Directly Anodized Sulfur-Doped TiO2 Nanotubes as Improved Anodes for Li-ion Batteries
Abstract
1. Introduction
2. Results and Discussion
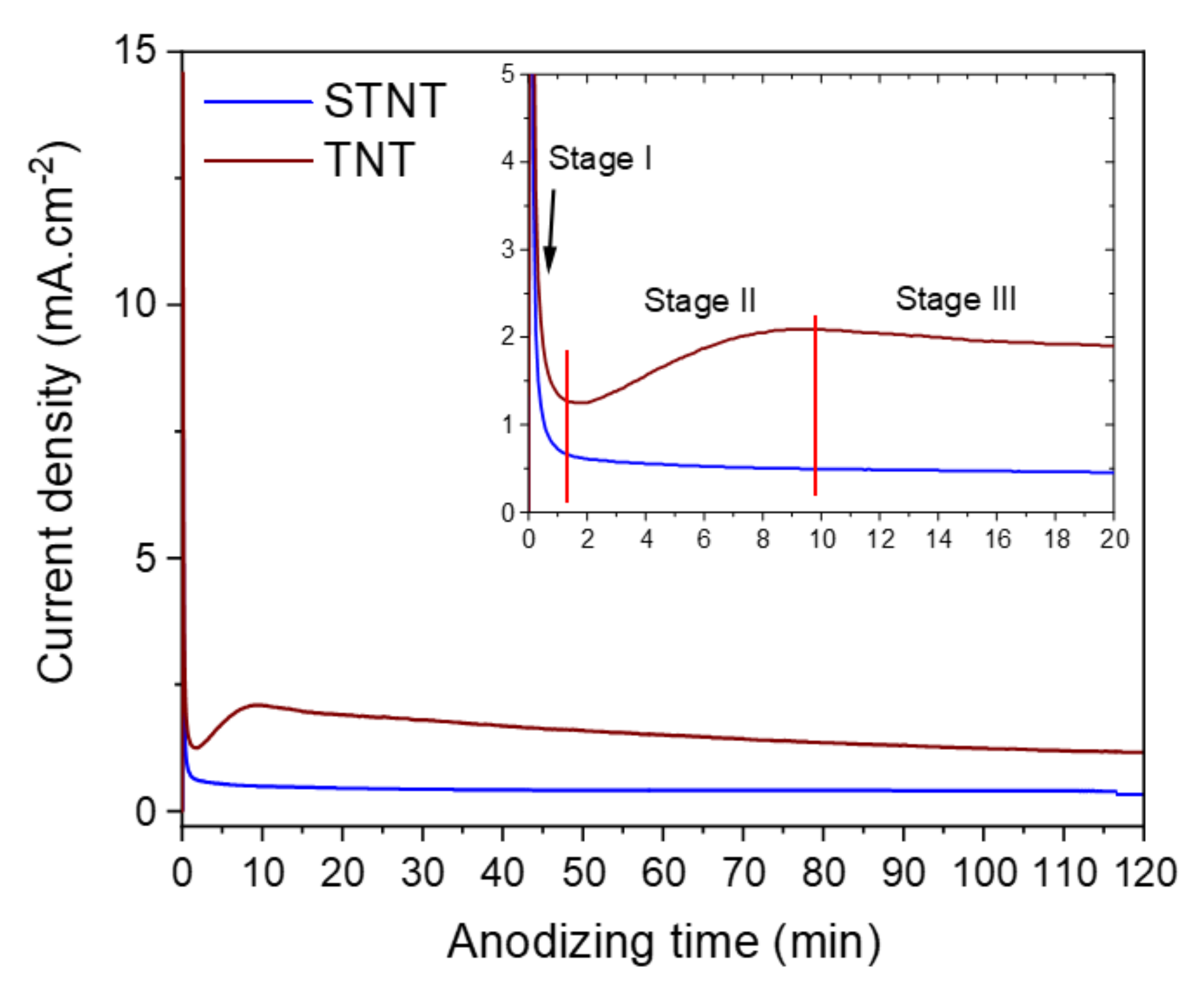
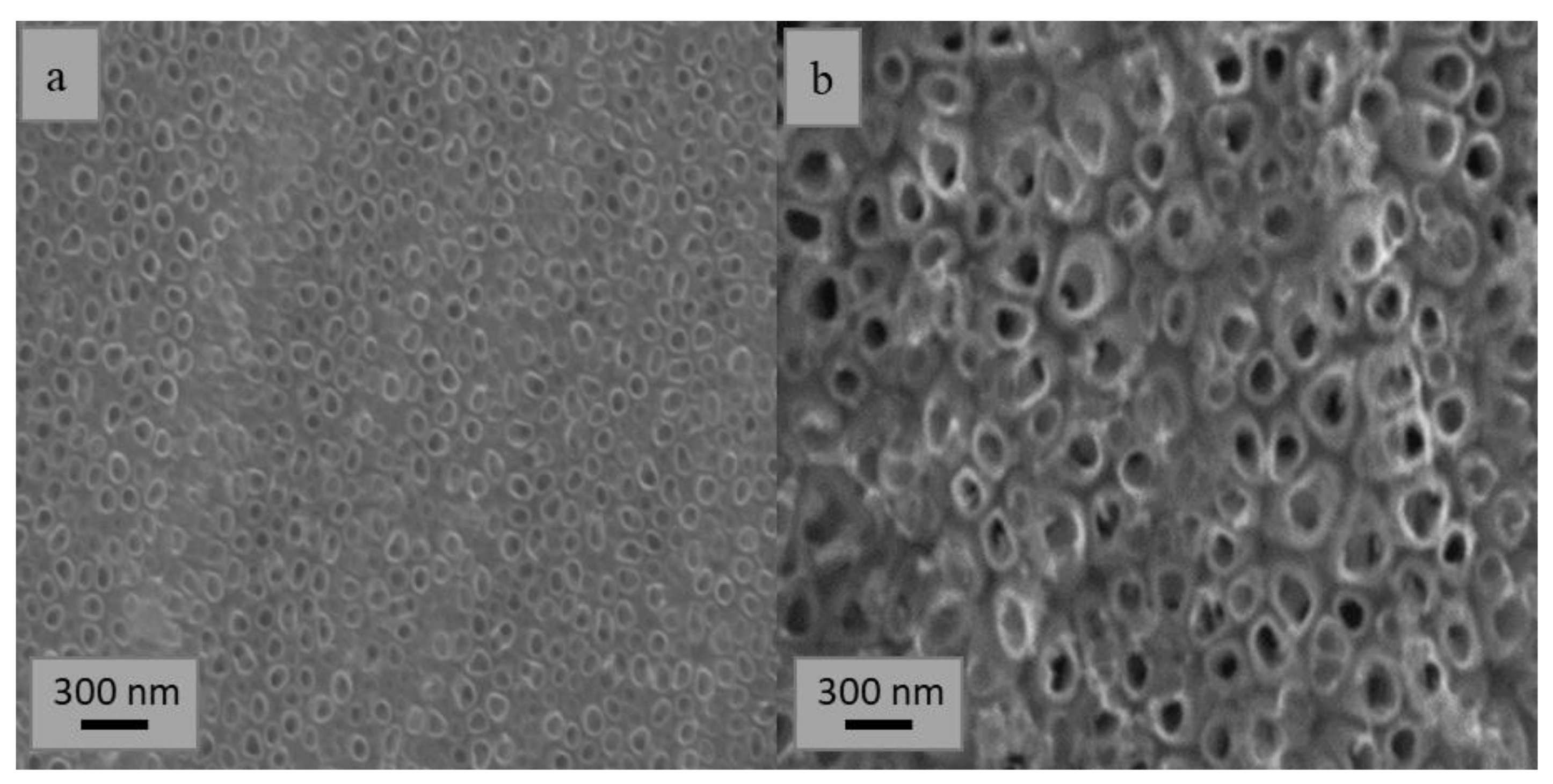
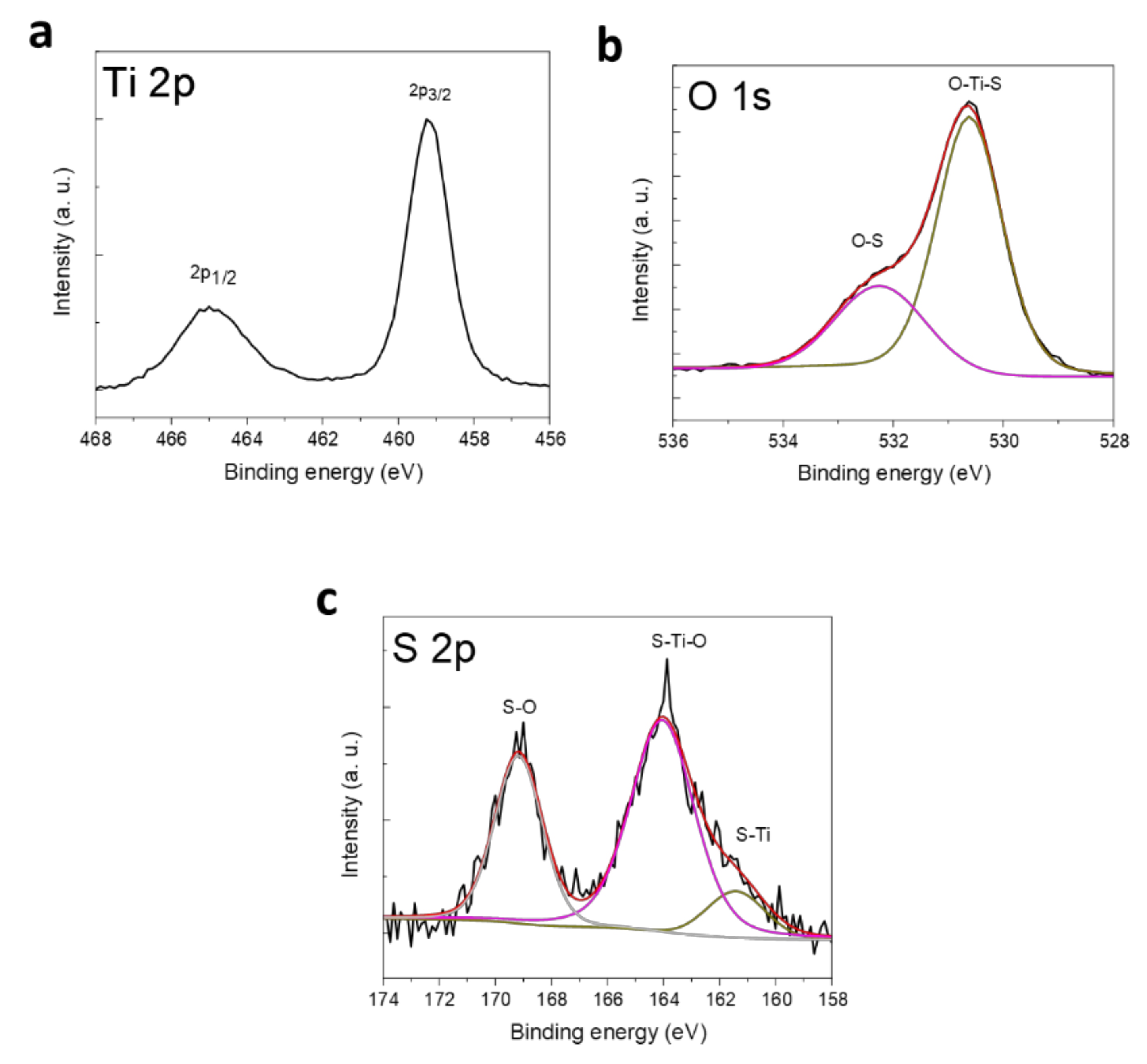
2.1. Preparation and Characterization of TiO2 Nanotubes
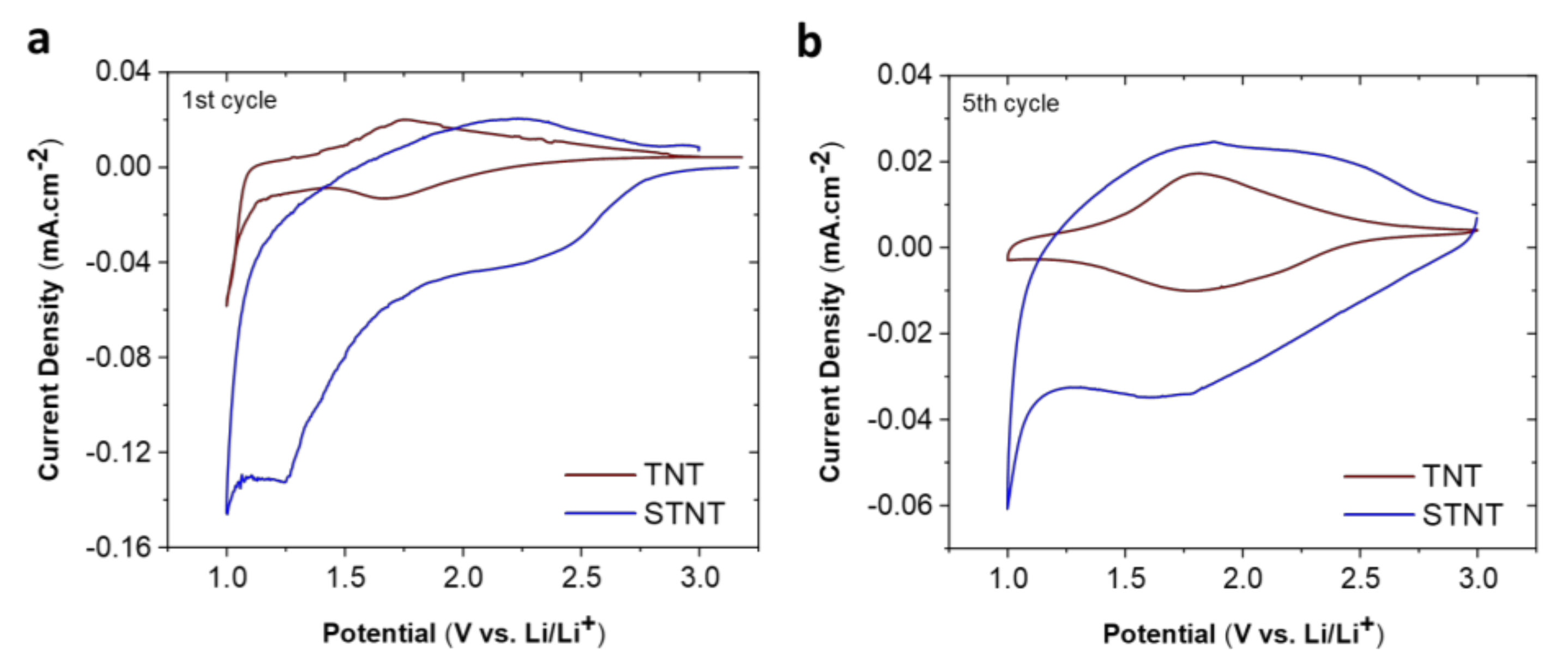
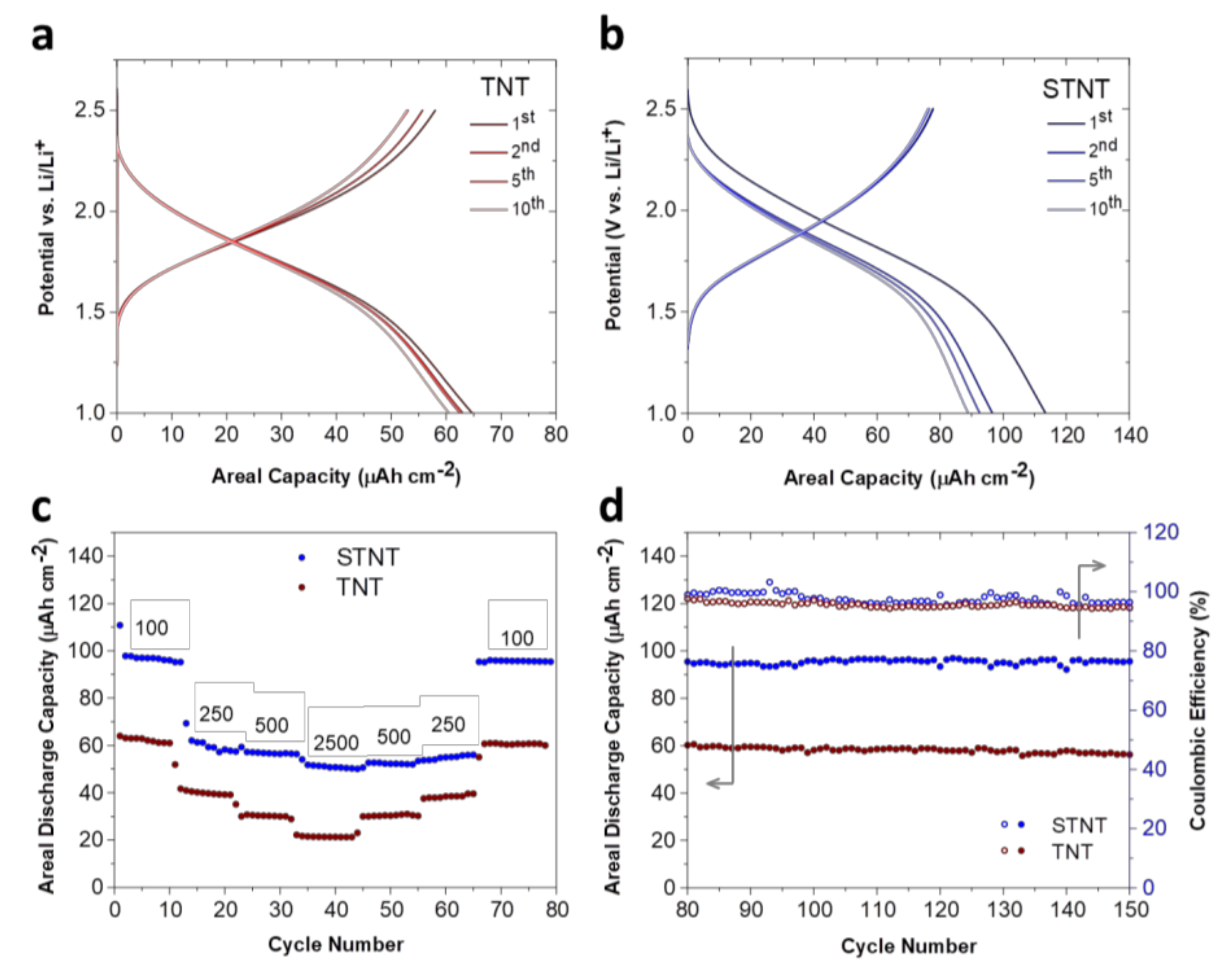
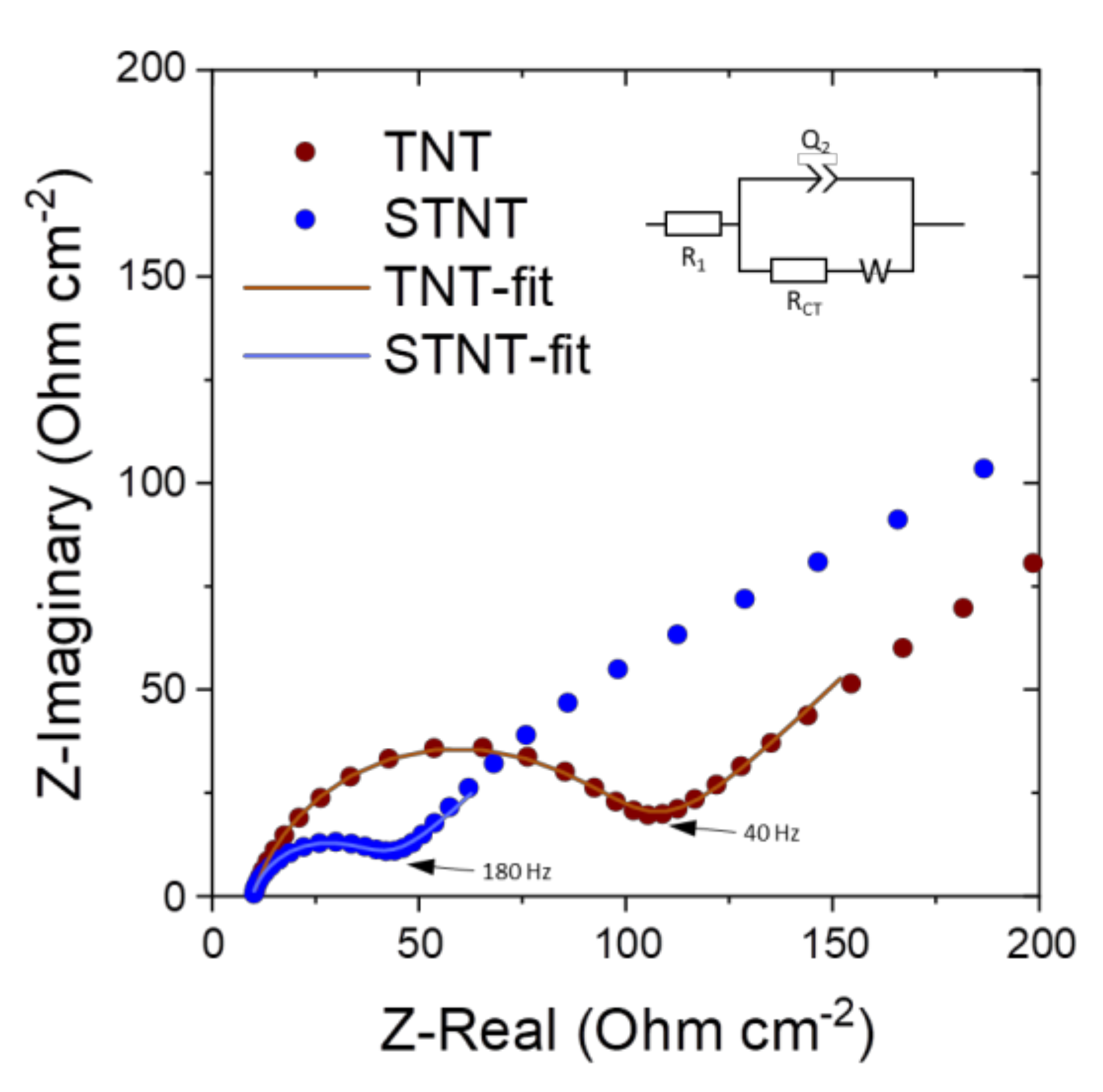
2.2. Electrochemical Properties of TNT and STNT
3. Materials and Methods
3.1. Fabrication of Pure TiO2 Nanotubes and S-Doped TiO2 Nanotubes
3.2. Characterization
3.3. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrog. Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sust. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on challenges and recent advances in the electrochemical performance of high capacity Li-and Mn-rich cathode materials for Li-ion batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Chen, C.; Hu, X.; Wang, Z.; Xiong, X.; Hu, P.; Liu, Y.; Huang, Y. Controllable growth of TiO2-B nanosheet arrays on carbon nanotubes as a high-rate anode material for lithium-ion batteries. Carbon 2014, 69, 302–310. [Google Scholar] [CrossRef]
- Opra, D.P.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Voit, E.I.; Sokolov, A.A.; Ustinov, A.Y.; Zheleznov, V.V. Zr4+/F–co-doped TiO2 (anatase) as high performance anode material for lithium-ion battery. Prog. Nat. Sci. Mater. Int. 2018, 28, 542–547. [Google Scholar] [CrossRef]
- Ming, H.; Li, X.; Su, L.; Liu, M.; Jin, L.; Bu, L.; Kang, Z.; Zheng, J. One step synthesis of C&N co-doped mesoporous TiO2 with enhanced performance in a lithium-ion battery. RSC Adv. 2013, 3, 3836–3839. [Google Scholar]
- Madian, M.; Eychmüller, A.; Giebeler, L. Current advances in TiO2-based nanostructure electrodes for high performance lithium ion batteries. Batteries 2018, 4, 7. [Google Scholar] [CrossRef]
- Madian, M.; Klose, M.; Jaumann, T.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J.; Giebeler, L. Anodically fabricated TiO2–SnO2 nanotubes and their application in lithium ion batteries. J. Mater. Chem. A 2016, 4, 5542–5552. [Google Scholar] [CrossRef]
- Zhu, G.N.; Wang, Y.G.; Xia, Y.Y. Ti-based compounds as anode materials for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6652–6667. [Google Scholar] [CrossRef]
- Feckl, J.M.; Fominykh, K.; Döblinger, M.; Fattakhova-Rohlfing, D.; Bein, T. Nanoscale porous framework of lithium titanate for ultrafast lithium insertion. Angew. Chem. Int. Ed. Engl. 2012, 51, 7459–7463. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Han, L.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Pan, L. TiO2 nanocrystals embedded in sulfur-doped porous carbon as high-performance and long-lasting anode materials for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 24224–24231. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; van Aken, P.A.; Maier, J.; Yu, Y. Self-supported Li4Ti5O12-C nanotube arrays as high-rate and long-life anode materials for flexible Li-ion batteries. Nano Lett. 2014, 14, 2597–2603. [Google Scholar] [CrossRef]
- Oh, Y.; Nam, S.; Wi, S.; Kang, J.; Hwang, T.; Lee, S.; Park, H.H.; Cabana, J.; Kim, C.; Park, B. Effective wrapping of graphene on individual Li4Ti5O12 grains for high-rate Li-ion batteries. J. Mater. Chem. A 2014, 2, 2023–2027. [Google Scholar] [CrossRef]
- Reddy, M.V.; Sharma, N.; Adams, S.; Rao, R.P.; Peterson, V.K.; Chowdari, B.V.R. Evaluation of undoped and M-doped TiO2, where M = Sn, Fe, Ni/Nb, Zr, V, and Mn, for lithium-ion battery applications prepared by the molten-salt method. RSC Adv. 2015, 5, 29535–29544. [Google Scholar] [CrossRef]
- Lan, T.; Zhang, W.; Wu, N.L.; Wei, M. Nb-Doped rutile TiO2 mesocrystals with enhanced lithium storage properties for lithium ion battery. Chem. Euro. J. 2017, 23, 5059–5065. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Sulfur-doped TiO2: Structure and surface properties. Catalysts 2017, 7, 214. [Google Scholar] [CrossRef]
- Jiao, W.; Li, N.; Wang, L.; Wen, L.; Li, F.; Liu, G.; Cheng, H.-M. High-rate lithium storage of anatase TiO2 crystals doped with both nitrogen and sulfur. Chem. Commun. 2013, 49, 3461–3463. [Google Scholar] [CrossRef]
- Liu, S.; Cai, Z.; Zhou, J.; Pan, A.; Liang, S. Nitrogen-doped TiO2 nanospheres for advanced sodium-ion battery and sodium-ion capacitor applications. J. Mater. Chem. A 2016, 4, 18278–18283. [Google Scholar] [CrossRef]
- Jung, H.-G.; Yoon, C.S.; Prakash, J.; Sun, Y.-K. Mesoporous anatase TiO2 with high surface area and controllable pore size by F−-ion doping: Applications for high-power Li-ion battery anode. J. Phys. Chem. C 2009, 113, 21258–21263. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
- Tang, X.; Li, D. Sulfur-doped highly ordered TiO2 nanotubular arrays with visible light response. J. Phys. Chem. C 2008, 112, 5405–5409. [Google Scholar] [CrossRef]
- Ksibi, M.; Rossignol, S.; Tatibouët, J.-M.; Trapalis, C. Synthesis and solid characterization of nitrogen and sulfur-doped TiO2 photocatalysts active under near visible light. Mater. Lett. 2008, 62, 4204–4206. [Google Scholar] [CrossRef]
- Li, F.; Liu, W.; Lai, Y.; Qin, F.; Zou, L.; Zhang, K.; Li, J. Nitrogen and sulfur co-doped hollow carbon nanofibers decorated with sulfur doped anatase TiO2 with superior sodium and lithium storage properties. J. Alloys. Compd. 2017, 695, 1743–1752. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-supported nanotube arrays of Sulfur-doped TiO2 enabling ultrastable and robust Sodium storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef]
- Guan, D.; Cai, C.; Wang, Y. Amorphous and crystalline TiO2 nanotube arrays for enhanced Li-ion intercalation properties. J. Nanosci. Nanotech. 2011, 11, 3641–3650. [Google Scholar] [CrossRef]
- Fang, H.T.; Liu, M.; Wang, D.W.; Sun, T.; Guan, D.S.; Li, F.; Zhou, J.; Sham, T.K.; Cheng, H.M. Comparison of the rate capability of nanostructured amorphous and anatase TiO2 for lithium insertion using anodic TiO2 nanotube arrays. Nanotechnology 2009, 20, 225701. [Google Scholar] [CrossRef]
- Ryu, W.H.; Nam, D.H.; Ko, Y.S.; Kim, R.H.; Kwon, H.S. Electrochemical performance of a smooth and highly ordered TiO2 nanotube electrode for Li-ion batteries. Electrochim. Acta 2012, 61, 19–24. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Ohtsu, N.; Semboshi, S.; Masahashi, N. Visible light responses of sulfur-doped rutile titanium dioxide photocatalysts fabricated by anodic oxidation. Appl. Catal. B Environ. 2009, 91, 152–156. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio. Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MI, USA, 1992; p. 261. [Google Scholar]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Madian, M.; Ummethala, R.; Abo El Naga, A.O.; Ismail, N.; Rümmeli, M.H.; Eychmüller, A.; Giebeler, L. Ternary CNTs@TiO2/CoO nanotube composites: Improved anode materials for high performance lithium ion batteries. Materials 2017, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Madian, M.; Wanga, Z.; Gonzalez-Martinez, I.; Oswald, S.; Giebeler, L.; Mikhailova, D. Ordered Ti-Fe-O nanotubes as additive-free anodes for lithium ion batteries. Appl. Mater. Today 2020, 20, 100676. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Wang, L.; Du, Z.; Fang, H.; Ling, Y.; Huang, Z. Coaxial SnO2@TiO2 nanotube hybrids: From robust assembly strategies to potential application in Li+ storage. J. Mater. Chem. 2012, 22, 11151. [Google Scholar] [CrossRef]
- Guan, D.; Li, J.; Gao, X.; Yuan, C. Controllable synthesis of MoO3-deposited TiO2 nanotubes with enhanced lithium-ion intercalation performance. J. Power Sources 2014, 246, 305–312. [Google Scholar] [CrossRef]
- Appadurai, T.; Subramaniyam, C.M.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952. [Google Scholar] [CrossRef] [PubMed]
- Madian, M.; Giebeler, L.; Klose, M.; Jaumann, T.; Uhlemann, M.; Gebert, A.; Oswald, S.; Ismail, N.; Eychmüller, A.; Eckert, J. Self-organized TiO2/CoO nanotubes as potential anode materials for lithium ion batteries. ACS Sustain. Chem. Eng. 2015, 3, 909–919. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabaghi, D.; Madian, M.; Omar, A.; Oswald, S.; Uhlemann, M.; Maghrebi, M.; Baniadam, M.; Mikhailova, D. Directly Anodized Sulfur-Doped TiO2 Nanotubes as Improved Anodes for Li-ion Batteries. Batteries 2020, 6, 51. https://doi.org/10.3390/batteries6040051
Sabaghi D, Madian M, Omar A, Oswald S, Uhlemann M, Maghrebi M, Baniadam M, Mikhailova D. Directly Anodized Sulfur-Doped TiO2 Nanotubes as Improved Anodes for Li-ion Batteries. Batteries. 2020; 6(4):51. https://doi.org/10.3390/batteries6040051
Chicago/Turabian StyleSabaghi, Davood, Mahmoud Madian, Ahmad Omar, Steffen Oswald, Margitta Uhlemann, Morteza Maghrebi, Majid Baniadam, and Daria Mikhailova. 2020. "Directly Anodized Sulfur-Doped TiO2 Nanotubes as Improved Anodes for Li-ion Batteries" Batteries 6, no. 4: 51. https://doi.org/10.3390/batteries6040051
APA StyleSabaghi, D., Madian, M., Omar, A., Oswald, S., Uhlemann, M., Maghrebi, M., Baniadam, M., & Mikhailova, D. (2020). Directly Anodized Sulfur-Doped TiO2 Nanotubes as Improved Anodes for Li-ion Batteries. Batteries, 6(4), 51. https://doi.org/10.3390/batteries6040051





