Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries
Abstract
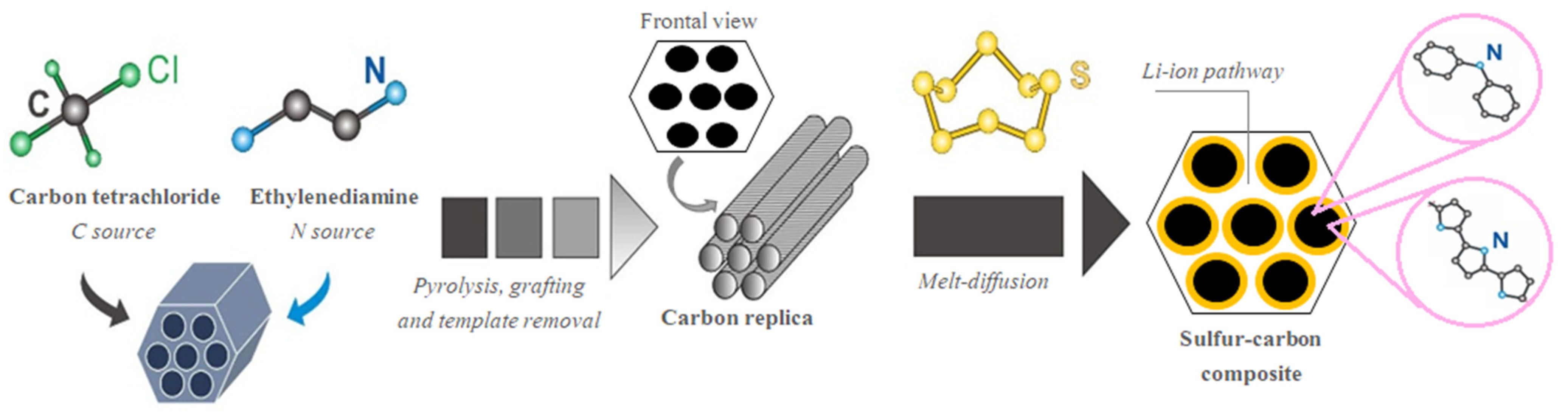
:1. Introduction
2. Results and Discussion
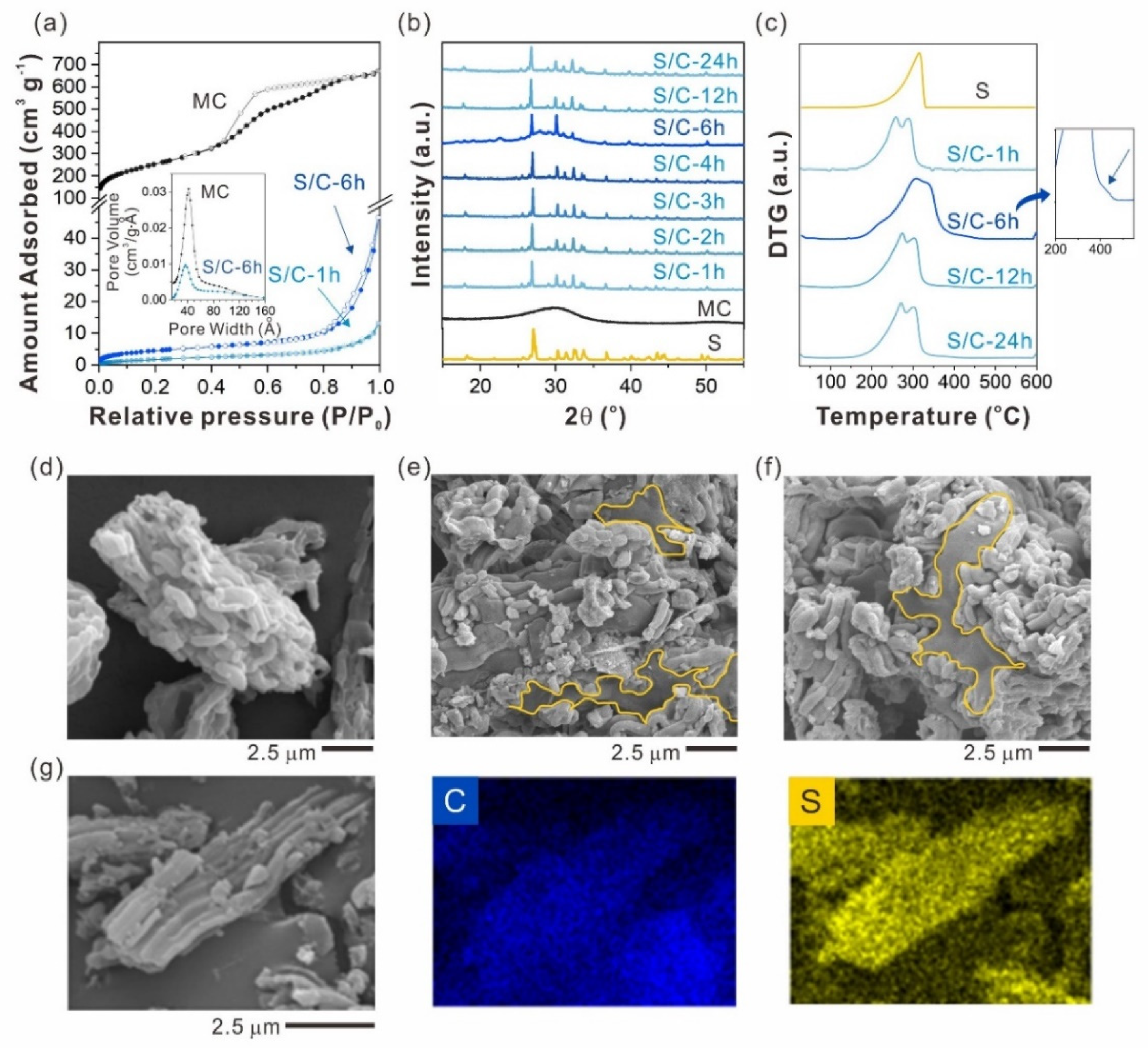
2.1. Sulfur Infiltration Time Effect
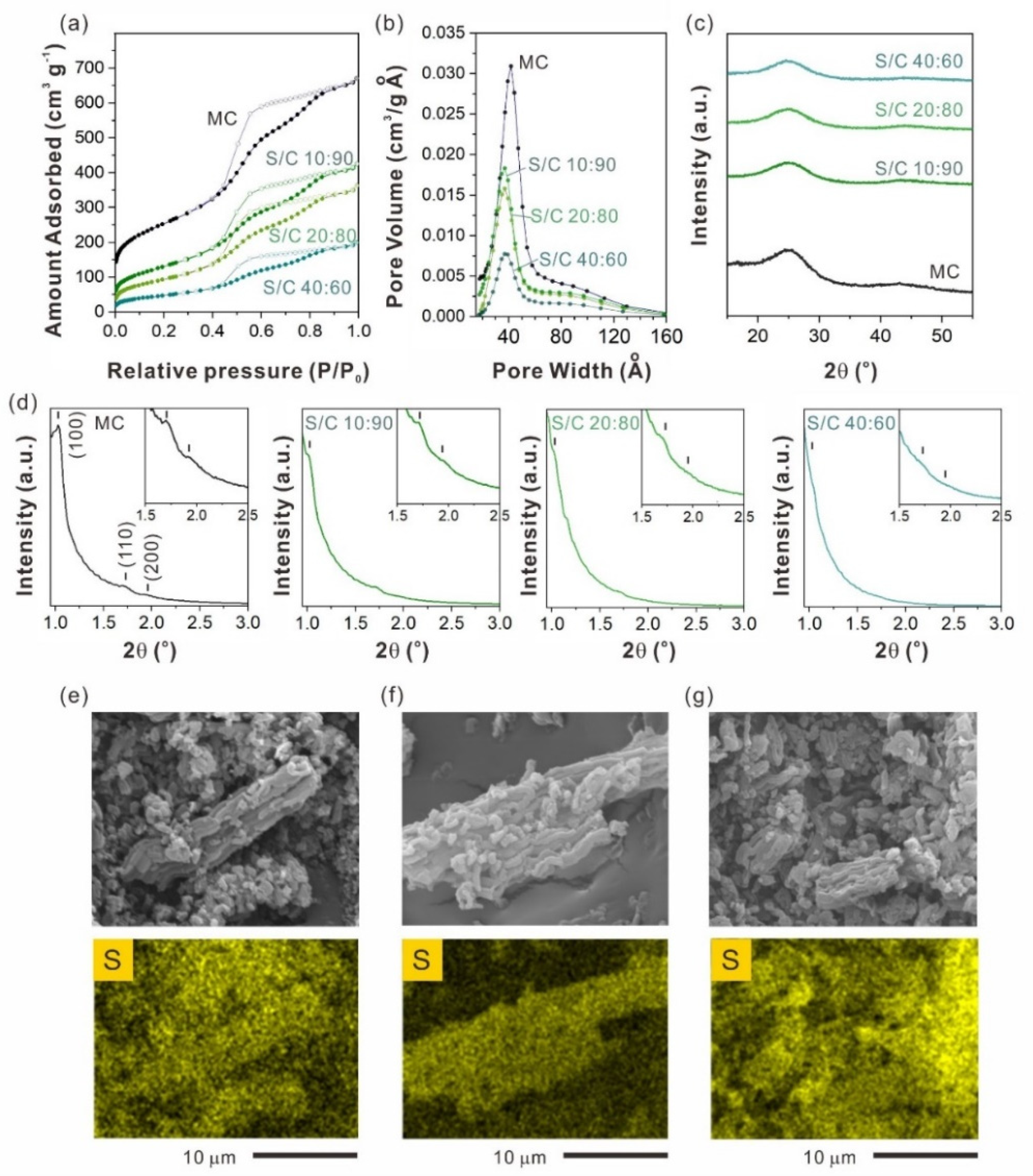
2.2. Sulfur Content Effect
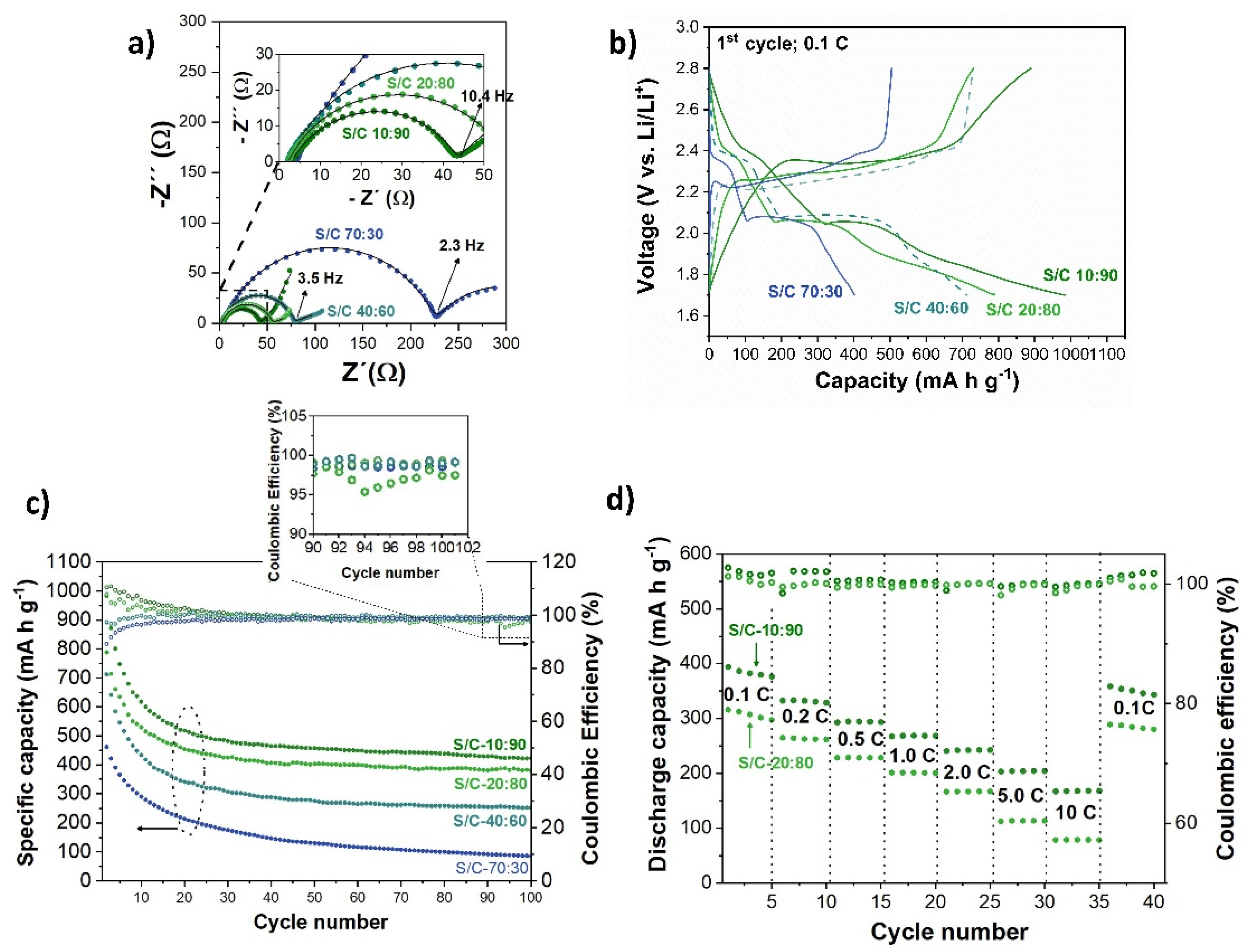
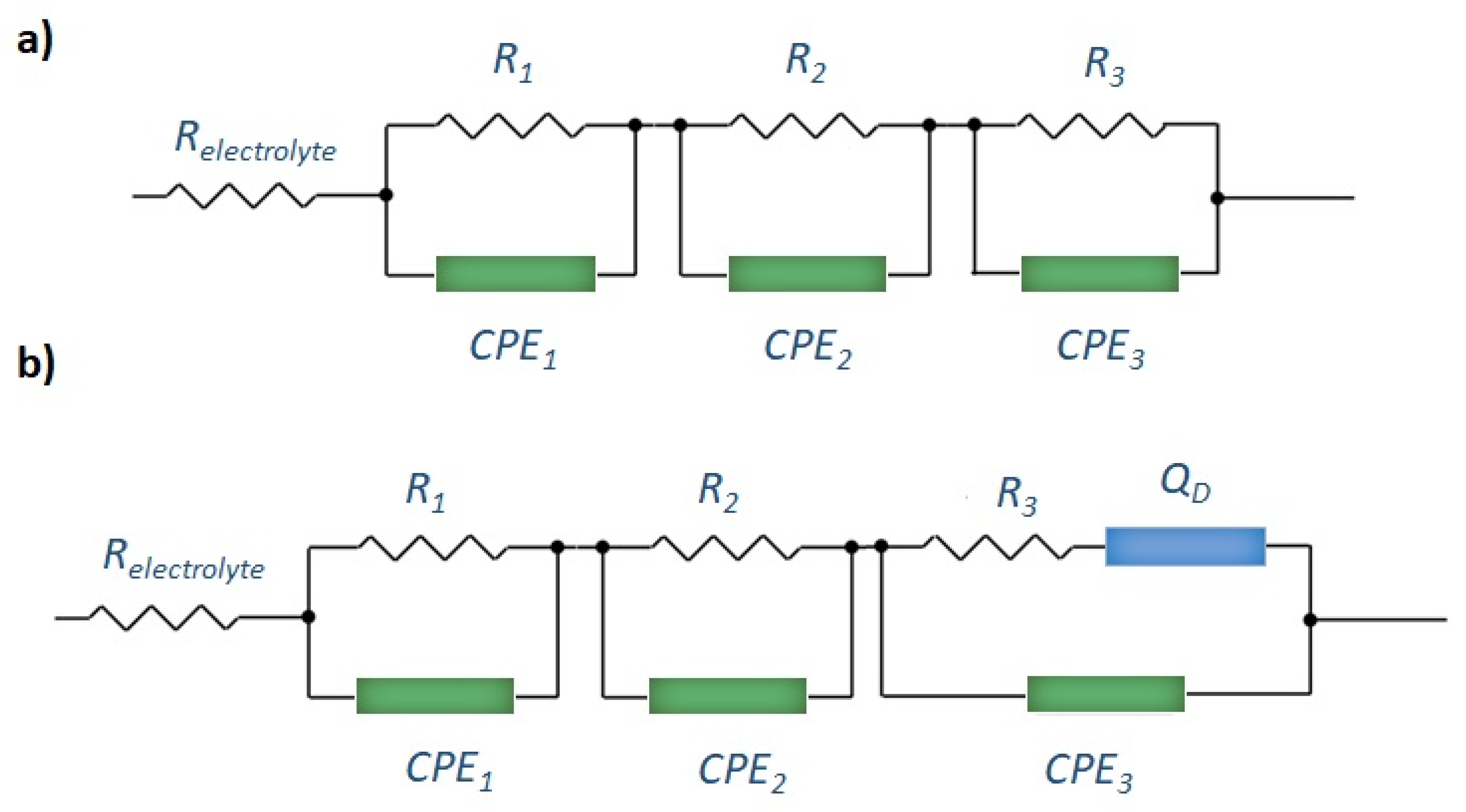
2.3. Electrochemical Properties
3. Materials and Methods
3.1. Preparation of S/C Composites
3.2. Characterization
3.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masoud, E.M. Nano α-Fe2O3 Synthesized Using EDTA-Aqueous Solution Simple and Novel Method: Improved Capacity Retention at 1 C Rate as Anode for High Rate Performance of Lithium-Ion Batteries. Ionics 2021, 27, 2847–2855. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of Long Stability of Sulfur Cathode by Encapsulating Sulfur into Micropores of Carbon Spheres. Energy Environ. Sci. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Xu, C.; Pan, L. Mesoporous Carbon/Sulfur Composite with N-Doping and Tunable Pore Size for High-Performance Li-S Batteries. J. Solid State Electrochem. 2017, 21, 1101–1109. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.; Park, E.; Scrosati, B.; Hassoun, J.; Park, M.; Kim, Y.; Kim, H.; Belharouak, I.; Sun, Y. Highly Cyclable Lithium-Sulfur Batteries with a Dual-Type Sulfur Cathode and a Lithiated Si/SiO. Nano Lett. 2015, 15, 2863–2868. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Zhang, B.; Lin, Z. Developments of Electrolyte Systems for Lithium—Sulfur Batteries: A Review. Front. Energy Res. 2015, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, X.; Chauhan, G.S.; Ahn, J.H. Nanostructured Nitrogen-Doped Mesoporous Carbon Derived from Polyacrylonitrile for Advanced Lithium Sulfur Batteries. Appl. Surf. Sci. 2016, 380, 151–158. [Google Scholar] [CrossRef]
- Patel, K. Lithium-Sulfur Battery: Chemistry, Challenges, Cost, and Future. J. Undergrad. Res. 2016, 9, 39–42. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, J.; Hwang, J.Y.; Sun, Y.K. Recent Research Trends in Li-S Batteries. J. Mater. Chem. A 2018, 6, 11582–11605. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.W. Cathode Materials for Lithium-Sulfur Batteries: A Practical Perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Dysart, A.D.; Cardoza, N.A.; Mitchell, G.; Ortalan, V.; Pol, V.G. Effect of Synthesis Method Using Varying Types of Micropore Level Sulfur Infiltration on Electrochemical Performance in Lithium–Sulfur Batteries. Energy Technol. 2019, 7, 1900194. [Google Scholar] [CrossRef]
- Yang, J.Y.; Han, H.J.; Repich, H.; Zhi, R.C.; Qu, C.Z.; Kong, L.; Kaskel, S.; Wang, H.Q.; Xu, F.; Li, H.J. Recent Progress on the Design of Hollow Carbon Spheres to Host Sulfur in Room-Temperature Sodium-Sulfur Batteries. Xinxing Tan Cailiao/New Carbon Mater. 2020, 35, 630–645. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Wagner, M.J.; Hays, K.A.; Li, X.; Zuo, P.; Wang, C.; Zhang, J.-G.; Liu, J.; Xiao, J. Revisit Carbon/Sulfur Composite for Li-S Batteries. J. Electrochem. Soc. 2013, 160, A1624–A1628. [Google Scholar] [CrossRef]
- Li, M.; Carter, R.; Douglas, A.; Oakes, L.; Pint, C.L. Sulfur Vapor-Infiltrated 3D Carbon Nanotube Foam for Binder-Free High Areal Capacity Lithium-Sulfur Battery Composite Cathodes. ACS Nano 2017, 11, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.G.; Chun, J.; Cho, B.W.; Lee, J.; Kim, Y.T. Enhanced Performance of Sulfur-Infiltrated Bimodal Mesoporous Carbon Foam by Chemical Solution Deposition as Cathode Materials for Lithium Sulfur Batteries. Sci. Rep. 2017, 7, 42238. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Tateishi, M.; Nagao, M.; Imade, B.Y.; Yokoi, T.; Hirayama, M.; Tatsumi, T.; Kanno, R. Synthesis, Structure, and Electrochemical Properties of a Sulfur-Carbon Replica Composite Electrode for All-Solid-State Li-Sulfur Batteries. J. Electrochem. Soc. 2017, 164, A6178–A6183. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon-Sulphur Cathode for Lithium-Sulphur Batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Ji, X.; Evers, S.; Black, R.; Nazar, L.F. Stabilizing Lithium-Sulphur Cathodes Using Polysulphide Reservoirs. Nat. Commun. 2011, 2, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Oschatz, M.; Wu, F. Towards Stable Lithium-Sulfur Battery Cathodes by Combining Physical and Chemical Confinement of Polysulfides in Core-Shell Structured Nitrogen-Doped Carbons. Carbon N. Y. 2020, 161, 162–168. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-Performance Lithium Sulfur Batteries Enabled by a Synergy between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Muthuraj, D.; Ghosh, A.; Kumar, A.; Mitra, S. Nitrogen and Sulfur Doped Carbon Cloth as Current Collector and Polysulfide Immobilizer for Magnesium-Sulfur Batteries. ChemElectroChem 2019, 6, 684–689. [Google Scholar] [CrossRef]
- Zhao, Y.; Lyu, H.; Liu, Y.; Liu, W.; Tian, Y.; Wang, X. Rational Design and Synthesis of Multimorphology Mesoporous Carbon@silica Nanoparticles with Tailored Structure. Carbon N. Y. 2021, 183, 912–928. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Zhao, H.; Krall, A.; Wen, Z.; Chen, J.; Hurley, P.; Jiang, J.; Li, Y. Sulfur-Infiltrated Porous Carbon Microspheres with Controllable Multi-Modal Pore Size Distribution for High Energy Lithium-Sulfur Batteries. Nanoscale 2014, 6, 882–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhu, K.; Tian, J.; Tang, Q.; Shan, Z. Preparation of Yolk-Shell Sulfur/Carbon Nanocomposite via an Organic Solvent Route for Lithium-Sulfur Batteries. J. Solid State Electrochem. 2014, 18, 2077–2085. [Google Scholar] [CrossRef]
- Hossain, M.D.; Zhang, Q.; Cheng, T.; Goddard, W.A.; Luo, Z. Graphitization of Low-Density Amorphous Carbon for Electrocatalysis Electrodes from ReaxFF Reactive Dynamics. Carbon N. Y. 2021, 183, 940–947. [Google Scholar] [CrossRef]
- Kim, N.I.; Cheon, J.Y.; Kim, J.H.; Seong, J.; Park, J.Y.; Joo, S.H.; Kwon, K. Impact of Framework Structure of Ordered Mesoporous Carbons on the Performance of Supported Pt Catalysts for Oxygen Reduction Reaction. Carbon N. Y. 2014, 72, 354–364. [Google Scholar] [CrossRef]
- Geng, X.; Rao, M.; Li, X.; Li, W. Highly Dispersed Sulfur in Multi-Walled Carbon Nanotubes for Lithium/Sulfur Battery. J. Solid State Electrochem. 2013, 17, 987–992. [Google Scholar] [CrossRef]
- Li, K.; Wang, B.; Su, D.; Park, J.; Ahn, H.; Wang, G. Enhance Electrochemical Performance of Lithium Sulfur Battery through a Solution-Based Processing Technique. J. Power Sources 2012, 202, 389–393. [Google Scholar] [CrossRef]
- Xue, M.; Chen, C.; Tan, Y.; Ren, Z.; Li, B.; Zhang, C. Mangosteen Peel-Derived Porous Carbon: Synthesis and Its Application in the Sulfur Cathode for Lithium Sulfur Battery. J. Mater. Sci. 2018, 53, 11062–11077. [Google Scholar] [CrossRef]
- He, X.; Hou, H.; Yuan, X.; Huang, L.; Hu, J.; Liu, B.; Xu, J.; Xie, J.; Yang, J.; Liang, S.; et al. Electrocatalytic Activity of Lithium Polysulfides Adsorbed into Porous TiO2 Coated MWCNTs Hybrid Structure for Lithium-Sulfur Batteries. Sci. Rep. 2017, 7, 40679. [Google Scholar] [CrossRef] [Green Version]
- Krishnaveni, K.; Subadevi, R.; Radhika, G.; Premkumar, T.; Raja, M.; Liu, W.R.; Sivakumar, M. Carbon Wrapping Effect on Sulfur/Polyacrylonitrile Composite Cathode Materials for Lithium Sulfur Batteries. J. Nanosci. Nanotechnol. 2018, 18, 121–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, F.; Zhang, Y.; Zhang, C.; Mentbayeva, A.; Umirov, N.; Xie, H.; Bakenov, Z. A Free-Standing Sulfur/Nitrogen-Doped Carbon Nanotube Electrode for High-Performance Lithium/Sulfur Batteries. Nanoscale Res. Lett. 2015, 10, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, W.; Kim, K.-B.; Jung, K.-N.; Shin, K.-H.; Jin, C.-S. Synthesis and Electrochemical Properties of a Sulfur-Multi Walled Carbon Nanotubes Composite as a Cathode Material for Lithium Sulfur Batteries. J. Power Sources 2012, 202, 394–399. [Google Scholar] [CrossRef]
- Yang, J.; Xie, J.; Zhou, X.; Zou, Y.; Tang, J.; Wang, S.; Chen, F.; Wang, L. Functionalized N-Doped Porous Carbon Nanofiber Webs for a Lithium-Sulfur Battery with High Capacity and Rate Performance. J. Phys. Chem. C 2014, 118, 1800–1807. [Google Scholar] [CrossRef]
- Kazazi, M. Synthesis and Elevated Temperature Performance of a Polypyrrole-Sulfur-Multi-Walled Carbon Nanotube Composite Cathode for Lithium Sulfur Batteries. Ionics 2016, 22, 1103–1112. [Google Scholar] [CrossRef]
- Wang, Y.X.; Huang, L.; Sun, L.C.; Xie, S.Y.; Xu, G.L.; Chen, S.R.; Xu, Y.F.; Li, J.T.; Chou, S.L.; Dou, S.X.; et al. Facile Synthesis of a Interleaved Expanded Graphite-Embedded Sulphur Nanocomposite as Cathode of Li-S Batteries with Excellent Lithium Storage Performance. J. Mater. Chem. 2012, 22, 4744–4750. [Google Scholar] [CrossRef] [Green Version]
- Hencz, L.; Gu, X.; Zhou, X.; Martens, W.; Zhang, S. Highly Porous Nitrogen-Doped Seaweed Carbon for High-Performance Lithium–Sulfur Batteries. J. Mater. Sci. 2017, 52, 12336–12347. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, C.; Li, R.; Fukuto, M.; Vogt, B.D. Sulfur Diffusion within Nitrogen-Doped Ordered Mesoporous Carbons Determined by in Situ X-Ray Scattering. Langmuir 2018, 34, 8767–8776. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ren, T.Z. Porous Carbon Modified with Sulfur in Energy Related Applications. Carbon N. Y. 2017, 118, 561–577. [Google Scholar] [CrossRef]
- Olchowski, R.; Zieba, E.; Giannakoudakis, D.A.; Anastopoulos, I.; Dobrowolski, R.; Barczak, M. Tailoring Surface Chemistry of Sugar-Derived Ordered Mesoporous Carbons towards Efficient Removal of Diclofenac from Aquatic Environments. Materials 2020, 13, 1625. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Nie, R.; Zhang, H.; Lu, X.; Zhou, D.; Xia, Q. Ordered Mesoporous N-Doped Carbon Supported Ru for Selective Adsorption and Hydrogenation of Quinoline. Microporous Mesoporous Mater. 2018, 256, 10–17. [Google Scholar] [CrossRef]
- Weinberger, C.; Ren, S.; Hartmann, M.; Wagner, T.; Karaman, D.; Rosenholm, J.M.; Tiemann, M. Bimodal Mesoporous CMK-5 Carbon: Selective Pore Filling with Sulfur and SnO2 for Lithium Battery Electrodes. ACS Appl. Nano Mater. 2018, 1, 455–462. [Google Scholar] [CrossRef]
- Liu, X.; Rahmatinejad, J.; Ye, Z. Encapsulation of Sulfur within Well-Defined Size-Tunable Ultrasmall Carbon Nanospheres for Superior High-Rate and Long-Stability Li–S Batteries. Chem. Eng. J. 2021, 422, 130129. [Google Scholar] [CrossRef]
- Luo, C.; Hu, E.; Gaskell, K.J.; Fan, X.; Gao, T.; Cui, C.; Ghose, S.; Yang, X.Q.; Wang, C. A Chemically Stabilized Sulfur Cathode for Lean Electrolyte Lithium Sulfur Batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 14712–14720. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.; Lee, S.; Eom, K.S.; Pak, C.; Kim, H.J. Advanced Ordered Mesoporous Carbon with Fast Li-Ion Diffusion for Lithium–Selenium Sulfide Batteries in a Carbonate-Based Electrolyte. Carbon N. Y. 2020, 170, 236–244. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Lv, X.J. N-Doped Ordered Mesoporous Carbon as a High Performance Anode Material in Sodium Ion Batteries at Room Temperature. RSC Adv. 2014, 4, 62673–62677. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and Characterization of Micro-Mesoporous MCM-41 Using Various Ionic Liquids as Co-Templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Sawant, D.P.; Mori, T.; Ariga, K.; Chang, J.S.; Jhung, S.H.; Balasubramanian, V.V.; Hwang, Y.K. Three-Dimensional Cage Type Mesoporous CN-Based Hybrid Material with Very High Surface Area and Pore Volume. Chem. Mater. 2007, 19, 4367–4372. [Google Scholar] [CrossRef]
- Wei, C.; Han, Y.; Liu, H.; Gan, R.; Li, Q.; Wang, Y.; Hu, P.; Ma, C.; Shi, J. Advanced Lithium–Sulfur Batteries Enabled by a SnS2-Hollow Carbon Nanofibers Flexible Electrocatalytic Membrane. Carbon N. Y. 2021, 184, 1–11. [Google Scholar] [CrossRef]
- Cañas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigations of Lithium-Sulfur Batteries Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Ding, N.; Schnell, J.; Li, X.; Yin, X.; Liu, Z.; Zong, Y. Electrochemical Impedance Spectroscopy Study of Sulfur Reduction Pathways Using a Flexible, Free-Standing and High-Sulfur-Loading Film. Chem. Eng. J. 2021, 412, 128559. [Google Scholar] [CrossRef]
- Waluś, S.; Barchasz, C.; Bouchet, R.; Alloin, F. Electrochemical Impedance Spectroscopy Study of Lithium–Sulfur Batteries: Useful Technique to Reveal the Li/S Electrochemical Mechanism. Electrochim. Acta 2020, 359, 136944. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Chang, C. Improved Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode Materials: Via Incorporation of Rubidium Cations into the Original Li Sites. RSC Adv. 2017, 7, 51721–51728. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Ryu, K.S. Study of the Lithium Diffusion Properties and High Rate Performance of TiNb6O17 as an Anode in Lithium Secondary Battery. Sci. Rep. 2017, 7, 16617. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Lv, W.; Zhang, C.; Li, F.; Tang, L.; He, Y.; Li, B.; Yang, Q.H.; Kang, F. A Carbon Sandwich Electrode with Graphene Filling Coated by N-Doped Porous Carbon Layers for Lithium-Sulfur Batteries. J. Mater. Chem. A 2015, 3, 20218–20224. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur Speciation in Li-S Batteries Determined by Operando X-Ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, D.; Harris, J.B.; Ding, T.; Si, J.; Andrew, S.; Qu, D.; Yang, X.-Q.; Qu, D. Investigation of Li-S Battery Mechanism by Real-Time Monitoring the Changes of Sulfur and Polysulfide Species during the Discharge and Charge. ACS Appl. Mater. Interfaces 2017, 9, 4326–4332. [Google Scholar] [CrossRef]
- Weng, Y.T.; Wang, H.; Lee, R.C.; Huang, C.Y.; Huang, S.S.; Abdollahifar, M.; Kuo, L.M.; Hwang, B.J.; Kuo, C.L.; Cui, Y.; et al. Efficient Synthesis of High-Sulfur-Content Cathodes for High-Performance Li–S Batteries Based on Solvothermal Polysulfide Chemistry. J. Power Sources 2020, 450, 227676. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ju, Z.; Yu, X.; Wang, Y.; Du, Y.; Bai, Z.; Dou, S.; Yu, G. Thickness-Independent Scalable High-Performance Li-S Batteries with High Areal Sulfur Loading via Electron-Enriched Carbon Framework. Nat. Commun. 2021, 12, 4519. [Google Scholar] [CrossRef]
- Gueon, D.; Ju, M.-Y.; Moon, J.H. Complete Encapsulation of Sulfur through Interfacial Energy Control of Sulfur Solutions for High-Performance Li−S Batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 12686–12692. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, P.; Wang, J.; Tang, X.; An, T.; Zhou, H.; Zhang, D.; Fan, T. Synergetic Pore Structure Optimization and Nitrogen Doping of 3D Porous Graphene for High Performance Lithium Sulfur Battery. Carbon N. Y. 2019, 143, 869–877. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Xia, Y.; Cheng, C.; Liang, C.; Gan, Y.; Zhang, J.; Tao, X.; Zhang, W. Supercritical Fluid Assisted Synthesis of Titanium Carbide Particles Embedded in Mesoporous Carbon for Advanced Li-S Batteries. J. Alloys Compd. 2017, 706, 227–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Wang, Y.; Li, J.; Li, H.; Zeng, J.; Wang, J.; Hwang, B.J.; Zhao, J. High Sulfur-Containing Carbon Polysulfide Polymer as a Novel Cathode Material for Lithium-Sulfur Battery. Sci. Rep. 2017, 7, 11386. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.G. Anodes for Rechargeable Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1402273. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef]
- Emerce, N.B.; Eroglu, D. Effect of Electrolyte-to-Sulfur Ratio in the Cell on the Li-S Battery Performance. J. Electrochem. Soc. 2019, 166, A1490–A1500. [Google Scholar] [CrossRef]
- Fan, F.Y.; Chiang, Y.-M. Electrodeposition Kinetics in Li-S Batteries: Effects of Low Electrolyte/Sulfur Ratios and Deposition Surface Composition. J. Electrochem. Soc. 2017, 164, A917–A922. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, G.; Lv, W.; Shi, H.; Luo, C.; He, Y.; Li, B.; Yang, Q.H.; Kang, F. Sulfur Confined in Nitrogen-Doped Microporous Carbon Used in a Carbonate-Based Electrolyte for Long-Life, Safe Lithium-Sulfur Batteries. Carbon N. Y. 2016, 109, 1–6. [Google Scholar] [CrossRef]
- Cheng, R.; Guan, Y.; Luo, Y.; Zhang, C.; Xia, Y.; Wei, S.; Zhao, M.; Lin, Q.; Li, H.; Zheng, S.; et al. Guanine-Assisted N-Doped Ordered Mesoporous Carbons as Efficient Capacity Decaying Suppression Materials for Lithium–Sulfur Batteries. J. Mater. Sci. Technol. 2022, 101, 155–164. [Google Scholar] [CrossRef]
- Pan, H.; Cheng, Z.; He, P.; Zhou, H. A Review of Solid-State Lithium-Sulfur Battery: Ion Transport and Polysulfide Chemistry. Energy Fuels 2020, 34, 11942–11961. [Google Scholar] [CrossRef]
- Kang, N.; Lin, Y.; Yang, L.; Lu, D.; Xiao, J.; Qi, Y.; Cai, M. Cathode Porosity Is a Missing Key Parameter to Optimize Lithium-Sulfur Battery Energy Density. Nat. Commun. 2019, 10, 4597. [Google Scholar] [CrossRef] [PubMed]

| Elemental Analysis | XPS | ||||||
|---|---|---|---|---|---|---|---|
| C (wt.%) | N (wt.%) | H (wt.%) | O (wt.%) | Nitrogen | |||
| Graphitic-N | Pyrrolic-N | Pyridinic-N | N-o- | ||||
| 74.6 | 7.34 | 1.14 | 16.9 | 39.6 | 10.1 | 24.3 | 26 |
| Samples | MC | S/C 10:90 | S/C 20:80 | S/C 40:60 |
|---|---|---|---|---|
| Sulfur content by TGA (%) | - | 9.80 | 21.5 | 41.0 |
| Specific surface area (m2/g) | 872.8 | 479.0 | 352.3 | 177.6 |
| Pore volume (cm3/g) | 1.04 | 0.70 | 0.60 | 0.40 |
| Lattice crystal parameter, a0 (Å) | 99.0 | 100 | 100 | 97.0 |
| Pore diameter Dp,1 (Å) | 42 | 38 | 37 | 38 |
| Pore diameter Dp,2 (Å) | 89 | 87 | 87 | 82 |
| Wall thickness variation, Wt1 (Å) | 57 | 63 | 63 | 59 |
| Sample | Resistances | Warburg Factor Ωs−1/2 | Diffusivity (cm2/s) | |
|---|---|---|---|---|
| Rs (Ω) | Rc (Ω) | |||
| S/C 10:90 | 2.91 | 35.0 | 3.41 | 7.05 × 10−22 |
| S/C 20:80 | 2.30 | 44.4 | 2.83 | 2.73 × 10−22 |
| S/C 40:70 | 2.24 | 77.1 | 4.01 | 3.67 × 10−23 |
| S/C 70:30 | 4.01 | 216 | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laverde, J.; Rosero-Navarro, N.C.; Miura, A.; Buitrago-Sierra, R.; Tadanaga, K.; López, D. Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries. Batteries 2022, 8, 58. https://doi.org/10.3390/batteries8060058
Laverde J, Rosero-Navarro NC, Miura A, Buitrago-Sierra R, Tadanaga K, López D. Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries. Batteries. 2022; 8(6):58. https://doi.org/10.3390/batteries8060058
Chicago/Turabian StyleLaverde, Jennifer, Nataly C. Rosero-Navarro, Akira Miura, Robison Buitrago-Sierra, Kiyoharu Tadanaga, and Diana López. 2022. "Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries" Batteries 8, no. 6: 58. https://doi.org/10.3390/batteries8060058
APA StyleLaverde, J., Rosero-Navarro, N. C., Miura, A., Buitrago-Sierra, R., Tadanaga, K., & López, D. (2022). Impact of Sulfur Infiltration Time and Its Content in an N-doped Mesoporous Carbon for Application in Li-S Batteries. Batteries, 8(6), 58. https://doi.org/10.3390/batteries8060058










