A Method for Monitoring State-of-Charge of Lithium-Ion Cells Using Multi-Sine Signal Excitation
Abstract
:1. Introduction
- Battery cells can be used longer by preventing overcharging and over-discharging, which can cause permanent damage.
- It enables more aggressive cell operation. If the reliability of the SoC estimation is low, it must be operated conservatively in order to avoid overcharging and over-discharging the cell and to make the operation as safe as possible.
- As it shows reliable estimation results for any usage profile of the cell, it improves the reliability of the use of the battery cell in the application system.
- As the battery pack design does not have to be overengineered, it enables the production of smaller and lighter battery packs. It eventually lowers the price of the battery pack. In addition, the reliable battery system reduces battery maintenance costs.
- SoC estimation method based on open circuit voltage (OCV)
- SoC estimation method based on ampere counting
- SoC estimation method based on heuristic data
- SoC estimation method based on adaptive control
- SoC estimation method based on equivalent circuit model
Proposed Cell State Estimation Method
2. Experiment
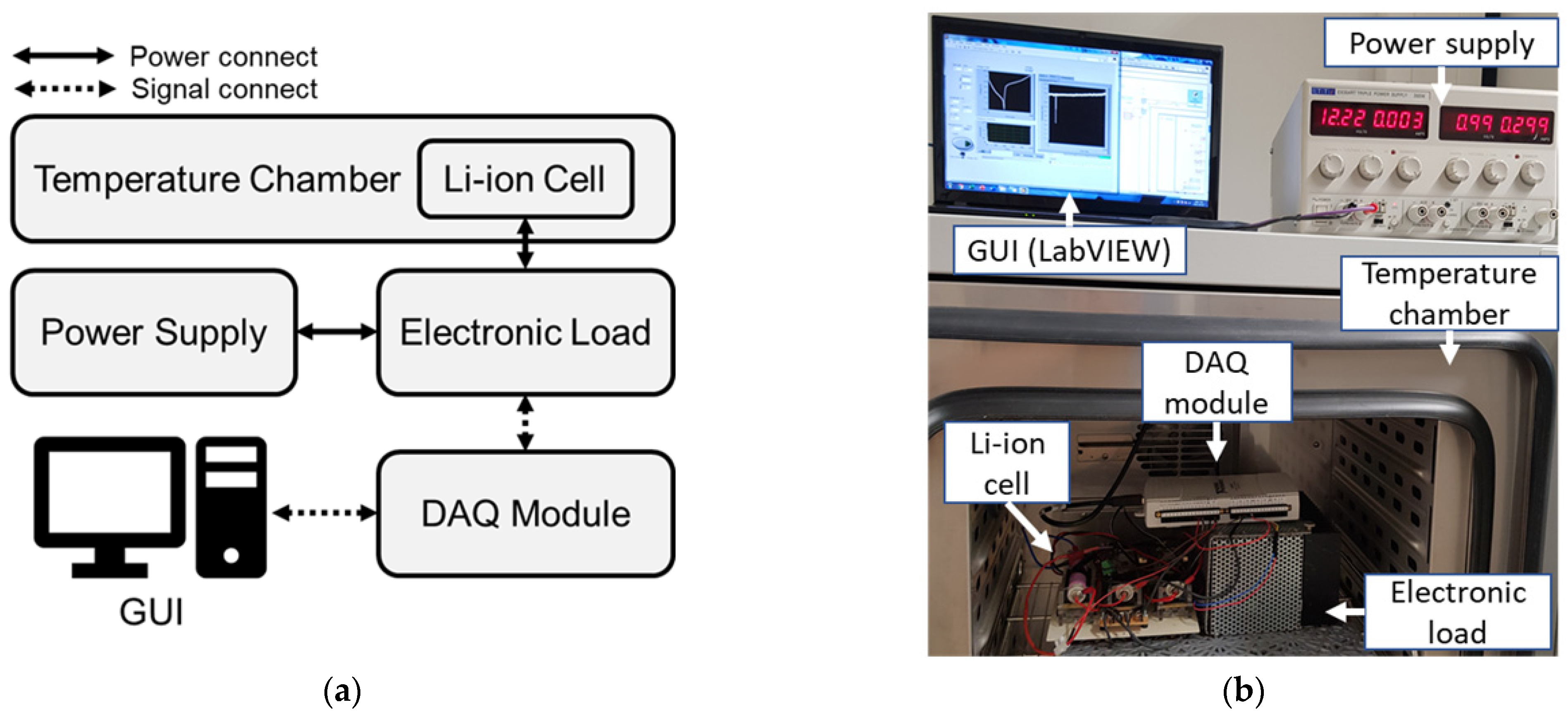
2.1. Measurement System
2.2. Definition of Key Terms
- Definition of C-rate
- Definition of SoC
- Definition of SoH
2.3. Measurement Parameters
2.3.1. Selection of Test Amplitude
2.3.2. Selection of Test Frequencies
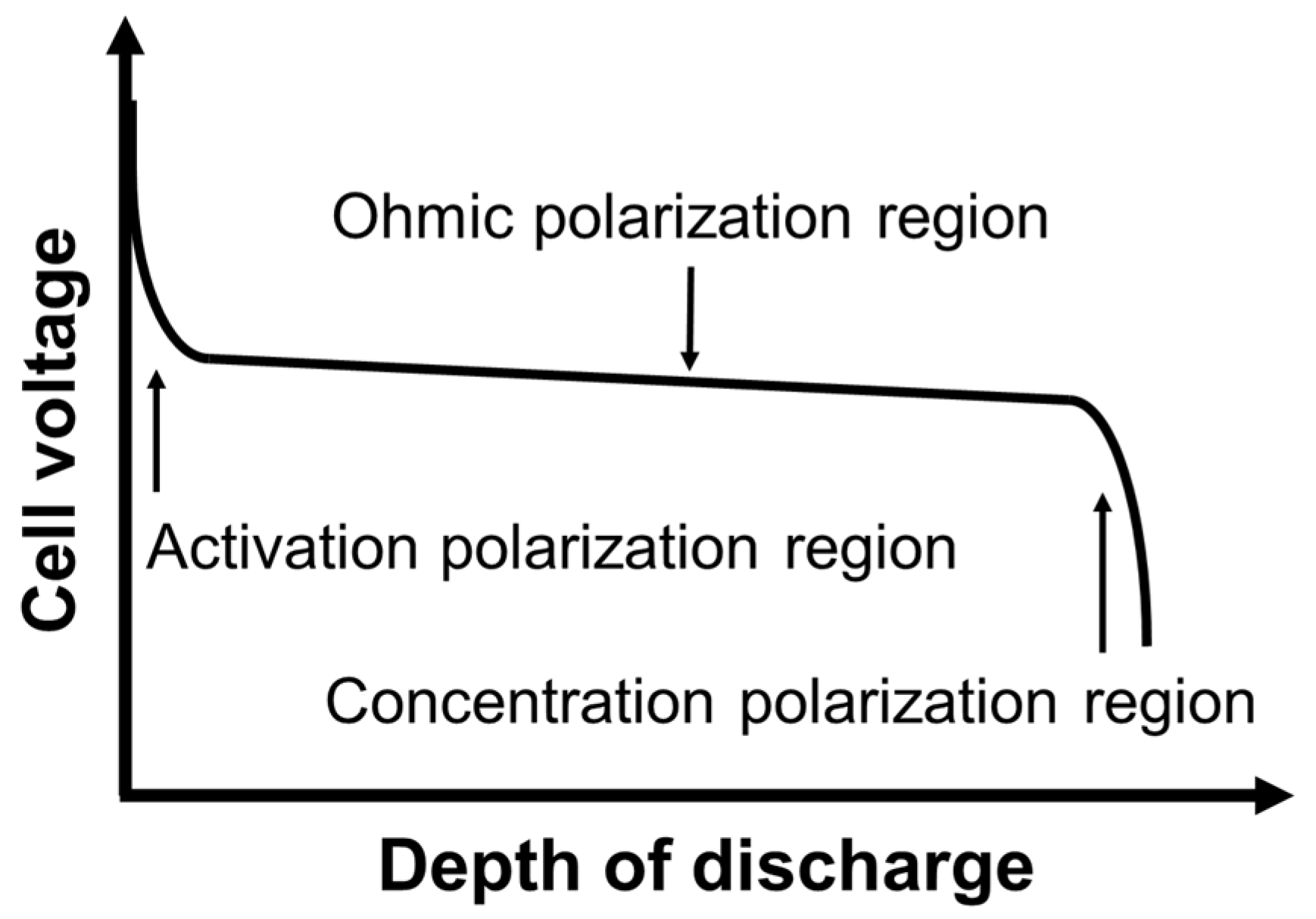
3. Measurement Results
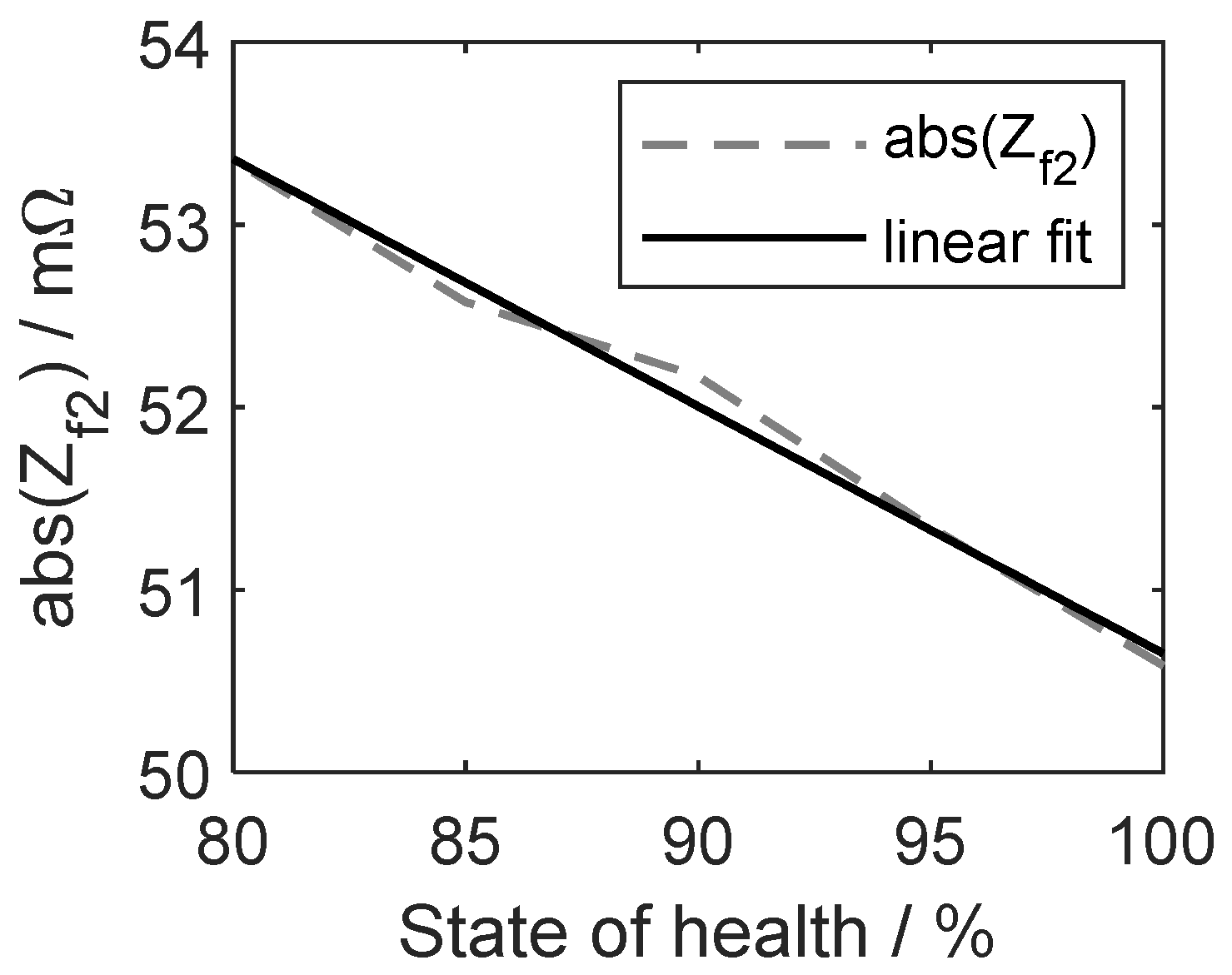
3.1. Cell SoH Estimation
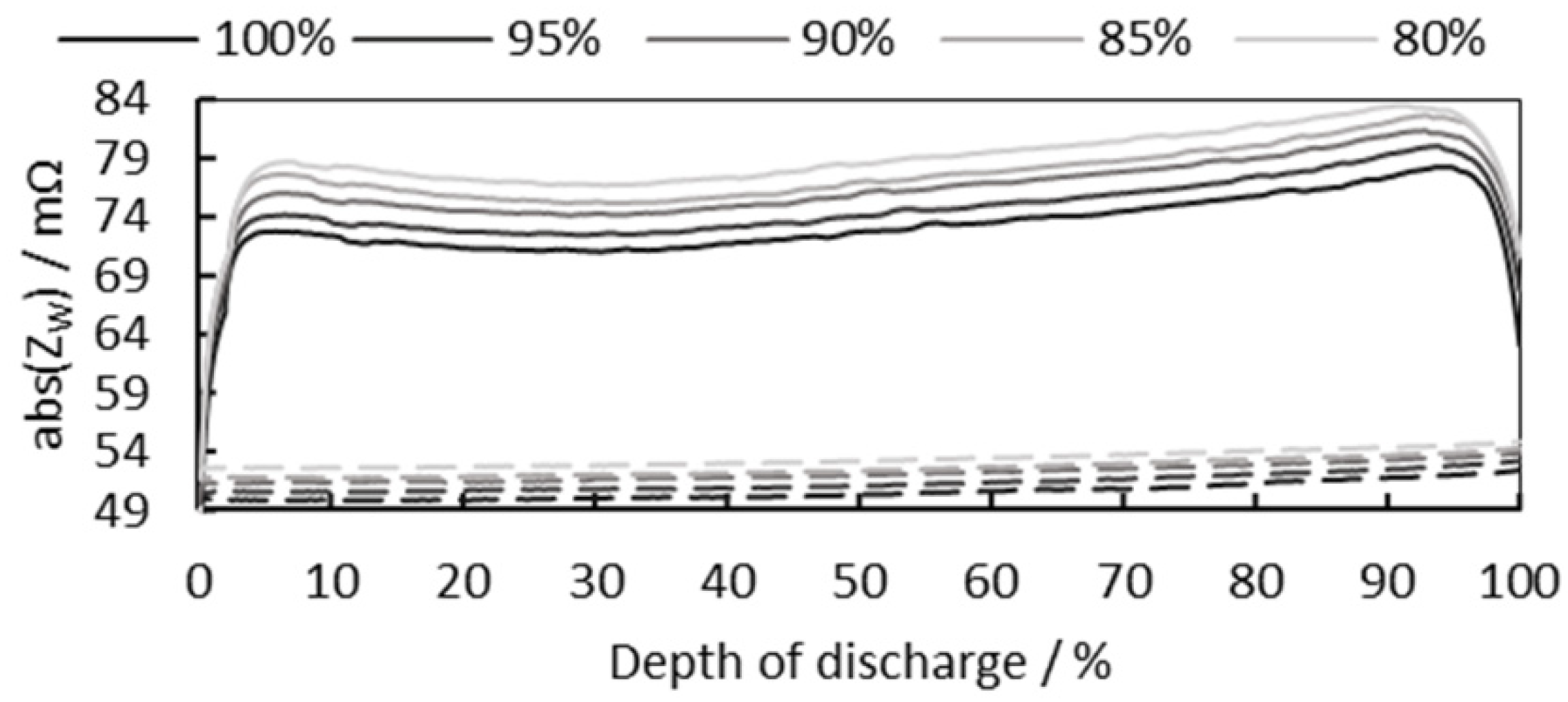
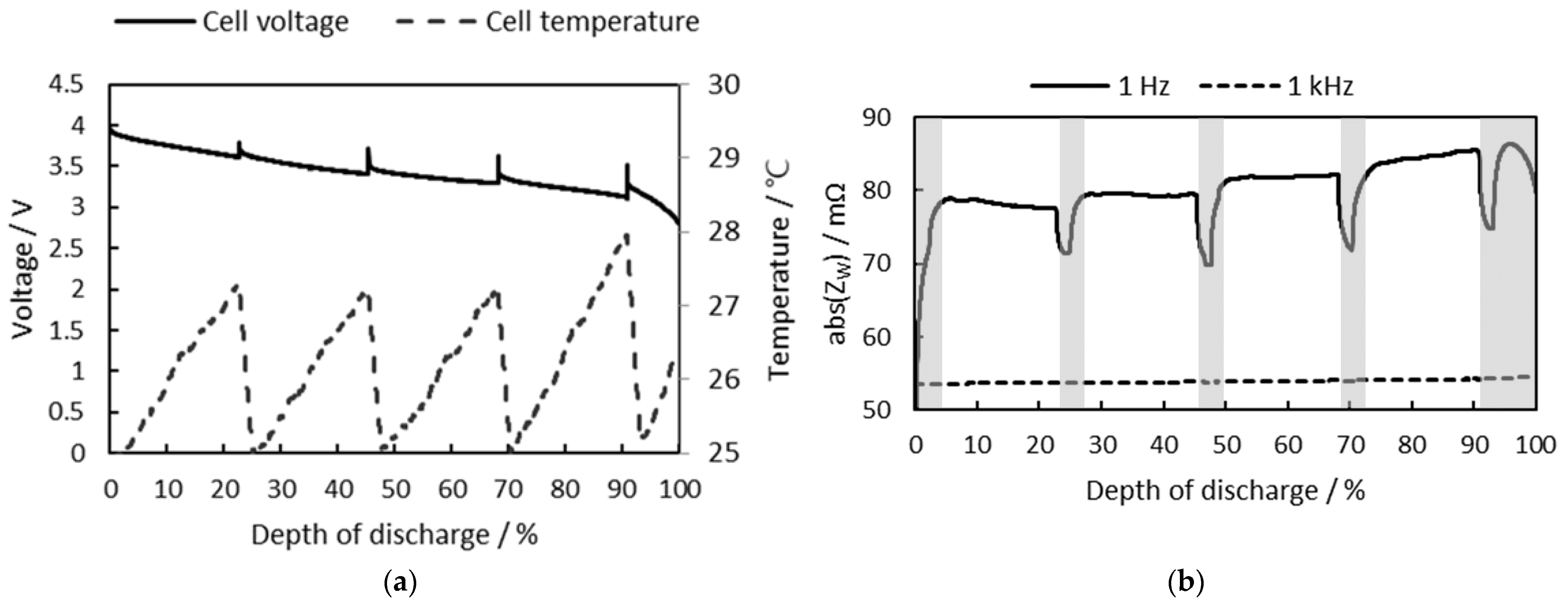
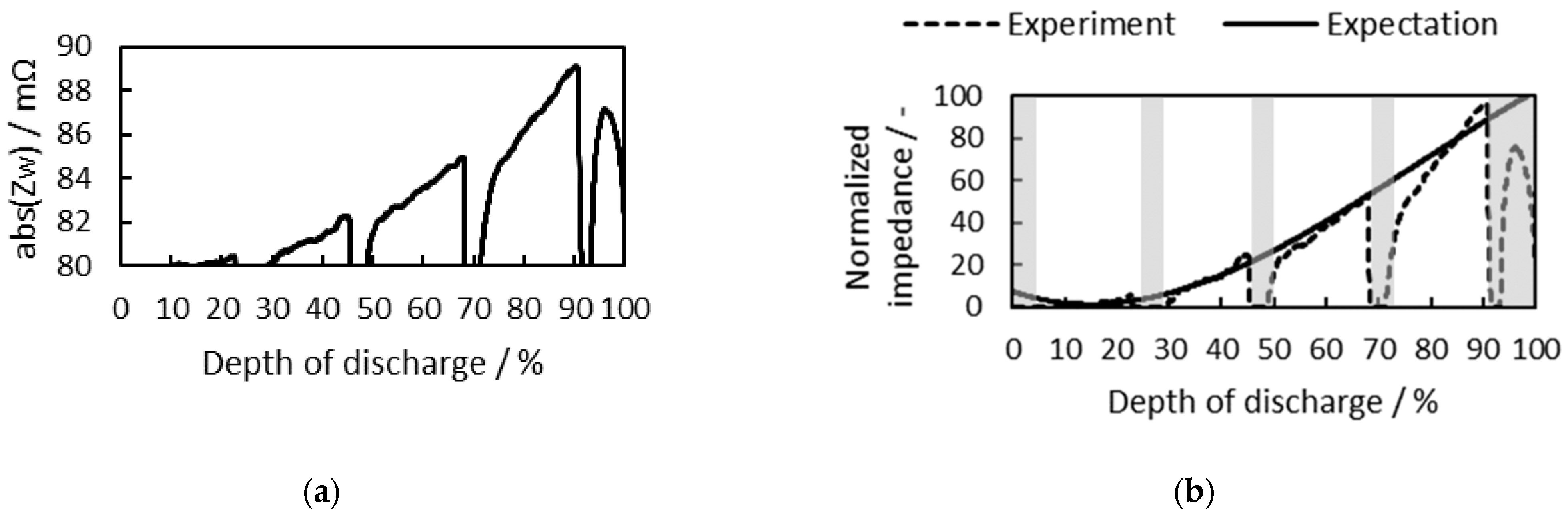
3.2. Cell SoC Estimation
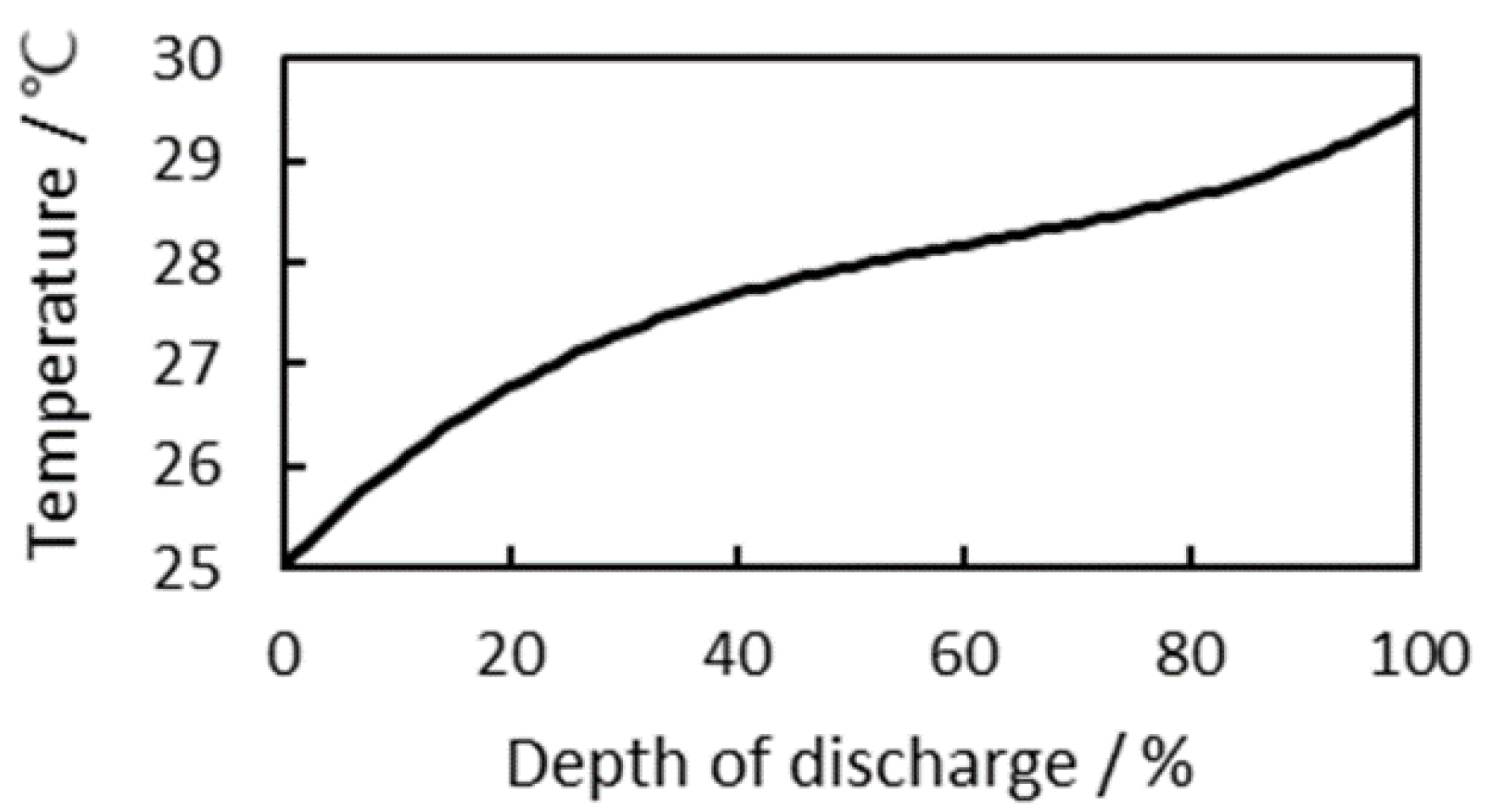
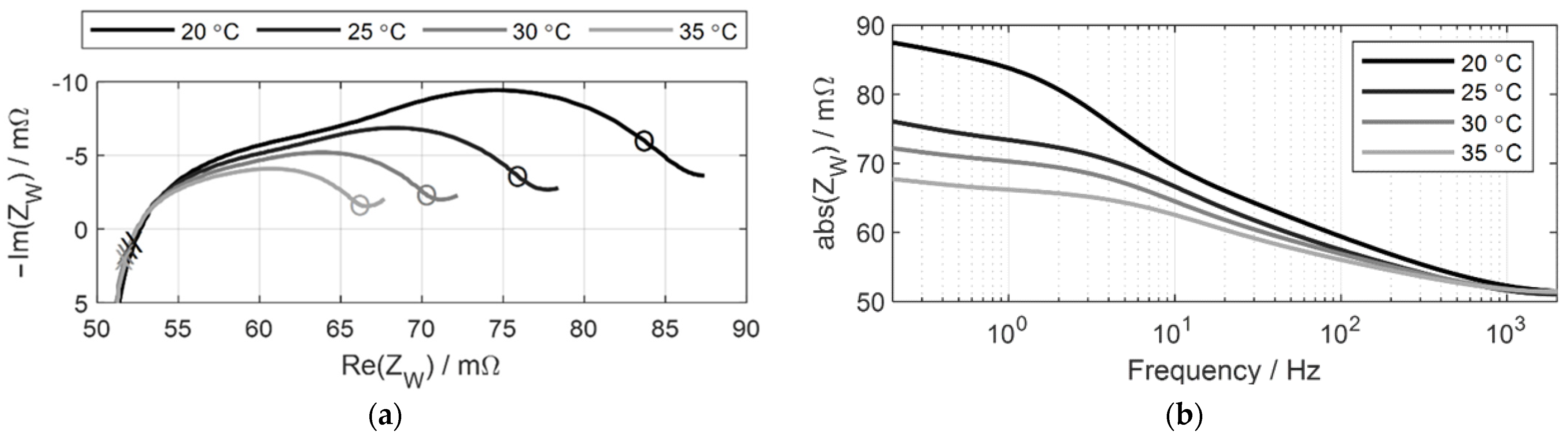
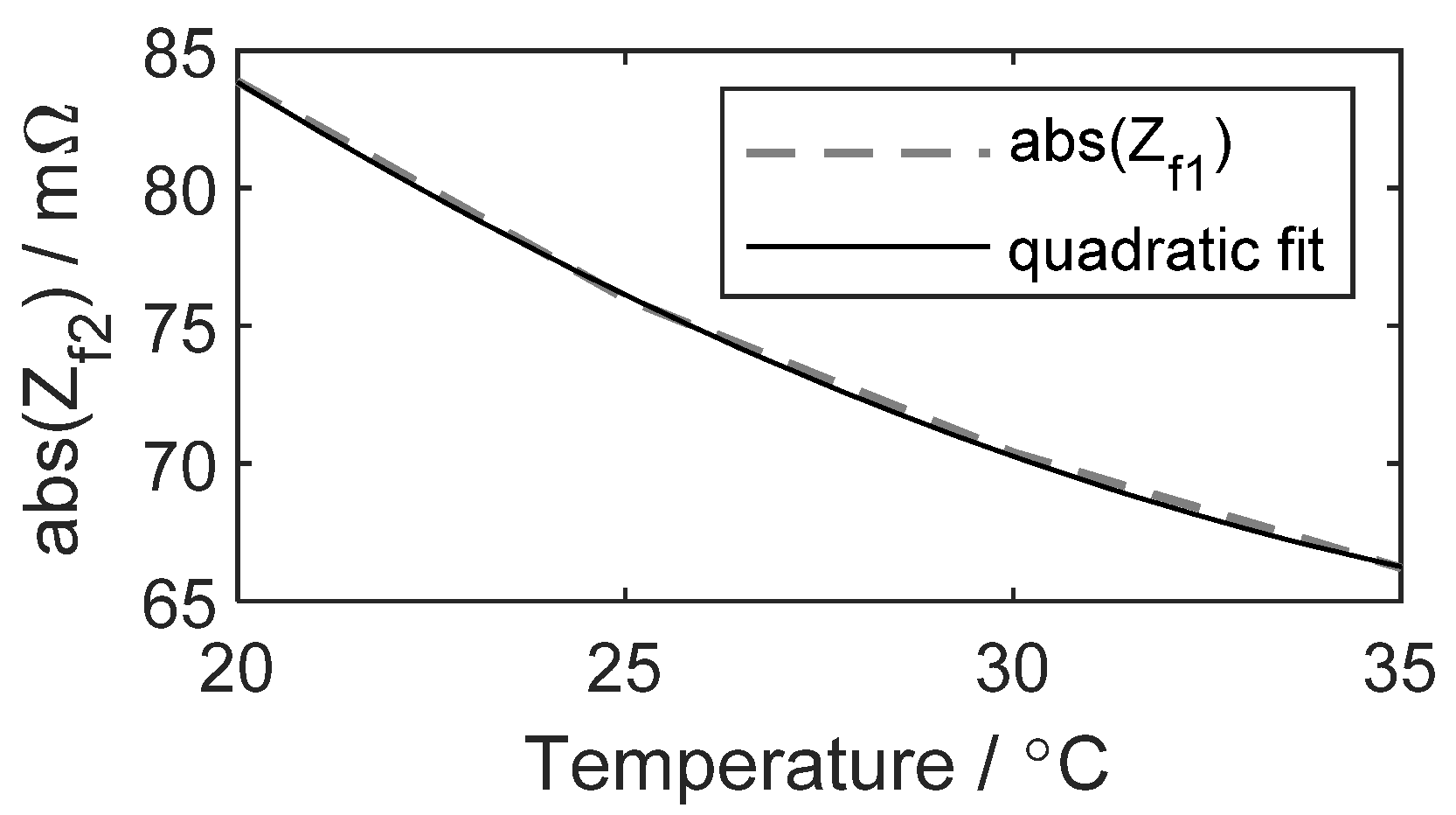
3.2.1. Consideration of Cell Temperature
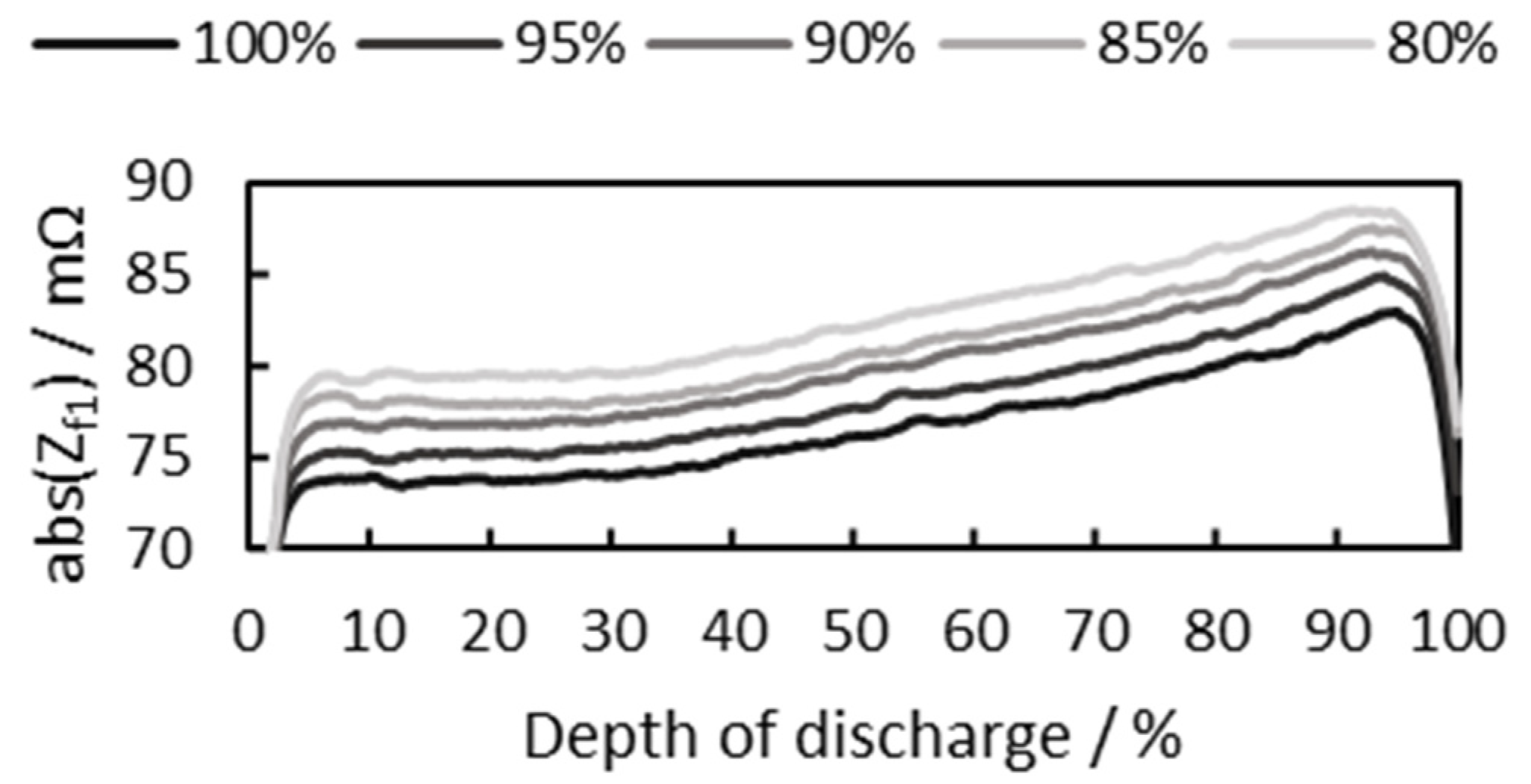
3.2.2. Consideration of Cell SoH
3.2.3. Battery Cell SoC Estimation at Different Initial SoCs
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy; Nature Publishing Group: Berlin, Germany, 2011; pp. 171–179. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Source 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Source 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Biensan, P.; Simon, B.; Peres, J.P.; de Guibert, A.; Broussely, M.; Bodet, J.M.; Perton, F. On safety of lithium-ion cells. J. Power Source 1999, 81, 906–912. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P. A general discussion of Li ion battery safety. Electrochem. Soc. Interface 2012, 21, 37. [Google Scholar]
- Belov, D.; Yang, M. Failure mechanism of Li-ion battery at overcharge conditions. J. Solid State Electrochem. 2008, 12, 885–894. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, F.; Wang, W.; Zhao, Y.; Liang, Z.; Yan, D. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Tobishima, S.; Yamaki, J. Aconsideration of lithium cell safety. J. Power Source 1999, 81, 882–886. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. Abuse testing of lithium-ion batteries: Characterization of the overcharge reaction of LiCoO2/graphite cells. J. Electrochem. Soc. 2001, 148, A838. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; di Michiel, M.; Hinds, G.; Brett, D.J.L.; Shearing, P.R. Investigating lithium-ion battery materials during overcharge-induced thermal runaway: An operando and multi-scale X-ray CT study. Phys. Chem. Chem. Phys. 2016, 18, 30912–30919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, J.; Zhou, T.; Yang, K.; Wei, S.; Tang, N.; Dang, N.; Li, H.; Qiu, X.; Chen, L. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by external heating, overcharge and short circuiting of commercial lithium-ion battery cells. J. Electrochem. Soc. 2014, 161, A1611. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Source 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Zheng, Y.; Qian, K.; Luo, D.; Li, Y.; Lu, Q.; Li, B.; He, Y.-B.; Wang, X.; Li, J.; Kang, F. Influence of over-discharge on the lifetime and performance of LiFePO4/graphite batteries. RSC Adv. 2016, 6, 30474–30483. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Cheng, X.; Du, C.; Guan, T.; Cui, Y.; Sun, S.; Zuo, P.; Gao, Y.; Yin, G. Capacity fading mechanism during long-term cycling of over-discharged LiCoO2/mesocarbon microbeads battery. J. Power Source 2015, 293, 1006–1015. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Effects of overdischarge on performance and thermal stability of a Li-ion cell. J. Power Source 2006, 160, 1395–1402. [Google Scholar] [CrossRef]
- Li, H.-F.; Gao, J.-K.; Zhang, S.-L. Effect of overdischarge on swelling and recharge performance of lithium ion cells. Chin. J. Chem. 2008, 26, 1585–1588. [Google Scholar] [CrossRef]
- Piller, S.; Perrin, M.; Jossen, A. Methods for state-of-charge determination and their applications. J. Power Source 2001, 96, 113–120. [Google Scholar] [CrossRef]
- Pop, V.; Bergveld, H.J.; Danilov, D.; Regtien, P.P.L.; Notten, P.H.L. State-of-the-art of battery state-of-charge determination. In Battery Management Systems: Accurate State-of-Charge Indication for Battery-Powered Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 11–45. [Google Scholar] [CrossRef]
- Rodrigues, S.; Munichandraiah, N.; Shukla, A.K. A review of state-of-charge indication of batteries by means of ac impedance measurements. J. Power Source 2000, 87, 12–20. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs. J. Power Source 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Kim, I.-S. The novel state of charge estimation method for lithium battery using sliding mode observer. J. Power Source 2006, 163, 584–590. [Google Scholar] [CrossRef]
- Kim, I.-S. Nonlinear State of Charge Estimator for Hybrid Electric Vehicle Battery. IEEE Trans. Power Electron. 2008, 23, 2027–2034. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Barsoukov, E., Macdonald, J.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-07408-3. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 383–389. [Google Scholar]
- Tröltzsch, U.; Kanoun, O.; Tränkler, H.-R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim. Acta 2006, 51, 1664–1672. [Google Scholar] [CrossRef]
- Buller, S.; Thele, M.; Karden, E.; de Doncker, R.W. Impedance-based non-linear dynamic battery modeling for automotive applications. J. Power Source 2003, 113, 422–430. [Google Scholar] [CrossRef]
- Buller, S. Impedance-Based Simulation Models for Energy Storage Devices in Advanced Automotive Power Systems; Shaker Verlag GmbH: Düren, Germany, 2003; ISBN 3832212256. [Google Scholar]
- Huet, F. A review of impedance measurements for determination of the state-of-charge or state-of-health of secondary batteries. J. Power Source 1998, 70, 59–69. [Google Scholar] [CrossRef]
- Fleischer, C.; Waag, W.; Heyn, H.M.; Sauer, D.U. On-line adaptive battery impedance parameter and state estimation considering physical principles in reduced order equivalent circuit battery models part 2. Parameter and state estimation. J. Power Source 2014, 262, 457–482. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Source 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Abraham, D.P.; Knuth, J.L.; Dees, D.W.; Bloom, I.; Christophersen, J.P. Performance degradation of high-power lithium-ion cells—Electrochemistry of harvested electrodes. J. Power Source 2007, 170, 465–475. [Google Scholar] [CrossRef]
- Thomas, E.V.; Bloom, I.; Christophersen, J.P.; Battaglia, V.S. Statistical methodology for predicting the life of lithium-ion cells via accelerated degradation testing. J. Power Source 2008, 184, 312–317. [Google Scholar] [CrossRef]
- Belt, J.R.; Ho, C.D.; Miller, T.J.; Habib, M.A.; Duong, T.Q. The effect of temperature on capacity and power in cycled lithium ion batteries. J. Power Source 2005, 142, 354–360. [Google Scholar] [CrossRef]
- Qahouq, J.A.A. Online battery impedance spectrum measurement method. In Proceedings of the 2016 IEEE Applied Power Electronics Conference and Exposition (APEC), Long Beach, CA, USA, 20–24 March 2016; pp. 3611–3615, ISBN 1467395501. [Google Scholar]
- Waag, W.; Fleischer, C.; Sauer, D.U. On-line estimation of lithium-ion battery impedance parameters using a novel varied-parameters approach. J. Power Source 2013, 237, 260–269. [Google Scholar] [CrossRef]
- Do, D.V.; Forgez, C.; Benkara, K.E.K.; Friedrich, G. Impedance observer for a Li-ion battery using Kalman filter. IEEE Trans. Veh. Technol. 2009, 58, 3930–3937. [Google Scholar]
- Huang, W.; Qahouq, J.A.A. An online battery impedance measurement method using DC–DC power converter control. IEEE Trans. Ind. Electron. 2014, 61, 5987–5995. [Google Scholar] [CrossRef]
- Howey, D.A.; Mitcheson, P.D.; Yufit, V.; Offer, G.J.; Brandon, N.P. Online measurement of battery impedance using motor controller excitation. IEEE Trans. Veh. Technol. 2013, 63, 2557–2566. [Google Scholar] [CrossRef]
- Ojarand, J.; Min, M.; Annus, P. Crest factor optimization of the multisine waveform for bioimpedance spectroscopy. Physiol. Meas. 2014, 35, 1019. [Google Scholar] [CrossRef]
- Sanchez, B.; Vandersteen, G.; Bragos, R.; Schoukens, J. Optimal multisine excitation design for broadband electrical impedance spectroscopy. Meas. Sci. Technol. 2011, 22, 115601. [Google Scholar] [CrossRef]
- Breugelmans, T.; Tourwé, E.; van Ingelgem, Y.; Wielant, J.; Hauffman, T.; Hausbrand, R.; Pintelon, R.; Hubin, A. Odd random phase multisine EIS as a detection method for the onset of corrosion of coated steel. Electrochem. Commun. 2010, 12, 2–5. [Google Scholar] [CrossRef]
- Kim, J.; Krüger, L.; Kowal, J. On-line state-of-health estimation of Lithium-ion battery cells using frequency excitation. J. Energy Storage 2020, 32, 101841. [Google Scholar] [CrossRef]
- Barai, A.; Gael, H.C.; Guo, Y.; McGordon, A.; Jennings, P. A study on the impact of lithium-ion cell relaxation on electrochemical impedance spectroscopy. J. Power Source 2015, 280, 74–80. [Google Scholar] [CrossRef]
- Frank, M.K.; Noel, A.; Simon, V.E.; Jossen, A. Long-term equalization effects in Li-ion batteries due to local state of charge inhomogeneities and their impact on impedance measurements. Electrochim. Acta 2015, 185, 107–116. [Google Scholar] [CrossRef]
- Wood, E.; Alexander, M.; Thomas, H.B. Investigation of battery end-of-life conditions for plug-in hybrid electric vehicles. J. Power Source 2011, 196, 5147–5154. [Google Scholar] [CrossRef]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; de Aníbal, T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Source 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. A comparative study of commercial lithium ion battery cycle life in electric vehicle: Capacity loss estimation. J. Power Source 2014, 268, 658–669. [Google Scholar] [CrossRef]
- Stoynov, Z.B.; Vladjkova, D.E. Measurement Methods | Electrochemical: Impedance spectroscopy. In Encyclopedia of Electrochemical Power Source; Elsevier: Amsterdam, The Netherlands, 2009; pp. 632–642. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Zhu, J.; Dai, H.; Zheng, Y.; Xu, X.; Chen, Q. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. eTransportation 2021, 7, 100093. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, X.; Lou, G.; Wen, Z.; Liu, L. Parameter sensitivity analysis and cathode structure optimization of a non-aqueous Li–O2 battery model. J. Power Source 2020, 451, 227821. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M. Modeling of SEI Layer Growth and Electrochemical impedance spectroscopy response using a thermal-electrochemical model of li-ion batteries. ECS Trans. 2014, 61, 43–61. [Google Scholar] [CrossRef]
- Liu, L.; Guan, P. Phase-field modeling of solid electrolyte interphase (SEI) evolution: Considering cracking and dissolution during battery cycling. ECS Trans. 2019, 89, 101–111. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Zhang, M.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4–NMC/graphite lithium ion batteries part II: Calendar aging. J. Power Source 2014, 258, 61–75. [Google Scholar] [CrossRef]
- Kim, J.-H.; Woo, S.C.; Park, M.-S.; Kim, K.J.; Yim, T.; Kim, J.-S.; Kim, Y.-J. Capacity fading mechanism of LiFePO4-based lithium secondary batteries for stationary energy storage. J. Power Source 2013, 229, 190–197. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Fundamentals of Electrochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 9780471741985. [Google Scholar]
- Liu, S.; Liu, X.; Dou, R.; Zhou, W.; Wen, Z.; Liu, L. Experimental and simulation study on thermal characteristics of 18,650 lithium–iron–phosphate battery with and without spot–welding tabs. Appl. Therm. Eng. 2020, 166, 114648. [Google Scholar] [CrossRef]


| Item | Description |
|---|---|
| Anode | Based on intercalation graphite |
| Cathode | Based on lithiated metal oxide 1 |
| Product name | Samsung ICR 18650-26F |
| Battery system | Li-ion |
| Nominal voltage | 3.7 V |
| Rated capacity | 2.6 Ah |
| Parameter | Description |
|---|---|
| Chamber temperature | 25 °C |
| Depth of discharge | From 0 to 100% |
| DC bias | 2600 mA (1C) |
| 1 Hz, 1 kHz | |
| 65 mA (each) |
| Parameter | Description |
|---|---|
| Cell temperature | 20, 25, 30, 35 °C |
| State of health | 80% |
| State of charge | 50% |
| DC bias | 0 mA |
| Frequency range | 0.2 to 2 kHz |
| AC amplitude | 100 mA |
| SoH | RMSE | |
|---|---|---|
| 100% | 0.99 | 2.42 |
| 95% | 0.99 | 2.66 |
| 90% | 0.99 | 2.02 |
| 85% | 0.99 | 2.51 |
| 80% | 0.98 | 3.85 |
| Average | 0.99 | 2.69 |
| Parameter | Description |
|---|---|
| State of health | 80% |
| Chamber temperature | 25 °C |
| Discharge time | 10 min (each) |
| Relaxation time | 60 min (each) |
| Depth of discharge | From 0 to 100% |
| DC bias | 2600 mA (1C) |
| 1, 1000 Hz | |
| 65 mA (each) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kowal, J. A Method for Monitoring State-of-Charge of Lithium-Ion Cells Using Multi-Sine Signal Excitation. Batteries 2021, 7, 76. https://doi.org/10.3390/batteries7040076
Kim J, Kowal J. A Method for Monitoring State-of-Charge of Lithium-Ion Cells Using Multi-Sine Signal Excitation. Batteries. 2021; 7(4):76. https://doi.org/10.3390/batteries7040076
Chicago/Turabian StyleKim, Jonghyeon, and Julia Kowal. 2021. "A Method for Monitoring State-of-Charge of Lithium-Ion Cells Using Multi-Sine Signal Excitation" Batteries 7, no. 4: 76. https://doi.org/10.3390/batteries7040076
APA StyleKim, J., & Kowal, J. (2021). A Method for Monitoring State-of-Charge of Lithium-Ion Cells Using Multi-Sine Signal Excitation. Batteries, 7(4), 76. https://doi.org/10.3390/batteries7040076







