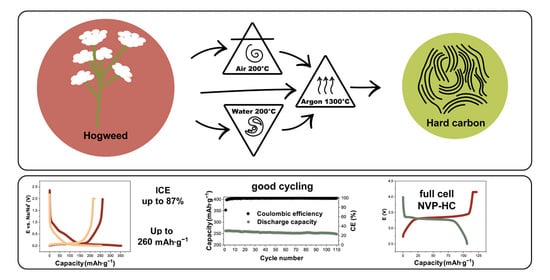
Sosnowskyi Hogweed-Based Hard Carbons for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.1.1. Hard Carbons (HCs)
2.1.2. Na3V2(PO4)3
2.2. Materials Characterization
2.3. Electrode Preparation
2.4. Electrochemical Characterization
2.4.1. Half Cells
2.4.2. Full Cells
3. Results and Discussion
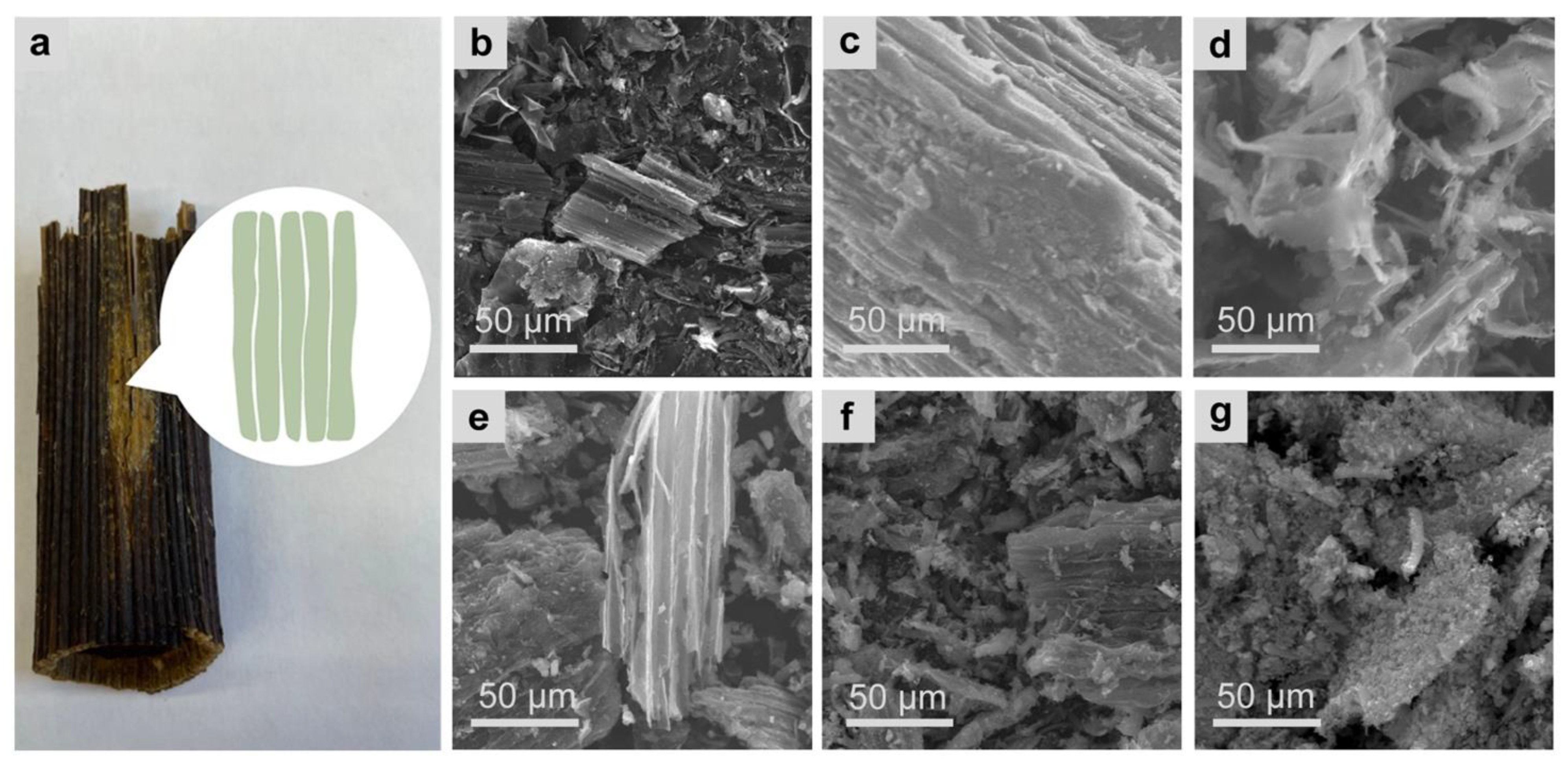
3.1. Pretreatment of Biomass
3.2. Structure and Morphology of Giant Hogweed-Based Hard Carbons
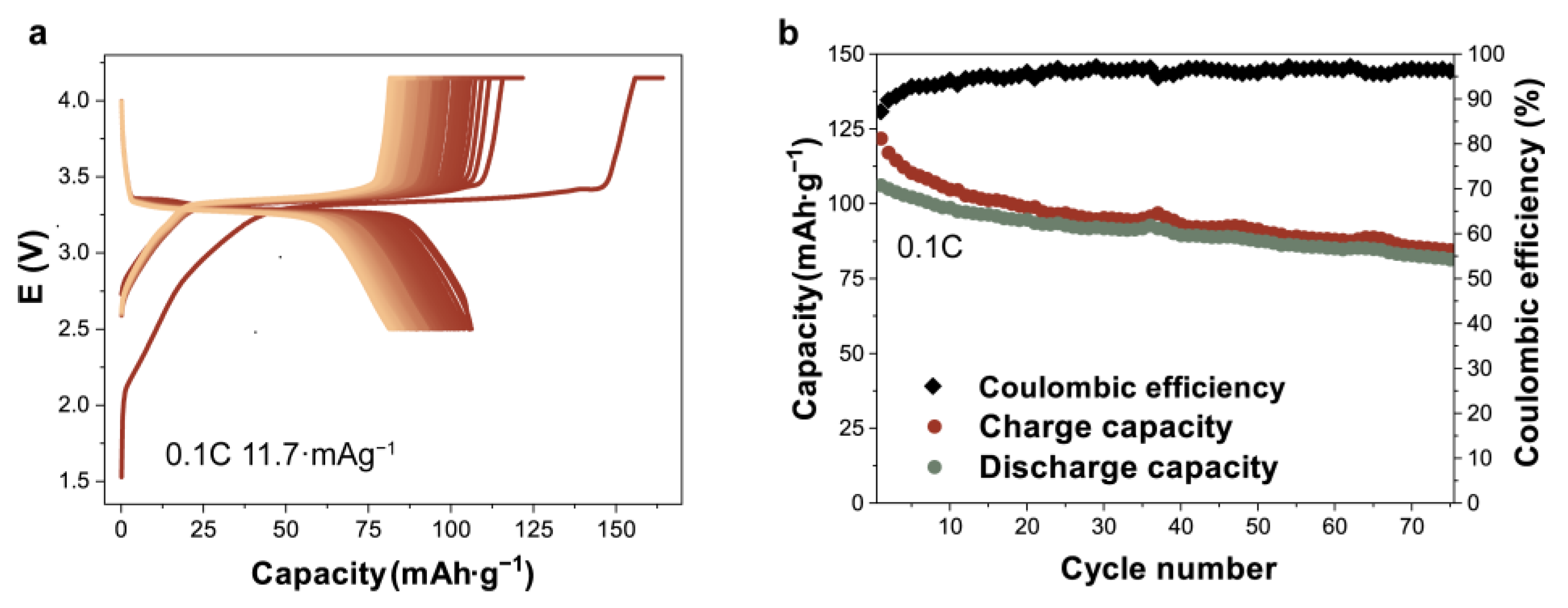
3.3. Electrochemical Performance of Half and Full Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M. Is Lithium the New Gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium Market Research—Global Supply, Future Demand and Price Development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of Hard Carbon from Argan Shells for Na-Ion Batteries. J. Mater. Chem. A Mater. 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Pei, L.; Cao, H.; Yang, L.; Liu, P.; Zhao, M.; Xu, B.; Guo, J. Hard Carbon Derived from Waste Tea Biomass as High-Performance Anode Material for Sodium-Ion Batteries. Ionics 2020, 26, 5535–5542. [Google Scholar] [CrossRef]
- Cao, L.; Hui, W.; Xu, Z.; Huang, J.; Zheng, P.; Li, J.; Sun, Q. Rape Seed Shuck Derived-Lamellar Hard Carbon as Anodes for Sodium-Ion Batteries. J. Alloy. Compd. 2017, 695, 632–637. [Google Scholar] [CrossRef]
- Nita, C.; Zhang, B.; Dentzer, J.; Matei Ghimbeu, C. Hard Carbon Derived from Coconut Shells, Walnut Shells, and Corn Silk Biomass Waste Exhibiting High Capacity for Na-Ion Batteries. J. Energy Chem. 2021, 58, 207–218. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, T.; Yuan, X.; Zhang, L.; Liu, Y.; Huang, J.; Guo, S. Enhanced Performance by Enlarged Nano-Pores of Holly Leaf-Derived Lamellar Carbon for Sodium-Ion Battery Anode. Sci. Rep. 2016, 6, 26246. [Google Scholar] [CrossRef]
- Muruganantham, R.; Wang, F.M.; Yuwono, R.A.; Sabugaa, M.; Liu, W.R. Biomass Feedstock of Waste Mango-Peel-Derived Porous Hard Carbon for Sustainable High-Performance Lithium-Ion Energy Storage Devices. Energy Fuels 2021, 35, 10878–10889. [Google Scholar] [CrossRef]
- Saavedra Rios, C.d.M.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from Various Biomass Types as Precursors for Hard Carbon Anodes in Sodium-Ion Batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Guo, Z.; Xie, F.; Liu, H.; Yadegari, H.; Tebyetekerwa, M.; Ryan, M.P.; Hu, Y.S.; Titirici, M.M. The Role of Hydrothermal Carbonization in Sustainable Sodium-Ion Battery Anodes. Adv. Energy Mater. 2022, 12, 2200208. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, S.; Xu, X.; Liu, J. Wheat Straw Carbon Matrix Wrapped Sulfur Composites as a Superior Cathode for Li–S Batteries. RSC Adv. 2015, 5, 100089–100096. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Energy Storage Materials from Nature through Nanotechnology: A Sustainable Route from Reed Plants to a Silicon Anode for Lithium-Ion Batteries. Angew. Chem. 2015, 127, 9768–9772. [Google Scholar] [CrossRef]
- Koldasbayeva, D.; Tregubova, P.; Shadrin, D.; Gasanov, M.; Pukalchik, M. Large-Scale Forecasting of Heracleum Sosnowskyi Habitat Suitability under the Climate Change on Publicly Available Data. Sci. Rep. 2022, 12, 6128. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, O.A.; Ageev, V.P.; Brodovskaya, E.P.; Shlyapkina, V.I.; Petrov, P.S.; Zharkov, M.N.; Yakobson, D.E.; Maev, I.V.; Sukhorukov, G.B.; Pyataev, N.A. Evaluation of Photocytotoxicity Liposomal Form of Furanocoumarins Sosnowsky’s Hogweed. Chem. Biol. Interact. 2022, 357, 109880. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.G.; Bedford, J.; Kanitkar, S. Keeping Pace with the Media; Giant Hogweed Burns—A Case Series and Comprehensive Review. Burns 2017, 43, 933–938. [Google Scholar] [CrossRef]
- Grzędzicka, E. Impact of Invasive Weeds on the Diversity and Dissimilarity of Bird Communities in Forested Areas. Diversity 2022, 14, 229. [Google Scholar] [CrossRef]
- Ustinova, E.N.; Savina, K.A.; Lysenkov, S.N. New Data on Consortive Associations of Sosnowsky’s Hogweed with Anthophilous Insects. Russ. J. Biol. Invasions 2017, 8, 375–385. [Google Scholar] [CrossRef]
- Thiele, J. Patterns and Processes of Heracleum Mantegazzianum Invasion into German Cultural Landscapes on the Local, Landscape and Regional Scale. Ph.D. Thesis, Justus-Liebig University Gießen, Gießen, Germany, 2007. [Google Scholar]
- Renčo, M.; Jurová, J.; Gömöryová, E.; Čerevková, A. Long-Term Giant Hogweed Invasion Contributes to the Structural Changes of Soil Nematofauna. Plants 2021, 10, 2103. [Google Scholar] [CrossRef]
- Menshchikov, A.; Shadrin, D.; Prutyanov, V.; Lopatkin, D.; Sosnin, S.; Tsykunov, E.; Iakovlev, E.; Somov, A. Real-Time Detection of Hogweed: UAV Platform Empowered by Deep Learning. IEEE Trans. Comput. 2021, 70, 1175–1188. [Google Scholar] [CrossRef]
- Kornilov, T.; Terentev, A.; Kekelidze, V. Use of a Hardware-Software Complex for Phytosanitary Monitoring and Chemical Treatments of the Sosnowski Hogweed. BIO Web. Conf. 2020, 18, 00015. [Google Scholar] [CrossRef]
- Andreeva, L.v. Sosnowsky Hogweed: New Ways to Use. IOP Conf. Ser. Earth Environ. Sci. 2020, 613, 012006. [Google Scholar] [CrossRef]
- Voznyakovskii, A.P.; Neverovskaya, A.Y.; Voznyakovskii, A.A.; Karmanov, A.P.; Shugalei, I.V. Hogweed Biomass as a Raw Material for Producing 2D Nanocarbons: An Environmental Aspect. Russ. J. Gen. Chem. 2020, 90, 2627–2631. [Google Scholar] [CrossRef]
- S. WinXPOW. Version 1.2; STOE & Cie GmbH: Darmstadt, Germany, 2000.
- Lin, X.; Liu, Y.; Tan, H.; Zhang, B. Advanced Lignin-Derived Hard Carbon for Na-Ion Batteries and a Comparison with Li and K Ion Storage. Carbon 2020, 157, 316–323. [Google Scholar] [CrossRef]
- Du, Y.F.; Sun, G.H.; Li, Y.; Cheng, J.Y.; Chen, J.P.; Song, G.; Kong, Q.Q.; Xie, L.J.; Chen, C.M. Pre-Oxidation of Lignin Precursors for Hard Carbon Anode with Boosted Lithium-Ion Storage Capacity. Carbon 2021, 178, 243–255. [Google Scholar] [CrossRef]
- del Mar Saavedra Rios, C.; Simonin, L.; Ghimbeu, C.M.; Vaulot, C.; da Silva Perez, D.; Dupont, C. Impact of the Biomass Precursor Composition in the Hard Carbon Properties and Performance for Application in a Na-Ion Battery. Fuel Process. Technol. 2022, 231, 107223. [Google Scholar] [CrossRef]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice Husk Research: From Environmental Pollutant to a Promising Source of Organo-Mineral Raw Materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; el Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; Zavareze, E.D.R. Cellulose Fibers Extracted from Rice and Oat Husks and Their Application in Hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Characterization of Adsorbent Developed from Rice Husk: Effect of Surface Functional Group on Phenol Adsorption. J. Appl. Sci. 2010, 10, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Abdelkafi, F.; Ammar, H.; Rousseau, B.; Tessier, M.; el Gharbi, R.; Fradet, A. Structural Analysis of Alfa Grass (Stipa tenacissima L.) Lignin Obtained by Acetic Acid/Formic Acid Delignification. Biomacromolecules 2011, 12, 3895–3902. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, L.; Ren, Q.; Fan, L.; Zhang, F.; Shi, Z. Facile Hydrothermal Treatment Route of Reed Straw-Derived Hard Carbon for High Performance Sodium Ion Battery. Electrochim. Acta 2018, 291, 188–196. [Google Scholar] [CrossRef]
- Kumar, H.; Detsi, E.; Abraham, D.P.; Shenoy, V.B. Fundamental Mechanisms of Solvent Decomposition Involved in Solid-Electrolyte Interphase Formation in Sodium Ion Batteries. Chem. Mater. 2016, 28, 8930–8941. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; He, X.; Qiao, Y.; Li, L.; Chou, S.-L. Hard Carbon Derived from Hazelnut Shell with Facile HCl Treatment as High-Initial-Coulombic-Efficiency Anode for Sodium Ion Batteries. Sustain. Mater. Technol. 2022, 33, e00446. [Google Scholar] [CrossRef]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Fujii, Y. Angelicin as the Principal Allelochemical in Heracleum sosnowskyi Fruit. Nat. Prod. Commun. 2015, 10, 767–770. [Google Scholar] [CrossRef]
- Susanti, R.F.; Alvin, S.; Kim, J. Toward High-Performance Hard Carbon as an Anode for Sodium-Ion Batteries: Demineralization of Biomass as a Critical Step. J. Ind. Eng. Chem. 2020, 91, 317–329. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, H.; Zheng, Y.; Zhang, H.; Shi, J.; Liu, W.; Huang, M.; Kan, J.; Zhao, X.; et al. High-Performance Sodium-Ion Capacitor Constructed by Well-Matched Dual-Carbon Electrodes from a Single Biomass. ACS Sustain. Chem. Eng. 2019, 14, 12188–12199. [Google Scholar] [CrossRef]
- Ren, X.; Xu, S.D.; Liu, S.; Chen, L.; Zhang, D.; Qiu, L. Lath-Shaped Biomass Derived Hard Carbon as Anode Materials with Super Rate Capability for Sodium-Ion Batteries. J. Electroanal. Chem. 2019, 841, 63–72. [Google Scholar] [CrossRef]
- Arie, A.A.; Tekin, B.; Demir, E.; Demir-Cakan, R. Utilization of The Indonesian’s Spent Tea Leaves as Promising Porous Hard Carbon Precursors for Anode Materials in Sodium Ion Batteries. Waste Biomass Valor. 2020, 6, 3121–3131. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Jiang, E.; Amiralian, N.; Annamalai, P.K.; Martin, D.J.; Kumar, N.A.; Zhao, X.S. Spinifex Nanocellulose Derived Hard Carbon Anodes for High-Performance Sodium-Ion Batteries. Sustain. Energy Fuels 2017, 5, 1090–1097. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Meng, Q.; Jensen, A.C.S.; Zhang, Q.; Zhang, Q.; Tong, Y.; Qi, Y.; Gu, L.; Titirici, M.-M.; et al. Regulating Pore Structure of Hierarchical Porous Waste Cork-Derived Hard Carbon Anode for Enhanced Na Storage Performance. Adv. Energy Mater. 2019, 48, 1902852. [Google Scholar] [CrossRef]


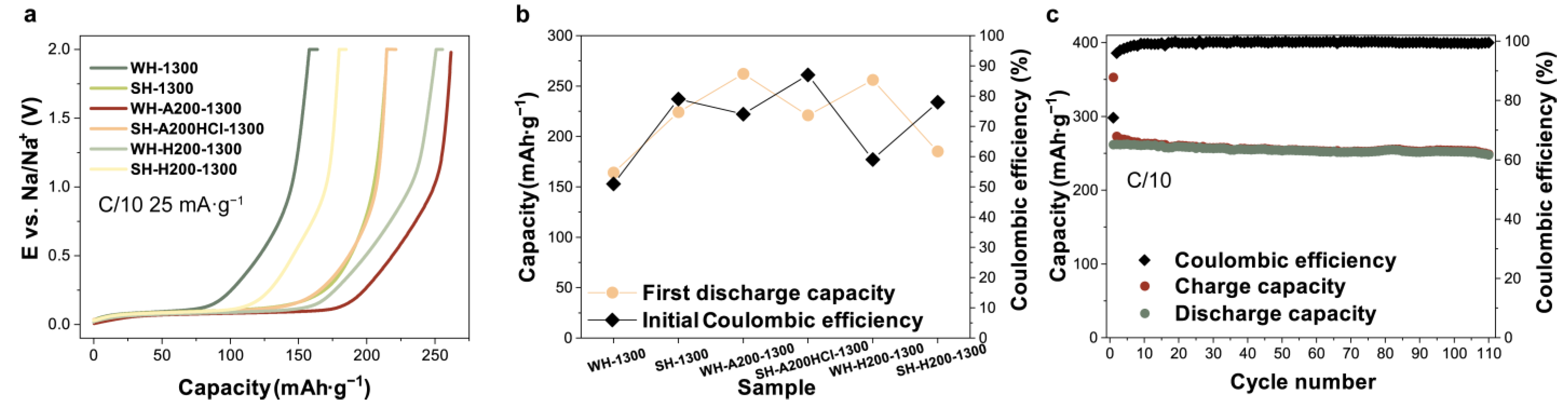
| Sample | Discharge Specific Capacity, mAh g−1 | Coulombic Efficiency (%) | SBET (m2 g−1) |
|---|---|---|---|
| WH-1300 | 164 | 51 | 337 |
| SH-1300 | 224 | 79 | 57 |
| WH-A200-1300 | 262 | 74 | 29 |
| SH-A200HCl-1300 | 221 | 87 | 7 |
| WH-H200-1300 | 256 | 59 | 127 |
| SH-H200-1300 | 185 | 78 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakienko, G.P.; Bobyleva, Z.V.; Apostolova, M.O.; Sultanova, Y.V.; Dyakonov, A.K.; Zakharkin, M.V.; Sobolev, N.A.; Alekseeva, A.M.; Drozhzhin, O.A.; Abakumov, A.M.; et al. Sosnowskyi Hogweed-Based Hard Carbons for Sodium-Ion Batteries. Batteries 2022, 8, 131. https://doi.org/10.3390/batteries8100131
Lakienko GP, Bobyleva ZV, Apostolova MO, Sultanova YV, Dyakonov AK, Zakharkin MV, Sobolev NA, Alekseeva AM, Drozhzhin OA, Abakumov AM, et al. Sosnowskyi Hogweed-Based Hard Carbons for Sodium-Ion Batteries. Batteries. 2022; 8(10):131. https://doi.org/10.3390/batteries8100131
Chicago/Turabian StyleLakienko, Grigorii P., Zoya V. Bobyleva, Maria O. Apostolova, Yana V. Sultanova, Andrey K. Dyakonov, Maxim V. Zakharkin, Nikita A. Sobolev, Anastasia M. Alekseeva, Oleg A. Drozhzhin, Artem M. Abakumov, and et al. 2022. "Sosnowskyi Hogweed-Based Hard Carbons for Sodium-Ion Batteries" Batteries 8, no. 10: 131. https://doi.org/10.3390/batteries8100131