One-Pot Spray Engineering to Design Na0.44MnO2 Cathode Electrodes for High-Rate and Cycle-Stable Na-Ion Batteries
Abstract
1. Introduction
2. Experimental
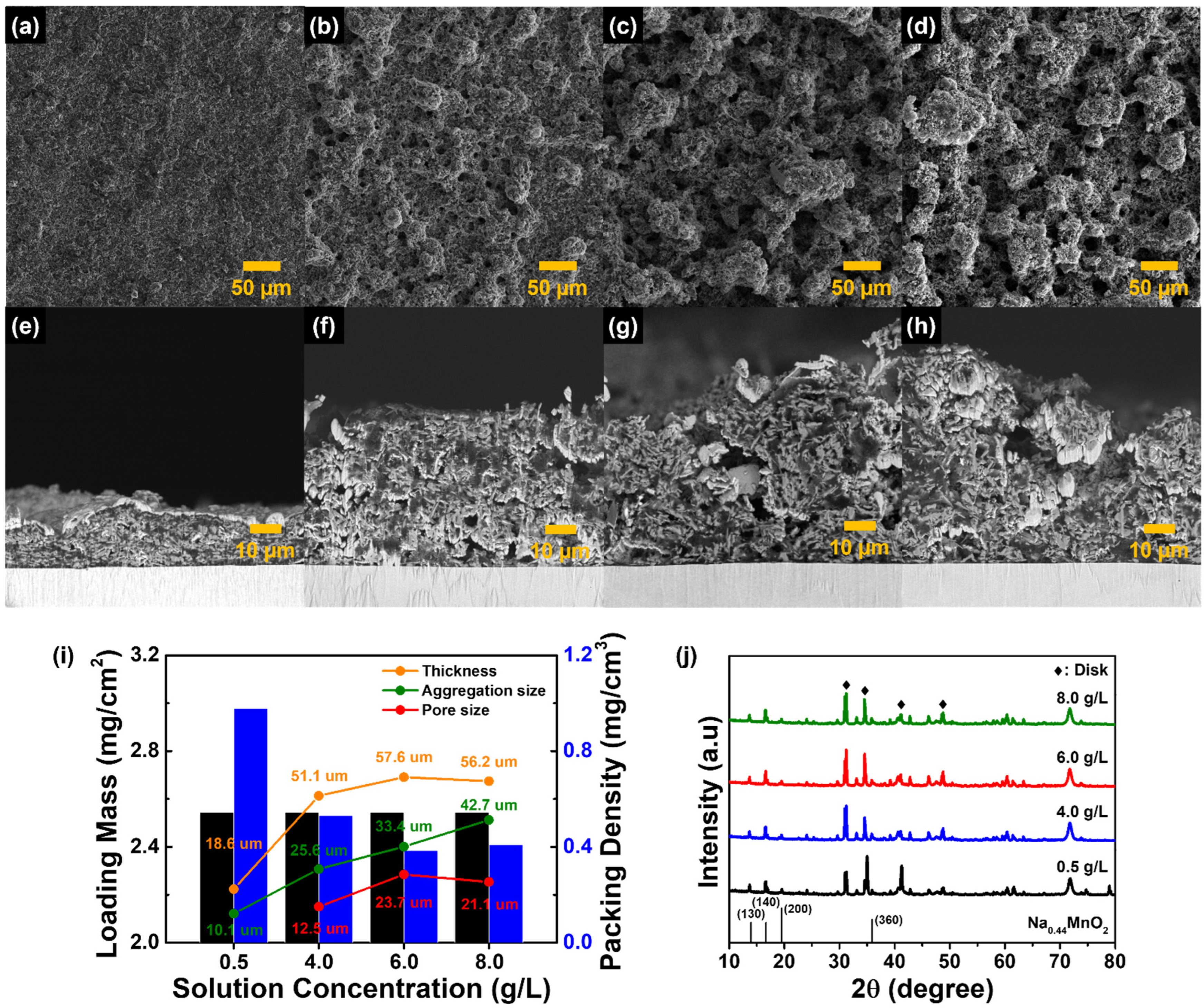
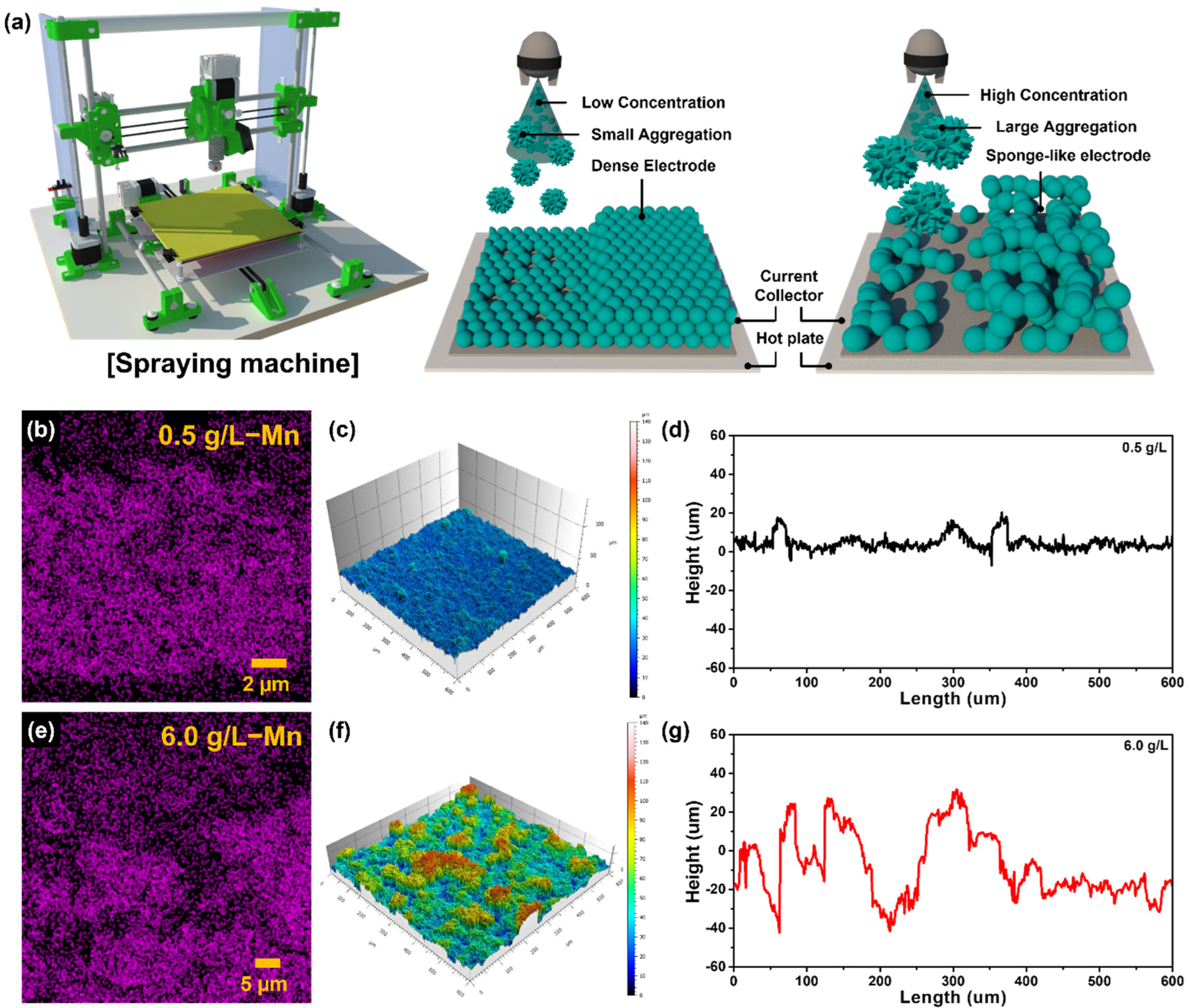
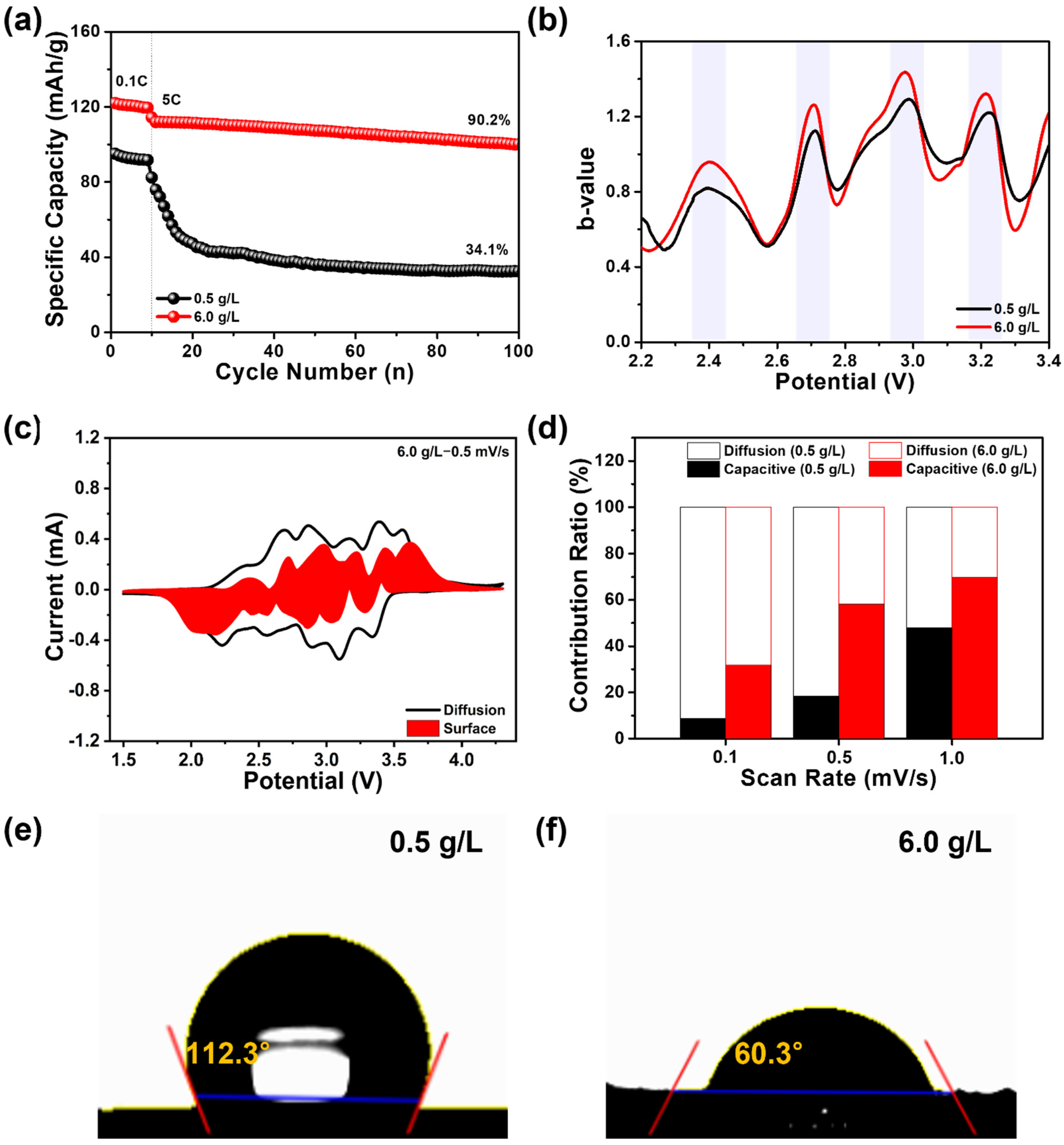
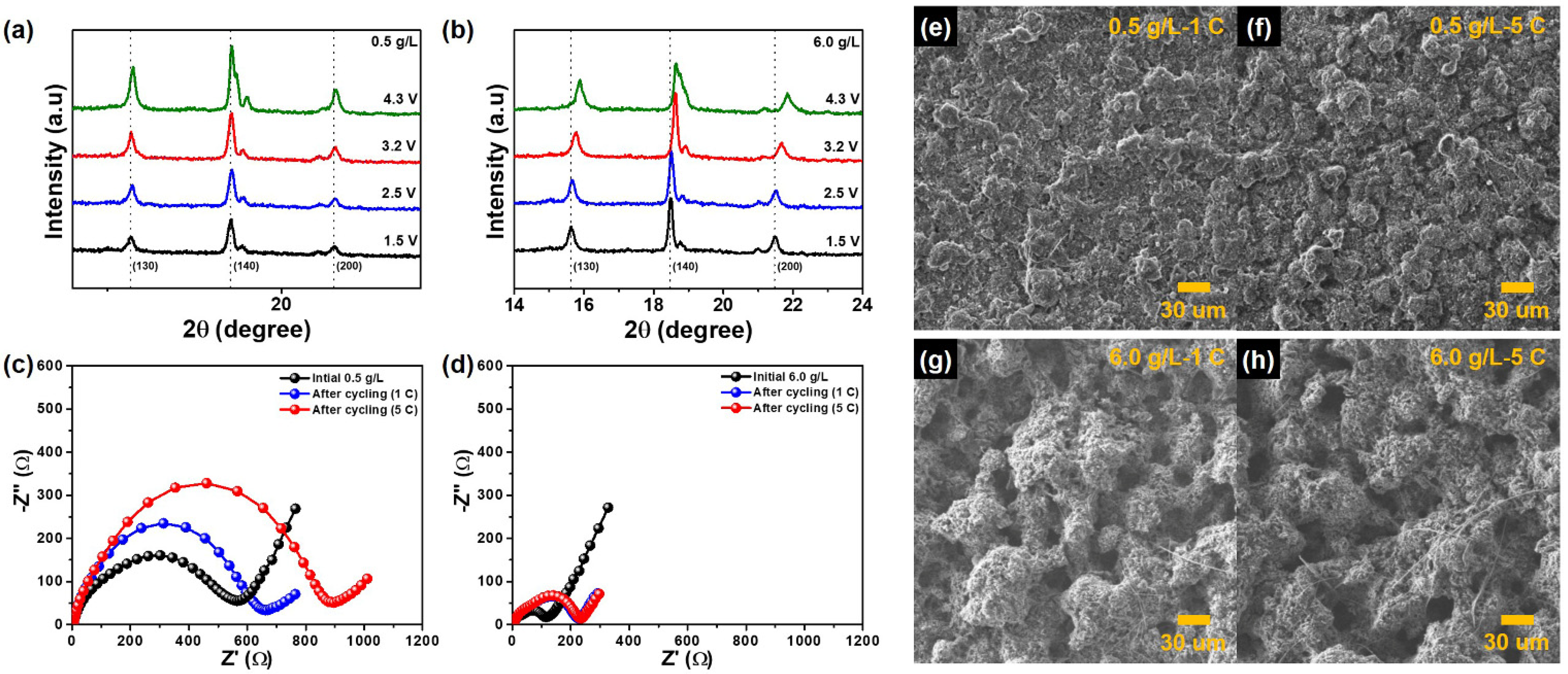
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.; Leung, C.L.A.; Leung, P.; Grant, P.S. A solid-state battery cathode with a polymer composite electrolyte and low tortuosity microstructure by directional freezing and polymerization. Adv. Energy Mater. 2021, 11, 2002387. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium metal extraction from seawater. Joule 2018, 2, 1648. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L.; Liu, Y.; Dou, S.-X. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus prussian blue analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Shukla, R.; Rashid, M.; Gupta, A.; Basu, S.; Dhaka, R.S. Synthesis and physical properties of NaxTO2 (T = Mn, Co) nanostructures for cathode materials in Na–ion batteries. Mater. Res. Bull. 2018, 105, 178. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent advances and prospects of cathode materials for sodium-ion batteries. Adv. Mater. 2015, 27, 5343. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RCS Adv. 2014, 4, 3633. [Google Scholar] [CrossRef]
- Xiao, B.; Soto, F.A.; Gu, M.; Han, K.S.; Song, J.; Wang, H.; Engelhard, M.H.; Murugesan, V.; Mueller, K.T.; Reed, D.; et al. Lithium-pretreated hard carbon as high-performance sodium-ion battery anodes. Adv. Energy Mater. 2018, 8, 1801441. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Cui, J.; Yao, S.; Ihsan-UI-Haq, M.; Mubarak, N.; Quattrocchi, E.; Ciucci, F.; Kim, J.-K. Dual-phase MoS2 as a high-performance sodium-ion battery anode. J. Mater. Chem. A 2020, 8, 2114. [Google Scholar] [CrossRef]
- Ran, L.; Luo, B.; Gentle, L.R.; Lin, T.; Sun, Q.; Li, M.; Rana, M.M.; Wang, L.; Knibbe, R. Biomimetic Sn4P3 anchored on carbon nanotubes as an anode for high-performance sodium-ion batteries. ACS Nano 2020, 14, 8826. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, J.; Xiao, L.; Ai, X.; Cao, Y.; Yang, H. Phosphate framework electrode materials for sodium ion batteries. Adv. Sci. 2017, 4, 1600392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, L.; Wen, J.; Zeng, Y.R. Structural stability and ionic transport property of NaMPO4 (M = V, Cr, Mn, Fe, Co, Ni) as cathode material for Na-ion batteries. J. Power Sources 2019, 438, 227016. [Google Scholar] [CrossRef]
- Dacek, S.T.; Richards, W.D.; Kitchaev, D.A.; Ceder, G. Structure and dynamics of fluorophosphate Na-ion battery cathodes. Chem. Mater. 2016, 28, 5450. [Google Scholar] [CrossRef]
- Chao, D.; Lai, C.-H.; Liang, P.; Wei, Q.; Wang, Y.-S.; Zhu, C.; Deng, G.; Doan-Nguyen, V.V.T.; Lin, J.; Mai, L.; et al. Sodium vanadium fluorophosphates (NVOPF) array cathode designed for high-rate full sodium ion storage device. Adv. Energy Mater. 2018, 8, 1800058. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian blue analogs for rechargeable batteries. iScience 2018, 3, 110. [Google Scholar] [CrossRef]
- Du, G.; Tao, M.; Li, J.; Yang, T.; Gao, W.; Deng, J.; Qi, Y.; Bao, S.J.; Xu, M. Low-operating temperature, high-rate and durable solid-state sodium-ion battery based on polymer electrolyte and prussian blue cathode. Adv. Energy Mater. 2020, 10, 1903351. [Google Scholar] [CrossRef]
- Yoo, G.; Koo, B.-R.; An, H.-R.; Huang, C.; An, G.-H. Enhanced and stabilized charge transport boosting by Fe-doping effect of V2O5 nanorod for rechargeable Zn-ion battery. J. Ind. Eng. Chem. 2021, 99, 344. [Google Scholar] [CrossRef]
- Tapia-Ruiz, N.; Dose, W.M.; Sharma, N.; Chen, H.; Heath, J.; Somerville, J.W.; Maitra, U.; Islam, M.S.; Bruce, P.G. High voltage structural evolution and enhanced Na-ion diffusion in P2-Na2/3Ni1/3−xMgxMn2/3O2 (0 ≤ x ≤ 0.2) cathodes from diffraction, electrochemical and ab initio studies. Energy Environ. Sci. 2018, 11, 1470. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A.J. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185. [Google Scholar] [CrossRef]
- Ma, G.; Zhao, Y.; Huang, K.; Ju, Z.; Liu, C.; Hou, Y.; Xing, Z. Effects of the starting materials of Na0.44MnO2 cathode materials on their electrochemical properties for Na-ion batteries. Electrochim. Acta 2016, 222, 36. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Qiu, B.; Paillard, E.; Ma, C.; Cao, X.; Liu, H.; Stan, M.C.; Liu, H.; Gallash, T.; et al. Durable high-rate capability Na0.44MnO2 cathode material for sodium-ion batteries. Nano Energy 2016, 27, 602. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, D.Y.W. Stabilizing Na0.7MnO2 cathode for Na-ion battery via a single-step surface coating and doping process. J. Power Sources 2018, 391, 106. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Liao, J.; Ding, X.; Hu, Q.; He, X.; Chen, C.-H. Suppressing the Unfavorable Surface Layer Growth on Na0.44MnO2 Cathode by a NaTi2(PO4)3 Coating To Improve Cycling Stability and Ultrahigh Rate Capability. ACS Appl. Energy Mater. 2019, 2, 7497. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, A.; Chen, Z.; Cao, Y. Research progress of tunnel-structural Na0.44MnO2 cathode for sodium-ion batteries: A mini review. Electrochem. Commum. 2021, 122, 106897. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commum. 2010, 12, 463. [Google Scholar] [CrossRef]
- Doeff, M.M.; Richardson, T.J.; Hwang, K.-T. Electrochemical and structural characterization of titanium-substituted manganese oxides based on Na0.44MnO2. J. Power Sources 2004, 135, 240. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, M.; Zhou, X.; Zhou, Z. Structural design for anodes of lithium-ion batteries: Emerging horizons from materials to electrodes. Mater. Horiz. 2015, 2, 553. [Google Scholar] [CrossRef]
- Wu, J.; Yang, S.; Cai, W.; Bi, Z.; Shang, G.; Yao, J. Multi-characterization of LiCoO2 cathode films using advanced AFM-based techniques with high resolution. Sci. Rep. 2017, 7, 11164. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett. 2008, 8, 265. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, M.; Zhou, X.; Luo, Y.; Wei, J.; Zhou, Z. Orderly packed anodes for high-power lithium-ion batteries with super-long cycle life: Rational design of MnCO3/Large-area graphene composites. Adv. Mater. 2015, 27, 806. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Johnston, C.; Grant, P.S. Spray printing and optimization of anodes and cathodes for high performance Li-Ion batteries. Electrochim. Acta 2018, 292, 546. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-tortuosity cathodes by ice and graded lithium ion battery templating. J. Mater. Chem. A 2019, 7, 21421. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Grant, P.S. Multi-layered composite electrodes of high power Li4Ti5O12 and high capacity SnO2 for smart lithium ion storage. Energy Storage Mater. 2021, 38, 70. [Google Scholar] [CrossRef]
- Sung, K.-W.; Koo, B.-R.; Ahn, H.-J. Hybrid nanocomposites of tunneled-mesoporous sulfur-doped carbon nanofibers embedded with zinc sulfide nanoparticles for ultrafast lithium storage capability. J. Alloys Compd. 2021, 854, 157206. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Grant, P.S. High energy lithium ion capacitors using hybrid cathodes comprising electrical double layer and intercalation host multi-layers. Energy Storage Mater. 2020, 33, 408. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Grant, P.S. Layer-by-layer printing of multi-layered heterostructures using Li4Ti5O12 and Si for high power Li-ion storage. Nano Energy 2019, 61, 96. [Google Scholar] [CrossRef]
- Huang, C.; Grant, P.S. Coral-like cathodes by ice directional porosity lithium ion battery templating. J. Mat. Chem. A 2018, 6, 14689. [Google Scholar] [CrossRef]
- Huang, C.; Young, N.P.; Zhang, J.; Snaith, H.J.; Grant, P.S. A two layer electrode structure for improved Li Ion diffusion and volumetric capacity in Li Ion batteries. Nano Energy 2017, 31, 377. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Yin, F.; Liu, P.; Cloetens, P.; Liu, Y.; Lin, F.; Zhao, K.J. Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling. Mech. Phys. Solids 2019, 129, 160. [Google Scholar] [CrossRef]
- Shasien, M.; Suzuki, M.; Tsutai, Y. Controlling the coating microstructure on axial suspension plasma spray process. Surf. Coat. Technol. 2018, 356, 96. [Google Scholar]
- Zhang, X.; Ju, Z.; Housel, L.M.; Wang, L.; Zhu, Y.; Singh, G.; Sadique, N.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; et al. Promoting transport kinetics in Li-ion battery with aligned porous electrode architectures. Nano Lett. 2019, 19, 8255. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-J.; Yan, Y.-W.; Chi, C.; Ma, X.-T.; Zhang, D.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Liu, S.-B. Fluorine anion doped Na0.44MnO2 with layer-tunnel hybrid structure as advanced cathode for sodium ion batteries. J. Power Sources 2019, 427, 129. [Google Scholar] [CrossRef]
- Zhong, W.; Huang, Q.; Zheng, F.; Deng, Q.; Pan, Q.; Liu, Y.; Li, Y.; Hu, J.; Yang, C.; Liu, M. Structural insight into the abnormal capacity of Co-substitution tunnel-type Na0.44MnO2 cathode for sodium ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 47548. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Martín, L.; Bayly, A.E.; Simmons, M.J.H. Particle aggregation in large counter-current spray drying towers: Nozzle configuration, vortex momentum and temperature. Procedia Eng. 2015, 102, 668. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical porous silicon structures with extraordinary mechanical strength as high performance lithium-ion battery anodes. Nat. Commum. 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-R.; Ahn, H.-J. Fast-switching electrochromic properties of mesoporous WO3 films with oxygen vacancy defects. Nanoscale 2017, 9, 17788. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Lu, B.; Zhao, J. Binder-free Si nanoparticle electrode with 3D porous structure prepared by electrophoretic deposition for lithium-ion batteries. J. ACS Appl. Mater. Interfaces 2015, 7, 7497. [Google Scholar] [CrossRef]
- Feng, F.; Chen, S.; Liao, X.-Z.; Ma, Z.-F. Hierarchical hollow prussian blue rods synthesized via self-sacrifice template as cathode for high performance sodium ion battery. Small Methods 2019, 3, 1800259. [Google Scholar] [CrossRef]
- Liu, C.; Guo, W.-L.; Wang, Q.-H.; Li, J.-G.; Yang, X.-P. Parametric study of hydrothermal soft chemical synthesis and application of Na0.44MnO2 nanorods for Li-ion battery cathode materials: Synthesis conditions and electrochemical performance. J. Alloys Compd. 2016, 658, 588. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, X.; Liu, J.; Liu, Q.; Lan, S.; Zhang, Q.; Liu, X.; Seo, J.K.; Chen, T.; Gu, L.; et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 2018, 9, 5100. [Google Scholar] [CrossRef]
- Shen, Q.; Zhao, X.; Liu, Y.; Li, Y.; Zhang, J.; Zhang, N.; Yang, C.; Chen, J. Dual-Strategy of Cation-Doping and Nanoengineering Enables Fast and Stable Sodium-Ion Storage in a Novel Fe/Mn-Based Layered Oxide Cathode. Adv. Sci. 2020, 2002199. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhu, Y.-F.; Yao, H.-R.; Wang, P.-F.; Zhang, X.-D.; Li, H.; Yang, X.; Gu, L.; Li, Y.-C.; Wang, T.; et al. A stable layered oxide cathode material for high-performance sodium-ion battery. Adv. Energy Mater. 2019, 9, 1803978. [Google Scholar] [CrossRef]
- Koo, B.-R.; Sung, K.-W.; Ahn, H.-J. Boosting Ultrafast Lithium Storage Capability of Hierarchical Core/Shell Constructed Carbon Nanofiber/3D Interconnected Hybrid Network with Nanocarbon and FTO Nanoparticle Heterostructures. Adv. Funct. Mater. 2020, 30, 2001863. [Google Scholar] [CrossRef]
- Yin, H.; Qu, H.-Q.; Liu, Z.; Jiang, R.-Z.; Li, C.; Zhu, M.-Q. Long cycle life and high rate capability of three dimensional CoSe2 grainattached carbon nanofibers for flexible sodium-ion batteries. Nano Energy 2019, 58, 715. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, J.; Zhuo, Y.; Hu, H.; Liu, W.; Liu, F.; Liu, P.; Yan, J.; Liu, K. An inactive metal supported oxide cathode material with high rate capability for sodium ion batteries. Energy Storage Mater. 2019, 20, 263. [Google Scholar] [CrossRef]
- Ye, J.; Baumgaertel, A.C.; Wang, Y.M.; Biener, J.; Biener, M.M. Structural Optimization of 3D Porous Electrodes for High-Rate Performance Lithium Ion Batteries. ACS Nano 2015, 9, 2194. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.-H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.-S.; Roh, K.C.; et al. Facile Synthesis of Nb2O5@Carbon Core-Shell Nanocrystals with Controlled Crystalline Structure for High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2015, 9, 7497. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, Y.; Qin, J.; Shi, C.; Liu, E.; Zhao, N. Capacitance controlled, hierarchical porous 3D ultra-thin carbon networks reinforced prussian blue for high performance Na-ion battery cathode. Nano Energy 2019, 58, 192. [Google Scholar] [CrossRef]
- Fang, G.; Wu, Z.; Zhou, J.; Zhu, C.; Cao, X.; Lin, Y.; Chen, Y.; Wang, C.; Pan, A.; Liang, S. Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Bu, F.; Zhao, X.; Wang, L.; Shen, Q.; Zhang, J.; Zhang, N.; Jiao, L.; Fan, L.-Z. Boosting fast and durable sodium-ion storage by tailoring well-shaped Na0.44MnO2 nanowires cathode. Electrochim. Acta 2019, 313, 122. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Lee, B.; Qiao, R.; Yang, Z.; Xu, S.; Yu, X.; Gu, L.; Hu, Y.-S.; Yang, W.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhang, F.; Wu, N.; Tang, Y. A Dual-Carbon Battery Based on Potassium-Ion Electrolyte. Adv. Energy Mater. 2017, 7, 1700920. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, B.-R.; Lee, Y.-G.; Lee, S.H.; An, G.-H.; Huang, C. One-Pot Spray Engineering to Design Na0.44MnO2 Cathode Electrodes for High-Rate and Cycle-Stable Na-Ion Batteries. Batteries 2022, 8, 181. https://doi.org/10.3390/batteries8100181
Koo B-R, Lee Y-G, Lee SH, An G-H, Huang C. One-Pot Spray Engineering to Design Na0.44MnO2 Cathode Electrodes for High-Rate and Cycle-Stable Na-Ion Batteries. Batteries. 2022; 8(10):181. https://doi.org/10.3390/batteries8100181
Chicago/Turabian StyleKoo, Bon-Ryul, Young-Geun Lee, Sang Ho Lee, Geon-Hyoung An, and Chun Huang. 2022. "One-Pot Spray Engineering to Design Na0.44MnO2 Cathode Electrodes for High-Rate and Cycle-Stable Na-Ion Batteries" Batteries 8, no. 10: 181. https://doi.org/10.3390/batteries8100181
APA StyleKoo, B.-R., Lee, Y.-G., Lee, S. H., An, G.-H., & Huang, C. (2022). One-Pot Spray Engineering to Design Na0.44MnO2 Cathode Electrodes for High-Rate and Cycle-Stable Na-Ion Batteries. Batteries, 8(10), 181. https://doi.org/10.3390/batteries8100181






