Phase-Transformation-Activated MnCO3 as Cathode Material of Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Results
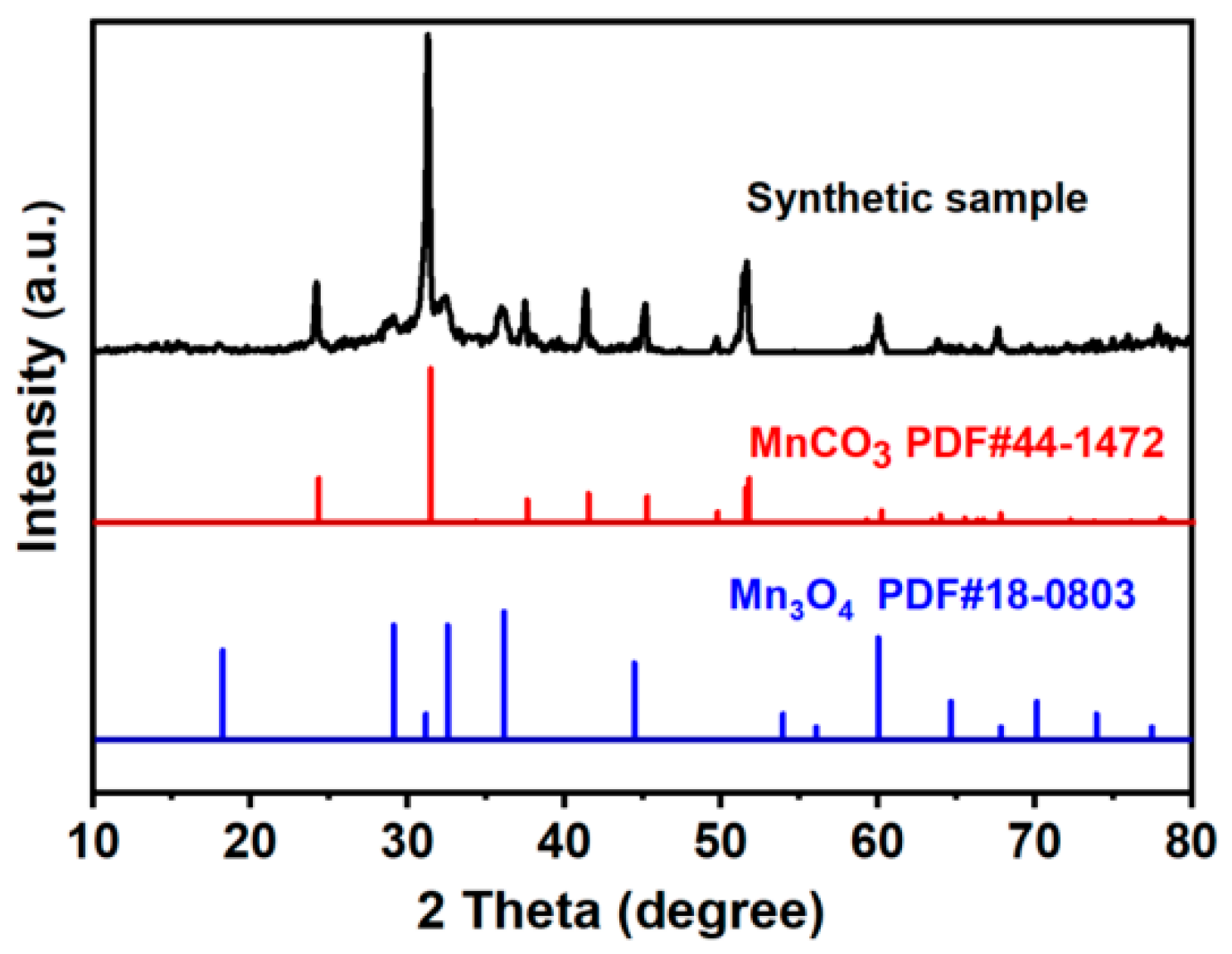
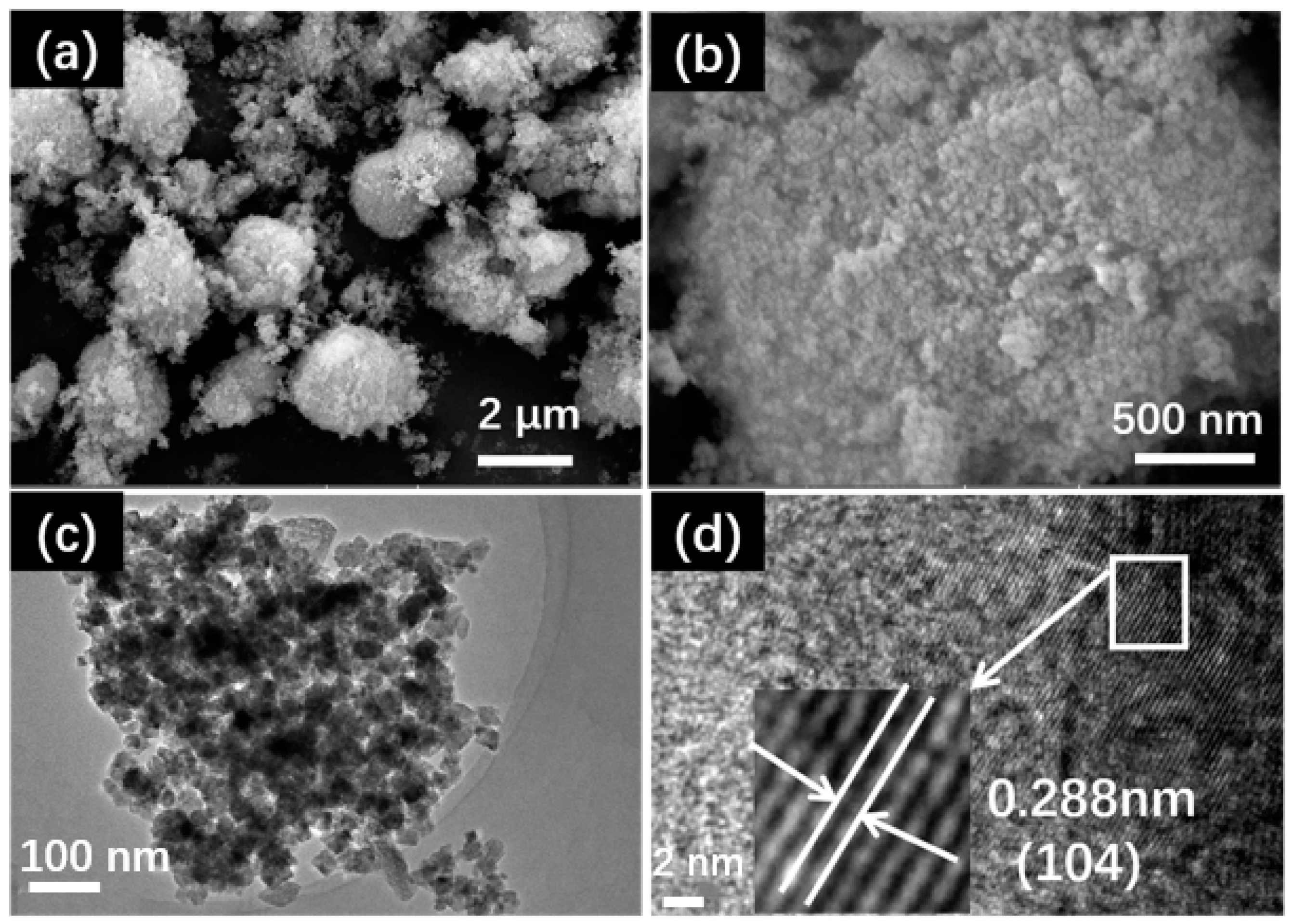
2.1. Structure Characterization Analysis of MnCO3
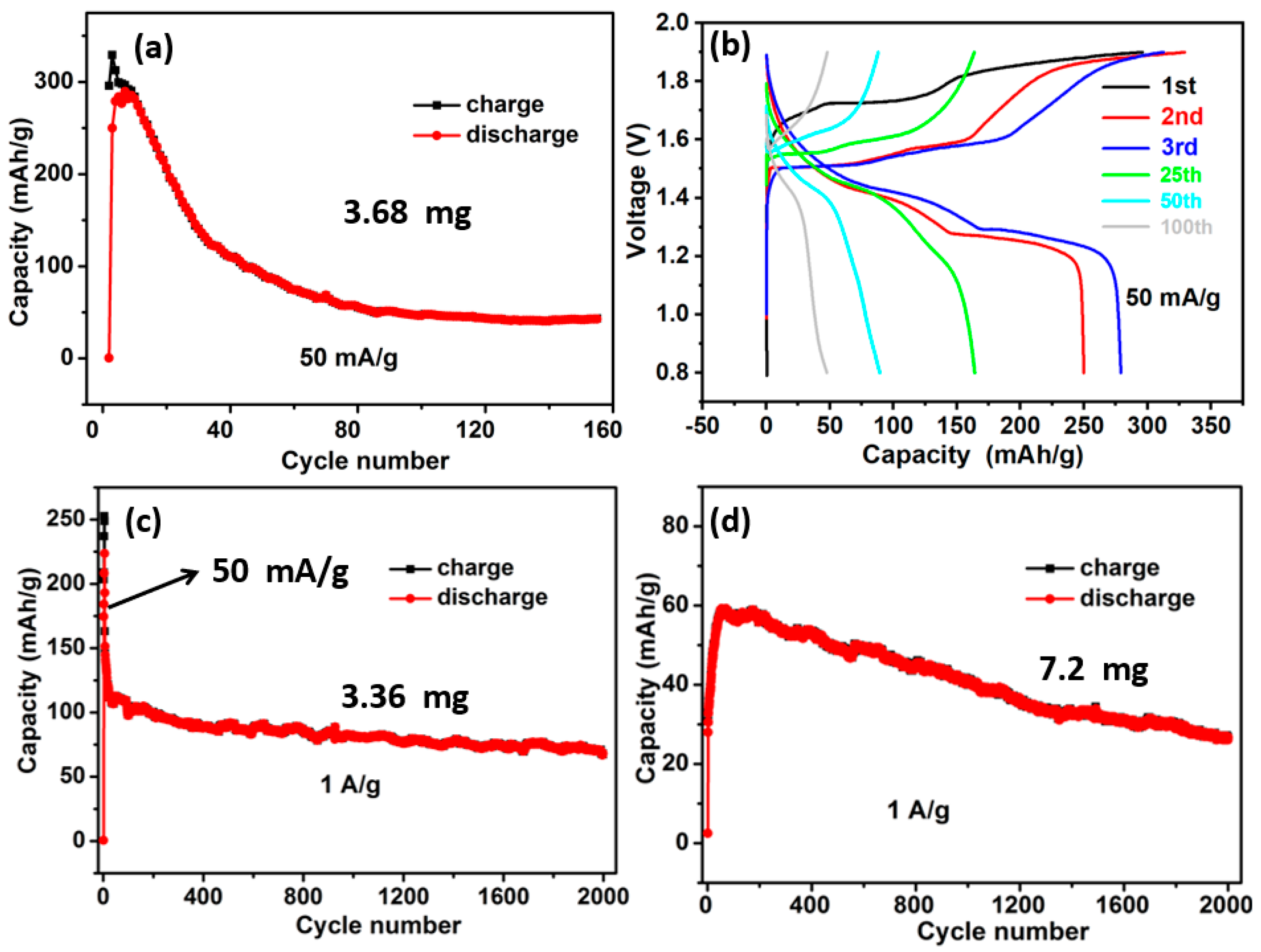
2.2. Electrochemical Performance Analysis of MnCO3
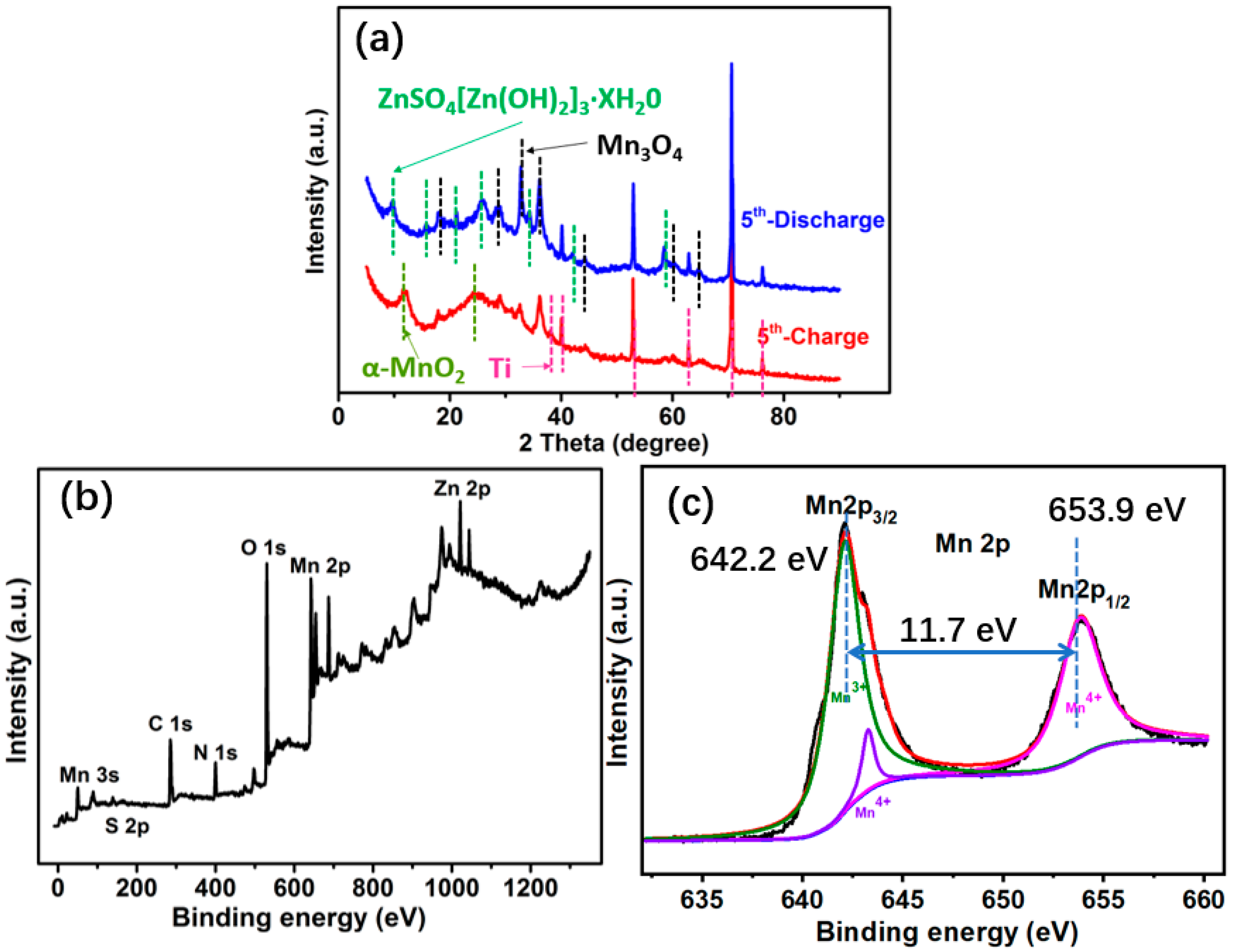
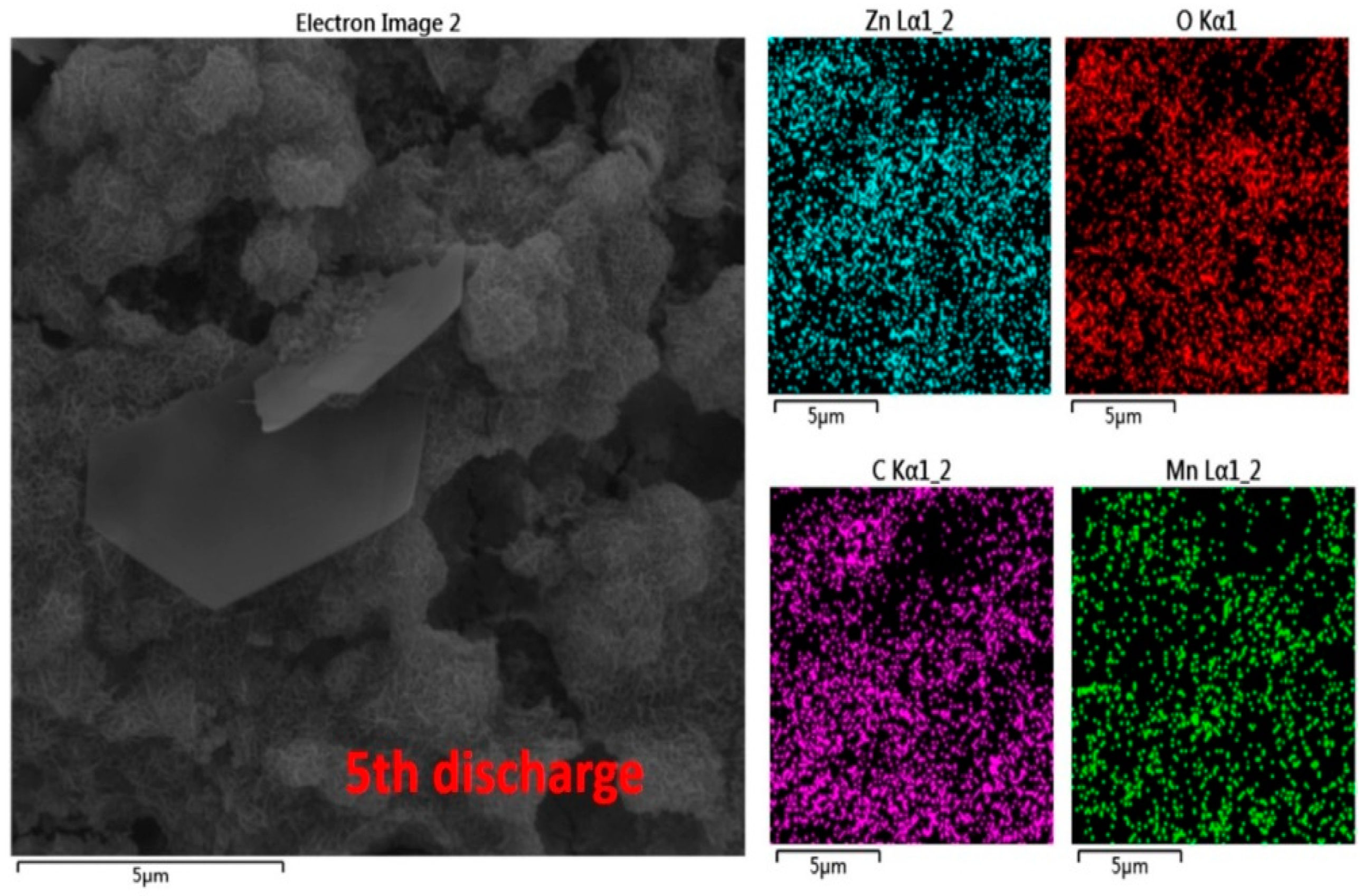
2.3. Reaction Mechanism Analysis
3. Discussion
4. Materials and Methods
4.1. Procedures of MnCO3 Preparation
4.2. Analysis and Characterization
4.3. Electrode-Preparation and Electrochemical-Performance Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Chen, B.; Wang, L.; Cui, G. Progress and prospect on failure mechanisms of solid-state lithium batteries. J. Power Sources 2018, 392, 94–115. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2008, 2, 148–173. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by Side Battery Technologies with Lithium Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089. [Google Scholar]
- Wendy, P.; Jaime Andres, P.-T.; Alba, A. Tug-of-War in the Selection of Materials for Battery Technologies. Batteries 2022, 8, 105. [Google Scholar]
- Shi, Y.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y.; Li, Y.; Sun, Z.; Ali, W.; et al. An Overview and Future Perspectives of Rechargeable Zinc Batteries. Small 2020, 16, 2000730. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Shang, W.; Yu, W.; Liu, Y.; Li, R.; Dai, Y.; Cheng, C.; Tan, P.; Ni, M. Rechargeable alkaline zinc batteries: Progress and challenges. Energy Storage Mater. 2020, 31, 44–57. [Google Scholar]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, M.; Chen, G.; Qu, Z.; Kohn, B.; Scheler, U.; Chu, X.; Fu, Y.; Schmidt, O.G.; Feng, X. An Anode-Free Zn-Graphite Battery. Adv. Mater. 2022, 34, e2201957. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Ji, Z.; Wang, H.; Liu, J.; Wang, J.; Hu, M.; Huang, Y. High-Voltage Flexible Aqueous Zn-Ion Battery with Extremely Low Dropout Voltage and Super-Flat Platform. Nano-Micro Lett. 2020, 12, 75. [Google Scholar]
- Cui, M.; Fei, J.; Mo, F.; Lei, H.; Huang, Y. Ultra-High-Capacity and Dendrite-Free Zinc–Sulfur Conversion Batteries Based on a Low-Cost Deep Eutectic Solvent. ACS Appl. Mater. Interfaces 2021, 13, 54981–54989. [Google Scholar] [PubMed]
- Lei, H.; Cui, M.; Huang, Y. S-Doping Promotes Pyridine Nitrogen Conversion and Lattice Defects of Carbon Nitride to Enhance the Performance of Zn–Air Batteries. ACS Appl. Mater. Interfaces 2022, 14, 34793–34801. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Yan, B.; Mo, F.; Wang, X.; Huang, Y.; Fan, J.; Zhi, C.; Li, H. In-situ grown porous protective layers with high binding strength for stable Zn anodes. Chem. Eng. J. 2022, 434, 134688. [Google Scholar] [CrossRef]
- Ling, W.; Yang, Q.; Mo, F.; Lei, H.; Wang, J.; Jiao, Y.; Qiu, Y.; Chen, T.; Huang, Y. An ultrahigh rate dendrite-free Zn metal deposition/striping enabled by silver nanowire aerogel with optimal atomic affinity with Zn. Energy Storage Mater. 2022, 51, 453–464. [Google Scholar] [CrossRef]
- Song, J.; Xu, K.; Liu, N.; Reed, D.; Li, X. Crossroads in the renaissance of rechargeable aqueous zinc batteries. Mater. Today 2021, 45, 191–212. [Google Scholar]
- Ma, L.; Chen, S.; Wang, D.; Yang, Q.; Mo, F.; Liang, G.; Li, N.; Zhang, H.; Zapien, J.A.; Zhi, C. Super Stretchable Zinc Air Batteries Based on an Alkaline Tolerant Dual Network Hydrogel Electrolyte. Adv. Energy Mater. 2019, 9, 1803046. [Google Scholar] [CrossRef]
- Ming, F.; Zhu, Y.; Huang, G.; Emwas, A.H.; Liang, H.; Cui, Y.; Alshareef, H.N. Co-Solvent Electrolyte Engineering for Stable Anode-Free Zinc Metal Batteries. J. Am. Chem. Soc. 2022, 144, 7160–7170. [Google Scholar] [CrossRef]
- Baishan, L. Transition Metal Dichalcogenides for High−Performance Aqueous Zinc Ion Batteries. Batteries 2022, 8, 62. [Google Scholar]
- Bi, S.; Wang, S.; Yue, F.; Tie, Z.; Niu, Z. A rechargeable aqueous manganese-ion battery based on intercalation chemistry. Nat. Commun. 2021, 12, 6991. [Google Scholar]
- Tian, H.; Li, Z.; Feng, G.; Yang, Z.; Fox, D.; Wang, M.; Zhou, H.; Zhai, L.; Kushima, A.; Du, Y.; et al. Stable, high-performance, dendrite-free, seawater-based aqueous batteries. Nat. Commun. 2021, 12, 237. [Google Scholar]
- Lyubomir, S.; Delyana, M.; Violeta, K.; Antonia, S.; Radostina, S. Comparison of the Properties of Ni–Mn Hydroxides/Oxides with Ni–Mn Phosphates for the Purpose of Hybrid Supercapacitors. Batteries 2022, 8, 51. [Google Scholar]
- Bi, S.; Zhang, Y.; Deng, S.; Tie, Z.; Niu, Z. Proton-Assisted Aqueous Manganese-Ion Battery Chemistry. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200809. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Ed. 2011, 51, 933–935. [Google Scholar]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene Scroll-Coated α-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, P.; Yao, J.; Gan, Y.; Li, J.; Wang, C.; Liu, X.; Rao, Y.; Ma, G.; Lv, L.; et al. Phase transformation mechanism of MnCO3 as cathode materials for aqueous zinc-ion batteries. Front. Chem. 2022, 10, 954592. [Google Scholar] [CrossRef]
- Liu, D.; Hur, S.H.; Chung, J.S.; Choi, W.M. Fabrication of g-C3N4 Quantum Dots/MnCO3 Nanocomposite on Carbon Cloth for Flexible Supercapacitor Electrode. Appl. Sci. 2020, 10, 7927. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Sakthivel, P.; Asaithambi, S.; Murugan, R.; Yuvakkumar, R.; Ravi, G. Formation of one dimensional nanorods with microsphere of MnCO3 using Ag as dopant to enhance the performance of pseudocapacitors. Mater. Chem. Phys. 2019, 228, 1–8. [Google Scholar]
- Lv, Y.; Dong, G.; Kang, J.; Han, W.; Li, L. MnF2-Ni and MnCO3-Ni derived from the same raw materials for high-performance supercapacitor. Mater. Lett. 2018, 215, 125–128. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, D.; Qin, L.; Wen, G.; Pan, H.; Zhang, Y.; Tian, N.; Zhou, Y.; Huang, X. MnCO3/Mn3O4/reduced graphene oxide ternary anode materials for lithium-ion batteries: Facile green synthesis and enhanced electrochemical performance. J. Mater. Chem. A 2017, 5, 17001–17011. [Google Scholar]
- Zhou, L.; Kong, X.; Gao, M.; Lian, F.; Li, B.; Zhou, Z.; Cao, H. Hydrothermal Fabrication of MnCO3@rGO Composite as an Anode Material for High-Performance Lithium Ion Batteries. Inorg. Chem. 2014, 53, 9228–9234. [Google Scholar] [PubMed]
- Xiao, L.; Wang, S.; Wang, Y.; Meng, W.; Deng, B.; Qu, D.; Xie, Z.; Liu, J. High-Capacity and Self-Stabilized Manganese Carbonate Microspheres as Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 25369–25378. [Google Scholar]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Y.; You, A.; Zhang, Z.; Li, G.; Lu, X.; Tong, Y. High-performance flexible quasi-solid-state Zn–MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 2017, 5, 14838–14846. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, A.; Du, L.; Yang, C.; Ye, L.; Wang, X.; Zhao, L.; Jiang, Q. Mn-Doped ZnO Microspheres as Cathode Materials for Aqueous Zinc Ion Batteries with Ultrastability up to 10 000 Cycles at A Large Current Density. Chem. Eng. J. 2020, 421, 127770. [Google Scholar]
- Chen, Y.; Qin, W.; Wang, J.; Chen, B. Fabrication and electrochemical performance of nanoflake MnO2@carbon fiber coaxial nanocables for supercapacitors. J. Appl. Electrochem. 2015, 46, 241–249. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, F.; Cui, M.; Yang, L.; Lei, H.; Chen, S.; Wei, J.; Kang, L. Phase-Transformation-Activated MnCO3 as Cathode Material of Aqueous Zinc-Ion Batteries. Batteries 2022, 8, 239. https://doi.org/10.3390/batteries8110239
Mo F, Cui M, Yang L, Lei H, Chen S, Wei J, Kang L. Phase-Transformation-Activated MnCO3 as Cathode Material of Aqueous Zinc-Ion Batteries. Batteries. 2022; 8(11):239. https://doi.org/10.3390/batteries8110239
Chicago/Turabian StyleMo, Funian, Mangwei Cui, Liangliang Yang, Hao Lei, Sheng Chen, Jun Wei, and Litao Kang. 2022. "Phase-Transformation-Activated MnCO3 as Cathode Material of Aqueous Zinc-Ion Batteries" Batteries 8, no. 11: 239. https://doi.org/10.3390/batteries8110239
APA StyleMo, F., Cui, M., Yang, L., Lei, H., Chen, S., Wei, J., & Kang, L. (2022). Phase-Transformation-Activated MnCO3 as Cathode Material of Aqueous Zinc-Ion Batteries. Batteries, 8(11), 239. https://doi.org/10.3390/batteries8110239






