Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes
Abstract
:1. Introduction
1.1. Motivation
1.2. Relevant Literature
1.3. Structure and Technical Contribution
2. Materials and Methods
2.1. Material and Cell Preparation
2.2. Cell Degradation Experiments
3. Results
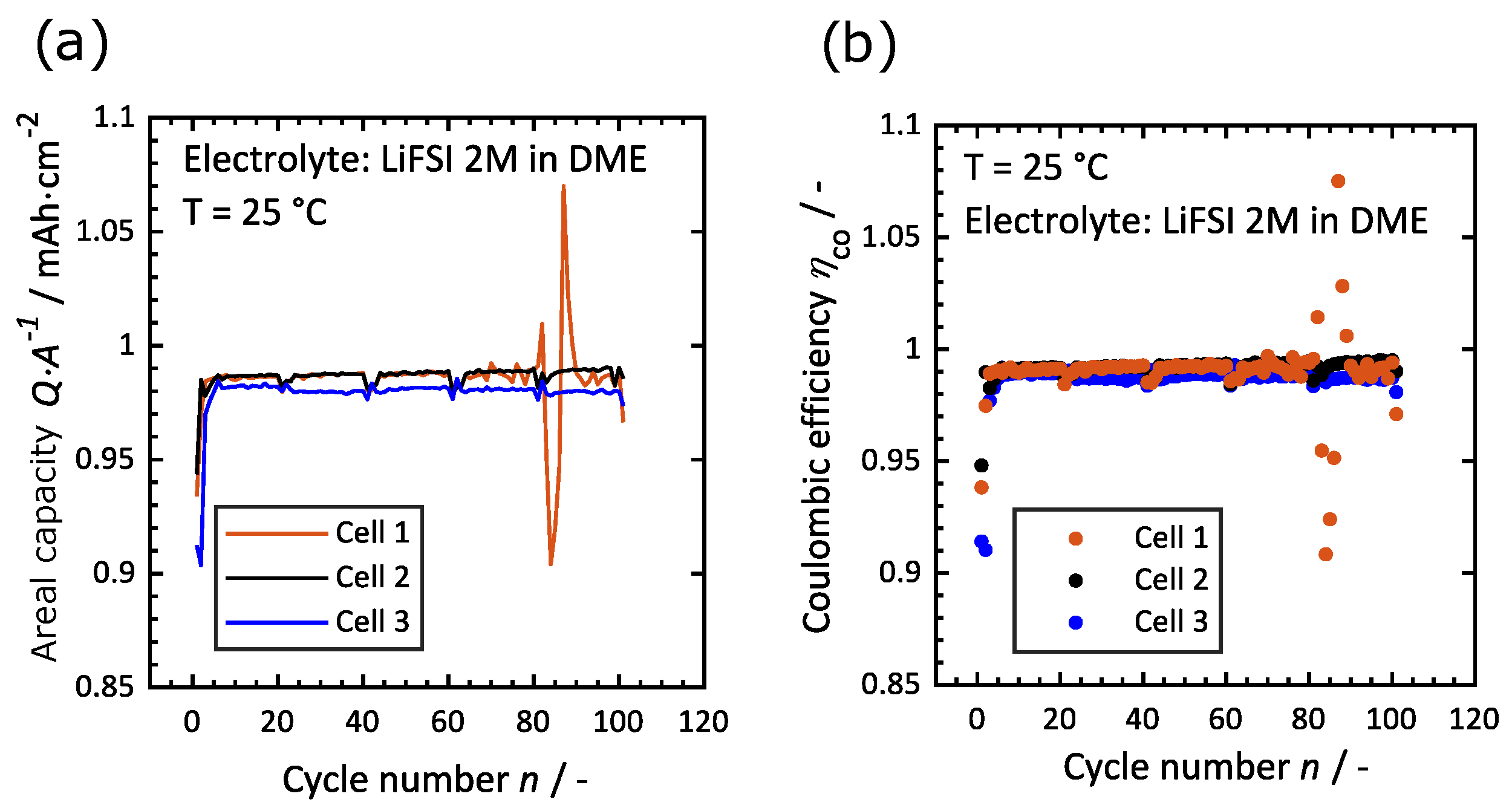
3.1. Reproducibility of Measurements
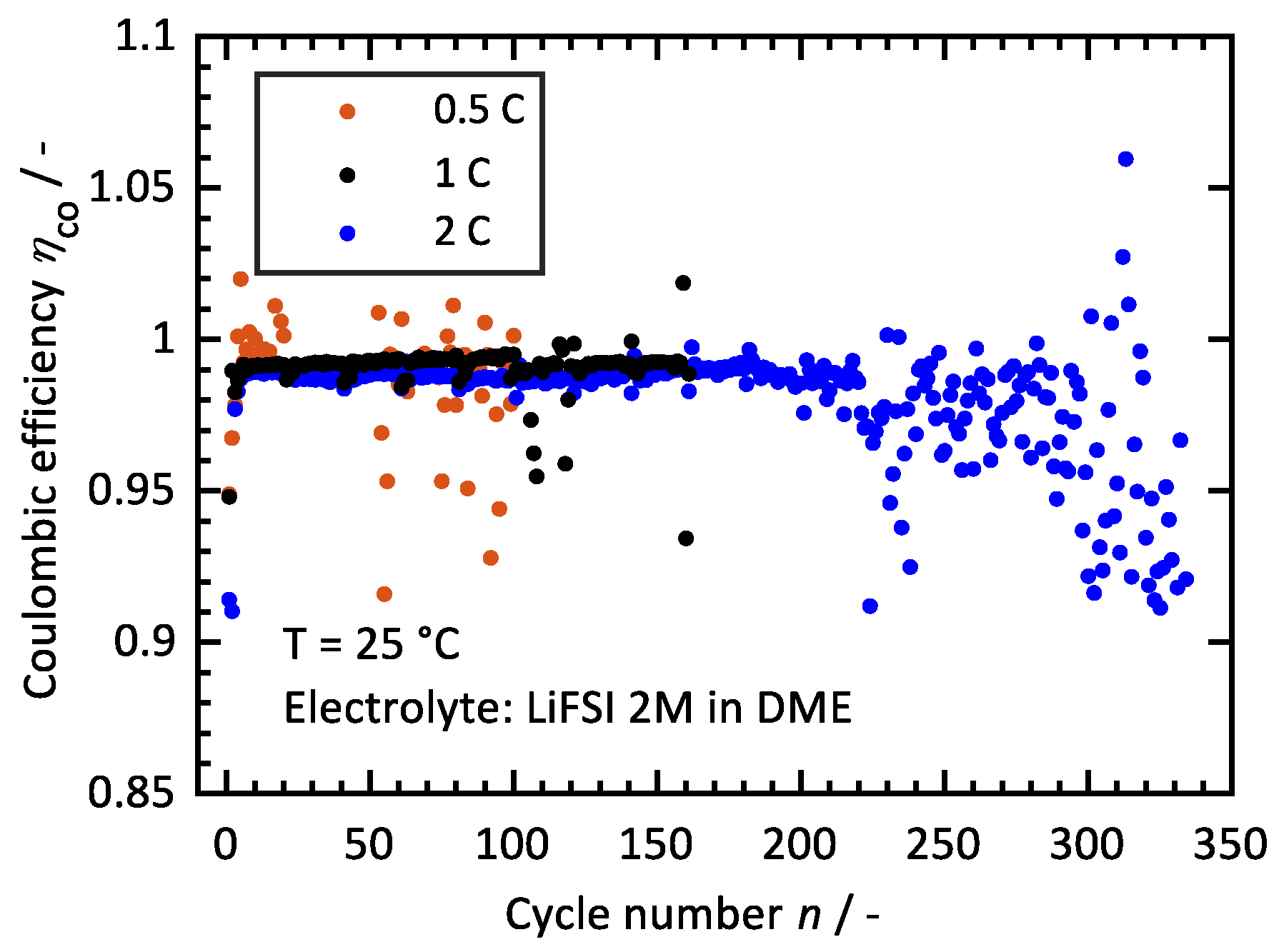
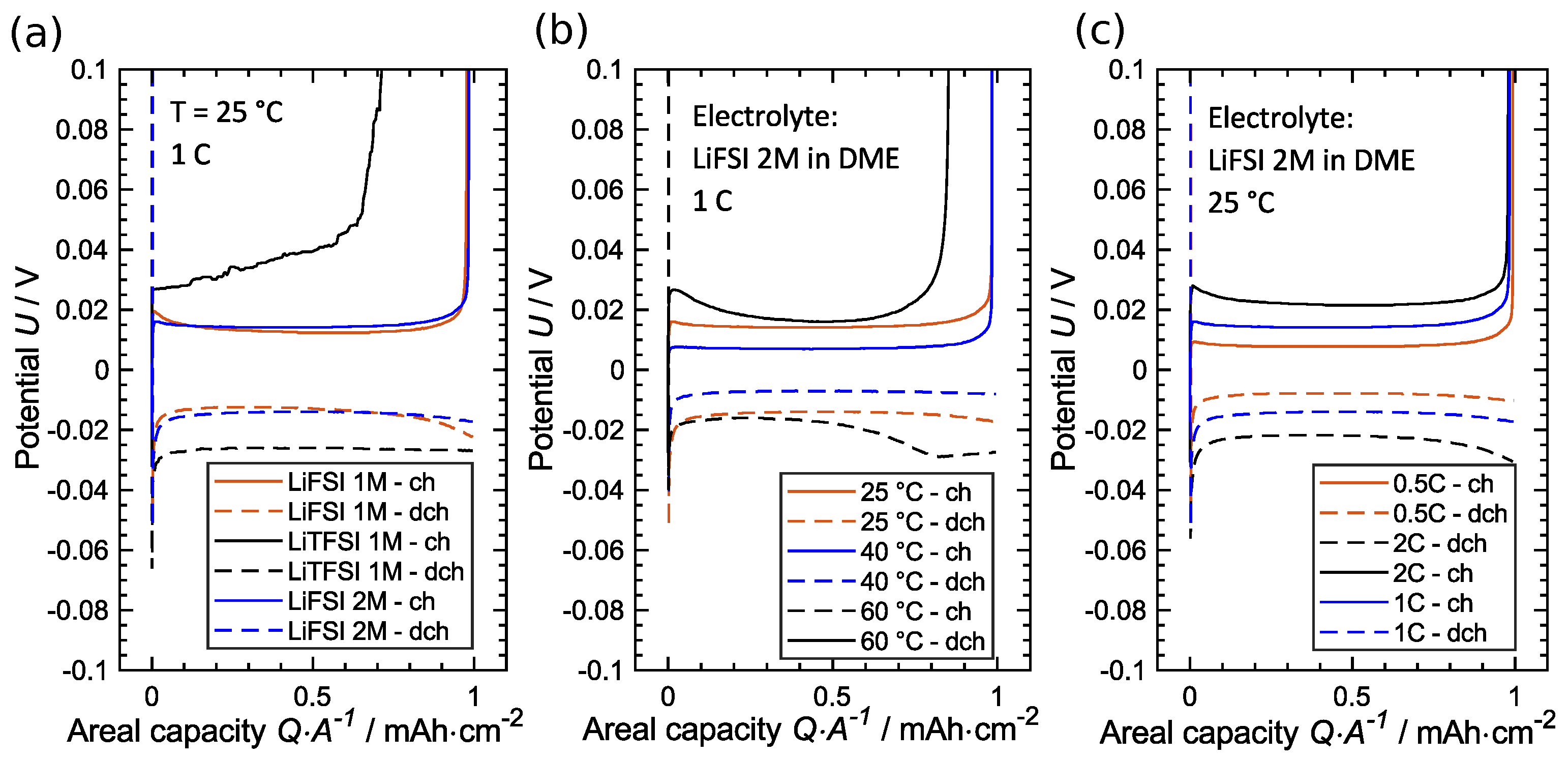
3.2. Influence of C-Rate
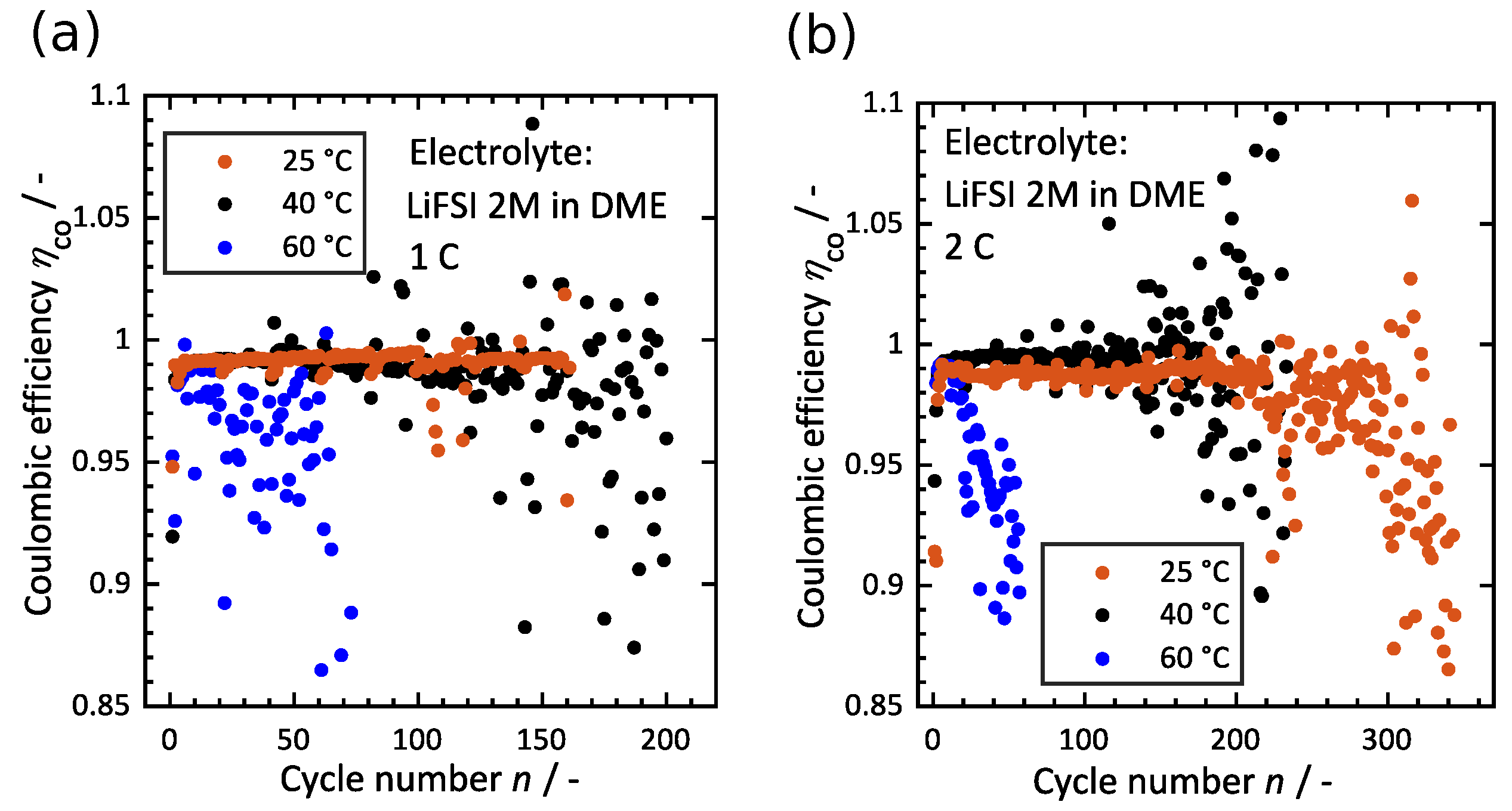
3.3. Influence of Temperature
3.4. Influence of Salt, Concentration and Temperature
4. Discussion
5. Future Work
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Effective surface area |
| c | Molar concentration |
| water concentration | |
| oxygen concentration | |
| CC | Current Collector |
| CE | Coulombic efficiency |
| Cu | Copper |
| Diameter of the current collector | |
| Diameter of the Lithium foil | |
| DME | 1,2-dimethoxyethane |
| DOE | US Department of Energy |
| e | electron |
| EIS | Electrochemical Impedance Spectroscopy |
| Maximum frequency of EIS | |
| Minimum frequency of EIS | |
| Hydrogen | |
| Water | |
| Thickness of the Copper foil | |
| Thickness of the Lithium foil | |
| Cell current | |
| j | Current density |
| Li | Lithium |
| Lithium Ion | |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LiTFSI | Lithium bis(trifluoromethanesul-fonyl)imide |
| n | Number of cycles |
| Maximum number of cycles | |
| Oxygen | |
| OCP | Open Circuit Potential |
| Charge transfer resistance | |
| Ohmic resistance | |
| SEI | Solid Electrolyte Interface |
| SPE | Solid Polymer Electrolyte |
| t | Time |
| cell temperature | |
| Time of drying | |
| Temperature of drying | |
| Lower voltage limit | |
| Upper voltage limit | |
| Amount of electrolyte | |
| Purity of LiTFSI and LiFSI | |
| XRD | X-ray diffraction |
| Imaginary part of the impedance | |
| Real part of the impedance | |
| Coulombic efficiency | |
| Average Coulombic efficiency | |
| overpotential of Li deposition nucleation | |
| overpotential of particle growth | |
| Potential of Li versus | |
| Standard deviation of the Coulombic efficiency | |
| Standard deviation of the areal capacity |
References
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- USABC Goals for Advanced Batteries for EVs—CY 2023 Commercialization (USABC, 2017). 2018.
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 2018, 3, 16–21. [Google Scholar] [CrossRef]
- Brandt, K. Historical development of secondary lithium batteries. Solid State Ionics 1994, 69, 173–183. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.D.; Deng, Z.Q.; Chen, M.C. Interaction of nitrogen with lithium in lithium ion batteries. Solid State Ionics 2009, 180, 212–215. [Google Scholar] [CrossRef]
- Furukawa, T.; Hirakawa, Y.; Kondo, H.; Kanemura, T.; Wakai, E. Chemical reaction of lithium with room temperature atmosphere of various humidities. Fusion Eng. Des. 2015, 98, 2138–2141. [Google Scholar] [CrossRef] [Green Version]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—The solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Sergi, F.; Arista, A.; Agnello, G.; Ferraro, M.; Andaloro, L.; Antonucci, V. Characterization and comparison between lithium iron phosphate and lithium-polymers batteries. J. Energy Storage 2016, 8, 235–243. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Nagpure, S.C.; Tanim, T.R.; Dufek, E.J.; Viswanathan, V.V.; Crawford, A.J.; Wood, S.M.; Xiao, J.; Dickerson, C.C.; Liaw, B. Impacts of lean electrolyte on cycle life for rechargeable Li metal batteries. J. Power Sources 2018, 407, 53–62. [Google Scholar] [CrossRef]
- Christensen, J.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2011, 159, R1. [Google Scholar] [CrossRef]
- Yang, T.; Liu, J.; Dai, J.; Han, Y. Shaping particles by chemical diffusion and reaction. CrystEngComm 2017, 19, 72–79. [Google Scholar] [CrossRef]
- Han, Y.; Jie, Y.; Huang, F.; Chen, Y.; Lei, Z.; Zhang, G.; Ren, X.; Qin, L.; Cao, R.; Jiao, S. Enabling stable lithium metal anode through electrochemical kinetics manipulation. Adv. Funct. Mater. 2019, 29, 1904629. [Google Scholar] [CrossRef]
- Li, L.; Basu, S.; Wang, Y.; Chen, Z.; Hundekar, P.; Wang, B.; Shi, J.; Shi, Y.; Narayanan, S.; Koratkar, N. Self-heating–induced healing of lithium dendrites. Science 2018, 359, 1513–1516. [Google Scholar] [CrossRef] [Green Version]
- Aryanfar, A.; Brooks, D.J.; Colussi, A.J.; Merinov, B.V.; Goddard, W.A., III; Hoffmann, M.R. Thermal relaxation of lithium dendrites. Phys. Chem. Chem. Phys. 2015, 17, 8000–8005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Xie, J.; Pei, A.; Liu, B.; Wu, Y.; Lin, D.; Li, J.; Wang, H.; Chen, H.; Xu, J.; et al. Fast lithium growth and short circuit induced by localized-temperature hotspots in lithium batteries. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Adair, K.R.; Banis, M.N.; Zhao, Y.; Bond, T.; Li, R.; Sun, X. Temperature-Dependent Chemical and Physical Microstructure of Li Metal Anodes Revealed through Synchrotron-Based Imaging Techniques. Adv. Mater. 2020, 32, 2002550. [Google Scholar] [CrossRef]
- Geng, Z.; Lu, J.; Li, Q.; Qiu, J.; Wang, Y.; Peng, J.; Huang, J.; Li, W.; Yu, X.; Li, H. Lithium metal batteries capable of stable operation at elevated temperature. Energy Storage Mater. 2019, 23, 646–652. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A review of solid electrolyte interphases on lithium metal anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Andersson, A.; Edström, K. Chemical composition and morphology of the elevated temperature SEI on graphite. J. Electrochem. Soc. 2001, 148, A1100. [Google Scholar] [CrossRef]
- Aurbach, D.; Weissman, I.; Zaban, A.; Chusid, O. Correlation between surface chemistry, morphology, cycling efficiency and interfacial properties of Li electrodes in solutions containing different Li salts. Electrochim. Acta 1994, 39, 51–71. [Google Scholar] [CrossRef]
- Kim, H.; Wu, F.; Lee, J.T.; Nitta, N.; Lin, H.T.; Oschatz, M.; Cho, W.I.; Kaskel, S.; Borodin, O.; Yushin, G. In situ formation of protective coatings on sulfur cathodes in lithium batteries with LiFSI-based organic electrolytes. Adv. Energy Mater. 2015, 5, 1401792. [Google Scholar] [CrossRef]
- Dornbusch, D.A.; Hilton, R.; Lohman, S.D.; Suppes, G.J. Experimental validation of the elimination of dendrite short-circuit failure in secondary lithium-metal convection cell batteries. J. Electrochem. Soc. 2014, 162, A262. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Yu, P.; Ding, F.; Li, X.; Wang, Z.; Lv, Z.; Wang, X.; Liu, Z.; Huang, X. Ion association tailoring SEI composition for Li metal anode protection. J. Energy Chem. 2020, 45, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jurng, S.; Brown, Z.L.; Kim, J.; Lucht, B.L. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ. Sci. 2018, 11, 2600–2608. [Google Scholar] [CrossRef]
- Bärmann, P.; Mohrhardt, M.; Frerichs, J.E.; Helling, M.; Kolesnikov, A.; Klabunde, S.; Nowak, S.; Hansen, M.R.; Winter, M.; Placke, T. Mechanistic Insights into the Pre-Lithiation of Silicon/Graphite Negative Electrodes in “Dry State” and After Electrolyte Addition Using Passivated Lithium Metal Powder. Adv. Energy Mater. 2021, 11, 2100925. [Google Scholar] [CrossRef]
- Blanchard, D.; Slagter, M. In operando Raman and optical study of lithium polysulphides dissolution in Lithium-Sulfur Cells with carrageenan binder. J. Phys. Energy 2021, 3, 044003. [Google Scholar] [CrossRef]
- Chladil, L.; Kunický, D.; Vanýsek, P.; Čech, O. In-Situ X-Ray Study of Carbon Coated LiFePO4 for Li-Ion Battery in Different State of Charge. ECS Trans. 2018, 87, 107–114. [Google Scholar] [CrossRef]
- Merryweather, A.J.; Schnedermann, C.; Jacquet, Q.; Grey, C.P.; Rao, A. Operando optical tracking of single-particle ion dynamics in batteries. Nature 2021, 594, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, F.; Modrzynski, C.; Roscher, V.; Danilov, D.L.; Notten, P.H.L.; Riemschneider, K.-R. Investigation of charge carrier dynamics in positive lithium-ion battery electrodes via optical in situ observation. J. Power Sources 2021, 482, 228943. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Diemant, T.; Lin, X.-M.; Cambaz, M.A.; Golla-Schindler, U.; Kaiser, U.; Behm, R.J.; Fichtner, M. Nitrogen Rich Hierarchically Organized Porous Carbon/Sulfur Composite Cathode Electrode for High Performance Li/S Battery: A Mechanistic Investigation by Operando Spectroscopic Studies. Adv. Mater. Interfaces 2016, 3, 1600372. [Google Scholar] [CrossRef] [Green Version]
- Vinayan, B.P.; Euchner, H.; Zhao-Karger, Z.; Cambaz, M.A.; Li, Z.; Diemant, T.; Behm, R.J.; Gross, A.; Fichtner, M. Insights into the electrochemical processes of rechargeable magnesium–sulfur batteries with a new cathode design. Adv. Mater. Chem. A 2019, 7, 25490–25502. [Google Scholar] [CrossRef]
- Zou, J.; Sole, C.; Drewett, N.E.; Velický, M.; Hardwick, L.J. In Situ Study of Li Intercalation into Highly Crystalline Graphitic Flakes of Varying Thicknesses. J. Phys. Chem. Lett. 2016, 7, 4291–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]

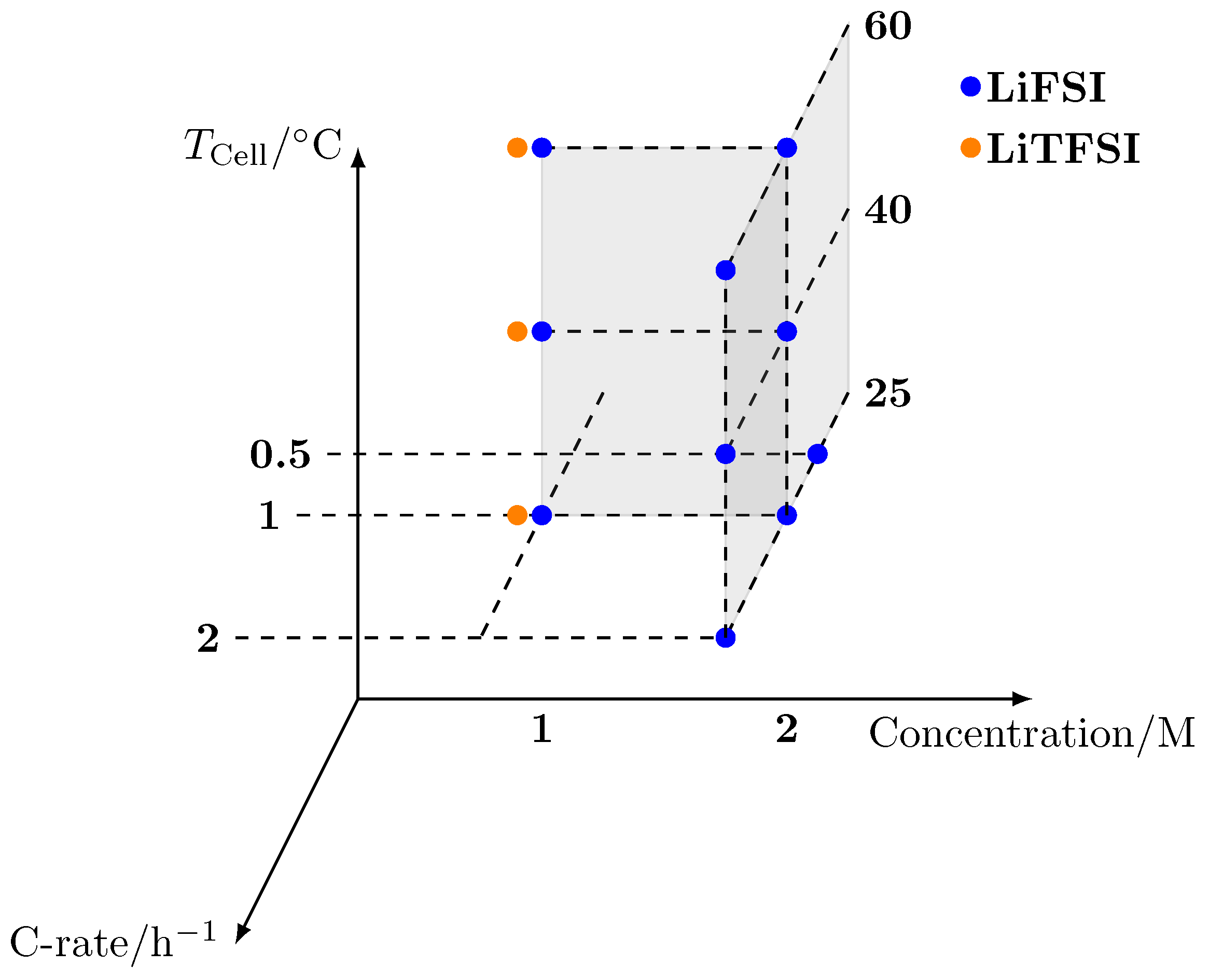


| LiFSI | LiTFSI | ||||
|---|---|---|---|---|---|
| 2 C | 1 C | 0.5 C | 1 C | ||
| 2 M | 25 C | #1 | #3 | #3 | |
| 40 C | #1 | #2 | |||
| 60 C | #1 | #2 | |||
| 1 M | 25 C | - | #1 | - | #1 |
| 40 C | - | #2 | - | #1 | |
| 60 C | - | #2 | - | #2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroujeni, S.M.; Fill, A.; Ridder, A.; Birke, K.P. Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes. Batteries 2021, 7, 67. https://doi.org/10.3390/batteries7040067
Boroujeni SM, Fill A, Ridder A, Birke KP. Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes. Batteries. 2021; 7(4):67. https://doi.org/10.3390/batteries7040067
Chicago/Turabian StyleBoroujeni, Sanaz Momeni, Alexander Fill, Alexander Ridder, and Kai Peter Birke. 2021. "Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes" Batteries 7, no. 4: 67. https://doi.org/10.3390/batteries7040067
APA StyleBoroujeni, S. M., Fill, A., Ridder, A., & Birke, K. P. (2021). Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes. Batteries, 7(4), 67. https://doi.org/10.3390/batteries7040067








