Case Study of Repeatability, Different Speeds, and Different SOCs on Battery Squeeze Test
Abstract
1. Introduction
2. Experiments and Methods
2.1. Research Subjects
2.2. Experiment Equipment
2.3. Experiment Steps
- (1)
- Charge and discharge the battery through a tester, and set the battery to an SOC of 0%, 20%, 40%, 60%, 80%, or 100%.
- (2)
- Connect the electric and thermocouple wires using the HIOKI data acquisition instrument to collect the battery electrodes, surface temperature, and voltage.
- (3)
- Start the experiment machine and data acquisition instrument, set the loading speed and termination displacement conditions. Place the battery under test on the base, and control the pressure head to reach the position where it will be in contact with the battery.
- (4)
- Conduct the same test three times, and apply an extrusion test to verify the repeatability of the same SOC of the battery.
- (5)
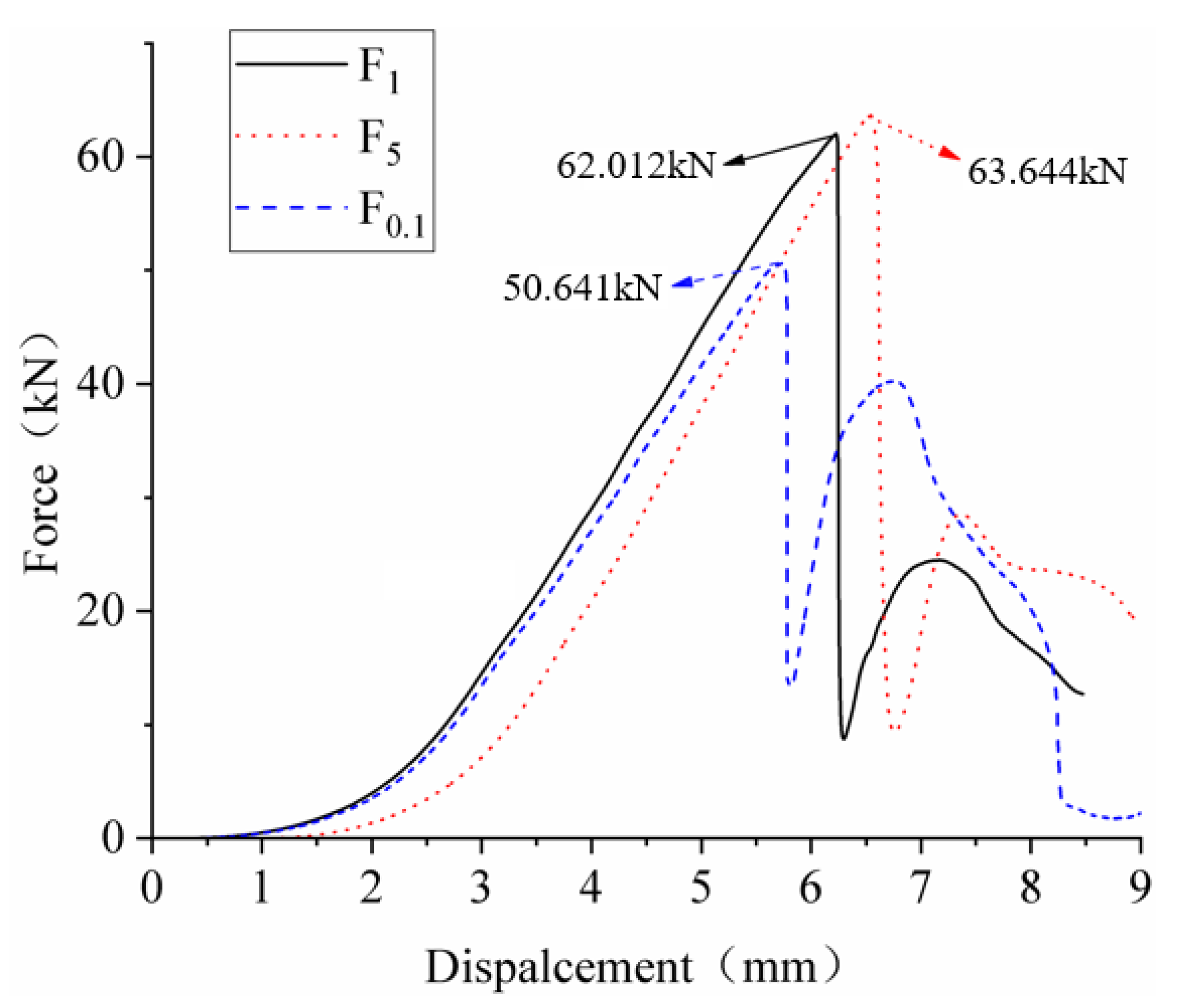
- Use 0.1, 1, and 5 mm/min loading speeds to squeeze the battery in the same SOC and study the influence of the squeeze speed on the battery response characteristics.
- (6)
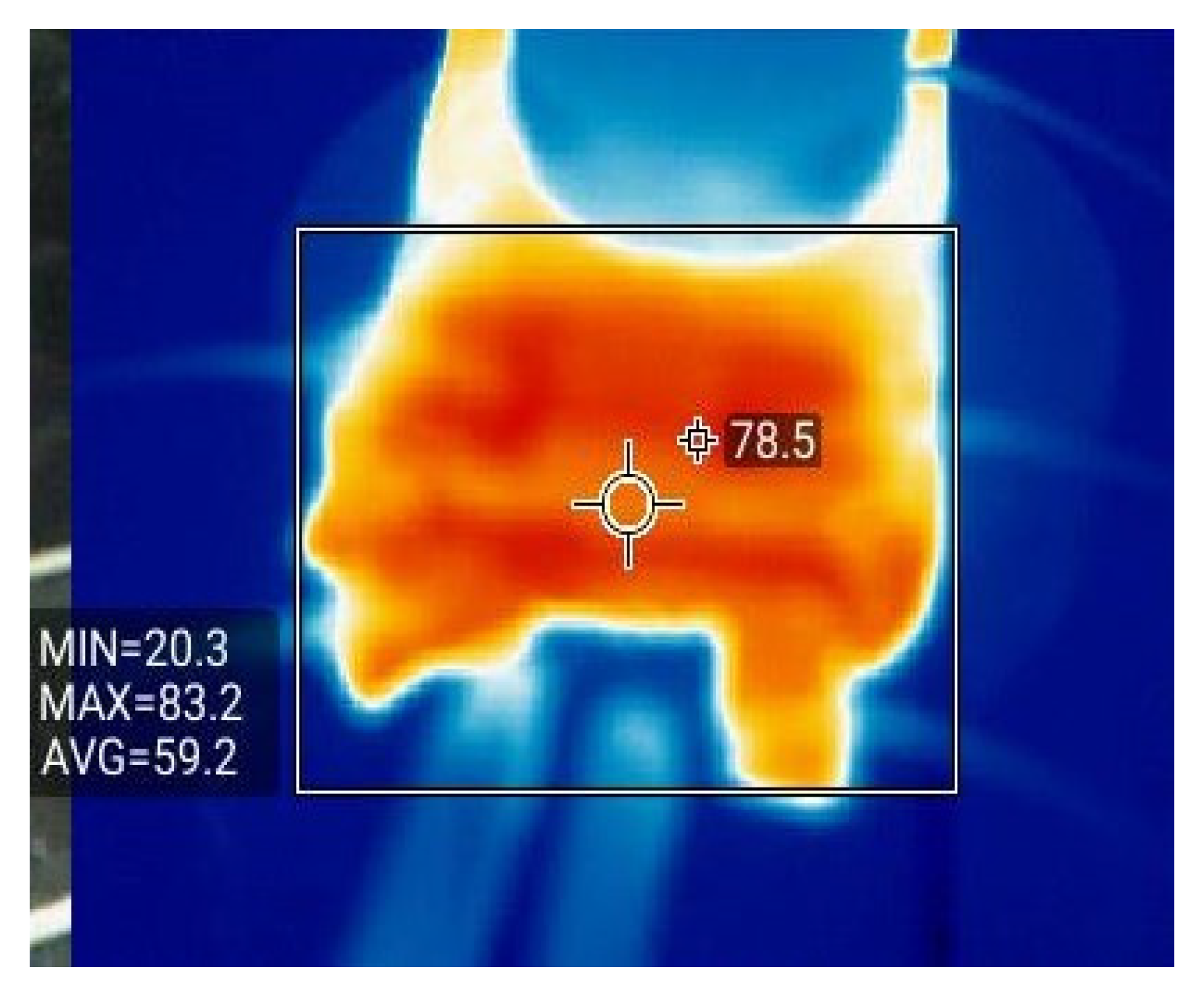
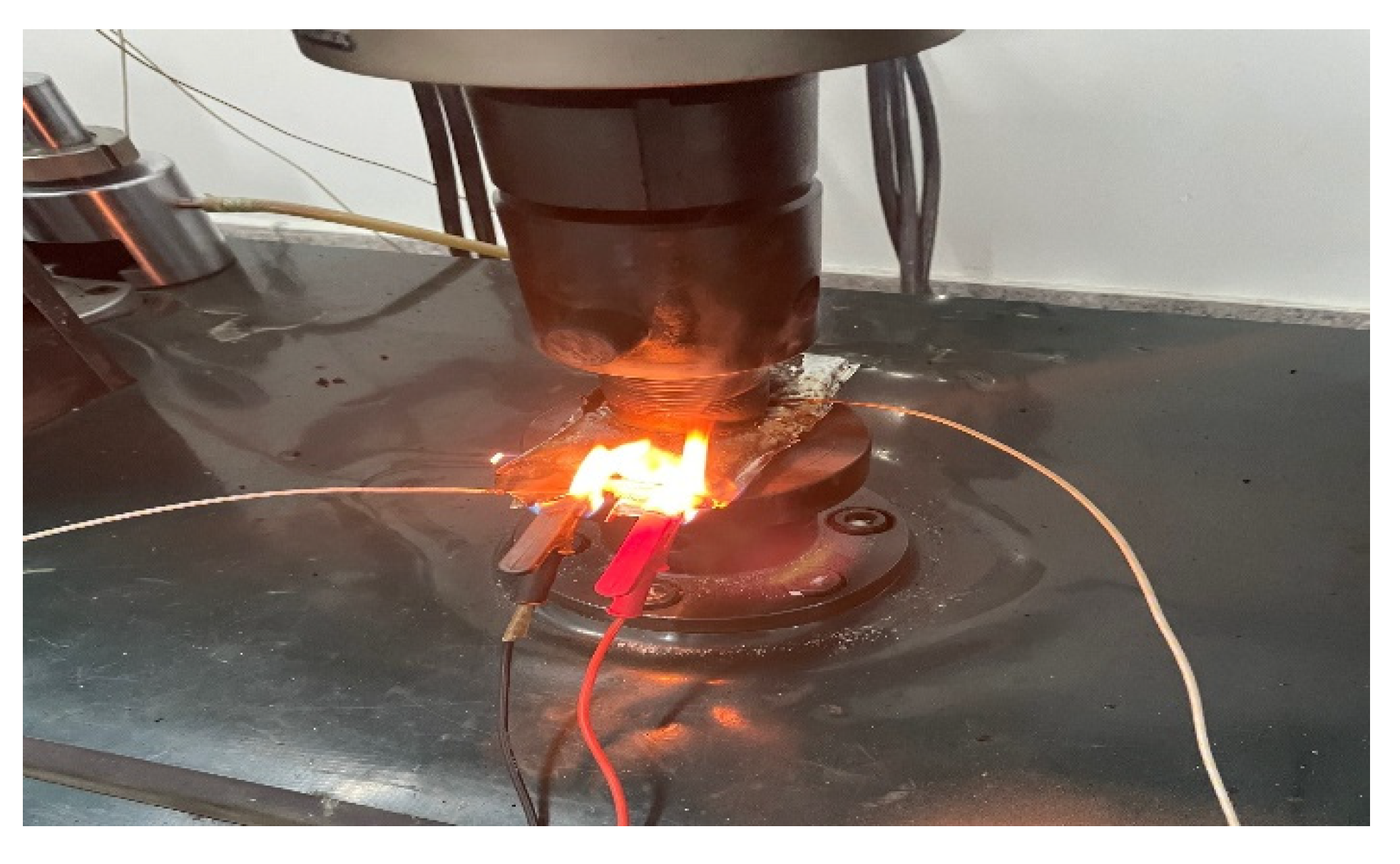
- Conduct an extrusion test of different SOCs of the battery and battery thermal runaway and study the response characteristics in a different SOC, i.e., a Fluke thermal imager thermography photographed cell image, when a thermal runaway occurs.
3. Battery Squeeze Experiment
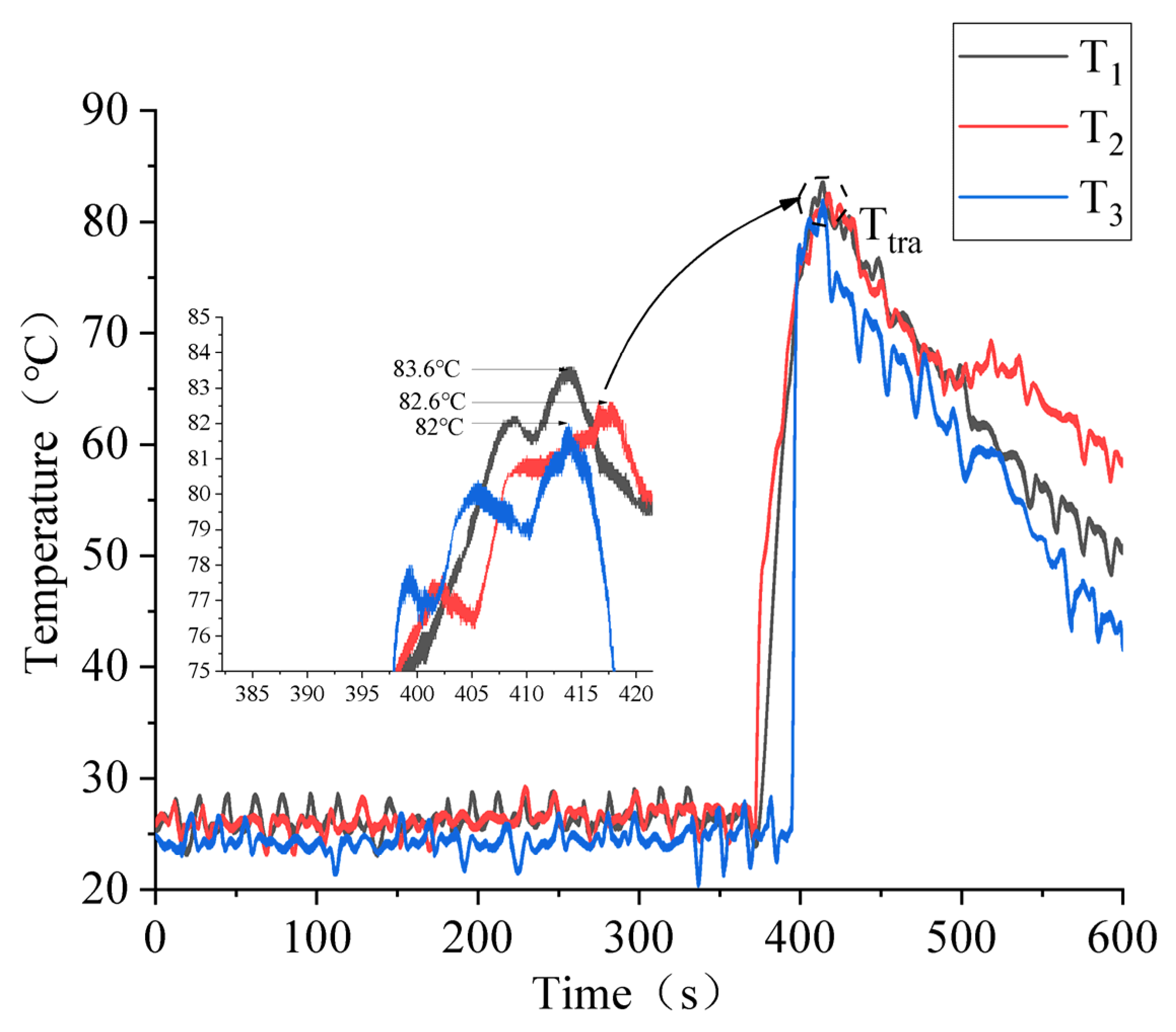
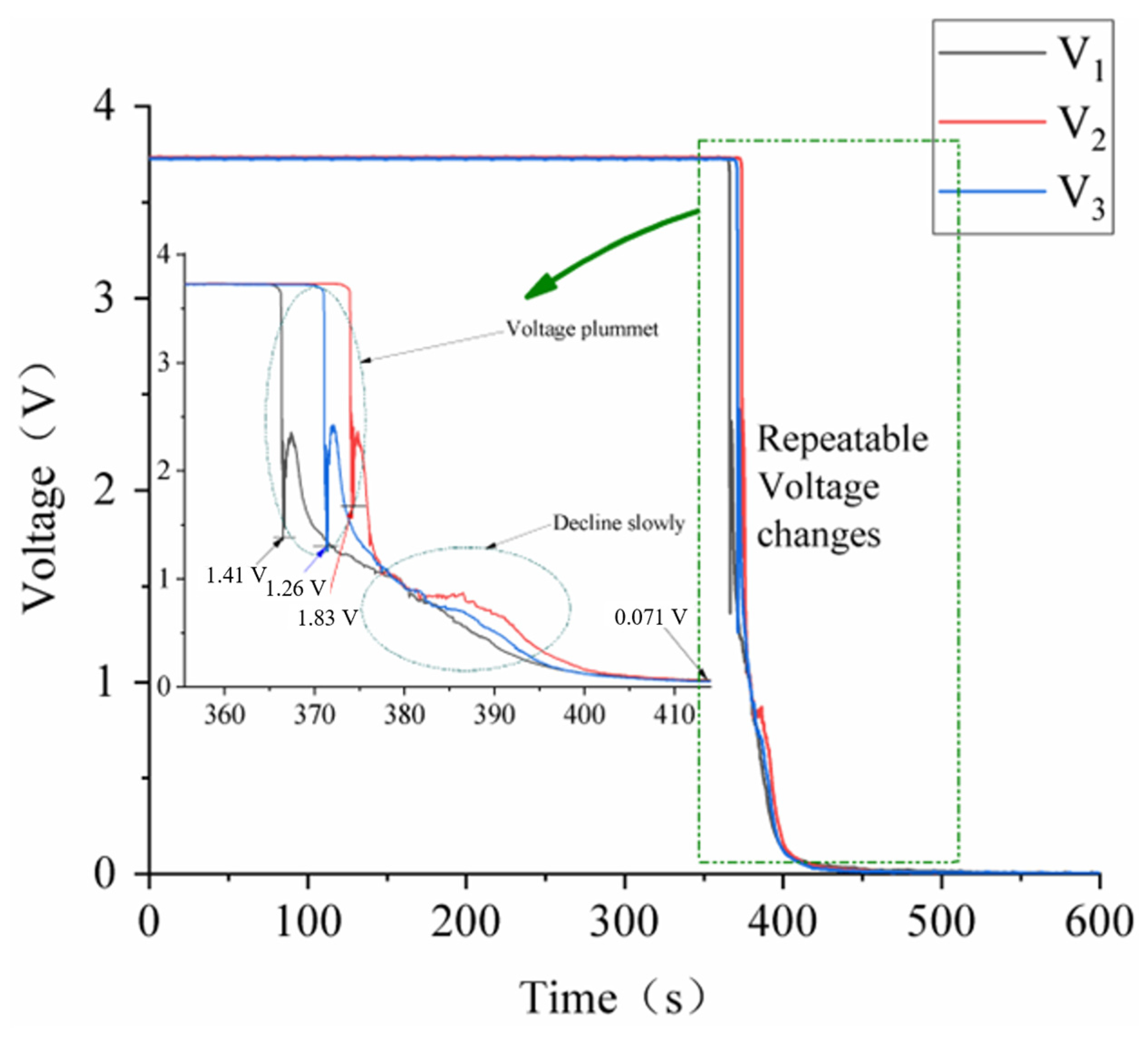
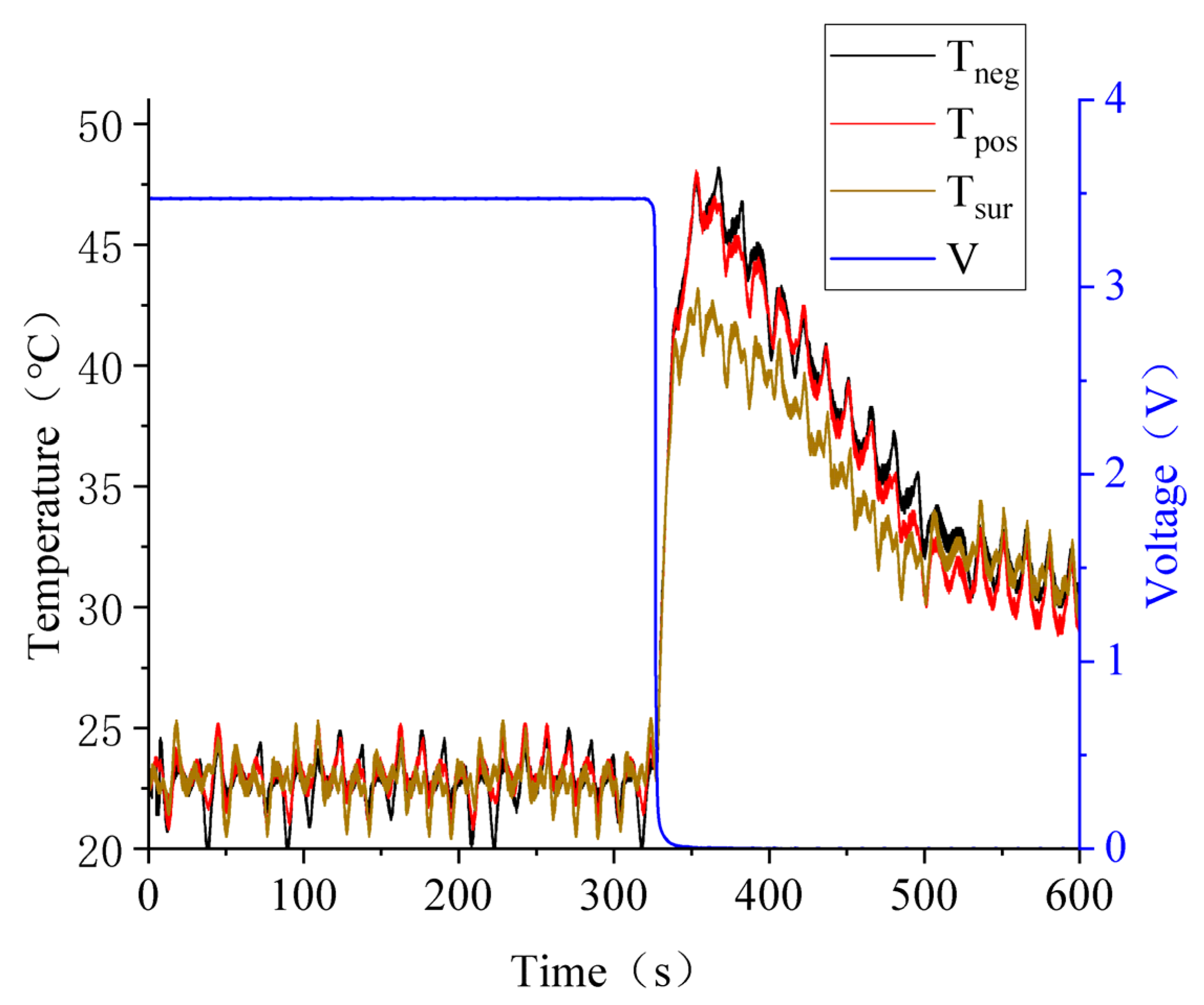
3.1. Test Repeatability Verification
3.2. Battery Extrusion Experiments under Different Loading Speeds
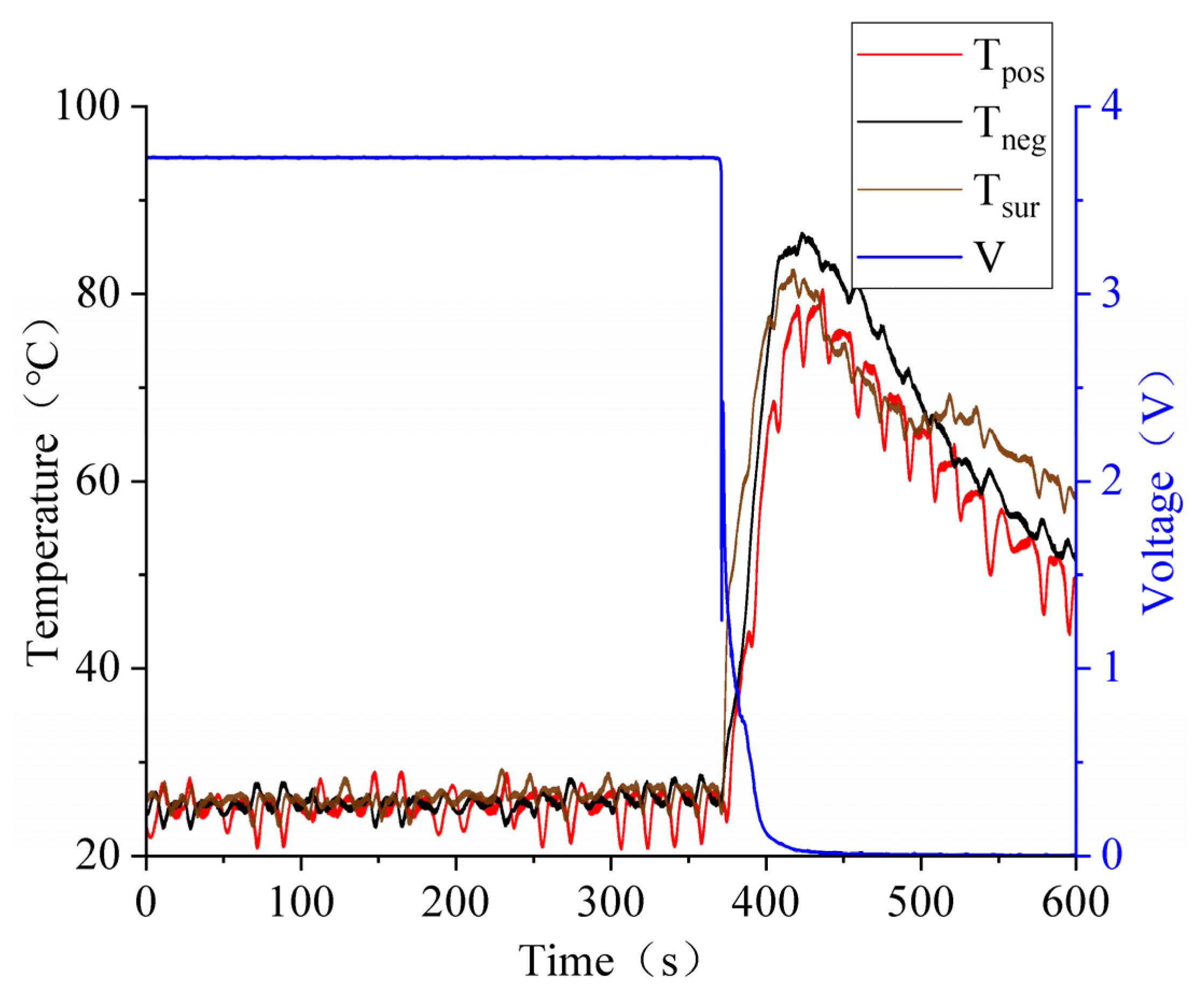
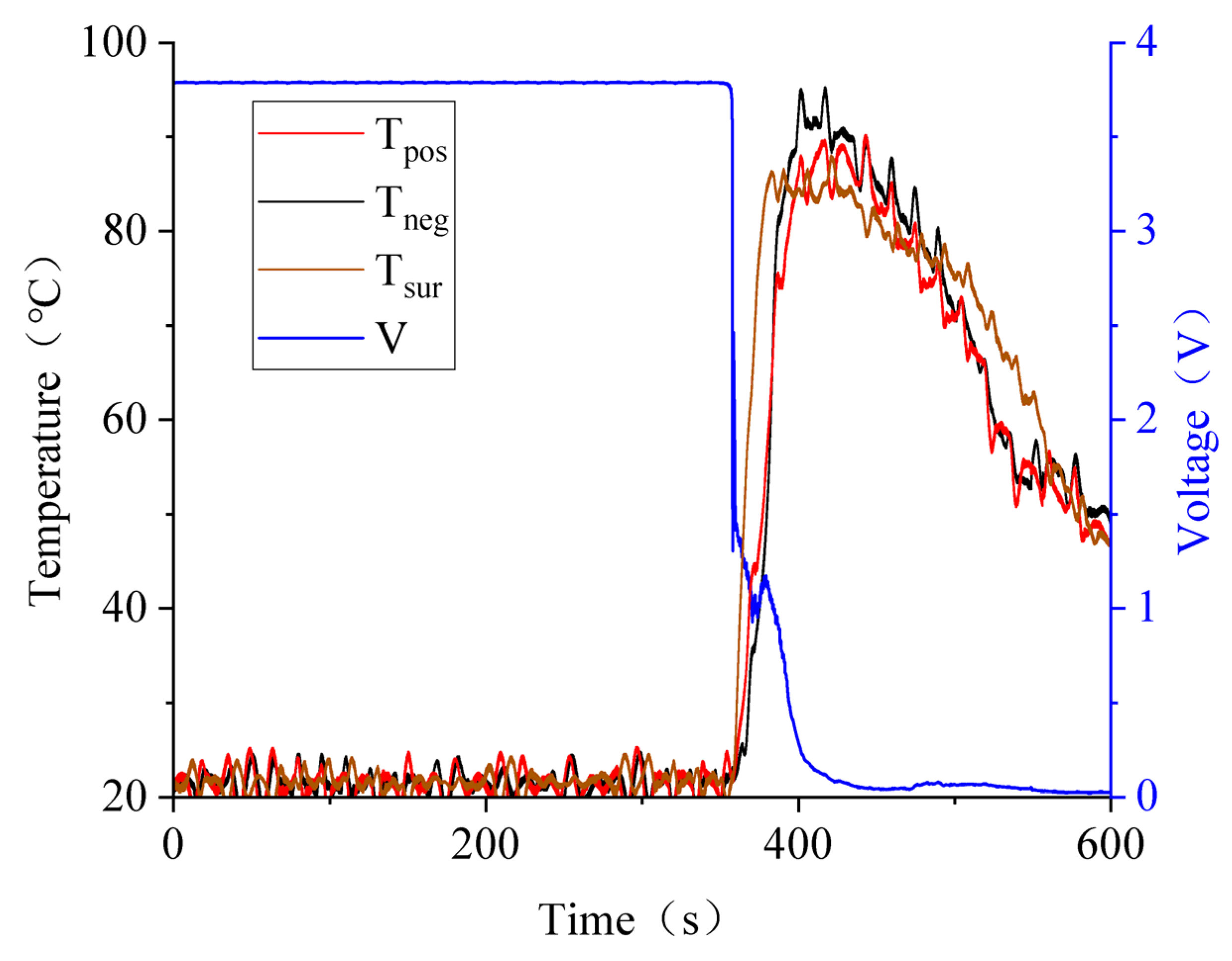
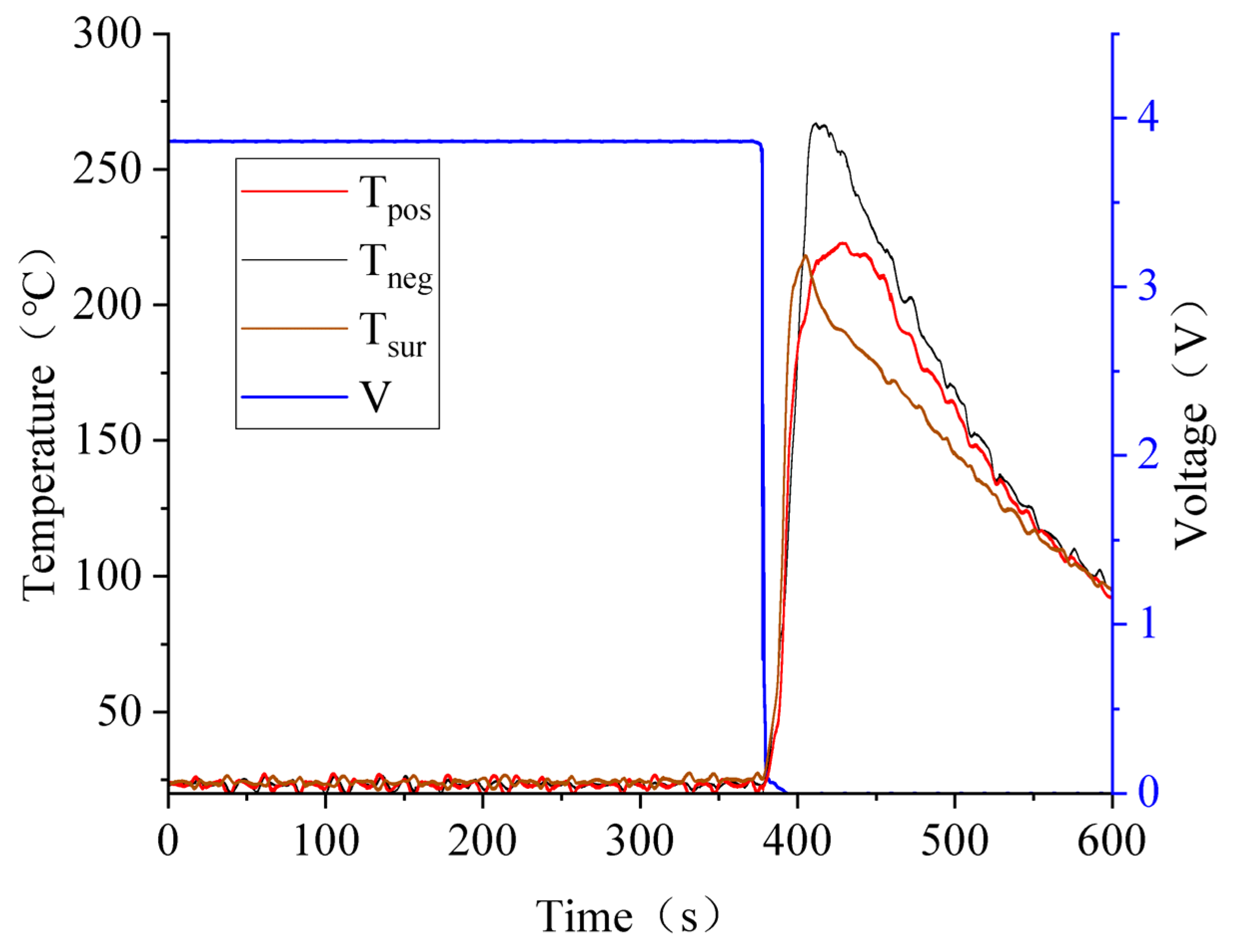
3.3. Battery Squeezing Experiment with Different SOCs
- (1)
- Battery SOC = 0%.
- (2)
- Battery SOC = 20%.
- (3)
- Battery SOC = 40%.
- (4)
- Battery SOC = 60%.
- (5)
- Battery SOC = 80%.
- (6)
- Battery SOC = 100%.
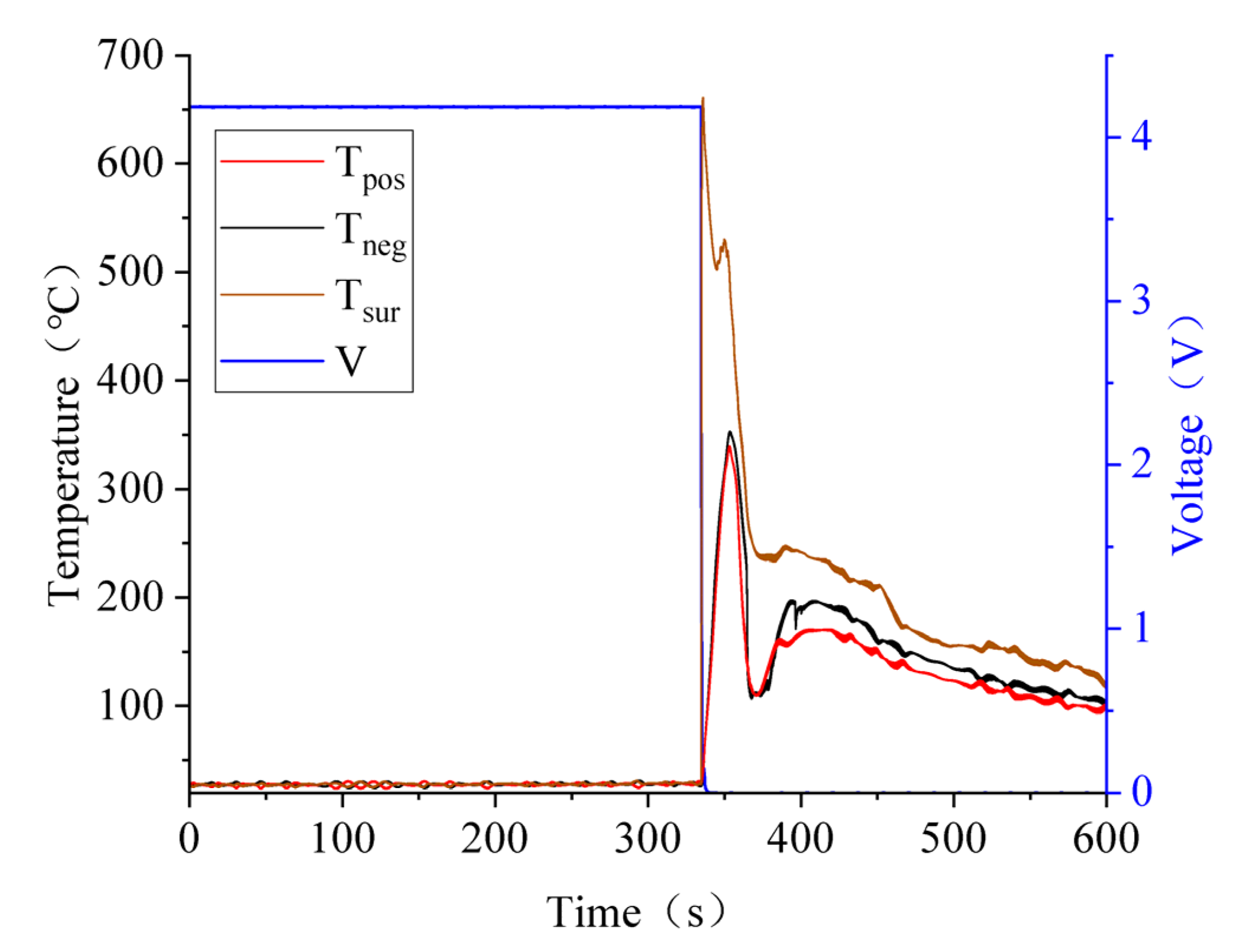
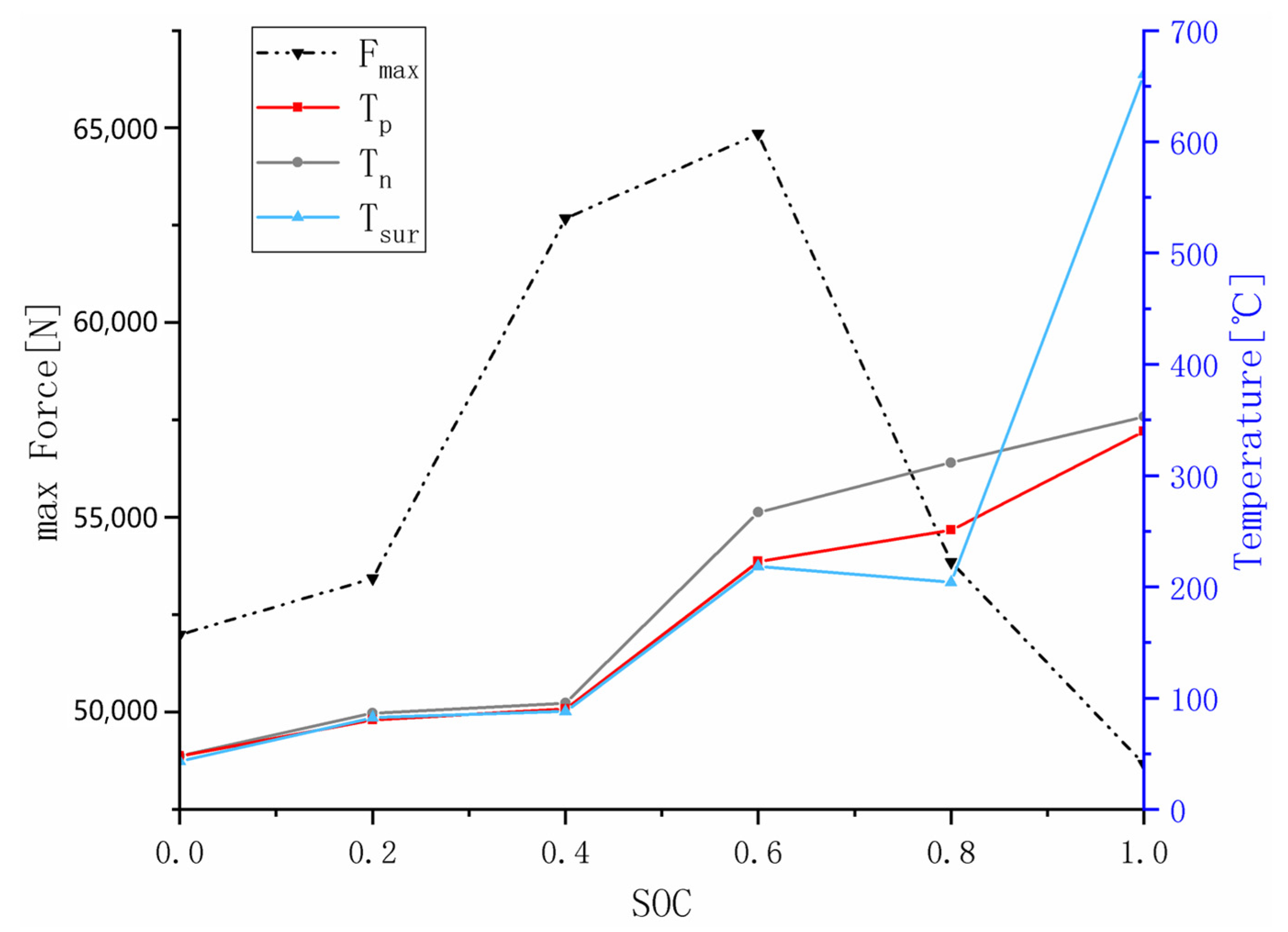
3.4. Effect of SOC Variation on Battery Thermal Runaway Response
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Wang, Z.; Zhang, L.; Zhu, X.; Dorrellc, D.D. TR behavior during overcharge for large-format Lithium-ion batteries with different packaging patterns. J. Energy Storage 2019, 25, 100811. [Google Scholar] [CrossRef]
- Feng, X.; Xu, C.; He, X.; Wang, L.; Zhang, G.; Ouyang, M. Mechanisms for the evolution of cell variations within a LiNixCoyMnzO2/graphite lithium-ion battery pack caused by temperature non-uniformity. J. Clean. Prod. 2018, 205, 447–462. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. TR mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 20, 246–267. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.L.; Zhuang, M.; Han, G.; Lu, L.; Han, X.; Chu, Z.; et al. TR of lithium-ion batteries without internal short circuit. Joule 2 2018, 10, 2047–2064. [Google Scholar] [CrossRef]
- Liu, P.; Sun, Z.; Wang, Z.; Zhang, J. Entropy-based voltage fault diagnosis of battery systems for electric vehicles. Energies 2018, 11, 136. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.M.; Ahn, S. Battery dimensional changes occurring during charge/discharge cycles—Thin rectangular lithium ion and polymer cells. J. Power Source 2003, 119, 833–837. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and characteristic analysis of powder ejected from a lithium ion battery during thermal runaway at elevated temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, C.; Huang, L. Overcharge investigation of large format lithium-ion pouch cells with Li (Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles: Degradation and failure mechanisms. J. Electrochem. Soc. 2018, 165, A3613–A3629. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large format lithium-ion pouch cells with Li (Ni0.6Co0.2Mn0.2) O2 cathode for electric vehicles: TR features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, K.; Wang, Z.; Tong, X.; Guo, L. Theoretical analysis of lithium-ion battery failure characteristics under different states of charge. Fire Mater. 2018, 42, 680–686. [Google Scholar] [CrossRef]
- Yang, R.; Xiong, R.; Shen, W.; Lin, X. Extreme Learning Machine-Based Thermal Model for Lithium-Ion Batteries of Electric Vehicles under External Short Circuit. Engineering 2020, 7, 395–405. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, R.; Lu, J.; Li, X. Temperature rise prediction of lithium-ion battery suffering external short circuit for all-climate electric vehicles application. Appl. Energy 2018, 213, 375–383. [Google Scholar] [CrossRef]
- Meng, J.; Boukhnifer, M.; Delpha, C.; Diallo, D. Incipient short-circuit fault diagnosis of lithium-ion batteries. J. Energy Storage 2020, 31, 101658. [Google Scholar] [CrossRef]
- Mao, N.; Wang, Z.-R.; Chung, Y.-H.; Shu, C.-M. Overcharge cycling effect on the thermal behavior, structure, and material of lithium-ion batteries. Appl. Therm. Eng. 2019, 163, 114147. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; He, X.; Ouyang, M. Overcharge behaviors and failure mechanism of lithium-ion batteries under different test conditions. Appl. Energy 2019, 250, 323–332. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Effects of overdischarge on performance and thermal stability of a Li-ion cell. J. Power Source 2006, 160, 1395–1402. [Google Scholar] [CrossRef]
- Dong, T.; Peng, P.; Jiang, F. Numerical modeling and analysis of the thermal behavior of NCM lithium-ion batteries subjected to very high C-rate discharge/ charge operations. Int. J. Heat Mass Tran. 2018, 117, 261–272. [Google Scholar] [CrossRef]
- Guo, R.; Lu, L.; Ouyang, M.; Feng, X. Mechanism of the entire overdischarge process and overdischarge-induced internal short circuit in lithium-ion batteries. Sci. Rep. 2016, 6, 30248. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Zhang, C.; Xu, J. Mechanical integrity of 18650 lithium-ion battery module: Packing density and packing mode. Eng. Fail. Anal. 2018, 91, 315–326. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611–A1617. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. TR features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Wang, H.; Lara-Curzio, E.; Rule, E.T.; Winchester, C.S. Mechanical abuse simulation and TR risks of large-format Li-ion batteries. J. Power Source 2017, 342, 913–920. [Google Scholar] [CrossRef]
- Kim, G.H. Numerical and Experimental Investigation of Internal Short Circuits in a Li-Ion Cell (Presentation), National Renewable Energy Lab. Available online: https://www.nrel.gov/transportation/assets/pdfs/50917.pdf (accessed on 18 May 2022).
- Keyser, M.; Long, D.; Jung, Y.S.; Pesaran, A.; Darcy, E.; McCarthy, B.; Patrick, L.; Kruger, C. Development of a novel test method for on-demand internal short circuit in a Li-ion cell. In Proceedings of the Large Lithium Ion Battery Technology and Application Symposium—Advanced Automotive Battery Conference, Pasadena, CA, USA, 8 June 2011; Available online: https://www.osti.gov/servlets/purl/1021805 (accessed on 19 May 2022).
- Maleki, H.; Howard, J.N. Internal short circuit in Li-ion cells. J. Power Source 2009, 191, 568–574. [Google Scholar] [CrossRef]
- Sahraei, E.; Campbell, J.; Wierzbicki, T. Modeling and short circuit detection of 18650 Li-ion cells under mechanical abuse conditions. J. Power Source 2012, 220, 360–372. [Google Scholar] [CrossRef]
- Cai, W.; Wang, H.; Maleki, H.; Howard, J.; Lara-Curzio, E. Experimental simulation of internal short circuit in Li-ion and Li-ion-polymer cells. J. Power Source 2011, 196, 7779–7783. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; Wang, F.; He, X.; Lu, L.; Peng, H. Characterization of penetration induced TR propagation process within a large format lithium ion battery module. J. Power Source 2015, 275, 261–273. [Google Scholar] [CrossRef]
- Sahraei, E.; Kahn, M.; Meier, J.; Wierzbicki, T. Modelling of cracks developed in lithium-ion cells under mechanical loading. RSC Adv. 2015, 5, 80369–80380. [Google Scholar] [CrossRef]
- Sahraei, E.; Meier, J.; Wierzbicki, T. Characterizing and modeling mechanical properties and onset of short circuit for three types of lithium-ion pouch cells. J. Power Source 2014, 247, 503–516. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Z.; Gao, T.; Bai, W.; Wang, J. Effect of mechanical extrusion force on thermal runaway of lithium-ion batteries caused by flat heating. J. Power Source 2021, 507, 230305. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Wang, X.; Gao, Y.; Allu, S.; Cakmak, E.; Wang, Z. Internal short circuit and failure mechanisms of lithium-ion pouch cells under mechanical indentation abuse conditions: An experimental study. J. Power Source 2020, 455, 227939. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; di Michiel, M.; Hinds, G.; Brett, D.J.L.; Shearing, P.R. Investigating lithium-ion battery materials during overcharge-induced thermal runaway: An operando and multi-scale X-ray CT study. Phys. Chem. Chem. Phys. 2016, 18, 30912–30919. [Google Scholar] [CrossRef]
- Srinivasan, R.; Demirev, P.A.; Carkhuff, B.G. Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention. J. Power Source 2018, 405, 30–36. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Source 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Yu, H.; Li, J.; Xu, J. Multiphysics computational framework for cylindrical lithium-ion batteries under mechanical abusive loading. Electrochim. Acta 2017, 256, 172–184. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, B.; Hong, Z.; Yin, S.; Finegan, D.P.; Xu, J. Safety issues of defective lithium-ion batteries: Identification and risk evaluation. J. Mater. Chem. A 2020, 8, 12472–12484. [Google Scholar] [CrossRef]
- Wang, L.; Duan, X.; Liu, B.; Li, Q.M.; Yin, S.; Xu, J. Deformation and failure behaviors of anode in lithium-ion batteries: Model and mechanism. J. Power Source 2019, 448, 227468. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Li, J.; Yin, S.; Yuan, C.; Hu, Z.; Wang, L.; Li, Y.; Xu, J. Safety issues caused by internal short circuits in lithium-ion batteries. J. Mater. Chem. A 2018, 6, 21475–21484. [Google Scholar] [CrossRef]
- Liu, B.; Yin, S.; Xu, J. Integrated computation model of lithium-ion battery subject to nail penetration. Appl. Energy 2016, 183, 278–289. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Cumming, D.J.; Brown, S.F. Pursuing safer batteries: Thermal abuse of LiFePO4 cells. J. Power Source 2019, 414, 557–568. [Google Scholar] [CrossRef]











| Battery Characteristic Parameter Table | ||
|---|---|---|
| Battery cathode material | LiCoO2 | |
| Nominal capacity | 6.3 Ah | |
| Nominal voltage | 3.7 V | |
| AC resistance (mΩ) | <2.0 | |
| weight (g) | 127 ± 3.0 | |
| Charging conditions | Maximum current | 12.6 A |
| Peak charge | 25.2 A | |
| Voltage | 4.15 V ± 0.03 V | |
| Discharge condition | Maximum current | 94.5 A |
| Peak discharge | 126 A | |
| Cutoff voltage | 3.0 V | |
| Cell size | thickness (mm) | 8.7 ± 0.3 |
| width (mm) | 56.0 ± 0.5 | |
| length (mm) | 116.5 ± 0.5 | |
| Tab spacing (mm) | 28 ± 1.5 | |
| Tab size | Tab material | copper |
| Lug width (mm) | 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Wang, J.; Yang, N.; Shi, M.; Liu, F.; Wang, F. Case Study of Repeatability, Different Speeds, and Different SOCs on Battery Squeeze Test. Batteries 2022, 8, 243. https://doi.org/10.3390/batteries8110243
Ren X, Wang J, Yang N, Shi M, Liu F, Wang F. Case Study of Repeatability, Different Speeds, and Different SOCs on Battery Squeeze Test. Batteries. 2022; 8(11):243. https://doi.org/10.3390/batteries8110243
Chicago/Turabian StyleRen, Xutong, Jianfeng Wang, Na Yang, Mengyu Shi, Fen Liu, and Fuqiang Wang. 2022. "Case Study of Repeatability, Different Speeds, and Different SOCs on Battery Squeeze Test" Batteries 8, no. 11: 243. https://doi.org/10.3390/batteries8110243
APA StyleRen, X., Wang, J., Yang, N., Shi, M., Liu, F., & Wang, F. (2022). Case Study of Repeatability, Different Speeds, and Different SOCs on Battery Squeeze Test. Batteries, 8(11), 243. https://doi.org/10.3390/batteries8110243









