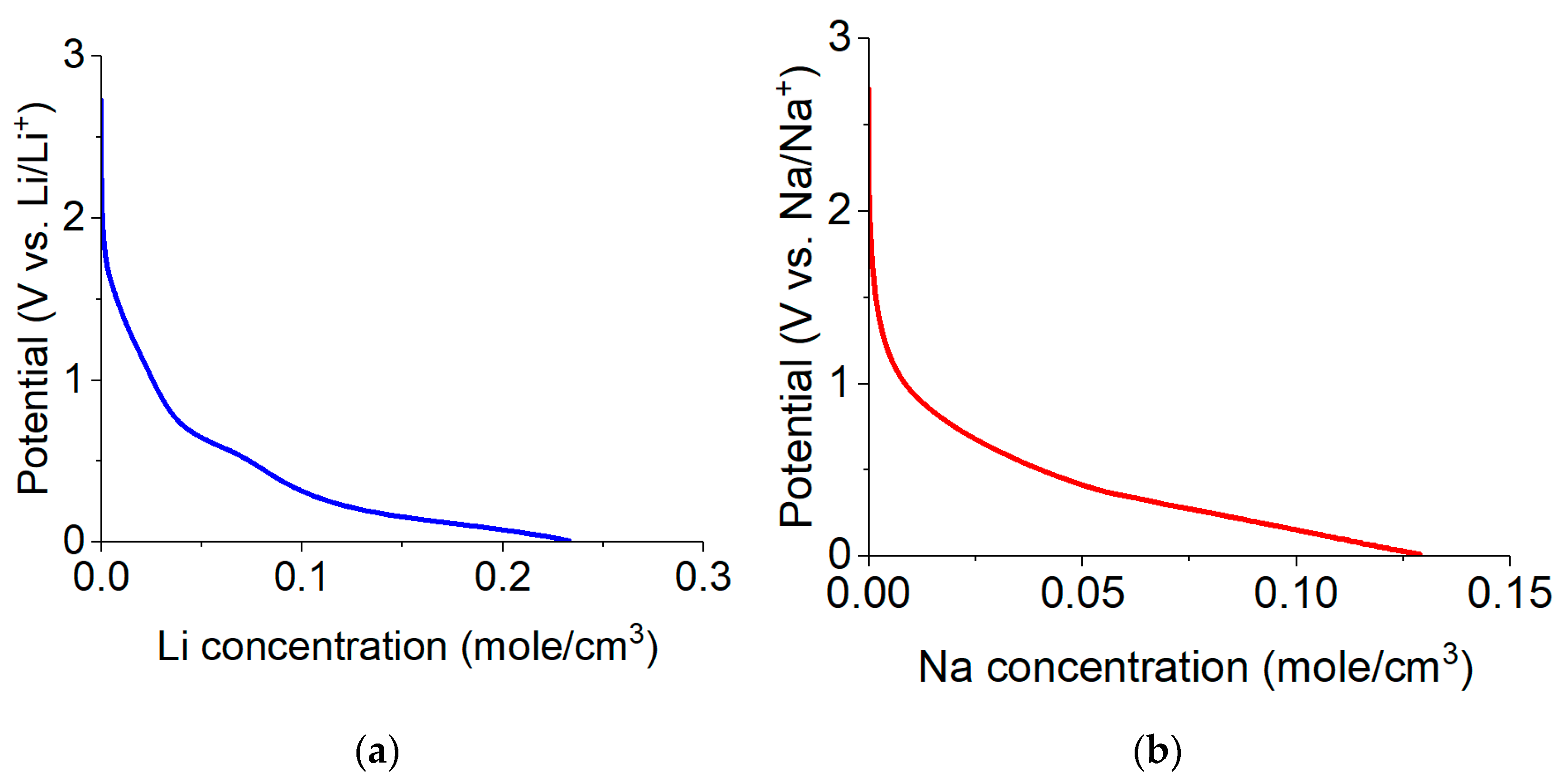
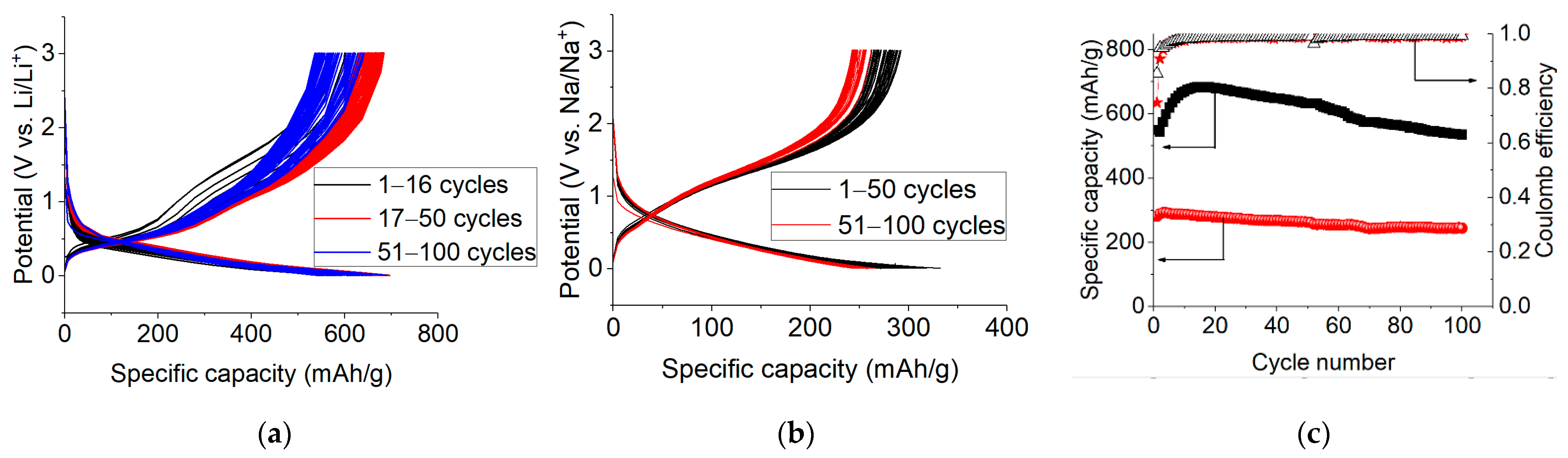
2.2. Electrochemical Studies of Lithium Insertion/Extraction
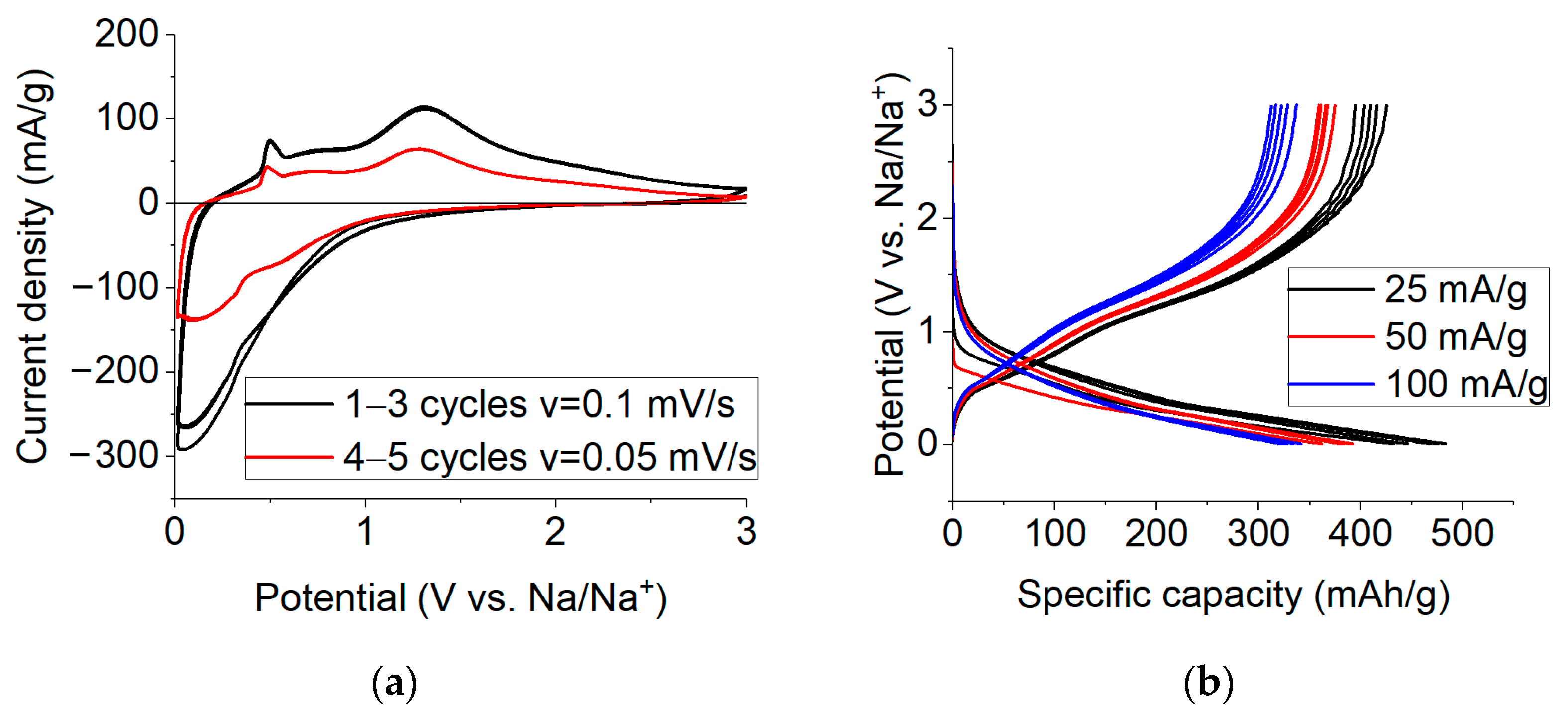
2.3. Electrochemical Studies of Sodium Insertion/Extraction
2.4. Impedance Measurements of CoGe2P0.1
2.5. Long-Term Cycling
3. Discussion
4. Materials and Methods
4.1. Samples Preparation
4.2. Samples Physical Characterization of Ge-Co-P Nanostructures
4.3. Electrochemical Characterization
4.3.1. Electrochemical Cells
4.3.2. Electrochemical Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Han, C.; Iocozzia, J.; Lu, M.; Ge, R.; Xu, R.; Lin, Z. Germanium-Based Nanomaterials for Rechargeable Batteries. Angew. Chem. Int. Ed. 2016, 55, 7898–7923. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Park, C.M. Co–Ge compounds and their electrochemical performance as high-performance Li-ion battery anodes. Mater. Today Energy 2020, 18, 100530. [Google Scholar] [CrossRef]
- Jing, Y.-Q.; Qu, J.; Jia, X.-Q.; Zhai, X.-Z.; Chang, W.; Zeng, M.-J.; Li, X.; Yu, Z.-Z. Constructing tunable core-shell Co5Ge3@Co nanoparticles on reduced graphene oxide by an interfacial bonding promoted Kirkendall effect for high lithium storage performances. Chem. Eng. J. 2021, 408, 127266. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, J.; Lei, Y.; Du, N.; Yang, D. A novel three-dimensional architecture of Co–Ge nanowires towards high-rate lithium and sodium storage. J. Alloys Compd. 2020, 815, 152281. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Yu, J.; Liao, J.; Zhao, B.; Huang, L.; Abdelhafiz, A.; Zhang, H.; Wang, J.-H.; Guo, Z.; et al. A self-healing layered GeP anode for high-performance Li-ion batteries enabled by low formation energy. Nano Energy 2019, 61, 594–603. [Google Scholar] [CrossRef]
- Shen, H.; Huang, Y.; Chang, Y.; Hao, R.; Ma, Z.; Wu, K.; Du, P.; Guo, B.; Lyu, Y.; Wang, P.; et al. Narrowing Working Voltage Window to Improve Layered GeP Anode Cycling Performance for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 17466–17473. [Google Scholar] [CrossRef]
- Yan, Y.; Ruan, J.; Xu, H.; Xu, Y.; Pang, Y.; Yang, J.; Zheng, S. Fast and Stable Batteries with High Capacity Enabled by Germanium−Phosphorus Binary Nanoparticles Embedded in a Porous Carbon Matrix via Metallothermic Reduction. ACS Appl. Mater. Interfaces 2020, 12, 21579–21585. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Hu, L.; Li, H.; Zhai, T. Aqueous Binder Enhanced High-Performance GeP5 Anode for Lithium-Ion Batteries. Front. Chem. 2018, 6, 21. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Lu, Z.; Gan, L.; Ke, L.; Zhai, T.; Zhou, H. Layered Phosphorus-Like GeP5: A Promising Anode Candidate with High Initial Coulombic Efficiency and Large Capacity for Lithium Ion Batteries. Energy Environ. Sci. 2015, 8, 3629–3636. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, X.; Fan, X.; Li, M.; Zhang, Y.; Zhang, W.; Chen, L. GeP5/C composite as anode material for high power sodium-ion batteries with exceptional capacity. J. Alloys Compd. 2018, 744, 15–22. [Google Scholar] [CrossRef]
- Li, W.; Ke, L.; Wei, Y.; Guo, S.; Gan, L.; Li, H.; Zhai, T.; Zhou, H. Highly reversible sodium storage in a GeP5/C composite anode with large capacity and low voltage. J. Mater. Chem. A 2017, 5, 4413–4420. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, S.; Nazarian-Samani, M.; Nazarian-Samani, M.; Roh, H.-K.; Chung, K.-Y.; Oh, S.-H.; Cho, B.-W.; Kashani-Bozorg, S.F.; Kim, K.-B. Exceptionally Reversible Li-/Na-Ion Storage and Ultrastable Solid-Electrolyte Interphase in Layered GeP5 Anode. ACS Appl. Mater. Interfaces 2019, 11, 32815–32825. [Google Scholar] [CrossRef]
- Shen, H.; Ma, Z.; Yang, B.; Guo, B.; Lyu, Y.; Wang, P.; Yang, H.; Li, Q.; Wang, H.; Liu, Z.; et al. Sodium storage mechanism and electrochemical performance of layered GeP as anode for sodium ion batteries. J. Power Sources 2019, 433, 126682. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Shen, P.; Yang, L.; Li, Y.; Shi, Z.; Zhang, H. Layered GeP-black P(Ge2P3): An advanced binary-phase anode for Li/Na storage. Ceram. Int. 2019, 45, 15711–15714. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, K.; Park, M.; Lau, V.W.; Wang, H.; Zhang, J.; Zhang, J.; Zhao, R.; Yamauchi, Y.; Kang, Y.-M. Highly Reversible and Rapid Sodium Storage in GeP3 with Synergistic Effect from Outside-In Optimization. ACS Nano 2020, 14, 4352–4365. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-H.; Jeon, K.-J.; Park, C.-M. Layered germanium phosphide-based anodes for high-performance lithium- and sodium-ion batteries. Energy Storage Mater. 2019, 17, 78–87. [Google Scholar] [CrossRef]
- Tseng, K.-W.; Huang, S.-B.; Chang, W.-C.; Tuan, H.-Y. Synthesis of Mesoporous Germanium Phosphide Microspheres for High-Performance Lithium-Ion and Sodium-Ion Battery Anodes. Chem. Mater. 2018, 30, 4440–4447. [Google Scholar] [CrossRef]
- Zeng, T.; He, H.; Guan, H.; Yuan, R.; Liu, X.; Zhang, C. Tunable Hollow Nanoreactors for In Situ Synthesis of GeP Electrodes towards High-Performance Sodium Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 12103–12109. [Google Scholar] [CrossRef]
- Yang, F.; Hong, J.; Hao, J.; Zhang, S.; Liang, G.; Long, J.; Liu, Y.; Liu, N.; Pang, W.K.; Chen, J.; et al. Ultrathin Few-Layer GeP Nanosheets via Lithiation-Assisted Chemical Exfoliation and Their Application in Sodium Storage. Adv. Energy Mater. 2020, 10, 1903826. [Google Scholar] [CrossRef]
- Usui, H.; Domi, Y.; Yamagami, R.; Fujiwara, K.; Nishida, H.; Sakaguchi, H. Sodiation−Desodiation Reactions of Various Binary Phosphides as Novel Anode Materials of Na-Ion Battery. ACS Appl. Energy Mater. 2018, 1, 306–311. [Google Scholar] [CrossRef]
- Kulova, T.; Gryzlov, D.; Skundin, A.; Gavrilin, I.; Kudryashova, Y.; Pokryshkin, N. Anode Material Synthesized from Red Phosphorus and Germanium Nanowires for Lithium-Ion and Sodium-Ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 211229. [Google Scholar] [CrossRef]
- Zhou, M.; Li, X.; Wang, B.; Zhang, Y.; Ning, J.; Xiao, Z.; Zhang, X.; Chang, Y.; Zhi, L. High-performance silicon battery anodes enabled by engineering graphene assemblies. Nano Lett. 2015, 15, 6222–6228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M. Conducting polymer-skinned electroactive materials of lithium-ion batteries: Ready for monocomponent electrodes without additional binders and conductive agents. ACS Appl. Mater. Interfaces 2014, 6, 12789–12797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, F.; Cheng, H.M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Lu, C.; Liu, W.; Li, H.; Tay, B.K. A binder-free CNT network-MoS2 composite as a high performance anode material in lithium ion batteries. Chem. Commun. 2014, 50, 3338–3340. [Google Scholar] [CrossRef]
- Yao, M.; Zeng, Z.; Zhang, H.; Yan, J.; Liu, X. Electrophoretic deposition of carbon nanofibers/silicon film with honeycomb structure as integrated anode electrode for lithium-ion batteries. Electrochim. Acta 2018, 281, 312–322. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Chen, D.; Zhao, J. A facile electrophoretic deposition route to the Fe3O4/CNTs/rGO composite electrode as a binder-free anode for lithium ion battery. ACS Appl. Mater. Interfaces 2016, 8, 26730–26739. [Google Scholar] [CrossRef]
- Sahay, R.; Suresh Kumar, P.; Aravindan, V.; Sundaramurthy, J.; Chui Ling, W.; Mhaisalkar, S.G.; Ramakrishna, S.; Madhavi, S. High aspect ratio electrospun CuO nanofibers as anode material for lithium-ion batteries with superior cycleability. J. Phys. Chem. C 2012, 116, 18087–18092. [Google Scholar] [CrossRef]
- Yang, M.; Yang, M.; Ko, S.; Im, J.S.; Choi, B.G. Free-standing molybdenum disulfide/graphenecomposite paper as a binder-and carbon-free anode for lithium-ion batteries. J. Power Sources 2015, 288, 76–81. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Jiang, Y.; Yu, Z.; Gu, L.; Yu, Y. Crystalline red phosphorus incorporated with porous carbon nanofibers as flexible electrode for high performance lithium-ion batteries. Carbon 2014, 78, 455–462. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhou, Q.; Wu, W.; Zhu, C.; Liu, Z.; Chang, S.; Pu, J.; Zhang, H. Ultra-flexible lithium ion batteries fabricated by electrodeposition and solvothermal synthesis. Electrochim. Acta 2017, 237, 119–126. [Google Scholar] [CrossRef]
- Xu, Y.; Menon, A.S.; Harks, P.P.R.M.L.; Hermes, D.C.; Haverkate, L.A.; Unnikrishnan, S.; Mulder, F.M. Honeycomb-like porous 3D nickel electrodeposition for stable Li and Na metal anodes. Energy Storage Mater. 2018, 12, 69–78. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, H.; Du, Y.; Pan, Y.; Peng, Y.; Zhou, P.; Premovic, M. Experimental Investigation of the Co-Ge Phase Diagram. J. Phase Equilib. Diffus. 2017, 38, 843–852. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kuz’mina, A.A.; Kulova, T.L.; Skundin, A.M.; Emets, V.V.; Volkov, R.L.; Dronov, A.A.; Borgardt, N.I.; Gavrilov, S.A. High-rate and low-temperature performance of germanium nanowires anode for lithium-ion batteries. J. Electroanalyt. Chem. 2021, 888, 115209. [Google Scholar] [CrossRef]
- Emets, V.V.; Gavrilin, I.M.; Kulova, T.L.; Skundin, A.M.; Sharafutdinova, A.M.; Gavrilov, S.A. Dynamics of changes in the kinetic parameters of germanium nanowires during lithiation/delithiation in a wide temperature range. J. Electroanalyt. Chem. 2021, 902, 115811. [Google Scholar] [CrossRef]
- Gavrilin, I.M.; Kudryashova, Y.O.; Kulova, T.L.; Skundin, A.M.; Gavrilov, S.A. The effect of growth temperature on the process of insertion/extraction of sodium into germanium nanowires formed by electrodeposition using indium nanoparticles. Mater. Lett. 2021, 287, 129303. [Google Scholar] [CrossRef]
- Kulova, T.L.; Gavrilin, I.M.; Kudryashova, Y.O.; Skundin, A.M.; Gavrilov, S.A. Cyclability enhancement and decreasing the irreversible capacity of anodes based on germanium nanowires for lithium-ion batteries. Mendeleev Commun. 2021, 31, 842–843. [Google Scholar] [CrossRef]
- Kuz’mina, A.A.; Kulova, T.L.; Tuseeva, E.K.; Chirkova, E.V. Specific Features in the Low-Temperature Performance of Electrodes of Lithium-Ion Battery. Russ. J. Electrochem. 2020, 56, 899–906. [Google Scholar] [CrossRef]
- Kulova, T.L.; Gavrilin, I.M.; Kudryashova, Y.O.; Skundin, A.M. A LiNi0.8Co0.15Al0.05O2/Ge electrochemical system for lithium-ion batteries. Mend. Commun. 2020, 30, 775–776. [Google Scholar] [CrossRef]
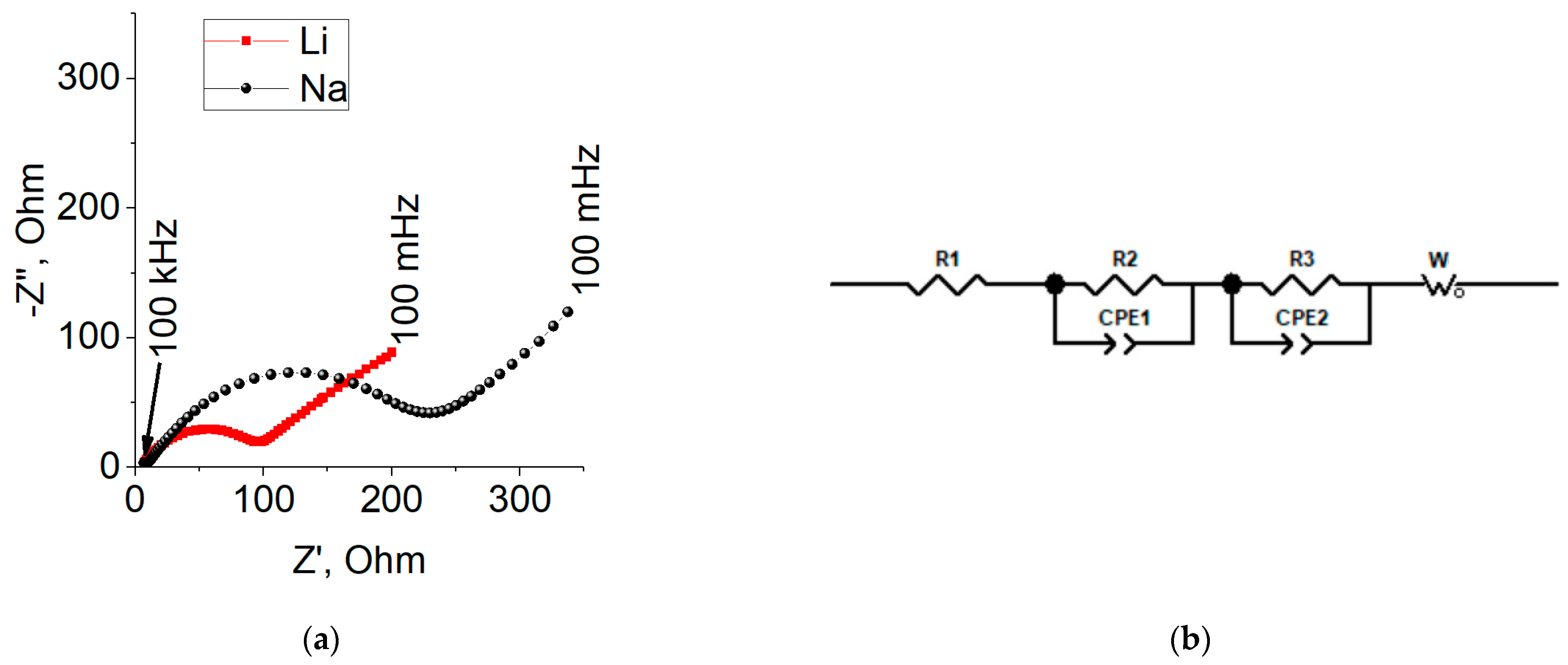


| R1, Ohm | R2, Ohm | Cf, F | R3, Ohm | Cdl, F | W, Ohm/s0.5 | |
|---|---|---|---|---|---|---|
| 3.76 | 5.19 | 3.82 × 10−6 | 76.29 | 2.79 × 10−6 | 40.66 | Li |
| 5.65 | 5.49 | 3.93 × 10−5 | 165.1 | 2.68 × 10−5 | 94.18 | Na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulova, T.L.; Skundin, A.M.; Gavrilin, I.M.; Kudryashova, Y.O.; Martynova, I.K.; Novikova, S.A. Binder-Free Ge-Co-P Anode Material for Lithium-Ion and Sodium-Ion Batteries. Batteries 2022, 8, 98. https://doi.org/10.3390/batteries8080098
Kulova TL, Skundin AM, Gavrilin IM, Kudryashova YO, Martynova IK, Novikova SA. Binder-Free Ge-Co-P Anode Material for Lithium-Ion and Sodium-Ion Batteries. Batteries. 2022; 8(8):98. https://doi.org/10.3390/batteries8080098
Chicago/Turabian StyleKulova, Tatiana L., Alexander M. Skundin, Il’ya M. Gavrilin, Yulia O. Kudryashova, Irina K. Martynova, and Svetlana A. Novikova. 2022. "Binder-Free Ge-Co-P Anode Material for Lithium-Ion and Sodium-Ion Batteries" Batteries 8, no. 8: 98. https://doi.org/10.3390/batteries8080098






