Abstract
Solid-state lithium batteries (SSLBs) have made significant progress in recent decades in response to increasing demands for improved safety and higher energy density. Nonetheless, the current state SSLBs are not suitable for wide commercial applications. The low ionic conductivity, lithium dendrites growth, and unstable interfaces between solid electrodes and electrolytes are some of the challenges that need to be overcome. Therefore, it is critical to fully comprehend the structural information of SSLBs at a nanometer scale. Neutron-based techniques (NBTs) are sensitive to light elements (H, Li, B, N, O, etc.) and can distinguish heavy metals (e.g., Mn, Fe, Co, Ni, etc.) containing close atomic numbers or even isotopes (e.g., 1H and 2H). Therefore, NBTs are important and powerful structural and analytical tools for SSLB research and have substantially improved our understanding of these processes. To provide real-time monitoring, researchers have explored many sophisticated in situ NBTs to investigate the underlying mechanisms of SSLBs. This minireview article is primarily dedicated to the investigation of SSLBs using in situ NBTs. In addition, it illustrates the capabilities of different in situ NBTs on SSLBs by illustrating the capabilities of different techniques in recently published works. Ultimately, some perspectives for the next evolution of in situ NBTs in SSLBs are highlighted.
1. Introduction
Rechargeable lithium-ion batteries (LIBs) have been widely used in portable electronics due to their high energy density [1,2] scale flexibility, and low maintenance cost [3]. However, for electric vehicles and large-scale energy storage systems, these applications demand more advanced LIBs with better safety characteristics and longer cycle life. Solid-state electrolytes (SSEs) in place of liquid electrolytes (LEs) in the LIBs could crucially resolve safety concerns [4]. Additionally, the electrochemical stability window of SSEs could be extended to as high as 5 V and eventually be utilized in high-voltage batteries [5,6].
Rechargeable (secondary) SSLBs are considered to be the next-generation high-performance power sources due to their remarkable advantages such as high-temperature stability, high energy density, and battery safety over traditional LIBs. Unfortunately, due to low electrolytes ionic conductivity (10−6–10−3 S cm−1), large interfacial resistance (between SSEs and electrodes), and lithium dendrites hazards, SSLBs always show poor specific capacity and cycling performance in the practical applications [7,8]. To solve these critical issues, many practical solutions have been developed. For example, new electrolytes synthesis strategies to improve ionic conductivity, surface modification to reduce interfacial resistance, and advanced characterization techniques to promote the mechanism understanding of SSLBs [9,10].
It is inevitable to develop SSLBs without developing advanced characterization techniques. Adequate characterization techniques can aid in tracking the evolution of active materials in the electrochemical reaction processes and thoroughly understanding the hidden reasons behind the existing problems. Accordingly, SSLBs can be better modified to improve their electrochemical performance [11,12]. Recently, under ex situ and in situ conditions, enormous progress has been made toward the development of advanced characterization techniques for SSLBs. Ex situ characterizations can reveal the properties of the electrode/SSEs interfaces, but they cannot provide real-time monitoring. Furthermore, its measurement may not fully reflect the true nature of SSLBs due to the sensitivity of electrodes and SSEs to air and moisture [13]. Thus, obtaining real-time information on SSLBs under in situ conditions is vital. In the past years, several in situ characterization techniques, such as X-ray diffraction (XRD), nuclear magnetic resonance (NMR), and in situ NBTs, have been developed [14]. In SSLBs, XRD probes crystal structures and phase transformations while NMR explores atomic sites. NBTs are powerful structural and analytical tools, especially for crystalline materials. In addition, unlike XRD, whose scattering cross section is increased proportionally with the atomic number, the scattering factors of neutron scattering are influenced by the nuclear forces of nuclei. Therefore, NBTs are more sensitive than X-rays in light elements [15,16,17]. NBTs can distinguish light elements (such as H, Li, B, N, O, etc.), isotopes (e.g., 1H and 2H, 6Li and 7Li, etc.), and transition metal elements (e.g., Mn, Fe, Co, Ni, etc.) with close atomic numbers [18,19]. Compared to XRD, NBTs provide complementary structural information to study SSLBs. This minireview describes in situ NBTs that were adopted to characterize the SSLBs and focuses on recent research to reveal the causes of capacity losses in SSLBs. Furthermore, it captures the intermediate phases during the synthesis of SSEs. Ultimately, it presents prospects to develop NBTs with a broad range of applications.
2. Neutron-Based Characterization Techniques
The working principle of NBTs is based on neutrons interacting with nuclei or electrons, with neutron–nucleus interactions as the most probable way. The neutrons-electrons interaction is also called magnetic scattering, which measures the spin–spin interaction between the magnetic moments of unpaired electrons and neutrons. The neutron–nucleus interactions can be classified into adsorption interactions and scattering interactions. Many NBTs have been applied in materials and batteries study, mainly including Neutron Powder Diffraction (NPD), Neutron Pair Distribution Function (NPDF), Small-Angle Neutron Scattering (SANS), Neutron Reflectometry (NR), Neutron Imaging (NI), Neutron Depth Profiling (NDP), Inelastic Neutron Scattering (INS), and Quasi Elastic Neutron Scattering (QENS). The detailed working principle and functions of these NBTs will be illustrated individually in the following section. For a more in-depth study of these methods, these reference books were recommended [20,21,22].
As a powerful tool to study materials and battery systems, NBTs have received increasing attention over the past decade. In 2006, the Commissioning of the Spallation Neutron Source (SNS) at the Oak Ridge National Laboratory drove the applications of NBTs in batteries [23]. Resolving fundamental mechanisms, including crystal structure’s progression during (de)lithiation, the real-time surface modification of active materials, and monitoring the distribution and deposition of lithium are among the most important contributions of NBTs. To gain a better understanding of the capacity decay processes, charge rate-phase change relationships, and safety information contained in SSLBs, in situ NPD, NPDF, SANS, NR, NI, NDP, INS, and QENS techniques were reviewed. In Figure 1, a summary of experimental setups, working mechanisms, functions, and advantages of these NBTs is illustrated.
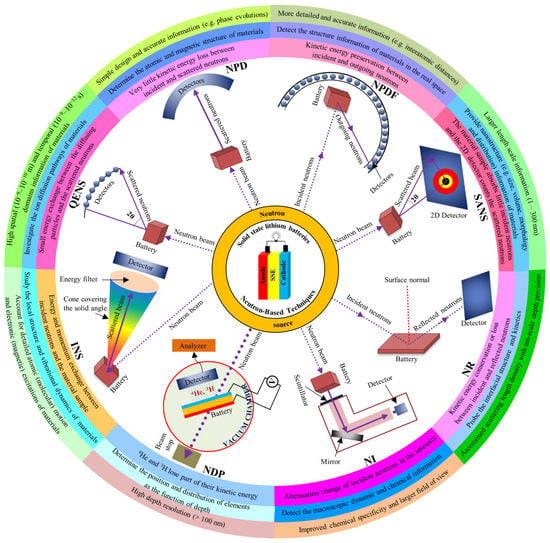
Figure 1.
A summary of experimental set-ups, working mechanisms, functions, and advantages of different neutron-based characterization techniques for solid-state lithium batteries.
2.1. Neutron Powder Diffraction (NPD)
The working principle of NPD is based on the coherent-interference pattern (i.e., Bragg peak) scattered in polycrystalline materials with well-defined lattice planes. The neutrons interact with the nucleus of atoms, which are non-destructive due to their low energy. As a result, this weak interaction leads to high penetration depths [24]. The measured NPD patterns can be used to determine the structure distortion, microscopic stress, phase composition, lattice defect, atomic occupation, and even minor dynamical information (e.g., Li-ion diffusion pathways) of materials [22]. The precision of this structure information is extremely reliant on the diffractometer’s resolution and the refinement process. It should also be noted that the inclusion of appropriate isotopes in the samples, such as 7Li, can increase the overall quality of the quantified NPD data [25]. Besides, compared to other NBTs, the NPD technique is fundamentally simpler, as depicted in Figure 2a, making it the ideal candidate for in situ/Operando research on SSLBs.
Polycrystalline materials are common in SSLB materials and NPD could be an effective method to observe these polycrystalline materials. Additionally, NPD can be used to accurately assess the Li-ion conductivity and dynamic of SSEs as well as their intrinsic crystal structure. For example, Kaup et al. [26] investigated the structure, and Li-ion diffusion pathways in Li3PS4 SSE using in situ high-resolution NPD at variable temperatures to reveal the root cause of the high ion conductivity and stability. The results showed that the fast-ion conductivity and good stability of nanoporous form β-Li3PS4 are strongly affected by the incorporation of amorphous hydrogen-containing components, which will not be released from the structure until very high temperatures. The lithium diffusion pathways of Li3PS4 are evaluated using the maximum entropy method and bond valence site energy calculations. The in situ NPD approach is a useful tool for revealing the processes that occur during electrochemical cycling in solid-state batteries but has not been widely used to research SSLBs. It is limited to battery materials with good crystallinity like argyrodite-types (e.g., Li6PS5X, X = Cl, Br, I) [27], Li7La3Zr3O12 (LLZO) [28,29,30], and other materials with longrange structural order [31]. Table 1 sheds light on recent research on in situ or Operando NBTs of different SSLBs, together with their findings.
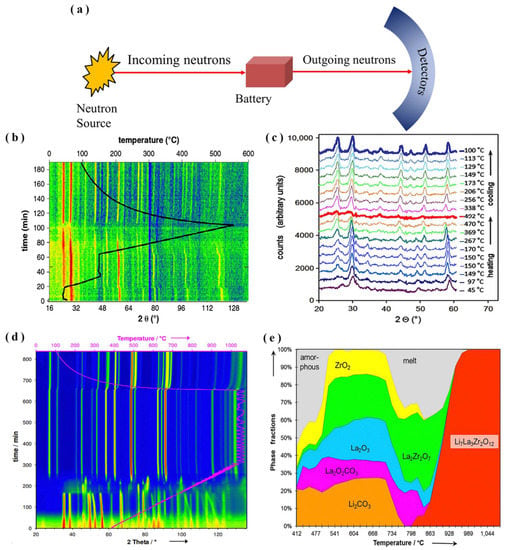
Figure 2.
(a) Simplified diagram for in situ/Operando NPD measurements. (b) Patterns of Li6PS5Cl SSE diffraction in two dimensions (2D), (c) NPD patterns for Li6PS5Cl SSE at various temperatures (adapted with permission from Ref. [27]. 2013 Elsevier B.V). (d) 2D of LLZO formation diffraction pattern intensity contour map, (e) Rietveld analysis weight fractions of LLZO sample phases during heating (adapted with permission from Ref. [32]. 2015 American Chemical Society).
For instance, Rao et al. [27] employed in situ NPD to study and better comprehend the phase formation of Li6PS5Cl SSE. The precursor mixture was amorphous at room temperature, with crystalline peaks of Li2S and LiCl. At 80 °C, crystalline argyrodite started its formation and stayed crystalline until it evolved into amorphous at 490 °C. Argyrodite recrystallized on cooling and stayed unchanged at a temperature of 90 °C. As shown in Figure 2b, the Li7PS6 crystalline argyrodite phase formed at a temperature range between 80 and 150 °C. However, it required an elevated annealing temperature (>430 °C) and fast cooling to reach the chemical composition Li6PS5Cl, as displayed in Figure 2c. Furthermore, using in situ NPD, Rao et al. [32] monitored the phase of LLZO formation and provided a thorough knowledge of its manufacturing processes, as displayed in Figure 2d. The results revealed that LLZO formation came as an effect of the decomposition of partially melted carbonate throughout the heating process, as demonstrated in Figure 2e. Besides, the cooling rate has a significant impact on the distribution and ordering of Li+-ions in LLZO, which is important for controlling ionic conductivity. Therefore, to achieve maximal LLZO conductivity, the pace of cooling must be modest enough to keep Li+ distribution equilibrium in different Li+-sites.
The NPD is an efficient instrument to demonstrate how element doping can influence the LLZO’s crystal structure and increase its ion conductivity [33]. Additionally, NPD has the potential to elucidate the reaction mechanism and coexisting intermediate phases that existed during garnet synthesis. As shown in Figure 3, using in situ NPD, Chen et al. [34] tracked stage transformations throughout the synthesis of Al- and Zn-doped garnets (LLZO-Al24 and LLZO-Zn60).
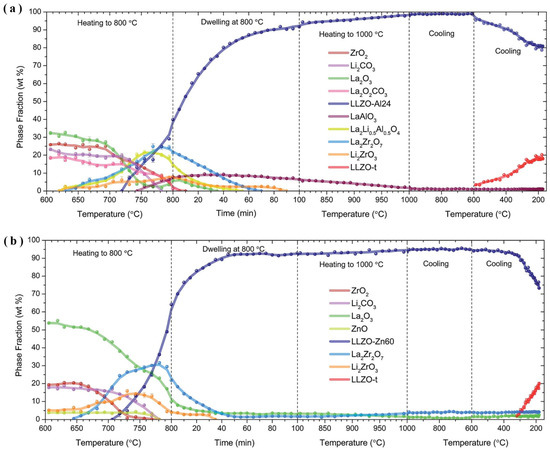
Figure 3.
In situ NPD synthesis of (a) LLZO-Al24 and (b) LLZO-Zn60 with intermediate phases La2Zr2O7 and Li2ZrO3 (adapted with permission Ref. [34]. 2015 The Royal Society of Chemistry).

Table 1.
In situ/Operando neutron-based techniques in SSLBs: capabilities, applications, and major research findings.
Table 1.
In situ/Operando neutron-based techniques in SSLBs: capabilities, applications, and major research findings.
| Composition | Applications | Main Findings | Refs. | ||
|---|---|---|---|---|---|
| Neutron-Based Techniques | Abilities | SSE | Cell Investigation | ||
| NPD | Examine the information contained in crystalline materials’ structures (space group, lattice constants, and atomic coordinates). | Li6PS5Cl | Li4Ti5O12:C:SSE/SSE/Li | The formation of Li7PS6 interphase at temperatures ranging from 80 to 150 °C during the synthesis of Li6PS5Cl crystalline argyrodite. | [27] |
| LLZO | No data | During the heating process, a partial carbonate melt starts to decompose, which induced the formation of LLZO. | [32] | ||
| LLZO-Al24, LLZO-Zn60 | No data | During the synthesis of Al24- and Zn60-doped LLZO, the Li source became non-stoichiometry, and the unreacted reagents caused the formation of La2Zr2O7 and Li2ZrO3 intermediate phases. | [34] | ||
| NPDF | Investigated the local structure of amorphous or polycrystalline materials (pair distribution function, the nearest neighbor distance, and coordination). | No data | No data | No data | |
| SANS | Provide the nanostructure information in bulk phases (nanoscale grain changes in volumetric and constants) of battery materials | Solid polymer electrolyte (SPE) | Li/SPE/LiFePO4 | The SPE lithium–metal batteries indicated no formation of dendrites and cell failure at 0.5 C and 80 °C but failed after more than 200 cycles when cycled at 0.7 C and 90 °C. | [35] |
| NR | High resolution for structure, composition, and magnetism investigations of battery material’s surface and interfaces. | LixPOyNz (LiPON) | Si/LiPON/SLEI/LE | The interphase formed between LiPON and different LEs interfaces consisted of a lithium-rich outer layer and an insulating layer directly in touch with LiPON. | [36] |
| NI | Contrast in neutron transmission (attenuation) to elucidate the bulk structure of materials visually. | No data | No data | No data | |
| NDP | Investigate the depth-dependent distribution of particular elements in SSLBs. | Nitrogen-doped Li3PO4 (N-LiPON) | LiCoO2/N-LiPON/Li | In situ NDP disclosed the thin-film all-SSLBs’ lithium position and mobility. | [37] |
| Li3PO4 | Si-Li3PO4-LiCoO2 | The lithium immobilization in SSE, originating from anode/SSE contact throughout the first charging, was the main source of Si-Li3PO4-LiCoO2 thin film batteries’ capacity loss. | [38] | ||
| Li6.4La3Zr1.4Ta0.6O12 (LLZTO) | Li/LLZTO/Ti | The majority of lithium deposited in the Ti 3D electrode’s void space significantly inhibited the extension of lithium dendrite and the deterioration of the SSE/electrode interface. | [39] | ||
| LLZO | Li/LLZO/Li, Li/LLZO/CNT | The poor physical contact caused Li ions to be only plated-stripped reversibly near the LLZO/CNT interface. | [40] | ||
| LLZO | Li/LLZO/Cu | Due to the high electronic conductivity of LLZO, quick deposition of Li dendrites occurred within the bulk LLZO. | [41] | ||
| Li3PS4 | Li/Li3PS4/Cu | Because of Li3PS4′s high electronic conductivity, Li dendrites were formed directly inside the bulk Li3PS4. | [41] | ||
| LiPON | LiCoO2/LiPON/Cu | LiPON’s lack of dendrite growth was due to its low electronic conductivity. | [41] | ||
| LLZO | NMC//CNT/Li6.75La3Zr1.75Ta0.25O12 (LLZTO)/Li | The LLZO’s reversible short-circuit characteristic was attributed to its low ionic conductivity and non-negligible electronic conductivity. | [42] | ||
| INS | Probe the local structure of materials as well as their dynamical vibrations (phonon dispersion and the density of states). | No data | No data | No data | |
| QENS | Examine the diffusional dynamics of battery materials (diffusion coefficient and atom jump length). | Li7P3S11 | No data | At 473 K, a signal for Li-ion self-diffusion was observed. | [43] |
LiPON: Lithium Phosphorous Oxide Nitride, SLEI: Solid–Liquid Electrolyte Interphases, SPE: Solid Polymer Electrolytes, NMC: Lithium Nickel Cobalt Manganese Oxide (LiNiCoMnO2), CNT: Carbon Nanotubes.
2.2. Neutron Pair Distribution Function (NPDF)
The NPDF has occasionally been termed neutron total scattering (NTS) because it detects all the scatterings throughout all reciprocal spaces, as displayed in Figure 4 [44,45]. It is quite similar to a standard NPD experiment, however, unlike NPD, NPDF takes into account both the Bragg and diffuse scatterings combined with pair distribution function (PDF) components obtained from the diffraction pattern and is a strong technique for detecting material structure on a small scale. The variable Q is typically utilized in NPDF studies to determine the location in reciprocal space. Q is the magnitude of the scattering vector, defined as:
where is half the scattering angle, and λ is the neutron beam wavelength. In an NPDF experiment, a likely minimum Q = 30 Å−1 can be quantified [45]. NPDF is particularly useful for studying the structure of nanomaterials, amorphous materials, and polycrystalline materials with poor crystallinity. Unlike the NPD technique, NPDF uses Fourier transformation of NTS data to directly identify material structure information in real space. The NPDF spectra can reveal real-space structure information about materials, such as interatomic distances, atomic coordination numbers, and so on. The average separation of atomic pairs, the coordination number, dynamic disorder, and structural coherence correspond to the NPDF peak position, intensity, width, and attenuation, respectively [46]. NPDF can be beneficial for investigating LIB materials [47,48,49,50,51,52,53]. However, based on our knowledge, there is no current research report on in situ/Operando NPDF investigating SSLBs. This could be owing to the general shortage of high energy and high flux radiation sources for NPDF experiments. Furthermore, NPDF has a low real-space resolution and makes it difficult to differentiate many components.
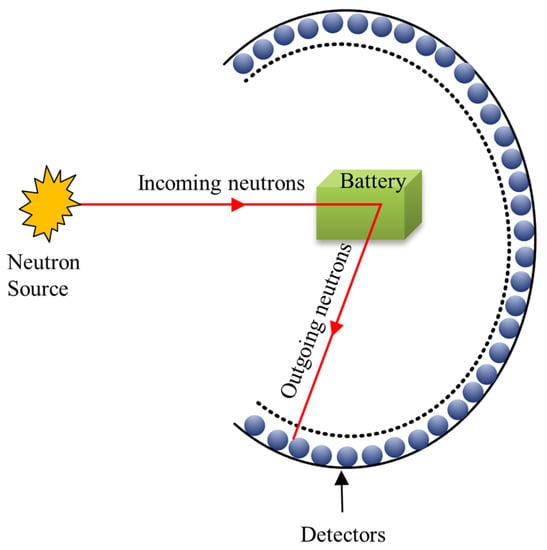
Figure 4.
Schematic experimental set-up of in situ/Operando NPDF (adapted with permission from Ref. [45]. 2019 The Royal Society of Chemistry).
2.3. Small-Angle Neutron Scattering (SANS)
The working mechanism of SANS is illustrated in Figure 5a. Incoming planar neutrons with wavelengths λ in the range of 5 to 20 Å hit the sample material and emit a spherical neutron wave, and a 2D area detector located at a sample detector distance (SDD) computes the scattered neutrons [22,54]. Neutron scattering can happen with or without energy exchange between scattered neutrons and the sample material. Especially, the scattered neutrons can have the same wavelength as incoming neutrons (elastic scattering) or a different wavelength than incoming neutrons (inelastic scattering). The relationship between the absolute values
where is the initial wavevector of the incident neutrons, kf is the resultant wavevector of the scattered neutrons, and λ is the wavelength of the neutrons. Only elastic scattering is taken into consideration in a SANS experiment, and the scattered intensity is plotted as a function of the absolute value of the scattering vector Q [22,54].
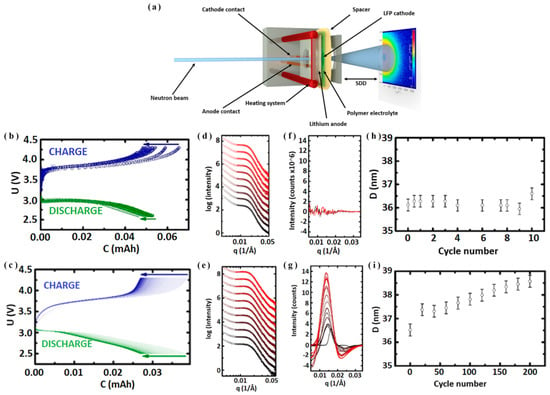
Figure 5.
(a) Graphic for complete battery Operando SANS configuration, battery capacity-voltage diagram cycled at a rate of (b) 0.5 C for 10 cycles from 2.6 to 4.2 V, and (c) 0.7 C for 200 cycles between 2.4 and 4.3 V, SANS radial profiles during battery cycling from (d) cycle 0 (black) to cycle 10 (red), and (e) cycle 0 (black) to cycle 200 (red), (f) initial frame subtracted SANS data shown in Figure 5d, (g) Gaussian fits the first frame of SANS data shown in (e), periodic distances of structures in polymer electrolyte SDBCE from SANS fits using hexagonally packed cylinder SPE morphology at (h) 0.5 C for 10 cycles between 2.6 to 4.2 V and (i) 0.7 C for 200 cycles between 2.4 and 4.3 V (adapted with permission from Ref. [35]. 2018 American Chemical Society).
Additionally, it is possible to get a wide range of Q (~0.001Å−1 to 0.5 Å−1), which covers a structural length scale from ~1 nm to ~200 nm, with different sample-to-detector distances [54]. As a result, in scattering experiments, the scale observed is inversely proportional to the wavelength.
To accurately achieve the sample scattering, several measurements are required, including direct beam intensity, sample transmission, background noise, empty cell scattering, and detector efficiency. In a distinctive SANS SSLB cell, neutron absorption by Li is minimal, and this is taken into consideration when quantitatively accounting for the sample transmission. Mostly, the resolution of the SANS measurement is determined by the dispersion of the neutron wavelength. The SANS is designed to detect the nanostructure information (e.g., size, volume, and distribution) of materials at a nanoscale level (1–300 nm) [55,56]. SANS quantifies two aspects: direct structure measurements, such as the swelling and recovery of nanoscale grains in a bulk assembly, and contrast changes caused by lithiation-induced scattering length density (SLD) fluctuation [54].
The SANS is highly perceptive of materials’ surface and interface structures [54,57,58,59]. However, until now, the applications of in situ SANS that address the challenges of SSLBs are limited. It is worthwhile to note that the Operando SANS has been employed to study the cycling performance of solid polymer electrolytes (SPE) batteries. For example, Möhl et al. [35] utilized Operando SANS to investigate whether the lithium metal batteries based on SPE (i.e., solid single-ion diblock copolymer electrolyte (SDBCE)) with lithium metal anodes and LFP cathodes are suitable for safe batteries, as shown in Figure 5b–i. There was neither dendrites formation nor cell failure after more than 200 cycles at 0.7 C. Furthermore, the cycling performances indicated that this unique SDBCE is the key to developing highly powerful and safe SSLBs [35].
2.4. Neutron Reflectivity (NR)
The NR working principle is based on the measurement of an incoming neutron beam that strikes a sample material’s surface at a grazing angle θ and is partially reflected at an angle θr and partially refracted into the specimen at an angle θt [22,60]. NR typically measures the SLD as a function of depth, which can be easily interpreted as a constituent material compositional profile [54,61]. The SLD is the sum of each isotope’s number density at a given depth multiplied by its bound coherent neutron scattering length. Normally, in an NR experiment, the desired information is the variation of sample composition along with the sample depth.
NR is a powerful neutron-based characterization tool used to examine the surface and interface structure and kinetics of solid materials. Additionally, via NR it is easy to interpret the compositional profile of the constituent materials from measurements of the SLD [54]. Nevertheless, NR necessitates samples that are very smooth with very little roughness [36]. Despite the application of in situ/Operando to investigate a wide variety of thin-film materials [62,63,64,65,66], the application of in situ NR to address the issues of SSLBs remains in the early stages.
However, Weiss et al. [36] used in situ NR to track and measure the formation and thickness of the SLEI between typical LEs (1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)) and LiPON SSE, as indicated in Figure 6a,d. Besides, the authors hypothesized a method of how the SLEI could form. The NR measurement results indicated that the formed SLEI consisted of a lithium-rich outer layer (HCH2OLi) and a nonconducting layer (Li2CO3) directly in touch with LiPON. The authors demonstrated that the SLEI grew as a result of the rapid deposition of a film that covered the LiPON with only a small amount of porosity remaining. The remaining void space was gradually filled, blocking the remaining channels for ionic conduction, and increasing the interphase resistance. Eventually, how to slow down or suppress highly resistive interphase formation can help improve hybrid battery performance with LE and SSE.
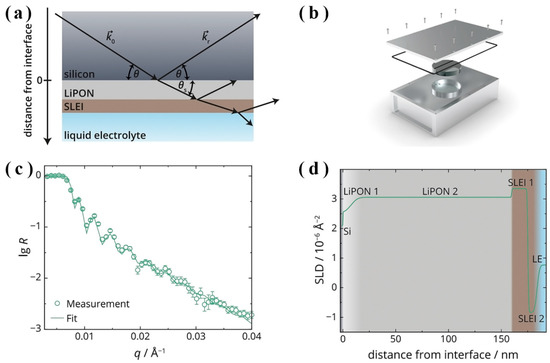
Figure 6.
Measurement set-up for: (a) NR studies and (b) airtight measurement cell. In situ NR curve of (c) sputtered LiPON film dipped into LE for 3 h and (d) changes of SLD throughout the Si/LiPON/SLEI/LE model (adapted with permission from Ref. [36]. 2019 American Chemical Society).
2.5. Neutron Imaging (NI)
NI, also known as neutron radiography or neutron tomography, is a non-destructive method for seeing the bulk structural information of battery materials and is a valuable technique for in situ quantifications. As presented in Figure 7, during an experiment using NI technique, scintillators convert neutrons transmitted through the object into light signals. A digital detector records the signal, which is subsequently used to create grayscale radiographs known as projections [67].
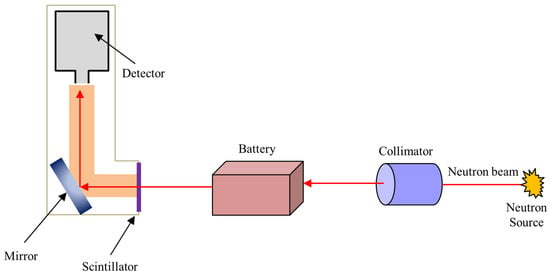
Figure 7.
Experimental set-up of the NI for in situ/Operando quantifications (adapted with permission from Ref. [67]. 2018 Elsevier).
The fundamental premise of the NI approach is to observe the perspective image of the measured battery by observing the neutron beam’s attenuation change in intensity as it travels through the battery. The number of transmitted neutrons is reduced due to neutron interactions with the battery [68]. Advanced NI techniques, including Bragg-edge imaging, three-dimensional tomography, phase-contrast imaging, etc., are now possible due to improved detector spatial and temporal resolution and computational methodologies. For example, Bragg-edge imaging has a greater spatial resolution than the standard NI approach but has significant hurdles in terms of experimental measurements and data interpretation.
NI has been regarded as a potent and direct technique for studying Li-ion transport, spatial distribution [67,69], and concentration evolution [68] during cycling processes, which is critical for optimizing overall battery performance. NI was utilized in conjunction with an in situ experiment to monitor the lithiation and the delithiation processes in the electrode during cycling. In situ/Operando NI proved to be an excellent instrument for observing and comprehending the gas dynamics in LIBs [70,71]. Furthermore, the use of Operando NI revealed the dynamic distribution of dendrites in real-time as well as the LIB short-circuiting [72]. Currently, Nazer et al. [73] used Operando neutron radiography and neutron tomography in a commercial ICR 10440 LIB containing a three-phase mixture of Li(Ni, Mn, Co)O2, LiCoO2, LiMn2O4, and graphite as the anode electrode, and revealed an uneven distribution of lithium, with the inner electrode windings undergoing more pronounced changes as current rates increased.
However, to the best of our knowledge, presently, there are no data regarding the study of SSLBs using in situ/Operando NI. This is partially due to the scarcity of high-intensity neutron sources. Furthermore, the reliance on nuclear reactor facilities, which are difficult to reach due to logistical constraints, might have hampered the application of NI [74]. Because only such facilities can supply enough neutrons for imaging, these services are now mostly provided to the non-destructive testing community through a small number of NI-capable nuclear research facilities that are open for commercial usage. However, with pulsed spallation sources, the fewer developed NI and diffraction instruments have become operational [75,76,77,78,79]. Therefore, by producing abundant and high-intensity neutron sources that do not require reactors, the NI characterization tool will gain increased reliability and accessibility.
2.6. Neutron Depth Profiling (NDP)
The operating principle of NDP characterization techniques is based on 6Li + n → 4He (2055 keV) + 3H (2727 keV) nuclear reaction. During the reaction, the generated particles, i.e., 4He and 3H, would lose a portion of their kinetic energy when passing through the sample before reaching the detector. By quantifying the surplus energies of the released 4He and 3H particles, the depth of the capture process may thus be rebuilt [37,38,80]. This technique can be considered non-destructive because the number of 6Li atoms involved in this nucleus reaction is insignificant in terms of the total amount of lithium [38]. However, with a larger flux of neutrons and detection capacity, NDP has the potential to become a useful tool to test the accuracy and longevity of SSLB in real-world scenarios. As a function of solid surface depth, NDP is a quantitative measurement technique for a variety of technologically relevant elements (Li, B, N, He, Na, and so on) [54]. The quantification of NDP would be especially beneficial for assessing SSLBs performance at high charging/discharging rates, which is crucial for automotive applications [61].
NDP characterization shows its capability in the study of Li dendrite growth phenomenon and interfacial behaviors of SSEs. Firstly, NDP is a non-destructive and widely used potent tool for analyzing the distribution and transportation behavior of Li ions as a function of depth profiles in SSLB systems [39]. Secondly, since NDP is solely sensitive to the 6Li isotope, the analyzed thin-film battery with a 6Li-enriched LiCoO2 cathode can lead to enhanced signals. Oudenhoven et al. [37] used in situ NDP to measure and analyze the LiCoO2/N-LiPON/Li thin-film battery. Because there was no remarkable lithium exchange between the LiCoO2 cathode and N-Li3PO4 SSE between the time of battery manufacture and the NDP quantification, it was concluded that during the cycling performance the enriched lithium was still present in the cathode as plotted in Figure 8a,b. Therefore, thin-film all-SSLBs can greatly benefit from the NDP technique, because it displays the location and transport of lithium-ion within the thin-film stack.
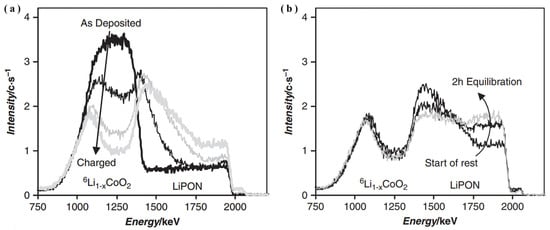
Figure 8.
In situ NDP schemes of 6LiCoO2/N-LiPON/Li battery at (a) various stages of charging and (b) equilibration in the charged state after 0, 1, and 2 h (adapted with permission from Ref. [37]. 2011 Wiley-VCH).
The degradation mechanisms in all-SSLBs are different from traditional LIBs, thus long-term degradation mechanisms of all-SSLBs must be elucidated. For example, Chen et al. [38] applied Operando NDP to study and characterize the aging mechanisms of Si-Li3PO4-LiCoO2 all-solid-state thin-film batteries. As displayed in Figure 9a,b, the authors explained that as the Si-Li3PO4-LiCoO2 battery was initially charged, lithium immobilization in the Li3PO4 SSE initiated capacity degradations of the Si-Li3PO4-LiCoO2 battery, primarily due to anode growth at the anode/Li3PO4 SSE interface. The insertion of silicon (from the anode) into Li3PO4 SSE induced the Li-immobilization layer in Li3PO4 SSE, which grew at a slower rate during the following cycling.
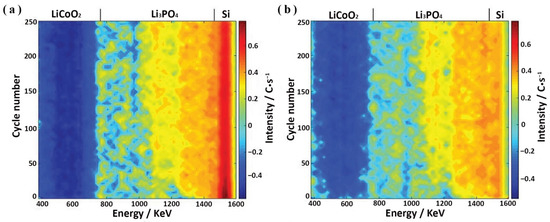
Figure 9.
The evolution of the measured NDP spectra as an energy function of (a) fully charged and (b) discharged Si-Li3PO4-LiCoO2 battery after 250 cycles (adapted with permission from Ref. [38]. 2018 Wiley-VCH).
The resistance to dendrite growth and the high interfacial impedance against electrodes are two major issues that have yet to be addressed [81]. By the means of in situ NDP, Wang et al. [40] monitored the Li plating-stripping processes to elucidate garnet SSE’s interfacial behavior in contact with metallic Li. They fabricated symmetric and asymmetric cells to simulate the performance of lithium metal and lithium-free lithium metal anodes, as shown in Figure 10a–c. As indicated in Figure 10d–m, the obtained data revealed that the source of lithium dendrites was the Li that failed to plating-striping reversibly. Eventually, to solve this interfacial contact problem, the authors proposed that ionically conductive materials, such as liquid or polymer-based electrolytes, may be necessary to enhance Li-ion transport. Alternately, the authors suggested the application of a 3D mixed electron-ion conductor strategy, which can serve as a Li metal host with improved contact area, minimized Li diffusion distance, and compensated space for volume change.
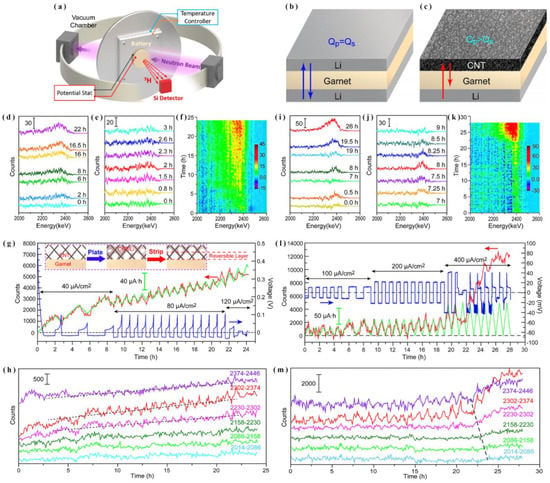
Figure 10.
(a) The set-up for the NDP measurement, schematic of Li plating-stripping in a (b) Li/garnet/Li symmetric cell and (c) Li/garnet/CNT asymmetric cell, (d–h) in situ NDP measurement of a Li/garnet/CNT asymmetric cell while cycling, (i–m) in situ NDP measurement of a Li/garnet/Li symmetric cell during Li plating-stripping (adapted with permission from Ref. [40]. 2017 American Chemical Society).
Similarly, Li et al. [39] used in situ the NDP characterization system to probe the lithium electroplating behavior. Crack propagation and lithium dendrites growth were observed in the Li/LLZTO/Ti SSLBs with 3D Ti electrodes, as displayed in Figure 11a–c. The results revealed that most of the lithium components preferred to deposit within the gap space of the Ti 3D active material, which reduced SSE/active materials contact deterioration and suppressed lithium dendrite growth, as shown in Figure 11d,e. Thus, this study indicated that during lithium plating, a cathode with a well-designed 3D framework could tackle the huge volume expansion and subdue the expansion of lithium dendrites by regulating lithium deposition behavior.
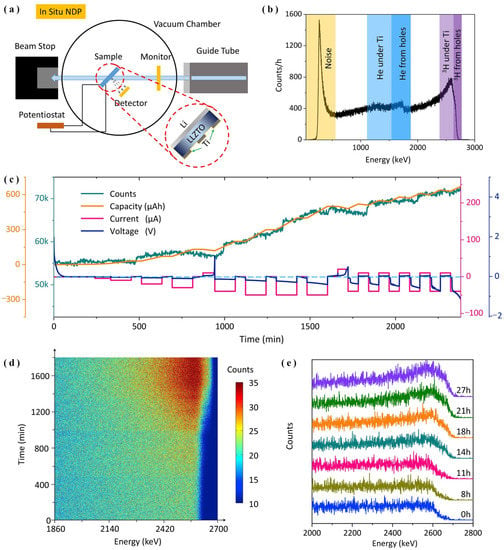
Figure 11.
(a) The experimental set-up of the NDP system, (b) ASSLB NDP spectrum with a 3D Ti electrode, (c) records of in situ measurements in terms of voltages, capacity, and the total number of NDP signal, (d) NDP spectra collected at 3 min intervals during cycling and (e) NDP data integrated curves collected every 1 h during Li plating-stripping of Li/LLZO/Ti SSLBs (adapted with permission from Ref. [39]. 2019 Elsevier).
Due to their stiff nature, engineer researchers believed that SSEs in all-SSLBs could block lithium dendrite deposition and its propagation, hence clearing up the short-circuit issue [81]. However, several types of research illustrated that lithium dendrites can facilely be deposited in LLZO [81,82,83,84] and Li2S-P2S5 SSEs [85], but with a crucially different mechanism compared with LEs. For example, Han et al. [41] utilized time-resolved Operando NDP to track and detect the dynamically evolving LiPON, LLZO, and amorphous Li3PS4 SSE lithium concentrations. The authors envisaged Li plating inside LLZO and Li3PS4 bulk, and there were no clear lithium concentration changes in LiPON. The authors believed that the dendrite extension in LLZO and Li3PS4 SSEs was due to their high electronic conductivities. Ultimately, it is important to reduce the electronic conductivity while keeping the high ionic conductivity of SSEs to achieve the successful application of SSLBs.
For the first time, as shown in Figure 12a, Ping et al. [42] performed real-time in situ NDP to shed light on the reversible nature of short-circuiting behavior induced by the dendrite formation in NMC//CNT/LLZTO/Li all-SSLBs, as revealed in Figure 12b. During the charging process of NMC//CNT/LLZTO/Li all-SSLB, voltage decays on the CNT electrode, demonstrating the generation of Li dendrites within the LLZTO bulk. The smooth voltage profile observed during the discharging process, on the other hand, revealed the vanishing of the short circuit, as indicated in Figure 12c,d. These experimental results are consistent with those of Han et al. [41]. As evidenced in Figure 12e,f, the electrochemical impedance spectroscopy (EIS) measurement also confirmed the reversible short circuit behavior in SSLB. The raised gap between ionic and electronic conductivities at room temperature and 60 °C effectively inhibited the electrically conductive Li dendrite [42]. Thus, to inhibit the short circuit in SSLBs, it is essential and effective to decrease electronic conductivity while increasing ionic conductivity [41,42]. To realize a rechargeable SSLB an efficient design of a 3D framework is extremely significant. In addition, to control lithium’s depositional behavior and stop the growth of lithium dendrites, the 3D framework can also withstand the huge volume enlargement in the process of lithium deposition [39,40].
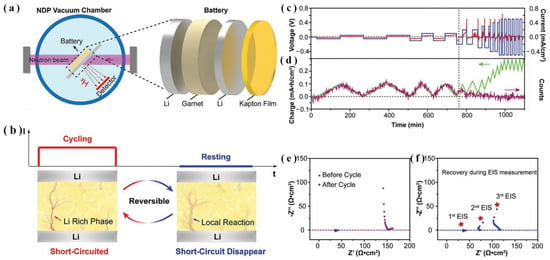
Figure 12.
Schematic of (a) in situ NDP set-up for quantitative Li monitoring, (b) symmetric Li/LLZTO/Li all-SSLBs with reversible short-circuit behavior, (c,d) in situ NDP quantification of a Li/LLZTO/Li symmetric all-SSLB cycling at various current densities, (e,f) pre-NDP and post-NDP impedance spectra of the Li/LLZTO/Li cell and EIS quantifications recovery after NDP cycling (adapted with permission from Ref. [42]. 2020, Wiley-VCH).
2.7. Inelastic Neutron Scattering (INS)
INS can elucidate atomic/molecular movements and electronic/magnetic excitations in great detail. The INS technique is normally equivalent to vibrational spectroscopy with neutrons, which is commonly used to investigate the local structure and vibrational dynamics of materials. Generally, INS refers to scattering processes that entail the exchange of energy and momentum between the neutron and the scatterer (or the sample material) [22,86]. As depicted in Figure 13, an energy filter, which picks neutrons with a limited distribution of energies and/or spins over a collimated solid angle, must be placed in the incident or scattered beam, or both, to differentiate the net energy change for a scattering event [86]. INS peaks, as opposed to quasi-elastic broadening, typically appear at greater energy levels. In particular, inelastic peaks are linked with a periodic motion, and the forces that govern this movement are stronger than those that govern diffusive motion.
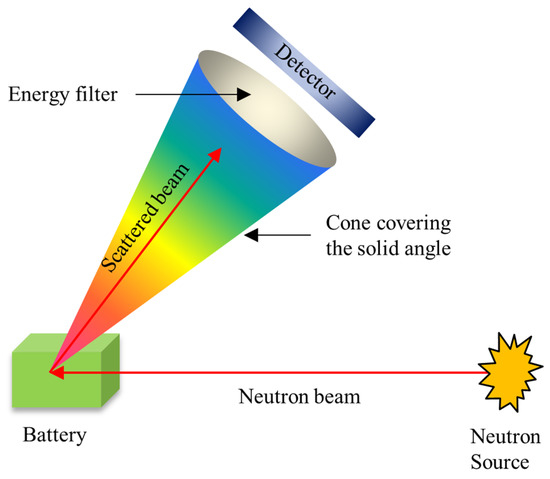
Figure 13.
Scheme of experimental set-up for in situ/Operando INS (adapted with permission from Ref. [86]. 2006 Mineralogical Society of America).
The ion mobility and stability of SSEs play an important role in the electrochemical performance and lifetime of SSLBs. According to Muy et al. [87], the lattice dynamics of LISICON (Lithium superionic conductors) SSEs can have a significant impact on lithium-ion mobility and stability. The use of density functional theory (DFT), in conjunction with INS quantifications, revealed that there is a trade-off between the mobility and stability of fast lithium-ion conductors. To overcome this obstacle and achieve lithium-ion conductors with both high ion conductivity and stability, conductors with low lithium band centers but high anion band centers like Olivines have great potential. These findings offered new ways for manipulating lattice dynamics to create new lithium-ion conductors with improved conductivity and stability [87]. Currently, there is no report on in situ/Operando INS to probe SSEs for all-SSLBs. This may be brought about by the low intensity of INS which is frequently flux-limited. Nevertheless, this condition would be improved with new-generation high-flux neutron sources like SNS. Additionally, experimentalists may find it difficult to interpret INS data. Today, researchers must use theoretical modeling and simulation methodologies that need a high level of sophistication and a significant number of processing resources.
2.8. Second-Quasi-Elastic Neutron Scattering (QENS)
The QENS method is a direct method for studying ion dynamics using neutrons [88,89]. As represented in Figure 14, an incident neutron with an initial wavevector of interacts with the sample’s atomic nucleus and scatters at a 2θ, resulting in a wavevector . The difference between and is defined as the scattering vector q.
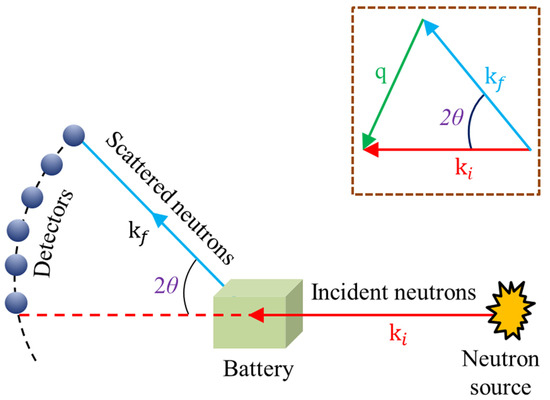
Figure 14.
Model for in situ/Operando QENS experiment (Inset represents the scattering geometry) (adapted with permission from Ref. [90]. 2019 Cambridge University Press).
The incoming neutron can lose or obtain energy as a result of its interaction with the sample, and the quantity of energy transfer is tiny enough to make the elastic approximation [90]. As a result, the scattering vector q, which is defined as the difference between the outgoing and entering wavevector, can be securely approximated to:
Specifically, in QENS experiments, the working mechanism is based on the small energy exchanges between the diffusing particles and the scattered neutrons. This usually causes the elastic peak to spread out a little more. QENS is a powerful instrument that delivers atoms with diffusive dynamic information in the spatial (10−9 to 10−10 nm) and temporal (10−9 to 10−12 s) domains [22]. Besides, QENS provides access to the diffusion dynamics on time and length scales that are consistent with the local diffusion jumps. The incoherent scattering cross-section (σinc) of the target atoms determines the intensity of the QENS signal [88,91,92].
Using QENS ion dynamics of different SSEs has been studied [93,94,95,96,97,98]. Mori et al. [43] used state-of-the-art QENS to directly observe the self-diffusion of Li ions in a metastable Li7P3S11 crystal. The broad peak (the quasielastic component) was unclear at 297 K, while at 473 K it was possible to observe a signal for Li-ion self-diffusion. However, its use in in situ/Operando SSLBs study is limited, but it is critical to explore the behavior of Li+ conductivity and ion dynamic pathways of SSEs.
3. Conclusions and Future Perspectives
In this minireview, research progresses on in situ/Operando NBTs in SSLB materials were primarily discussed with particular examples. Significant advances have been made in techniques using NPD, NPDF, SANS, NR, NI, NDP, INS, and QENS. These techniques could help to explore the correlation between structural properties and electrochemical performances and deterioration of SSLBs, thereby leading to safer and more affable SSLBs. However, it is worth noting that every NBT possesses advantages and disadvantages for SSLBs research. As an example, NPD can provide very accurate information about the 3D structure of atoms. However, this is applicable only for crystallinity and long-range structural order battery materials. The NPDF technique can compensate for the NPD’s shortcomings by analyzing battery materials at the local level, but a lack of real-space resolution and difficulty in distinguishing multiple components are evident shortcomings of NPDF. While SANS can be used to analyze the structure of both crystalline and non-crystalline materials, the low-resolution data produced by SANS techniques are often hard to interpret. The surface (interface) structure of materials is sensitive to NR, but the need for a relatively vast and microscopically smooth surface is the predominant constraint for its quantification. Although the NI can contrast in neutron transmission (attenuation), its resolving power is currently barely restricted to microns, making it a limited imaging tool for battery-related data visualization. The concentration change of Li-ion with the depth of battery materials is very sensitive to NDP with nanoscale spatial precision, but its operating principle precludes its widespread application for structural research. For battery materials, QENS and INS can furnish information on ionic and lattice dynamics. The decreased resoluteness of QENS and INS, on the other hand, hamper the development of these in situ measurements. Because producing nanostructured-SSLBs for in situ characterization is a time-consuming and sophisticated operation, and most Operando equipment is quite expensive, NBTs are not extensively employed for SSLB research. Therefore, to understand the interfacial reactions during the charging/discharging of SSLBs, it is encouraged to develop fairer and more affordable in situ equipment for SSLB research. However, in situ NBTs were applied to track and monitor the evolution of intermediate phases during the synthesis of SSEs for SSLBs and clear up the interfacial processes such as lithium dendrites, which cause the fading of SSLBs. Besides, techniques to prevent lithium dendrites were suggested.
All in all, to get a comprehensive grasp of the active materials and SSLB cell, it is critical to understand the constraints of each NBT and to choose appropriate methods carefully. Additionally, the individual method can only reveal limited information, which may lead to inconsistencies in the understanding of the SSLBs mechanism. Henceforth, an all-SSLB mechanism understanding may benefit from a combination of collective in situ and Operando NBTs. It is anticipated that, with a better understanding of characterization techniques, SSLBs will achieve more practical use shortly.
Author Contributions
M.A.: Conceptualization, Formal analysis, Writing—original draft; X.Y. and C.S.D.: Editing; Y.Z.: Conceptualization; Y.Y.: Conceptualization, Writing—reviewing and editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the National Key R&D Program of China (No. 2018YFE0181300), the National Natural Science Foundation of China (21905035), Liaoning Revitalization Talents Program (XLYC1907093), and the Liaoning Natural Science Foundation (20180510043).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M. Building a Better Battery. Science 2010, 330, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From Nanoscale Interface Characterization to Sustainable Energy Storage Using All-Solid-State Batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Pan, H.; Li, C.; Mu, X.; Du, Y.; Zhang, F.; Zhang, X.; He, P.; Zhou, H. An in Situ Solidifying Strategy Enabling High-Voltage All-Solid-State Li-Metal Batteries Operating at Room Temperature. J. Mater. Chem. A 2020, 8, 25217–25225. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, F.; Wang, X.; Zheng, L.; Yang, X.; Zhao, H.; Sun, Y.; Zhao, W.; Bai, Y.; Wu, C. An Ion-Dipole-Reinforced Polyether Electrolyte with Ion-Solvation Cages Enabling High–Voltage-Tolerant and Ion-Conductive Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2107764. [Google Scholar] [CrossRef]
- Zhao, E.; Ma, F.; Guo, Y.; Jin, Y. Stable LATP/LAGP Double-Layer Solid Electrolyte Prepared: Via a Simple Dry-Pressing Method for Solid State Lithium Ion Batteries. RSC Adv. 2016, 6, 92579–92585. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.; Kang, F. Challenges and Perspectives of Garnet Solid Electrolytes for All Solid-State Lithium Batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-Polymer Composites for Solid-State Lithium Batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, S.; Guan, M.; Ding, B.; Yu, J.; Yan, J. Integrated Solid-State Li-Metal Batteries Mediated by 3D Mixed Ion-Electron Conductive Anodes and Deformable Molten Interphase. Chem. Eng. J. 2022, 442, 136227. [Google Scholar] [CrossRef]
- Yang, S.; Min, X.; Fan, H.; Xiao, J.; Liu, Y.; Mi, R.; Wu, X.; Huang, Z.; Xi, K.; Fang, M. In Situ Characterization of Lithium-Metal Anodes. J. Mater. Chem. A Rev. View 2022, 10, 17917–17947. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X.; Cheng, Y.; Sun, X.; Yang, Y. Advanced Characterization Techniques for Solid State Lithium Battery Research. Mater. Today 2020, 36, 139–157. [Google Scholar] [CrossRef]
- Linsmeier, C.; Fu, C.; Kaprolat, A.; Nielsen, S.F.; Mergia, K.; Schäublin, R.; Lindau, R.; Bolt, H.; Buffière, J.; Caturla, M.J.; et al. Advanced Materials Characterization and Modeling Using Synchrotron, Neutron, TEM, and Novel Micro-Mechanical Techniques—A European Effort to Accelerate Fusion Materials Development. J. Nucl. Mater. 2013, 442, S834–S845. [Google Scholar] [CrossRef]
- Manke, B.I.; Marko, H.; To, C.; Kardjilov, N.; Grothausmann, R.; Dawson, M.; Hartnig, C.; Haas, S.; Thomas, D.; Hoell, A.; et al. Investigation of Energy-Relevant Materials with Synchrotron X-Rays and Neutrons. Adv. Eng. Mater. 2011, 13, 712–729. [Google Scholar] [CrossRef]
- Gu, Q.; Kimpton, J.A.; Brand, H.E.A.; Wang, Z.; Chou, S. Solving Key Challenges in Battery Research Using in Situ Synchrotron and Neutron Techniques. Adv. Energy Mater. 2017, 1, 1602831. [Google Scholar] [CrossRef]
- Ren, Y.; Zuo, X. Synchrotron X-ray and Neutron Diffraction, Total Scattering, and Small-Angle Scattering Techniques for Rechargeable Battery Research. Small Methods 2018, 2, 1800064. [Google Scholar] [CrossRef]
- Balagurov, A.M.; Bobrikov, I.A.; Samoylova, N.Y.; Drozhzhin, O.A.; Antipov, E.V. Neutron Scattering for Analysis of Processes in Lithium-Ion Batteries. Russ. Chem. Rev. 2014, 83, 1120–1134. [Google Scholar] [CrossRef]
- Liang, L.; Rinaldi, R.; Schober, H. Neutron Scattering Applications and Techniques. In Neutron Applications in Earth, Energy and Environmental Sciences; Liang, L., Rinaldi, R., Schober, H., Eds.; Springer: Boston, MA, USA, 2009; pp. 1–14. [Google Scholar] [CrossRef]
- Squires, G.L. Introduction to the Theory of Thermal Neutron Scattering, 3rd ed.; Squires, G.L., Ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 2–9. ISBN 9781139107808. [Google Scholar]
- Fritzsche, H.; Huot, J.; Fruchart, D. Neutron Scattering and Other Nuclear Techniques for Hydrogen in Materials, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–276. ISBN 978-3-319-22792-4. [Google Scholar]
- Mason, T.E.; Abernathy, D.; Anderson, I.; Ankner, J.; Egami, T.; Ehlers, G.; Ekkebus, A.; Granroth, G.; Hagen, M.; Herwig, K.; et al. The Spallation Neutron Source in Oak Ridge: A Powerful Tool for Materials Research. Phys. B Condens. Matter 2006, 385, 955–960. [Google Scholar] [CrossRef]
- Wang, X.L.; An, K.; Cai, L.; Feng, Z.; Nagler, S.E.; Daniel, C.; Rhodes, K.J.; Stoica, A.D.; Skorpenske, H.D.; Liang, C.; et al. Visualizing the Chemistry and Structure Dynamics in Lithium-Ion Batteries by in Situ Neutron Diffraction. Sci. Rep. 2012, 2, 747. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Peterson, V.K. In Situ Neutron Powder Diffraction Studies of Lithium-Ion Batteries. J. Solid State Electrochem. 2012, 16, 1849–1856. [Google Scholar] [CrossRef]
- Kaup, K.; Zhou, L.; Huq, A.; Nazar, L.F. Impact of the Li Substructure on the Diffusion Pathways in Alpha and Beta Li3PS4: An In Situ High Temperature Neutron Diffraction Study. J. Mater. Chem. A 2020, 8, 12446–12456. [Google Scholar] [CrossRef]
- Rao, R.P.; Sharma, N.; Peterson, V.K.; Adams, S. Formation and Conductivity Studies of Lithium Argyrodite Solid Electrolytes Using in Situ Neutron Diffraction. Solid State Ion. 2013, 230, 72–76. [Google Scholar] [CrossRef]
- Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and Dynamics of the Fast Lithium Ion Conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef] [PubMed]
- Meesala, Y.; Liao, Y.; Jena, A.; Yang, N.; Pang, W.K.; Hu, S.; Chang, H.; Liu, C.; Liao, S.; Chen, J.; et al. An Efficient Multi-Doping Strategy to Enhance Li- Ion Conductivity in the Garnet-Type Solid Electrolyte Li7La3Zr2O12. J. Mater. Chem. A 2019, 7, 8589–8601. [Google Scholar] [CrossRef]
- Wagner, R.; Redhammer, G.J.; Rettenwander, D.; Senyshyn, A.; Schmidt, W.; Wilkening, M.; Amthauer, G. Crystal Structure of Garnet-Related Li-Ion Conductor Li7−3xGaxLa3Zr2O12: Fast Li-Ion Conduction Caused by a Different Cubic Modification? Chem. Mater. 2016, 28, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.A.; Martínez-Lope, M.J.; Aguadero, A.; Daza, L. Neutron Powder Diffraction as a Characterization Tool of Solid Oxide Fuel Cell Materials. Prog. Solid State Chem. 2008, 36, 134–150. [Google Scholar] [CrossRef]
- Rao, R.P.; Gu, W.; Sharma, N.; Peterson, V.K.; Avdeev, M.; Adams, S. In Situ Neutron Diffraction Monitoring of Li7La3Zr2O12 Formation: Toward a Rational Synthesis of Garnet Solid Electrolytes. Chem. Mater. 2015, 27, 2903–2910. [Google Scholar] [CrossRef]
- Baklanova, Y.V.; Tyutyunnik, A.P.; Tarakina, N.V.; Fortes, A.D.; Maksimova, L.G.; Korona, D.V.; Denisova, T.A. Stabilization of Cubic Li7La3Hf2O12 by Al-Doping. J. Power Sources 2018, 391, 26–33. [Google Scholar] [CrossRef]
- Chen, Y.; Rangasamy, E.; Dela Cruz, C.R.; Liang, C.; An, K. A Study of Suppressed Formation of Low-Conductivity Phases in Doped Li7La3Zr2O12 Garnets by in Situ Neutron Diffraction. J. Mater. Chem. A 2015, 3, 22868–22876. [Google Scholar] [CrossRef]
- Möhl, G.E.; Metwalli, E.; Bouchet, R.; Phan, T.N.T.; Cubitt, R.; Müller-Buschbaum, P. In Operando Small-Angle Neutron Scattering Study of Single-Ion Copolymer Electrolyte for Li-Metal Batteries. ACS Energy Lett. 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Weiss, M.; Seidlhofer, B.K.; Geiß, M.; Geis, C.; Busche, M.R.; Becker, M.; Vargas-Barbosa, N.M.; Silvi, L.; Zeier, W.G.; Schröder, D.; et al. Unraveling the Formation Mechanism of Solid-Liquid Electrolyte Interphases on LiPON Thin Films. ACS Appl. Mater. Interfaces 2019, 11, 9539–9547. [Google Scholar] [CrossRef]
- Oudenhoven, J.F.M.; Labohm, F.; Mulder, M.; Niessen, R.A.H.; Mulder, F.M.; Notten, P.H.L. In Situ Neutron Depth Profiling: A Powerful Method to Probe Lithium Transport in Micro-Batteries. Adv. Mater. 2011, 23, 4103–4106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Oudenhoven, J.F.M.; Danilov, D.L.; Vezhlev, E.; Gao, L.; Li, N.; Mulder, F.M.; Eichel, R.; Notten, P.H.L. Origin of Degradation in Si-Based All-Solid-State Li-Ion Microbatteries. Adv. Energy Mater. 2018, 8, 1801430. [Google Scholar] [CrossRef]
- Li, Q.; Yi, T.; Wang, X.; Pan, H.; Quan, B.; Liang, T. In Situ Visualization of Lithium Plating in All-Solid-State Lithium-Metal Battery. Nano Energy 2019, 63, 103895. [Google Scholar] [CrossRef]
- Wang, C.; Gong, Y.; Dai, J.; Zhang, L.; Xie, H.; Pastel, G.; Liu, B.; Wachsman, E.; Wang, H.; Hu, L. In Situ Neutron Depth Profiling of Lithium Metal-Garnet Interfaces for Solid State Batteries. J. Am. Chem. Soc. 2017, 139, 14257–14264. [Google Scholar] [CrossRef]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High Electronic Conductivity as the Origin of Lithium Dendrite Formation within Solid Electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Ping, W.; Wang, C.; Lin, Z.; Hitz, E.; Yang, C.; Wang, H.; Hu, L. Reversible Short-Circuit Behaviors in Garnet-Based Solid-State Batteries. Adv. Energy Mater. 2020, 10, 2000702. [Google Scholar] [CrossRef]
- Mori, K.; Enjuji, K.; Murata, S.; Shibata, K.; Kawakita, Y.; Yonemura, M.; Onodera, Y.; Fukunaga, T. Direct Observation of Fast Lithium-Ion Diffusion in a Superionic Conductor: Li7P3S11 Metastable Crystal. Phys. Rev. Appl. 2015, 4, 054008. [Google Scholar] [CrossRef]
- Monteforte, M.; Cockcroft, J. Neutron Pair Distribution Function Analysis and 3D Modelling: Structural Investigation of FePt Nanoparticle-Ligand Systems. Master’s Thesis, University of London, London, UK, 2013. [Google Scholar]
- Billinge, S.J.L. The Rise of the X-ray Atomic Pair Distribution Function Method: A Series of Fortunate Events. Philos. Trans. R. Soc. A 2019, 377, 20180413. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yang, Y.; Yang, Q.; Liu, Y.; Wang, L.; Chen, C.; Cheng, R. The Effect of Sintering Process on Lithium Ionic Conductivity of Li6.4Al0.2La3Zr2O12 Garnet Produced by Solid-State Synthesis. RSC Adv. 2018, 8, 13083–13088. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Cabana, J.; Bréger, J.; Yoon, W.S.; Grey, C.P. Cation Ordering in Li[NixMnxCo(1-2x)]O2-Layered Cathode Materials: A Nuclear Magnetic Resonance (NMR), Pair Distribution Function, X-Ray Absorption Spectroscopy, and Electrochemical Study. Chem. Mater. 2007, 19, 6277–6289. [Google Scholar] [CrossRef]
- Idemoto, Y.; Kitamura, N.; Ueki, K.; Vogel, S.C.; Uchimoto, Y. Average and Local Structure Analyses of Li(Mn1/3Ni1/3Co1/3−xAlx)O2 Using Neutron and Synchrotron X-Ray Sources. J. Electrochem. Soc. 2012, 159, A673–A677. [Google Scholar] [CrossRef]
- Idemoto, Y.; Ueki, K.; Kitamura, N. Dependence of Average and Local Structures and Cathode Performance on Synthetic Condition of Lix(Mn1/3Co1/3Ni1/3)O2 as a Cathode Active Material for Li Ion Battery. Electrochemistry 2010, 78, 475–481. [Google Scholar] [CrossRef][Green Version]
- Idemoto, Y.; Sera, Y.; Ishida, N.; Kitamura, N. Average and Local Crystal Structure and Electronic Structure of 0.4Li2MnO3-0.6LiMn1/3Ni1/3Co1/3O2 Using First-Principles Calculations and Neutron Beam and Synchrotron X-Ray Sources. Electrochemistry 2015, 83, 879–884. [Google Scholar] [CrossRef]
- Idemoto, Y.; Tejima, F.; Ishida, N.; Kitamura, N. Average, Electronic, and Local Structures of LiMn2−AlxO4 in Charge-Discharge Process by Neutron and Synchrotron X-ray. J. Power Sources 2019, 410, 38–44. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, M.; Wang, X.; Hu, E.; Liu, J.; Yu, X.; Olguin, M.; Wynn, T.A.; Meng, Y.S.; Page, K.; et al. Local Structure Adaptability through Multi Cations for Oxygen Redox Accommodation in Li-Rich Layered Oxides. Energy Storage Mater. 2020, 24, 384–393. [Google Scholar] [CrossRef]
- Zhao, E.; Li, Q.; Meng, F.; Liu, J.; Wang, J.; He, L.; Jiang, Z.; Zhang, Q.; Yu, X.; Gu, L.; et al. Stabilizing the Oxygen Lattice and Reversible Oxygen Redox Chemistry through Structural Dimensionality in Lithium-Rich Cathode Oxides. Angew. Chem. Int. Ed. 2019, 58, 4323–4327. [Google Scholar] [CrossRef]
- Wang, H.; Downing, R.G.; Dura, J.A.; Hussey, D.S. In Situ Neutron Techniques for Studying Lithium Ion Batteries. In Polymers for Energy Storage and Delivery: Polyelectrolytes for Batteries and Fuel Cells; Page, K.A., Soles, C.L., Runt, J., Eds.; ACS Symposium Series: Washington, DC, USA, 2012; Volume 1096, pp. 91–106. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, M.; Zhang, Y.; Shao, H.; Zhu, Y.; Guo, X.; Li, L.; Wang, H.; Liu, W. Lithium Migration Pathways at the Composite Interface of LiBH4 and Two-Dimensional MoS2 Enabling Superior Ionic Conductivity at Room Temperature. Phys. Chem. Chem. Phys. 2020, 22, 4096–4105. [Google Scholar] [CrossRef]
- Seidlmayer, S.; Hattendorff, J.; Buchberger, I.; Karge, L.; Gasteiger, H.A.; Gilles, R. In Operando Small-Angle Neutron Scattering (SANS) on Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A3116–A3125. [Google Scholar] [CrossRef]
- Bridges, C.A.; Sun, X.G.; Zhao, J.; Paranthaman, M.P.; Dai, S. In Situ Observation of Solid Electrolyte Interphase Formation in Ordered Mesoporous Hard Carbon by Small-Angle Neutron Scattering. J. Phys. Chem. C 2012, 116, 7701–7711. [Google Scholar] [CrossRef]
- Sacci, R.L.; Baniuelos, J.L.; Veith, G.M.; Littrell, K.C.; Cheng, Y.Q.; Wildgruber, C.U.; Jones, L.L.; Ramirez-Cuesta, A.J.; Rother, G.; Dudney, N.J. Structure of Spontaneously Formed Solid-Electrolyte Interphase on Lithiated Graphite Determined Using Small-Angle Neutron Scattering. J. Phys. Chem. C 2015, 119, 9816–9823. [Google Scholar] [CrossRef]
- Paul, N.; Wetjen, M.; Busch, S.; Gasteiger, H.; Gilles, R. Contrast Matched SANS for Observing SEI and Pore Clogging in Silicon-Graphite Anodes. J. Electrochem. Soc. 2019, 166, A1051–A1054. [Google Scholar] [CrossRef]
- Cousin, F.; Menelle, A. Neutron Reflectivity. EPJ Web Conf. 2015, 104, 01005. [Google Scholar] [CrossRef]
- He, Y.; Wang, H. In Situ Neutron Techniques for Lithium Ion and Solid-State Rechargeable Batteries. In Handbook of Solid State Batteries, 2nd ed.; Dudney, N.J., West, W.C., Nanda, J., Eds.; World Scientific: New York, NY, USA, 2016; Volume 6, pp. 51–77. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, N.; Mi, L.; Wang, J.P.; Metwalli, E.; Bießmann, L.; Herold, C.; Yang, J.; Wu, G.P.; Xu, Z.K.; et al. Impact of Thermal History on the Kinetic Response of Thermoresponsive Poly(Diethylene Glycol Monomethyl Ether Methacrylate)- Block-Poly(Poly(Ethylene Glycol)Methyl Ether Methacrylate) Thin Films Investigated by in Situ Neutron Reflectivity. Langmuir 2020, 36, 6228–6237. [Google Scholar] [CrossRef]
- Wagemakera, M.; van de Krol, R.; van Well, A.A. Nano-Morphology of Lithiated Thin Film TiO2 Anatase Probed with in Situ Neutron Reflectometry. Physica B 2003, 336, 124–129. [Google Scholar] [CrossRef]
- Hirayama, M.; Yonemura, M.; Suzuki, K.; Torikai, N.; Smith, H.; Watkinsand, E.; Majewski, J.; Kanno, R. Surface Characterization of LiFePO4 Epitaxial Thin Films by X-ray/Neutron Reflectometry. Electrochemistry 2010, 78, 413–415. [Google Scholar] [CrossRef]
- Owejan, J.E.; Owejan, J.P.; Decaluwe, S.C.; Dura, J.A. Solid Electrolyte Interphase in Li-Ion Batteries: Evolving Structures Measured in Situ by Neutron Reflectometry. Chem. Mater. 2012, 24, 2133–2140. [Google Scholar] [CrossRef]
- Jerliu, B.; Steitz, R.; Oberst, V.; Geckle, U.; Bruns, M.; Schmidt, H. Volume Expansion during Lithiation of Amorphous Silicon Thin Film Electrodes Studied by In-Operando Neutron Reflectometry. J. Phys. Chem. C 2014, 118, 9395–9399. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandran, K.S.R.; Bilheux, H.Z. Imaging of the Li Spatial Distribution within V2O5 Cathode in a Coin Cell by Neutron Computed Tomography. J. Power Sources 2018, 376, 125–130. [Google Scholar] [CrossRef]
- Siegel, J.B.; Lin, X.; Stefanopoulou, A.G.; Hussey, D.S.; Jacobson, D.L.; Gorsich, D. Neutron Imaging of Lithium Concentration in LFP Pouch Cell Battery. J. Electrochem. Soc. 2011, 158, A523–A529. [Google Scholar] [CrossRef]
- Owejan, J.P.; Gagliardo, J.J.; Harris, S.J.; Wang, H.; Hussey, D.S.; Jacobson, D.L. Direct Measurement of Lithium Transport in Graphite Electrodes Using Neutrons. Electrochim. Acta 2012, 66, 94–99. [Google Scholar] [CrossRef]
- Michalak, B.; Sommer, H.; Mannes, D.; Kaestner, A.; Brezesinski, T.; Janek, J. Gas Evolution in Operating Lithium-Ion Batteries Studied in Situ by Neutron Imaging. Sci. Rep. 2015, 5, 15627. [Google Scholar] [CrossRef]
- Starke, B.; Soc, J.E.; Starke, B.; Seidlmayer, S.; Schulz, M.; Dinter, A.; Revay, Z.; Gilles, R.; Pettinger, K. Gas Evolution and Capacity Fading in LiFexMn1−xPO4/Graphite Cells Studied by Neutron Imaging and Neutron Induced Prompt Gamma Activation Analysis. J. Electrochem. Soc. 2017, 164, A3943–A3948. [Google Scholar] [CrossRef]
- Song, B.; Dhiman, I.; Carothers, J.C.; Veith, G.M.; Liu, J.; Bilheux, H.Z.; Huq, A. Dynamic Lithium Distribution upon Dendrite Growth and Shorting Revealed by Operando Neutron Imaging. ACS Energy Lett. 2019, 4, 2402–2408. [Google Scholar] [CrossRef]
- Nazer, N.S.; Strobl, M.; Kaestner, A.; Vie, P.J.S.; Yartys, V.A. Operando Neutron Imaging Study of a Commercial Li-Ion Battery at Variable Charge-Discharge Current Densities. Electrochim. Acta 2022, 427, 140793. [Google Scholar] [CrossRef]
- Kardjilov, N.; Manke, I.; Hilger, A.; Strobl, M.; Banhart, J. Neutron Imaging in Materials Science. Mater. Today 2011, 14, 248–256. [Google Scholar] [CrossRef]
- Kockelmann, W.; Burca, G.; Kelleher, J.F.; Kabra, S.; Rhodes, N.J.; Schooneveld, E.M.; Sykora, J.; Pooley, D.E.; Jim, B.; Aliotta, F.; et al. Status of the Neutron Imaging and Diffraction Instrument IMAT. Phys. Procedia 2015, 69, 71–78. [Google Scholar] [CrossRef]
- Kiyanagi, Y.; Shinohara, T.; Kai, T.; Kamiyama, T.; Sato, H.; Kino, K. Present Status of Research on Pulsed Neutron Imaging in Japan. Phys. Procedia 2013, 43, 92–99. [Google Scholar] [CrossRef]
- Shinohara, T.; Kai, T. Commissioning Start of Energy-Resolved Neutron Imaging System, RADEN in J-PARC. Neutron News 2015, 26, 11–14. [Google Scholar] [CrossRef]
- Bilheux, H.; Herwig, K.; Keener, S.; Davis, L. Overview of the Conceptual Design of the Future VENUS Neutron Imaging Beam Line at the Spallation Neutron Source. Phys. Procedia 2015, 69, 55–59. [Google Scholar] [CrossRef]
- Strobl, M. The Scope of the Imaging Instrument Project ODIN at ESS. Phys. Procedia 2015, 69, 18–26. [Google Scholar] [CrossRef]
- Tomandl, I.; Vacik, J.; Kobayashi, T.; Mora Sierra, Y.; Hnatowicz, V.; Lavreniev, V.; Horak, P.; Ceccio, G.; Cannavo, A.; Baba, M.; et al. Analysis of Li Distribution in Ultrathin All-Solid-State Li-Ion Battery (ASSLiB) by Neutron Depth Profiling (NDP). Radiat. Eff. Defects Solids 2020, 175, 394–405. [Google Scholar] [CrossRef]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Frömling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.M. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Ke, X.; Wang, Y.; Dai, L.; Yuan, C. Cell Failures of All-Solid-State Lithium Metal Batteries with Inorganic Solid Electrolytes: Lithium Dendrites. Energy Storage Mater. 2020, 33, 309–328. [Google Scholar] [CrossRef]
- Liu, X.; Garcia-Mendez, R.; Lupini, A.R.; Cheng, Y.; Hood, Z.D.; Han, F.; Sharafi, A.; Idrobo, J.C.; Dudney, N.J.; Wang, C.; et al. Local Electronic Structure Variation Resulting in Li ‘Filament’ Formation within Solid Electrolytes. Nat. Mater. 2021, 20, 1485–1490. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Q.; Chen, J.; Li, Y.; Ye, H.; Zhao, J.; Geng, L.; Dai, Q.; Yang, T.; Li, H.; et al. In Situ Visualization of Lithium Penetration through Solid Electrolyte and Dead Lithium Dynamics in Solid-State Lithium Metal Batteries. ACS Nano 2021, 15, 19070–19079. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Zhu, X.; Wang, C. Suppressing Li Dendrite Formation in Li2S-P2S5 Solid Electrolyte by LiI Incorporation. Adv. Energy Mater. 2018, 8, 1703644. [Google Scholar] [CrossRef]
- Loong, C.K. Inelastic Scattering and Applications. Rev. Mineral. Geochem. 2006, 63, 233–254. [Google Scholar] [CrossRef]
- Muy, S.; Bachman, J.C.; Giordano, L.; Chang, H.H.; Abernathy, D.L.; Bansal, D.; Delaire, O.; Hori, S.; Kanno, R.; Maglia, F.; et al. Tuning Mobility and Stability of Lithium Ion Conductors Based on Lattice Dynamics. Energy Environ. Sci. 2018, 11, 850–859. [Google Scholar] [CrossRef]
- Heitmann, T.; Hester, G.; Mitra, S.; Calloway, T.; Miskowiec, A.; Diallo, S.; Osti, N.; Mamontov, E. Probing Li Ion Dynamics in Amorphous XLi2SO4⋅(1−x )LiPO3 by Quasielastic Neutron Scattering. Solid State Ion. 2019, 334, 95–98. [Google Scholar] [CrossRef]
- Shah, A.R.; Shutt, R.R.C.; Smith, K.; Hack, J.; Neville, T.P.; Headen, T.F.; Brett, D.J.L.; Howard, C.A.; Miller, T.S.; Cullen, P.L. Neutron Studies of Na-Ion Battery Materials. J. Phys. Mater. 2021, 4, 042008. [Google Scholar] [CrossRef]
- Grimaldo, M.; Roosen-Runge, F.; Zhang, F.; Schreiber, F.; Seydel, T. Dynamics of Proteins in Solution. Q. Rev. Biophys. 2019, 52, 1–63. [Google Scholar] [CrossRef]
- Bailey, E.J.; Griffin, P.J.; Tyagi, M.; Winey, K.I. Segmental Diffusion in Attractive Polymer Nanocomposites: A Quasi-Elastic Neutron Scattering Study. Macromolecules 2019, 52, 669–678. [Google Scholar] [CrossRef]
- Embs, J.P.; Juranyi, F.; Hempelmann, R. Introduction to Quasielastic Neutron Scattering. Z. Phys. Chem. 2010, 224, 5–32. [Google Scholar] [CrossRef]
- Sinha, K.; Wang, W.; Winey, K.I.; Maranas, J.K. Dynamic Patterning in PEO-Based Single Ion Conductors for Li Ion Batteries. Macromolecules 2012, 45, 4354–4362. [Google Scholar] [CrossRef]
- Sacci, R.L.; Lehmann, M.L.; Diallo, S.O.; Cheng, Y.Q.; Daemen, L.L.; Browning, J.F.; Doucet, M.; Dudney, N.J.; Veith, G.M. Lithium Transport in an Amorphous LixSi Anode Investigated by Quasi-Elastic Neutron Scattering. J. Phys. Chem. C 2017, 121, 11083–11088. [Google Scholar] [CrossRef]
- Ganapatibhotla, L.V.N.R.; Maranas, J.K. Interplay of Surface Chemistry and Ion Content in Nanoparticle-Filled Solid Polymer Electrolytes. Macromolecules 2014, 47, 3625–3634. [Google Scholar] [CrossRef]
- Nozaki, H.; Harada, M.; Ohta, S.; Watanabe, I.; Miyake, Y.; Ikedo, Y.; Jalarvo, N.H.; Mamontov, E.; Sugiyama, J. Li Diffusive Behavior of Garnet-Type Oxides Studied by Muon-Spin Relaxation and QENS. Solid State Ion. 2014, 262, 585–588. [Google Scholar] [CrossRef]
- Lefevr, J.; Cervini, L.; Griffin, J.M.; Blanchard, D. Lithium Conductivity and Ions Dynamics in LiBH4/SiO2 Solid Electrolytes Studied by Solid-State NMR and Quasi-Elastic Neutron Scattering and Applied in Lithium-Sulfur Batteries. J. Phys. Chem. C 2018, 122, 15264–15275. [Google Scholar] [CrossRef]
- Hester, G.; Heitmann, T.; Tyagi, M.; Rathore, M.; Dalvi, A.; Mitra, S. Neutron Scattering Studies of Lithium-Ion Diffusion in Ternary Phosphate Glasses. MRS Adv. 2016, 1, 3057–3062. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).