Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Material and Methods
4.1. Synthesis
4.2. Characterization Methods
4.3. Electrochemical Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, C.; Ashby, D.S.; Butts, D.M.; DeBlock, R.H.; Wei, G.; Lau, J.; Dunn, B. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 2020, 5, 5–19. [Google Scholar] [CrossRef]
- Chatterjee, D.; Nandi, A. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Xu, C.; Yang, H.; Li, Y.; Wang, J.; Lu, X. Surface engineering for advanced aqueous supercapacitors: A Review. ChemElectroChem 2020, 7, 586–593. [Google Scholar] [CrossRef]
- Zang, X.; Shen, C.; Sanghadasa, M.; Lin, L. High-voltage supercapacitors based on aqueous electrolytes. ChemElectroChem 2019, 6, 976–988. [Google Scholar] [CrossRef]
- Majumdar, D. Review on current progress of MnO2-based ternary nanocomposites for supercapacitor applications. ChemElectroChem 2021, 8, 291–336. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Lee, K.-T.; Lin, Y.-P.; Wu, N.-L.; Donne, S.W. Investigation on capacity fading of aqueous MnO2·nH2O electrochemical capacitor. J. Power Sources 2008, 177, 660–664. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Jiang, N.; Liu, A.; Gao, L.; Li, Y.H.; Wang, H.; Ma, T. In-situ grown Ni(OH)2 nanosheets on Ni foam for hybrid supercapacitors with high electrochemical performance. J. Electrochem. Soc. 2018, 165, A882. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Najafi, S.H.M.; Norouzi, Z. Hierarchical NiO@Ni(OH)2 nanoarrays as high-performance supercapacitor electrode material. Electrochim. Acta 2021, 368, 137633. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Yadav, M.H.; Lee, J.-J.; Kim, H.-S.; Kim, H.-S.; Kim, J.-H. Ni(OH)2-decorated nitrogen doped MWCNT nanosheets as an efficient electrode for high performance supercapacitors. Sci. Rep. 2019, 9, 6034. [Google Scholar] [CrossRef] [Green Version]
- Soserov, L.; Stoyanova, A.; Boyadzhieva, T.; Koleva, V.; Kalapsazova, M. Nickel-manganese structured and multiphase composites as electrodes for hybrid supercapacitors. Electrochim. Acta 2018, 283, 1063–1071. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Sankar, K.V.; Surendran, S.; Pandi, S.; Allin, M.; Nithya, V.D.; Lee, Y.S.; Selvan, R.K. Studies on the electrochemical intercalation/de-intercalation mechanism of NiMn2O4 for high stable pseudocapacitor electrodes. RSC Adv. 2015, 5, 27649–27656. [Google Scholar] [CrossRef]
- Dinesh, M.; Haldorai, Y.; Thangavelu, R.; Kumar, R. Mn–Ni binary metal oxide for high-performance supercapacitor and electro-catalyst for oxygen evolution reaction. Ceram. Int. 2020, 46, 28006–28012. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Sharma, S.R.; Singh, G. Enhanced supercapacitor performance by incorporating nickel in manganese oxide. RSC Adv. 2014, 4, 57192–57199. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Wang, J.; Liu, F.; Zhang, X.B. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Wang, R.; Wu, J. Structure and basic properties of ternary metal oxides and their prospects for application in supercapacitors. In Metal Oxides in Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–132. [Google Scholar]
- Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Hydrogenated NiO nanoblock architecture for high performance pseudocapacitor. ACS Appl. Mater. Interfaces 2014, 6, 4684–4692. [Google Scholar] [CrossRef]
- Lu, Q.; Lattanzi, M.W.; Chen, Y.P.; Kou, X.M.; Li, W.F.; Fan, X.; Unruh, K.M.; Chen, J.G.; Xiao, J.Q. Supercapacitor electrodes with high-energy and power densities prepared from monolithic NiO/Ni nanocomposites. Angew. Chem. Int. Ed. 2011, 50, 6847–6850. [Google Scholar] [CrossRef]
- Li, X.; Xin, M.; Guo, S.; Cai, T.; Du, D.; Xing, W.; Zhao, L.; Guo, W.; Xue, Q.; Yan, Z. Insight of synergistic effect of different active metal ions in layered double hydroxides on their electrochemical behaviors. Electrochim. Acta 2017, 253, 302–310. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef]
- Pang, H.; Yan, Z.; Wang, W.; Chen, J.; Zhang, J.; Zheng, H. Facile fabrication of NH4CoPO4·H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nanoscale 2012, 4, 5946–5953. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Li, Q.; Wei, J.; Xue, H.; Pang, H. Metal (M = Co, Ni) phosphate based materials for high-performance supercapacitors. Inorg. Chem. Front. 2018, 5, 11–28. [Google Scholar] [CrossRef]
- Prabaharan, S.R.S.; Anslin Star, R.; Kulkarni, A.R.; Michael, M.S. Nano-composite LiMnPO4 as new insertion electrode for electrochemical supercapacitors. Curr. Appl. Phys. 2015, 15, 1624–1633. [Google Scholar] [CrossRef]
- Michael, M.S.; Kulkarni, A.R.; Prabaharan, S.R.S. Design of monolayer porous carbon-embedded hybrid-LiMnPO4 for high energy density Li-ion capacitors. J. Nanosci. Nanotechnol. 2016, 16, 7314–7324. [Google Scholar] [CrossRef]
- Xu, L.; Wang, S.; Zhang, X.; He, T.; Lu, F.; Li, H.; Ye, J. A facile method of preparing LiMnPO4/reduced graphene oxide aerogel as cathodic material for aqueous lithium-ion hybrid supercapacitors. Appl. Surf. Sci. 2018, 428, 977–985. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Rupa Kasturi, P.; Shanmugavani, A.; Surendran, S.; Shanmugapriya, S.; Kalai Selvan, R. Effect of chelating agent on the sol-gel thermolysis synthesis of LiNiPO4 and its electrochemical properties for hybrid capacitors. J. Phys. Chem. Solids 2018, 119, 183–192. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Sankar, K.V.; Vasylechko, L.; Lee, Y.-S.; Selvan, R.K. Synthesis and electrochemical performances of maricite-NaMPO4 (M = Ni, Co, Mn) electrodes for hybrid supercapacitors. RSC Adv. 2014, 4, 53192–53200. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.; Jones, R.; Alenazey, F.; Watcharatharapong, T.; Chakrabortyf, S.; Ahuja, R. Synthesis, structural and electrochemical properties of sodium nickel phosphate for energy storage devices. Nanoscale 2016, 8, 11291–11305. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Mitchell, D.R.G. Dispersion of Ni2+ ions via acetate precursor in the preparation of NaNiPO4 nanoparticles: Effect of acetate vs. nitrate on the capacitive energy storage properties. Dalton Trans. 2017, 46, 13704–13713. [Google Scholar] [CrossRef]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/Activated carbon: Hybrid capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Watcharatharapong, T.; Chakraborty, S.; Ahuja, R.; Duraisamy, S.; Rao, T.; Munichandraiah, N. Synthesis, and crystal and electronic structure of sodium metal phosphate for use as a hybrid capacitor in non-aqueous electrolyte. Dalton Trans. 2015, 44, 20108–20120. [Google Scholar] [CrossRef] [Green Version]
- Koleva, V.; Stoyanova, R.; Zhecheva, E. Nano-crystalline LiMnPO4 prepared by a new phosphate–formate precursor method. Mater. Chem. Phys. 2010, 121, 370–377. [Google Scholar] [CrossRef]
- Koleva, V.; Boyadzhieva, T.; Zhecheva, E.; Nihtianova, D.; Simova, S.; Tyuliev, G.; Stoyanova, R. Precursor-based methods for low-temperature synthesis of defectless NaMnPO4 with an olivine- and maricite-type structure. Cryst. Eng. Comm. 2013, 15, 9080–9089. [Google Scholar] [CrossRef]
- Roberts, A.J.; Slade, R.C.T. Effect of specific surface area on capacitance in asymmetric carbon/α-MnO2 supercapacitors. Electrochim. Acta 2010, 55, 7460–7469. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Saha, S.; Ghosh, M.; Chowdhury, S.R.; Maiyalagan, T.; Bhattacharya, S.K.; Das, S. Das, Electrochemical energy storage properties of Ni-Mn-oxide electrodes for advance asymmetric supercapacitor application. Langmuir 2019, 35, 8257–8267. [Google Scholar]
- Liu, P.F.; Zhou, J.J.; Li, G.C.; Wu, M.K.; Tao, K.; Yi, F.Y.; Zhao, W.N.; Han, L. A hierarchical NiO/NiMn-layered double hydroxide nanosheet array on Ni foam for high performance supercapacitors. Dalton Trans. 2017, 46, 7388–7391. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ai, Y.; Liu, F.; Chang, X.; Xue, Y.; Huang, Q.; Wang, C.; Lin, H.; Han, S. Carbon-coated hierarchical Ni–Mn layered double hydroxide nanoarrays on Ni foam for flexible high-capacitance supercapacitors. Electrochim. Acta 2016, 213, 55–65. [Google Scholar] [CrossRef]
- Bucher, N.; Hartung, S.; Nagasubramanian, A.; Cheah, Y.L.; Hoster, H.E.; Madhavi, S. Layered NaxMnO2+z in sodium ion batteries–influence of morphology on cycle performance. ACS Appl. Mater. Interfaces 2014, 6, 8059–8065. [Google Scholar] [CrossRef]
- Yu, M.; Liu, R.; Liu, J.; Li, S.; Ma, Y. Polyhedral-like NiMn-layered double hydroxide/porous carbon as electrode for enhanced electrochemical performance supercapacitos. Small 2017, 13, 1702616. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yan, X.; Lyu, M.; Wang, L.; Bell, J.; Wang, H. 2-Methylimidazole-derived Ni–Co layered double hydroxide nanosheets as high rate capability and high energy ensity storage material in hybrid supercapacitors. ACS Appl. Mat. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Hussain, S.; Söderlind, F.; Käll, P.-O.; Abbasi, M.A.; Durrani, S.K. Honeycomb β-Ni(OH)2 films grown on 3D nickel foam substrates at low temperature. Mater. Lett. 2012, 69, 37–40. [Google Scholar] [CrossRef]
- Huang, J.; Xu, P.; Cao, D.; Zhou, X.; Wang, G. Asymmetric supercapacitors based on β-Ni(OH)2 nanosheets and activated carbon with high energy density. J. Power Sources 2014, 246, 371–376. [Google Scholar] [CrossRef]
- Pertlik, F. Structures of hydrothermally synthesized cobalt(II) carbonate and nickel(II) carbonate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 4. [Google Scholar] [CrossRef]
- Maslen, E.N.; Strel’tsov, V.A.; Strel’tsova, N.R.; Ishizawa, N. Electron density and optical anisotropy in rhombohedral carbonates.III.Synchrotron X-ray studies of CaCO3, MgCO3 and MnCO3. Acta Crystallogr. B 1995, 51, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Koleva, V.; Zhecheva, E.; Stoyanova, R. Ordered Olivine-Type Lithium–Cobalt and Lithium–Nickel Phosphates Prepared by a New Precursor Method. Eur. J. Inorg. Chem. 2010, 2010, 4091–4099. [Google Scholar] [CrossRef]


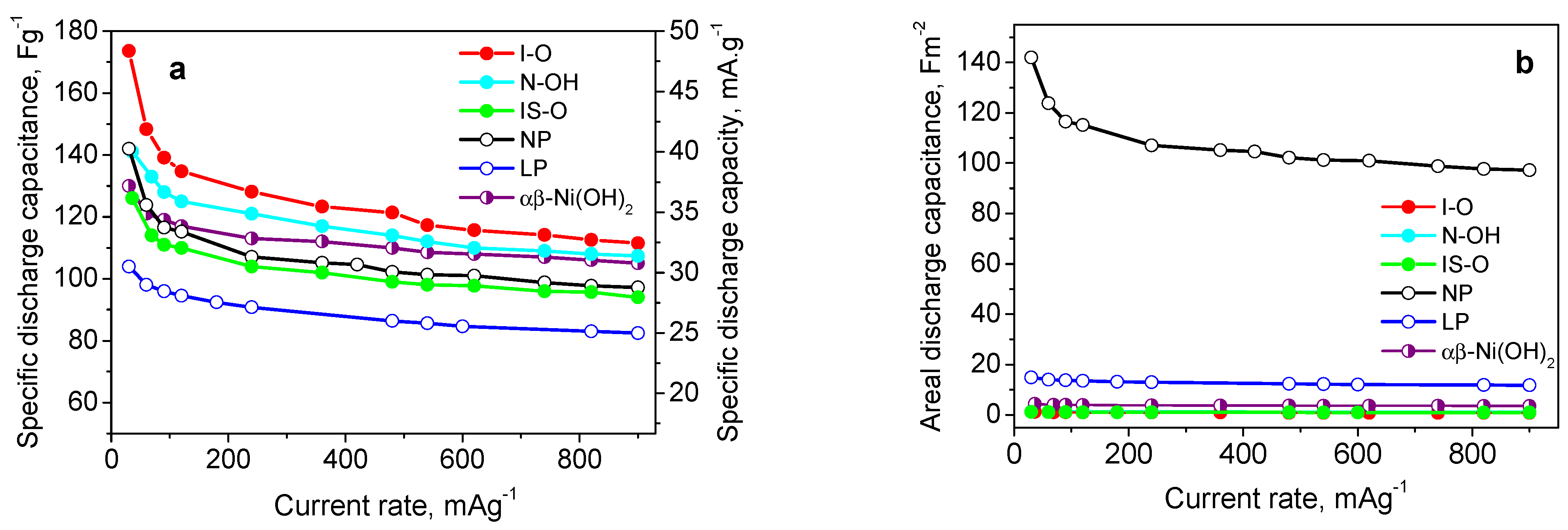

 ,
,  and
and  , respectively (right). The used references are given in the figure: [12,13,14,24,25,26,27,29,30,37,38,39,40].
, respectively (right). The used references are given in the figure: [12,13,14,24,25,26,27,29,30,37,38,39,40].
 ,
,  and
and  , respectively (right). The used references are given in the figure: [12,13,14,24,25,26,27,29,30,37,38,39,40].
, respectively (right). The used references are given in the figure: [12,13,14,24,25,26,27,29,30,37,38,39,40].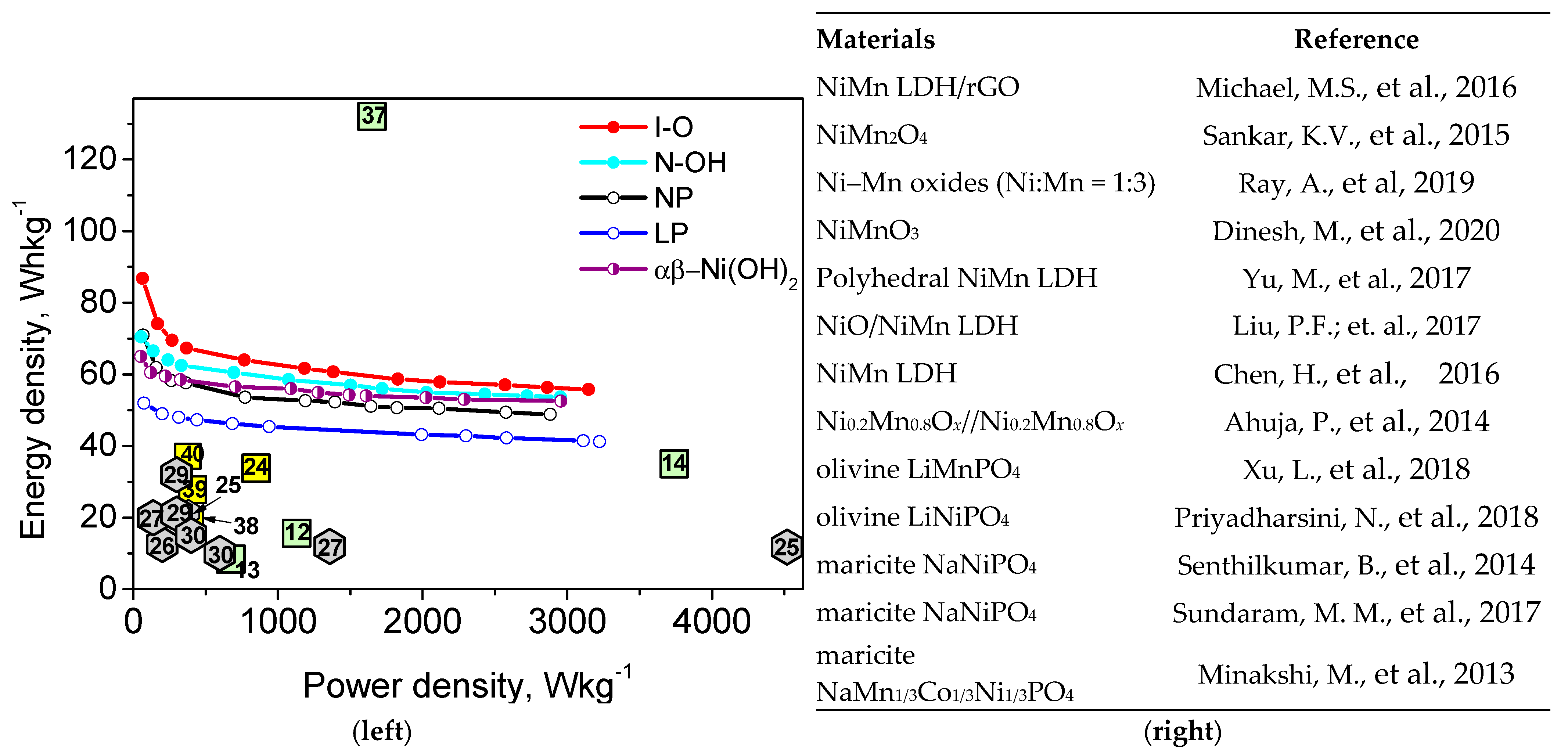
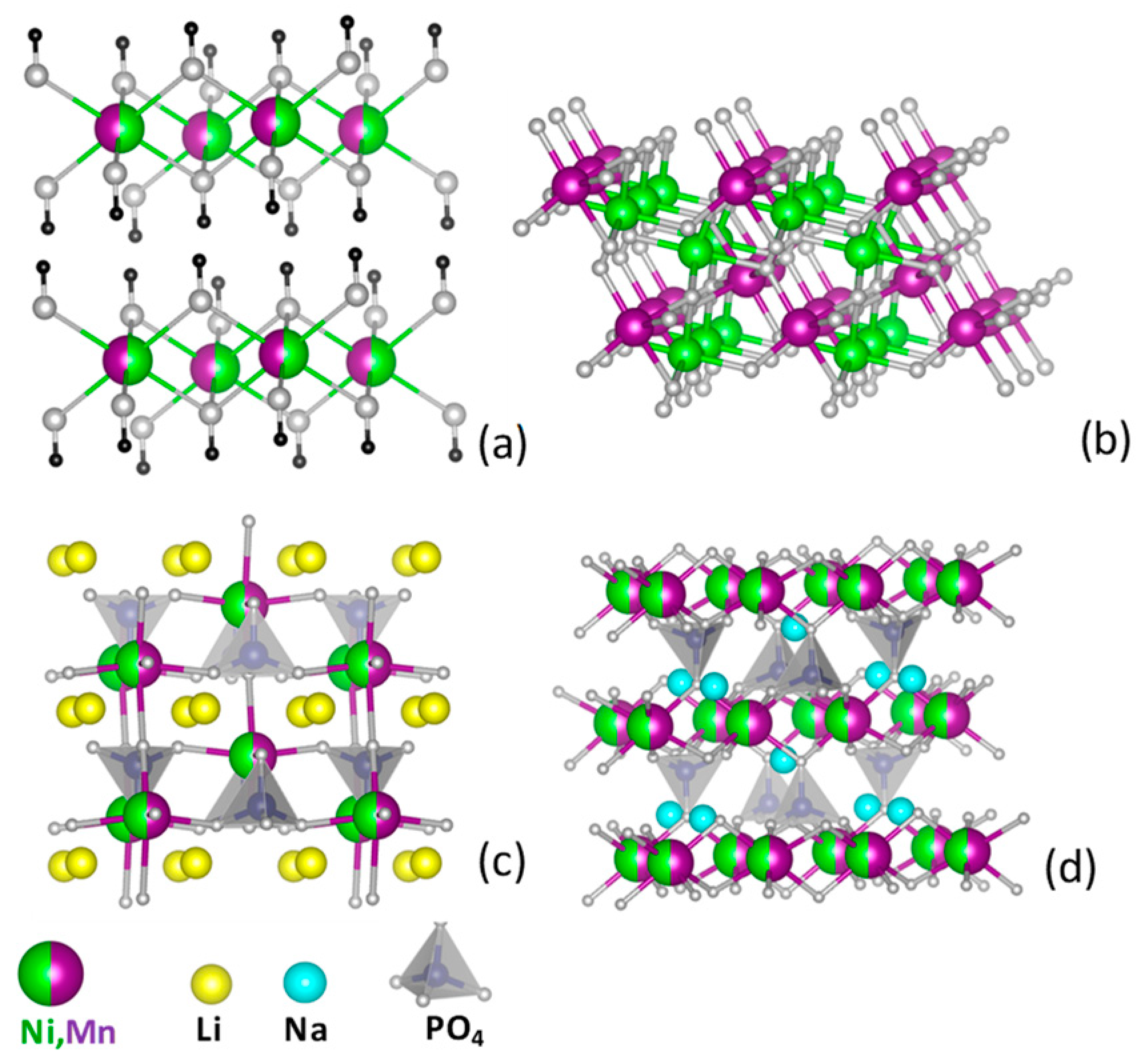
| Sample | Description | Preparation Method | T, °C | Annealing Time, hs | Phase Composition | Labeling |
|---|---|---|---|---|---|---|
| 1 | Ni–Mn hydroxide | Co-precipitation from nitrate salts | 25 | - | β-type Ni0.5Mn0.5(OH)2 | N-OH |
| 2 | Ni–Mn oxide | Thermal decomposition of hydroxides prepared from nitrates | 400 | 3 | Mixture of ilmenite NiMnO3 and spinel Ni1.5Mn1.5O4 | IS-O |
| 3 | Ni–Mn oxide | Thermal decomposition of Ni1/2Mn1/2CO3 | 400 | 3 | Single ilmenite NiMnO3 phase | I-O |
| 4 | Li–Ni–Mn phosphate | Li–Ni–Mn phosphate-formate precursor | 500 | 10 | Single olivine phase LiNi1/2Mn1/2PO4 | LP |
| 5 | Na–Ni–Mn phosphate | Na–Ni–Mn phosphate-formate precursor | 700 | 10 | Single maricite phase NaNi1/2Mn1/2PO4 | NP |
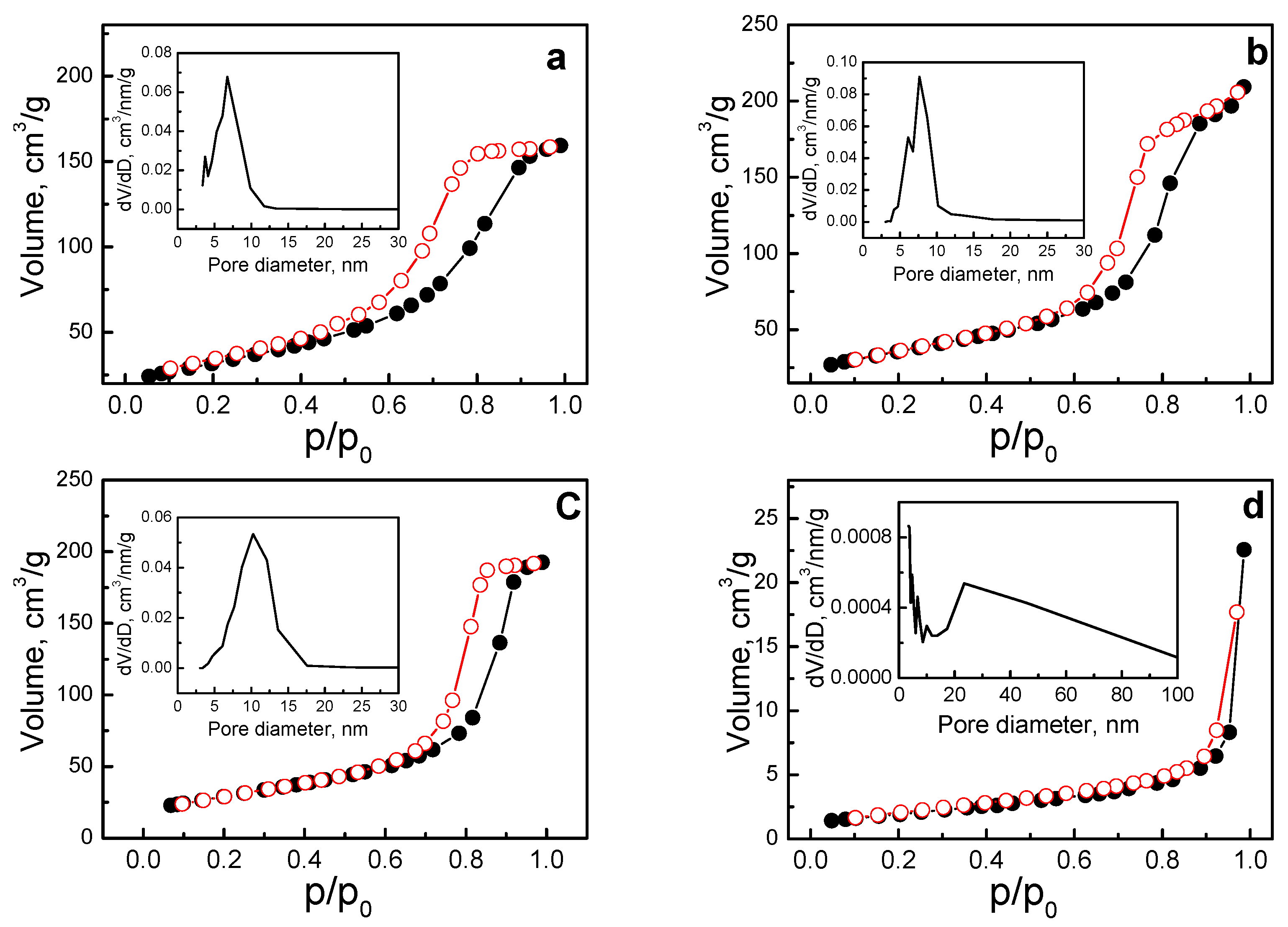
| Samples | Detailed Description | SBET, m2 g−1 | Vt, cm3 g−1 | Pore size Distribution, nm |
|---|---|---|---|---|
| N-OH | β-Ni1/2Mn1/2(OH)2 | 117 | 0.25 | Uniform narrow pore size distribution between 3 and 12 nm; mean pore size of 8 nm |
| I-O | NiMnO3 | 128 | 0.35 | Narrow pore size distribution between 3 and 12 nm; mean pore size of 10 nm |
| IS-O | NiMnO3 + Ni1.5Mn1.5O4 | 106 | 0.30 | Uniform narrow pore size distribution between 3 and 18 nm; mean pore size of 11 nm |
| LP | LiNi1/2Mn1/2PO4 | 7 | 0.04 | Broad pore size distribution between 5 and 100 nm, with mesopores between 10 and 50 nm being predominant |
| NP | NaNi1/2Mn1/2PO4 | ≈1 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soserov, L.; Marinova, D.; Koleva, V.; Stoyanova, A.; Stoyanova, R. Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors. Batteries 2022, 8, 51. https://doi.org/10.3390/batteries8060051
Soserov L, Marinova D, Koleva V, Stoyanova A, Stoyanova R. Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors. Batteries. 2022; 8(6):51. https://doi.org/10.3390/batteries8060051
Chicago/Turabian StyleSoserov, Lyubomir, Delyana Marinova, Violeta Koleva, Antonia Stoyanova, and Radostina Stoyanova. 2022. "Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors" Batteries 8, no. 6: 51. https://doi.org/10.3390/batteries8060051
APA StyleSoserov, L., Marinova, D., Koleva, V., Stoyanova, A., & Stoyanova, R. (2022). Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors. Batteries, 8(6), 51. https://doi.org/10.3390/batteries8060051








