Understanding Aqueous Organic Redox Flow Batteries: A Guided Experimental Tour from Components Characterization to Final Assembly
Abstract
1. Introduction
1.1. Redox-Active Materials—Requirements and Basic Characterization
1.1.1. Solubility
1.1.2. Redox Potential, E°
1.1.3. Chemical and Electrochemical Stability
1.1.4. Electrochemical Kinetics and Mass Transport
1.1.5. Further Optimization of Electrolyte Formulation
1.2. RFB Cell Components
1.2.1. Ion-Exchange Membrane
1.2.2. Porous Inert Electrodes
1.3. RFB Parameters
1.4. Capacity Fade Mechanisms
2. Experimental Methods
3. Results and Discussion
3.1. Experimental Characterization of the AQDS-Ferrocyanide RFB Single Cell
3.2. Effect of pH
3.3. Effect of Presence of Oxygen
3.4. Effect of Membrane Pretreatment
3.5. Effect of Capacity-Limiting Side
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Mazúr, P.; Krakowiak, J. Family Tree for Aqueous Organic Redox Couples for Redox Flow Battery Electrolytes: A Conceptual Review. Molecules 2022, 27, 560–599. [Google Scholar]
- Arevalo-Cid, P.; Dias, P.; Mendes, A.; Azevedo, J. Redox flow batteries: A new frontier on energy storage. Sustain. Energy Fuels 2021, 5, 5366–5419. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F.; Stella, A. Redox Flow Batteries for large scale energy storage. In Proceedings of the 2012 IEEE International Energy Conference and Exhibition (ENERGYCON), Florence, Italy, 9–12 September 2012; Volume 1, pp. 293–298. [Google Scholar]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Wu, M.C. Anion exchange membranes for aqueous acid-based redox flow batteries: Current status and challenges. Appl. Energy 2019, 1, 622–643. [Google Scholar] [CrossRef]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable redox flow batteries: Flow fields, stacks and design considerations. Chem. Soc. Rev. 2018, 47, 8721–8743. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Wedege, K.; Drazevic, E.; Bentien, A. Differential pH as a method for increasing cell potential in organic aqueous flow batteries. J. Mater. Chem. A 2017, 5, 21875–21882. [Google Scholar] [CrossRef]
- Hu, B.; Luo, J.; Hu, M.; Yuan, B.; Leo Liu, T. A pH-Neutral, Metal-Free Aqueous Organic Redox Flow Battery Employing an Ammonium Anthraquinone Anolyte. Angew. Chem. Int. Ed. 2019, 58, 16629–16636. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, T.; Ali, M.; Wu, C.; Chen, W. Recent Progress in Organic Species for Redox Flow Batteries. Energy Storage Mater. 2022, 50, 105–138. [Google Scholar] [CrossRef]
- Petrov, M.; Chikin, D.; Abunaeva, L.; Glazkov, A.; Pichugov, R.; Vinyukov, A.; Levina, I.; Motyakin, M.; Mezhuev, Y.; Konev, D.; et al. Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell. Membranes 2022, 12, 912. [Google Scholar] [CrossRef]
- Lloyd, D.; Magdalena, E.; Sanz, L.; Murtomaki, L.; Kontturi, K. Preparation of a cost-effective, scalable and energy efficient all-copper redox flow battery. J. Power Sources 2015, 292, 87–94. [Google Scholar] [CrossRef]
- Tucker, M.C.; Phillips, A.; Weber, A.Z. All-iron redox flow battery tailored for off-grid portable applications. ChemSusChem 2015, 8, 3996–4004. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jing, Y.; Wong, A.A.; Fell, E.M.; Jin, S.; Tang, Z.; Gordon, R.G.; Aziz, M.J. Extremely Stable Anthraquinone Negolytes Synthesized from Common Precursors. Chem 2020, 6, 1432–1442. [Google Scholar] [CrossRef]
- Suttil, J.A.; Kucharyson, J.F.; Escalante-Garcia, I.L.; Cabrera, P.J.; James, B.R.; Savinell, R.F.; Sanford, M.S.; Thompson, L.T. Metal acetylacetonate complexes for high energy density non-aqueous redox flow batteries. J. Mater. Chem. A 2015, 3, 7929–7938. [Google Scholar] [CrossRef]
- Luo, J.; Hu, B.; Hu, M.; Wu, W.; Leo Liu, T. An Energy Dense, Powerful, Robust Bipolar Zinc-Ferrocene Redox Flow Battery. Angew. Chem. Int. Ed. 2022, 61, e202204030. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama-Sato, K.; Nagano, T.; Noguchi, S.; Sugai, Y.; Du, J.; Nishide, H.; Oyaizu, K. Hydrophilic Organic Redox-Active Polymer Nanoparticles for Higher Energy Density Flow Batteries. ACS Appl. Polym. Mater. 2019, 1, 188–196. [Google Scholar] [CrossRef]
- Moklyak, V.; Hrubiak, A.; Gogitidze, Z.; Yavorski, Y. Biopolimer Peptide Batteries—A New Concept for Environmentally Friendly and Safer Energy Storage. Batteries 2021, 7, 50. [Google Scholar] [CrossRef]
- Singh, V.; Kim, S.; Kang, J.; Byon, H.R. Aqueous organic redox flow batteries. Nano Res. 2019, 12, 1988–2001. [Google Scholar] [CrossRef]
- Armstrong, C.G.; Toghill, K.E. Stability of molecular radicals in organic non-aqueous redox flow batteries: A mini review. Electrochem. Commun. 2018, 91, 19–24. [Google Scholar] [CrossRef]
- Mao, J.; Ruan, W.; Chen, Q. Understanding the Aqueous Solubility of Anthraquinone Sulfonate Salts: The Quest for High Capacity Electrolytes of Redox Flow Batteries. J. Electrochem. Soc. 2020, 167, 070522–070527. [Google Scholar] [CrossRef]
- Mazur, P.; Charvat, J.; Mrlik, J.; Procedic, J.; Akrman, J.; Kubac, L.; Rehakova, B.; Kosek, J. Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application. Molecules 2021, 26, 2484. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, B.; Debruler, C.; Bi, Y.; Zhao, Y.; Yuan, B.; Hu, M.; Wu, W.; Liu, T.L. Unprecedented Capacity and Stability of Ammonium Ferrocyanide Catholyte in pH Neutral Aqueous Redox Flow Batteries. Joule 2019, 3, 149–163. [Google Scholar] [CrossRef]
- Schwan, S.; Schroder, D.; Wegner, H.A.; Janek, J.; Mollenhauer, D. Substituent Pattern Effects on the Redox Potentials of Quinone-Based Active Materials for Aqueous Redox Flow Batteries. CheSusChem 2020, 13, 5480–5488. [Google Scholar] [CrossRef] [PubMed]
- Wedege, K.; Drazevic, E.; Konya, D.; Bentien, A. Organic Redox Species in Aqueous Flow Batteries: Redox Potentials, Chemical Stability and Solubility. Sci. Rep. 2016, 6, 39101–39114. [Google Scholar] [CrossRef] [PubMed]
- Goulet, M.A.; Aziz, M.J. Flow Battery Molecular Reactant Stability Determined by Symmetric Cell Cycling Methods. J. Electrochem. Soc. 2018, 165, A1466–A1479. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489. [Google Scholar] [CrossRef]
- Nolte, O.; Volodin, I.A.; Stolze, C.; Hager, M.D.; Schubert, U.S. Trust is good, control is better: A review on monitoring and characterization techniques for flow battery electrolytes. Mater. Horiz. 2021, 8, 1866–1925. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sayed, S.Y.; Luber, E.J.; Olsen, B.C.; Shirurkar, S.M.; Venkatakrishnan, S.; Tehafse, U.M.; Farquhar, A.K.; Smotkin, E.S.; McCreery, R.L.; et al. Redox Flow Batteries: How to Determine Electrochemical Kinetic Parameters. ACS Nano 2020, 14, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazakos, M. Membranes for Redox Flow Batteries Applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef]
- Tung, S.O.; Fisher, S.L.; Kotov, N.A.; Thompson, L.T. Nanoporous aramid nanofibre separators for nonaqueous redox flow batteries. Nat. Commun. 2018, 9, 4193–4202. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Jannasch, P. A practical method for measuring the ion exchange capacity decrease of hydroxide exchange membranes during intrinsic degradation. J. Power Sources 2018, 375, 361–367. [Google Scholar] [CrossRef]
- Vega, J.A.; Chartier, C.; Mustain, W.E. Effect of hydroxide and carbonate alkaline media on anion exchange membranes. J. Power Sources 2010, 195, 7176–7182. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef]
- Jiang, B.; Yu, L.; Wu, L.; Mu, D.; Liu, L.; Xi, J.; Xi, J.; Qiu, X. Insights into the Impact of the Nafion Membrane Pretreatment Process on Vanadium Flow Battery Performance. ACS Appl. Mater. Interfaces 2016, 8, 12228–12238. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, S.; Onggo, H. Effect of various concentration of sulfuric acid for Nafion membrane activation on the performance of fuel cell. AIP Conf. Proc. 2016, 1711, 060006. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, X.D. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electrochim. Acta 2007, 52, 6755–6762. [Google Scholar] [CrossRef]
- García-Acalde, L.; Gonzalez, Z.; Barreda, D.; Rocha, V.G.; Blanco, C.; Santamaria, R. Unraveling the relevance of carbon felts surface modification during electrophoretic deposition of nanocarbons on their performance as electrodes for the VO2+/VO2+ redox couple. Appl. Surf. Sci. 2021, 569, 151095–151102. [Google Scholar] [CrossRef]
- Ghimire, P.C.; Bhattari, A.; Lim, T.M.; Wai, N.; Skyllas-Kazacos, M.; Yan, Q. In-Situ Tools Used in Vanadium Redox Flow Battery. Batteries 2021, 7, 53. [Google Scholar] [CrossRef]
- Yao, Y.; Lei, J.; Ai, F.; Lu, Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Li, M.; Odom, S.A.; Pancoast, A.R.; Robertson, L.A.; Vaid, T.P.; Agarwal, G.; Doan, H.A.; Wang, Y.; Suduwella, T.M.; Bheemireddy, S.R.; et al. Experimental Protocols for Studying Organic Non-aqueous Redox Flow Batteries. ACS Energy Lett. 2021, 6, 3932–3943. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Zhang, X.; Ran, J.; Han, X.; Yang, Z.; Xu, T. Degradation of electrochemical active compounds in aqueous organic redox flow batteries. Curr. Opin. Electrochem. 2022, 32, 100895–100902. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, T.S.; Xu, Q.; Zhou, Q.X.; Zhang, Z.H. In-Situ investigation of hydrogen evolution behavior in vanadium redox flow batteries. Appl. Energy 2017, 190, 1112–1118. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, V.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Wiberg, C.; Busch, M.; Evenäs, L.; Ahlberg, E. The electrochemical response of core-functionalized naphthalene Diimides (NDI)—A combined computational and experimental investigation. Electrochim. Acta 2021, 367, 137480–137491. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Lin, K.; Ji, Y.; Kerr, E.F.; Goulet, M.A.; Porcellinis, D.D.; Tabor, D.P.; Pollack, D.A.; Aspuru-Guzik, A.; Gordon, R.G.; et al. Alkaline Quinone Flow Battery with Long Lifetime at pH 12. Joule 2018, 2, 1894–1906. [Google Scholar] [CrossRef]
- Mazur, P.; Mrlik, J.; Charvat, J.; Pocedic, J.; Vrana, J.; Dundalek, J.; Kosek, J. A complex four-point method for the evaluation of ohmic and faradaic losses within a redox flow battery single-cell. MehodsX 2019, 6, 534–539. [Google Scholar] [CrossRef]
- Fell, E.M.; De Porcellinis, D.; Jing, Y.; Gutierrez-Venegas, V.; Gordon, R.G.; Granados-Foncil, S.; Aziz, M.J. Long-Term Stability of Ferri-/Ferrocyanide as an Electroactive Component for Redox Flow 2 Battery Applications: On the Origin of Apparent Capacity Fade; Cambridge Open Engage: Cambridge, UK, 2022; submitted. [Google Scholar]
- Páez, T.; Martínez-Cueva, A.; Marcilla, R.; Palma, J.; Ventosa, E. Mitigating capacity fading in aqueous organic redox flow batteries through a simple electrochemical charge balancing protocol. J. Power Sources 2021, 512, 230516–230524. [Google Scholar] [CrossRef]
- Beh, E.S.; Porcellinis, D.D.; Garcia, R.L.; Xia, K.T.; Gordon, R.G.; Aziz, M.J. A Neutral pH Aqueous Organic—Organometallic Redox Flow Battery with Extremely High Capacity Retention. ACS Energy Lett. 2017, 2, 639–645. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538–118563. [Google Scholar] [CrossRef]
- Baricci, A.; Zago, M.; Casalegno, A. Modelling analysis of heterogenity og ageing in high temperature polymer electrolyte fuel cells. Insights into of electrochemical impedance spectra. Electrochim. Acta 2016, 222, 596–607. [Google Scholar]
- Zheng, Q.; Xing, F.; Li, X.; Ning, G.; Zhang, H. Flow feld design and optimization based on the mass transport polarization regulation in a fow-through type vanadium fow battery. J. Power Sources 2016, 324, 402–411. [Google Scholar]
- Wagner, N.; Gülzow, E. Change of electrochemical impedance spectra (EIS) with time during CO-poisoning of the Pt-anode in membrane fuel cell. J. Power Sources 2004, 127, 341–347. [Google Scholar]
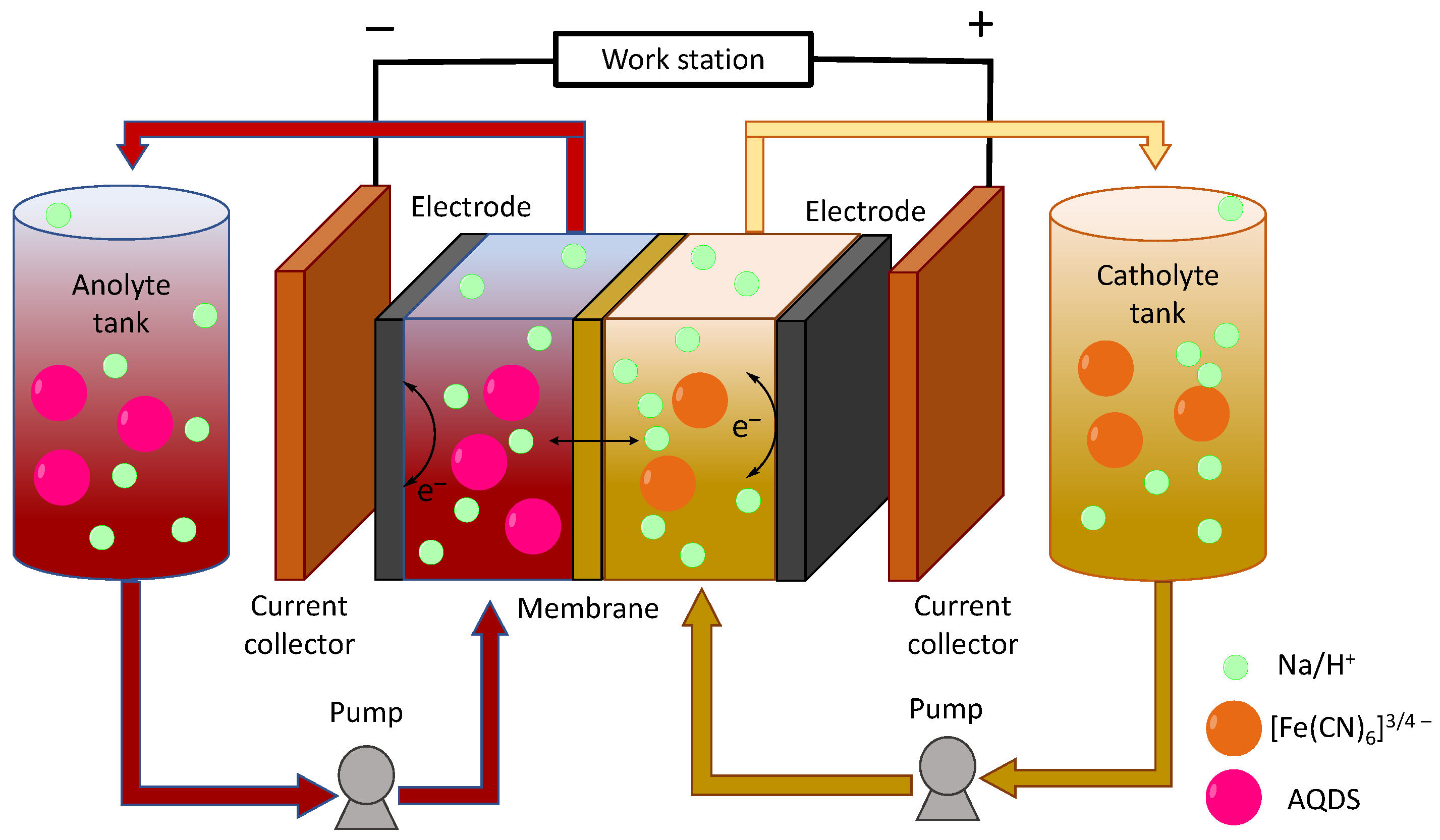

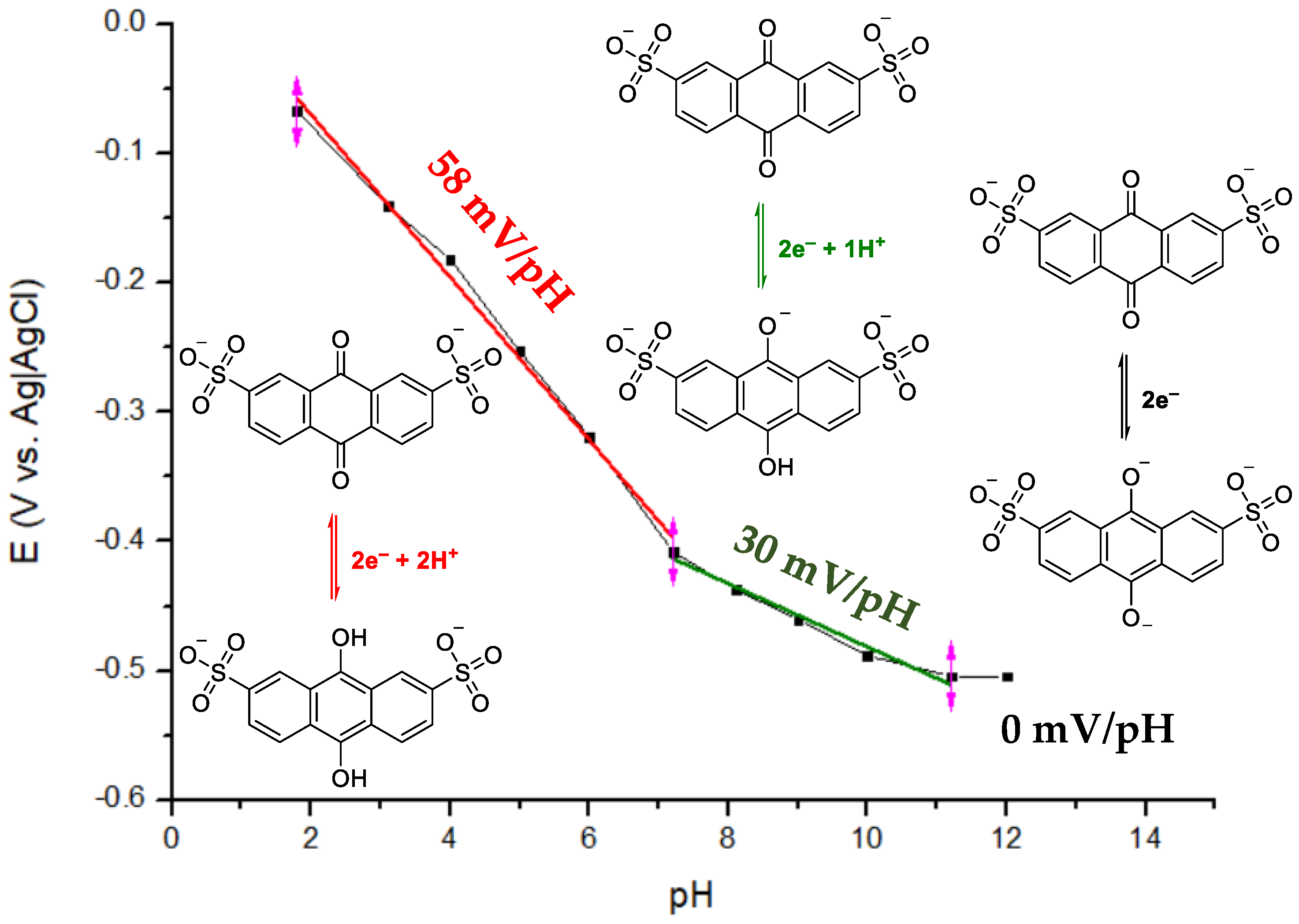
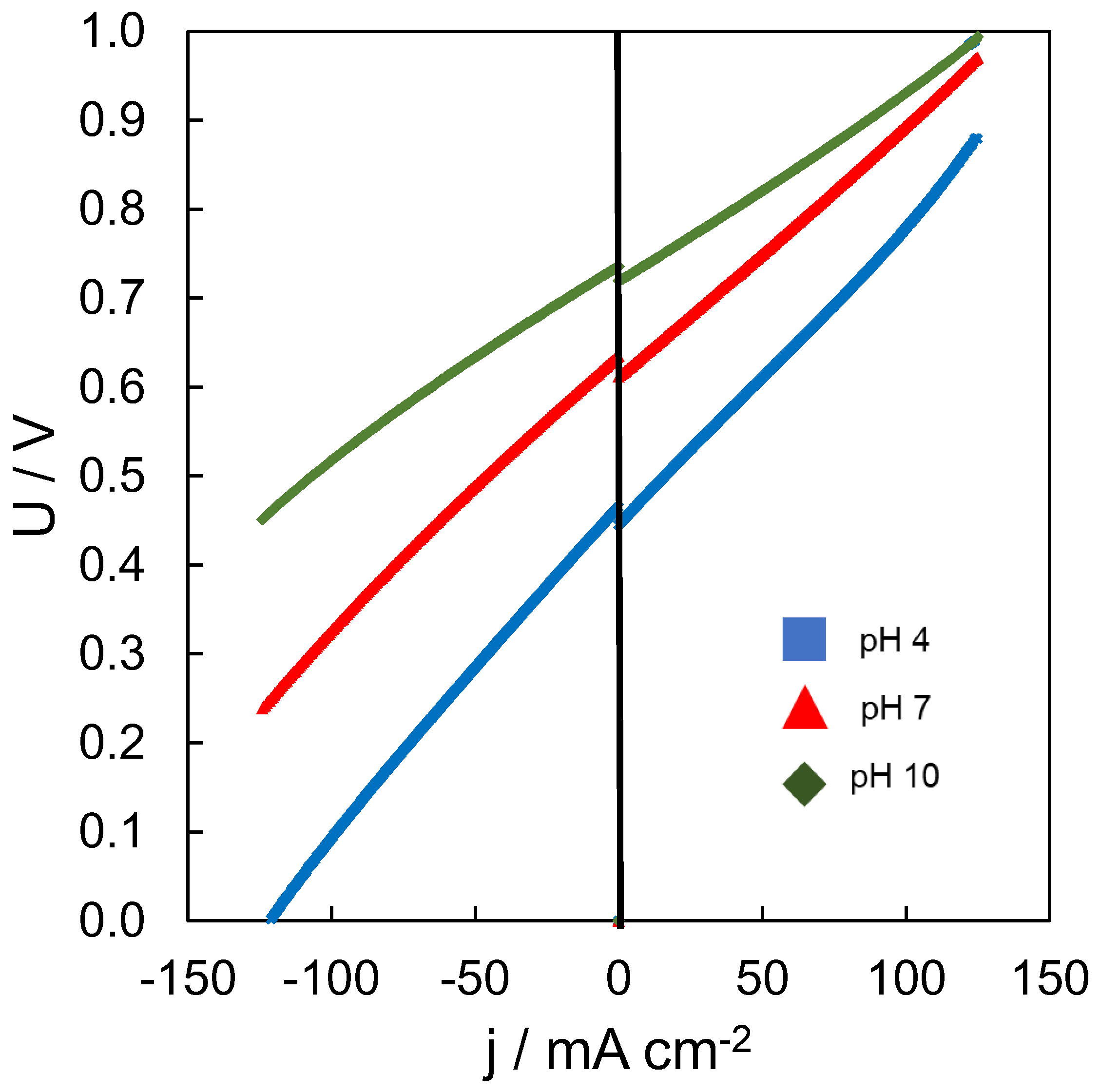


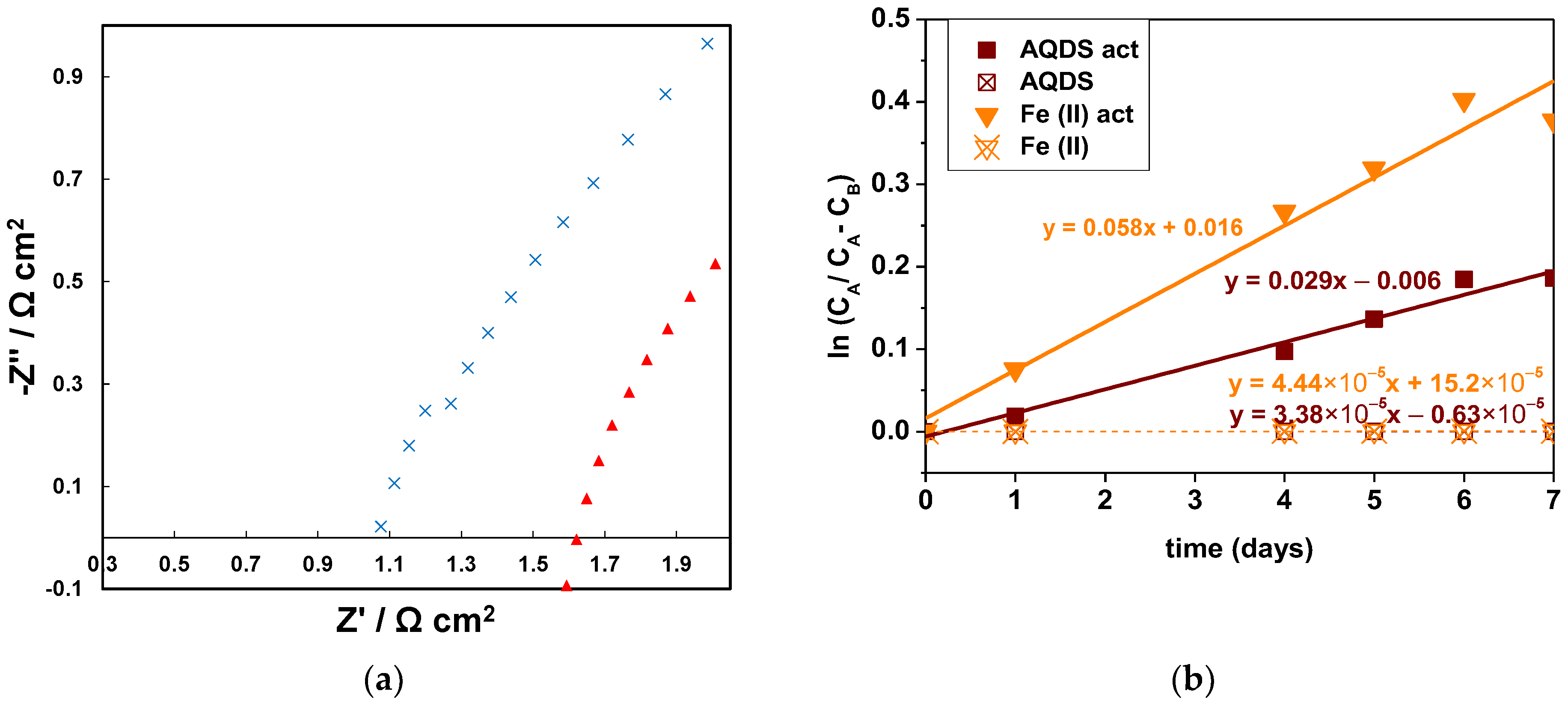
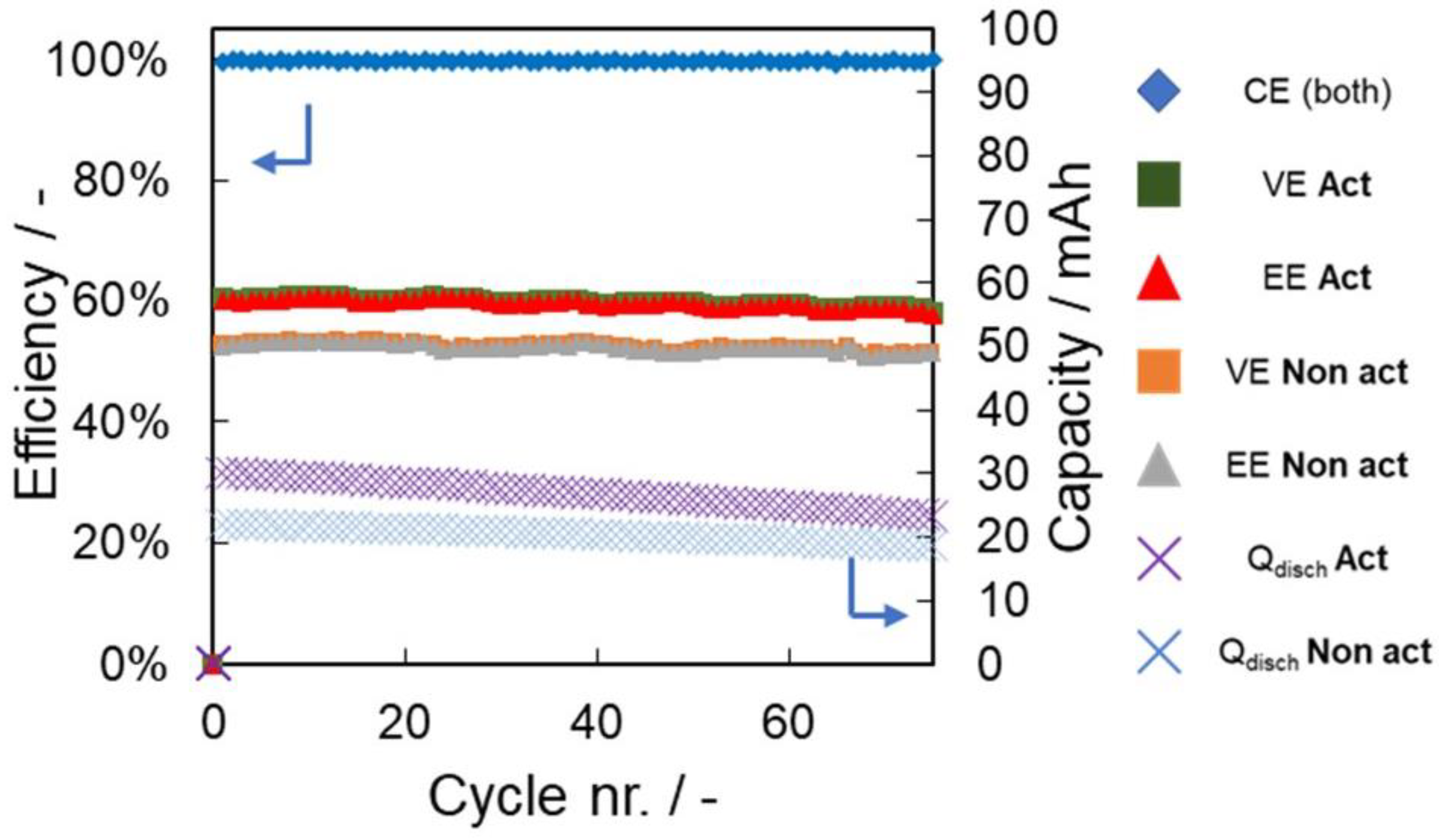
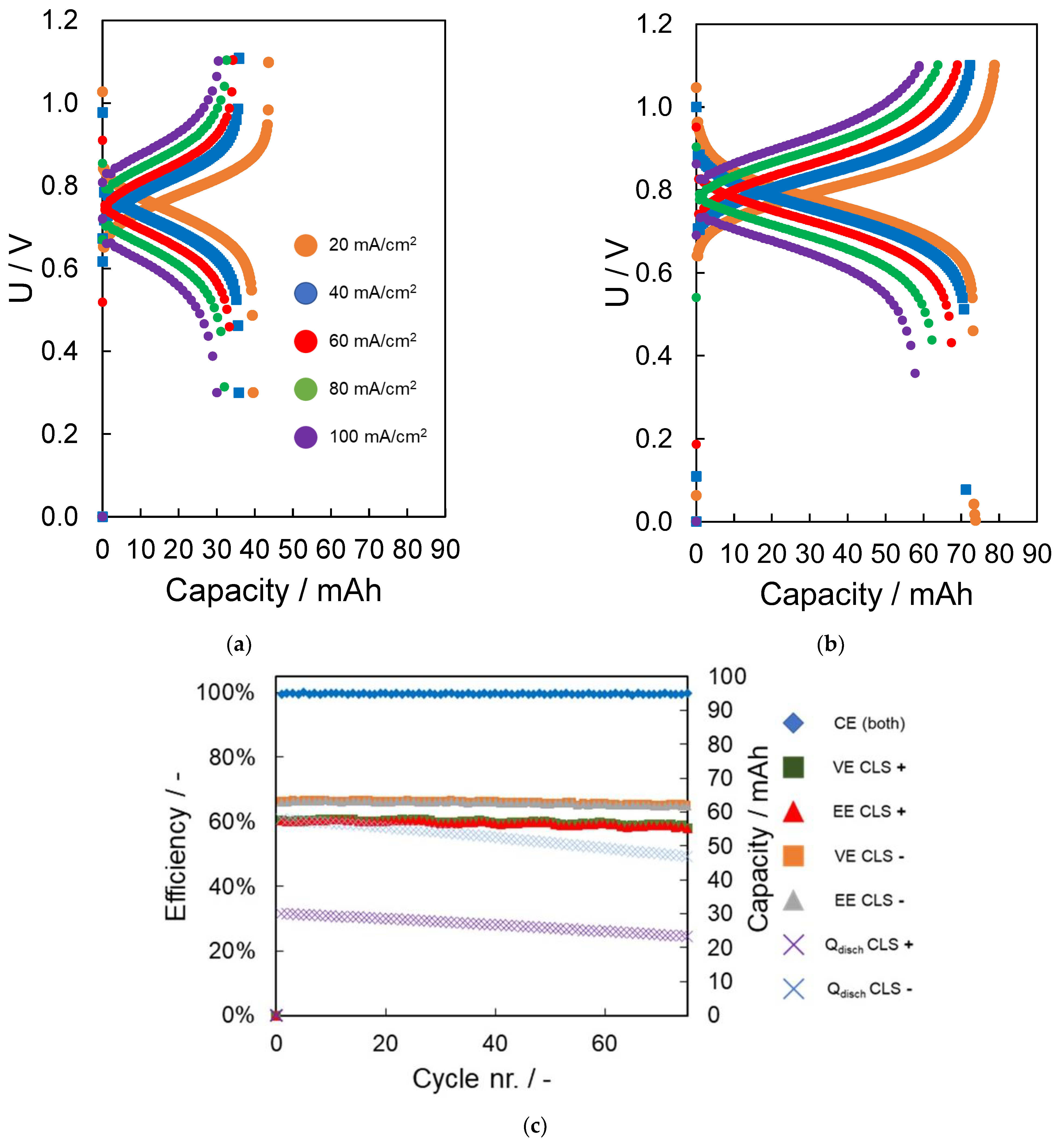
| Parameter | Unit | pH 4 | pH 7 | pH 10 | |||
|---|---|---|---|---|---|---|---|
| Ucell | V | 0.455 | 0.624 | 0.728 | |||
| Rohm (0% SOC) | Ohm·cm2 | 1.91 | 1.87 | 1.58 | |||
| Rohm (50% SOC) | Ohm·cm2 | 1.93 | 1.85 | 1.54 | |||
| Rdisch (50% SOC) | Ohm·cm2 | 3.83 | 3.21 | 2.30 | |||
| Rcharge (50% SOC) | Ohm·cm2 | 3.51 | 2.87 | 2.19 | |||
| Rohm (50% Soc)/Rdisch | - | 50.4 | 57.6 | 67.0 | |||
| Ionic conductivity | mS/cm | 21.2(A) | 41.9(C) | 21.2(A) | 54.7(C) | 41.9(A) | 54.7(C) |
| Energy efficiencies | % | 12.2 | 34.1 | 53.4 | |||
| Capacity decay | %Qtheo/h | −0.21 | −0.10 | −0.38 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asenjo-Pascual, J.; Salmeron-Sanchez, I.; Avilés-Moreno, J.R.; Mauleón, P.; Mazur, P.; Ocón, P. Understanding Aqueous Organic Redox Flow Batteries: A Guided Experimental Tour from Components Characterization to Final Assembly. Batteries 2022, 8, 193. https://doi.org/10.3390/batteries8100193
Asenjo-Pascual J, Salmeron-Sanchez I, Avilés-Moreno JR, Mauleón P, Mazur P, Ocón P. Understanding Aqueous Organic Redox Flow Batteries: A Guided Experimental Tour from Components Characterization to Final Assembly. Batteries. 2022; 8(10):193. https://doi.org/10.3390/batteries8100193
Chicago/Turabian StyleAsenjo-Pascual, Juan, Ivan Salmeron-Sanchez, Juan Ramón Avilés-Moreno, Pablo Mauleón, Petr Mazur, and Pilar Ocón. 2022. "Understanding Aqueous Organic Redox Flow Batteries: A Guided Experimental Tour from Components Characterization to Final Assembly" Batteries 8, no. 10: 193. https://doi.org/10.3390/batteries8100193
APA StyleAsenjo-Pascual, J., Salmeron-Sanchez, I., Avilés-Moreno, J. R., Mauleón, P., Mazur, P., & Ocón, P. (2022). Understanding Aqueous Organic Redox Flow Batteries: A Guided Experimental Tour from Components Characterization to Final Assembly. Batteries, 8(10), 193. https://doi.org/10.3390/batteries8100193






