1. Introduction
Lithium-ion batteries constitute the most reliable energy storage technology for electric applications where energy density is critical. This is the case of electric vehicles, smart devices and portable power tools. Lithium-ion is nowadays a mature technology: cost and lifetime were improved in a very sensitive way in the last decades.
However, studying the ageing of batteries is still necessary because the degradation of their features largely determines the cost, the performances and the environmental impact of electric vehicles, particularly of full electric vehicles. In this type of studies, battery ageing is typically classified in two types: calendar and cycling ageing. Calendar ageing occurs when a battery is at rest condition; this is when no current flows through the battery whereas cycling ageing occurs when the battery is charged or discharged.
Given that battery degradation occurs in a different way if the battery is in rest condition or if a current flows through, a major challenge is to determine how calendar and cycling ageing effects combine together. Electric cars spend most of the time (95% or more) parked and current rates of the battery are relatively low when they are used. In these applications the average current rates are frequently about
C/5 to
C/2 with peak values at about 3
C. Except for fast charge, the main ageing mechanism in this application is considered to be formation and growth of the Solid Electrolyte Interface (SEI) [
1,
2].
The chosen method in this work is divided in two distinct phases, namely characterisation and modelling: The characterisation phase is based on accelerated ageing testing of battery cells, the main results of this phase were reported in Reference [
3]. In this paper we are focusing in second phase: battery ageing modelling.
Battery ageing is modelled using the results obtained in the first phase. In our approach, we aim to establish ageing laws, that is to find the relations between ageing test conditions and performances decay and to quantify them. These ageing laws can be determined from test results and then generalised to predict the performance degradation of a battery subjected to different use conditions.
This paper is organised as follows: Main battery ageing mechanisms and modelling approaches are explained in
Section 2. The experimental setup and results are reported in
Section 3. In
Section 4, a calendar ageing model is developed and its parameters are identified to fit experimental results. Then, a combined ageing model (cycling + calendar) is developed in
Section 5. Finally, results are discussed and conclusions are drawn in
Section 6 and
Section 7.
The obtained ageing model is able to reproduce the non linear behaviour of different combinations of calendar and cycling periods. Other reliability approaches (for example event-oriented modelling) could not explain these non linearities in a simple manner. Our model is simple but effective: it lies in a low number of equations (2 differential equations) and 7 parameters and enables to simulate the capacity fade of a battery cell subjected to ageing conditions combining cycling and rest periods. This model can be used for example to optimise the design and use of the battery in a vehicle by minimising both energy consumption and battery degradation.
5. Combined Ageing Model
5.1. Model Formulation
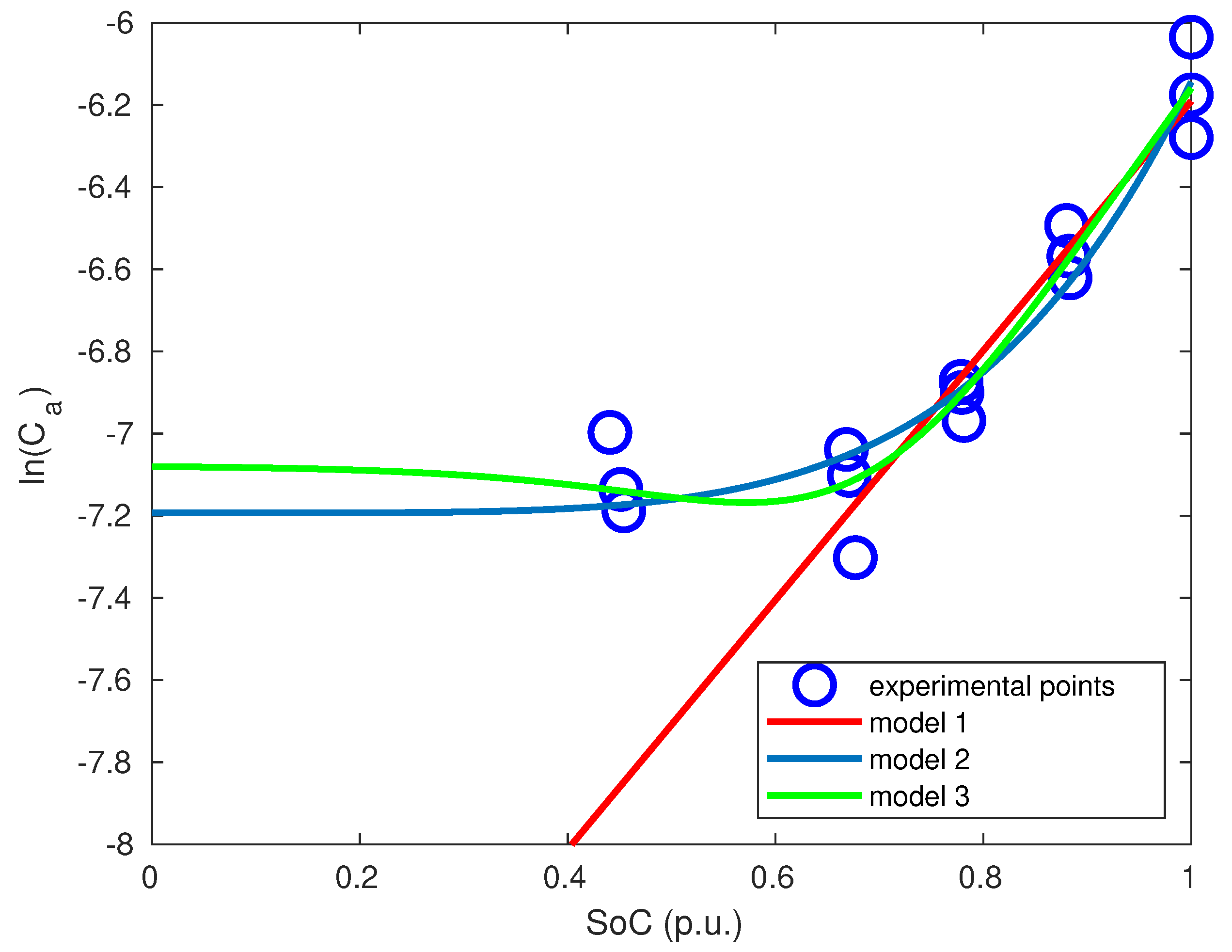
As explained above, the main hypothesis is to consider that ageing is mainly calendar and due to SEI growth. The effect of cycling on ageing is an acceleration of calendar ageing: this means that calendar ageing rate is modified by charges (and discharges).
The precise side reactions behind such ageing behaviour will remain unknown because a lack of analysis equipments. A possible explanation would be the following: cycling induces volume changes on the electrode; these dilatations make the SEI layer to be more propitious to further electrolyte reduction and thus to SEI growth. This should be validated by physico-chemical analyses.
To model this behaviour, we consider that SEI formation is a two-step reaction (Equation (
14)). This reaction is inspired by Reference [
26]. In that article, SEI formation is suggested to be formed by a two-step reaction. First step is a reversible reaction consisting in the formation of a complex between electrolyte solvent and lithium ions. The second one is the irreversible transformation of the previously formed complex to form SEI layer.
Consider the following multi-step reaction where X is irreversibly transformed into Z through the intermediary substance Y:
If reactions are zero order respect to X and first order respect to Y, the transformation rate is defined by the following ordinary differential equations (ODE) system, where
,
and
are the concentrations of X, Y and Z:
Reaction Equation (
14) can represent the lithium loss which is directly related to capacity fade, then let assume the following equivalences:
,
and
, where
Q,
and
are respectively the
capacity,
reversible capacity fade and
capacity fade.
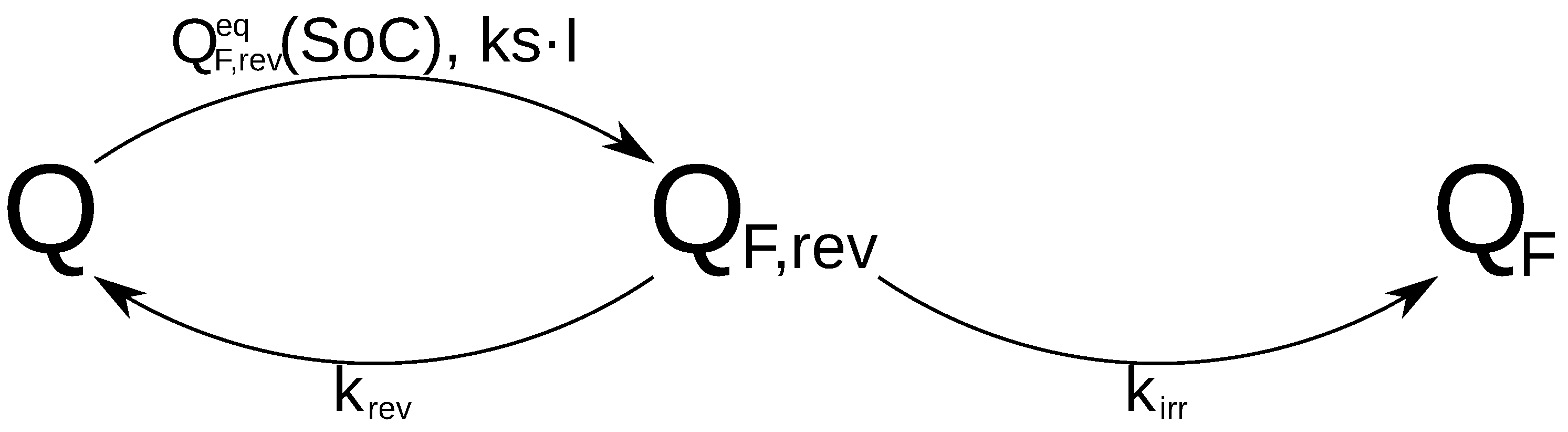
The operation of the proposed model is summarised by
Figure 4:
Q is first transformed in
depending of the values of
and
. After this, a part of
is reversibly transformed to
Q and the rest is irreversibly transformed to
(
).
Equations (
15)–(17) can be rewritten to put into evidence the influence of the model paramaters on the dynamic evolutions of
Q,
and
. To introduce the cycling effect into the model, we propose to add a term that modifies the
reversible capacity fade rate (
). This term is proportional to the current:
. The new ODE system is composed of Equations (
18)–(20):
The relations between the parameters of both ODE systems are:
The meaning of the new parameter set is the following: and define the distribution of decomposition into reversible and irreversible parts (). defines the response speed of the system. In other words, is the time constant of the system. Finally, is the equilibrium level of when the system is not forced ().
Since Q, and are capacities (quantities of charge), they are directly related to mass (quantity of lithium) and they are subjected to mass constraints:
- (i)
Mass is non-negative, then capacities must be non-negative:
- (ii)
Conservation of mass: the sum of capacities is constant and equal to initial capacity
:
Equation (
26) allows to reduce the number of ODE’s of the system given by Equations (
18) to (20). Thereafter, we will focus on Equations (19) and (20) enabling to calculate
and
.
Q can be calculated afterwards by using Equation (
26). Finally, the initial conditions are typically the following:
and
.
In conclusion, this model is defined by 2 independent ODE’s (Equations (19) and (20)), 2 constraint equations (Equations (
25) and (
26)), 4 independent (Note that
is directly related to
, Equation (24)) parameters (
,
,
and
) and a set of initial conditions (
,
and
).
In order to facilitate comparisons between different battery sizes, capacities are expressed in the p.u. system (capacity relative to nominal capacity of the battery, ). Thus, every capacity (Q, and ) can be valued between 0 and 1 p.u.
The chosen unit of time is the day, then capacity rates , and are expressed in p.u./day. In order to homogenise the units of the preceding equations, current (I), which is typically expressed in C-rate, will be expressed also in p.u./day. In fact, the C-rate system are units of p.u./hour: 1C is the current rate discharging (or charging) the battery in 1 hour. The conversion factor between C-rate (p.u./hour) and p.u./day is 24. For example, 1C is equivalent to 24 p.u./day and C/24 is equivalent to 1 p.u./day.
An important property of this model is that, in calendar conditions, it is equivalent to the calendar model proposed in
Section 4. When no current flows through the battery
, the term
of Equation (19) is 0. In these conditions,
evolution is that of a first order system, that is,
will converge to
from its initial value
. Then, after a certain time the equilibrium is found:
By using Equations (11) and (28),
can be expressed as a function of calendar ageing parameters obtained in the preceding Section (
and
B) and combined ageing parameters (
and
):
5.2. Parameter Identification
The parameter identification consists in solving a non-linear problem: to find the minimum of
, where
x is a parameter set (
,
,
,
) and
is the simulation error respect to experimental results. This process is iterative: it means that for each parameter set
, the error is evaluated and a new parameter set is established for next iteration until a satisfactory result is found (minimum of
). In this work,
is defined as the mean value of the errors on each profile (index
i indicates the profile number):
where the error is calculated as the mean absolute error of simulated capacity fade respect to measured capacity fade in RPT tests (index
j indicates the RPT number):
In
Section 4, a calendar ageing model based on the acceleration coefficient,
was found. As explained above, in calendar conditions, the combined ageing model is equivalent to the calendar ageing one. With this relation (Equation (
29)), the number of parameters to identify is decreased of one: at each iteration a three parameter set is established
and
have to be obtained from
,
and
. The iterative process is summarised by the following steps:
- (i)
establish a new parameter set:
- (ii)
calculate
(Equation (
29))
- (iii)
run simulation on each cycling profiles to obtain (Equations (19) and (20))
- (iv)
calculate the mean absolute error,
(Equation (
30))
- (v)
while a minimum is not found, go to step (i)
We have used Mathworks’
MATLAB and
Optimisation Toolbox to solve the parameter identification problem. The initial value of the parameter set, their constraints and the obtained parameter set are described in
Table 2.
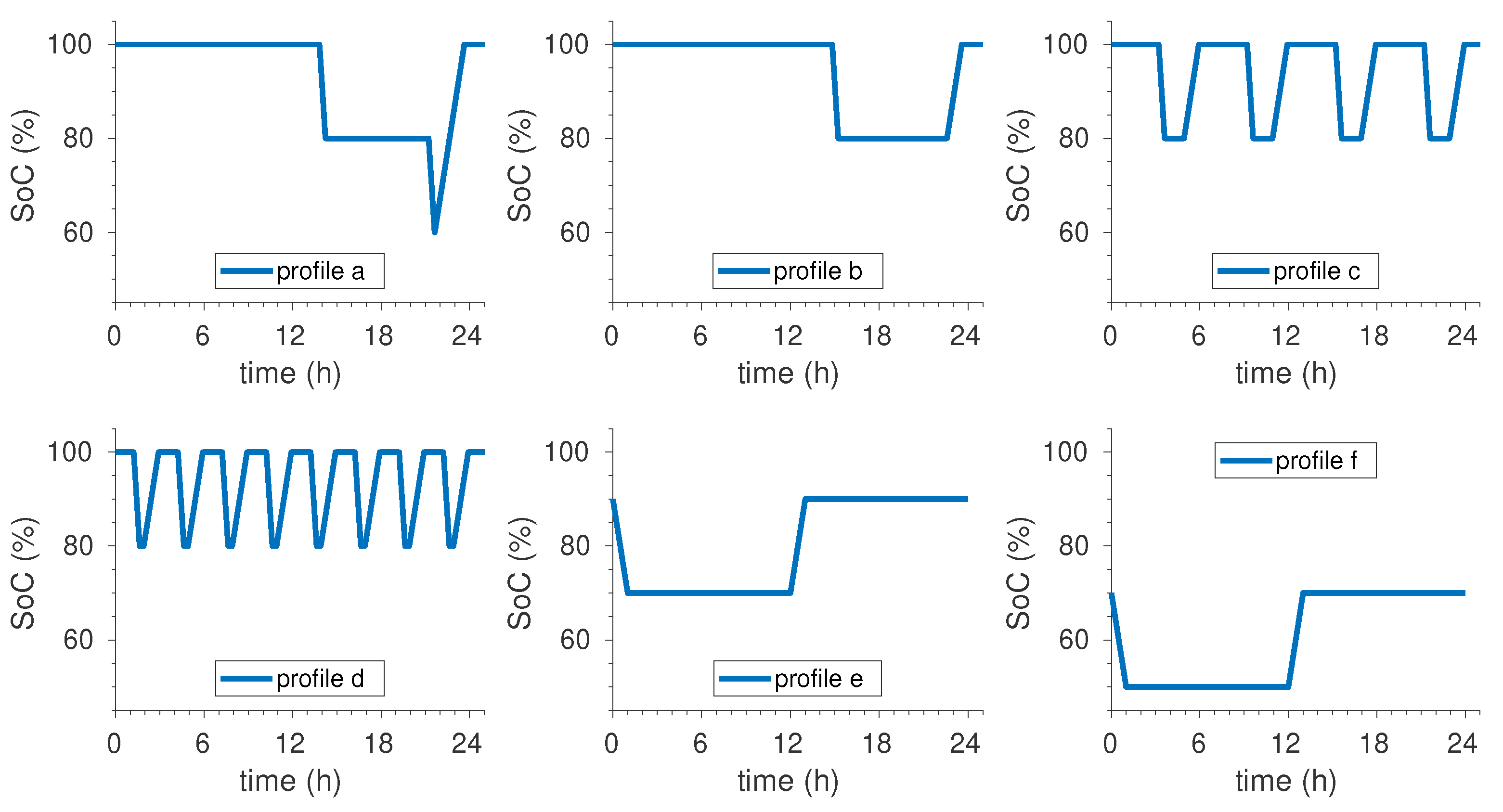
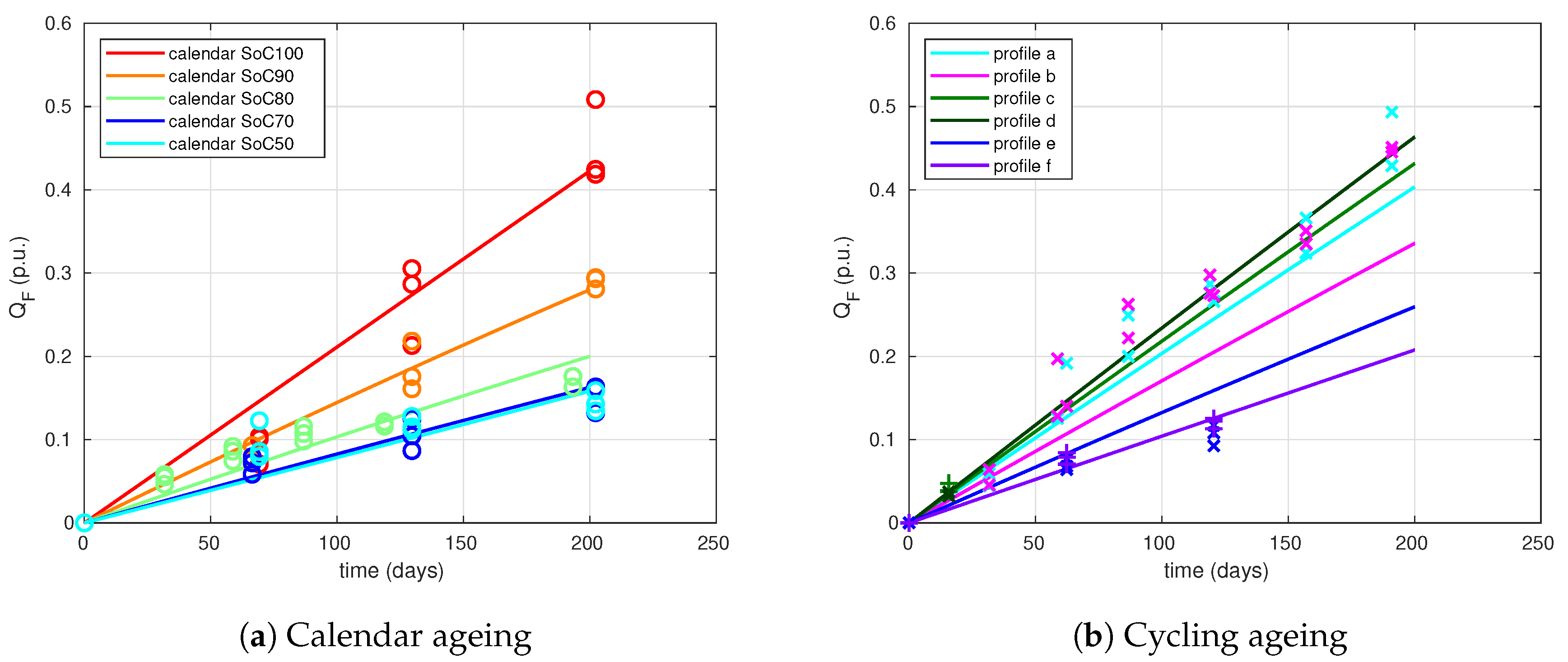
For the identification we have used profiles b to f. Profile a is the more complex one (see
Figure 1) and is used as model validation profile. The obtained combined ageing model is summarised by
Table 3. In this table, all the necessary equations and parameters are indicated.
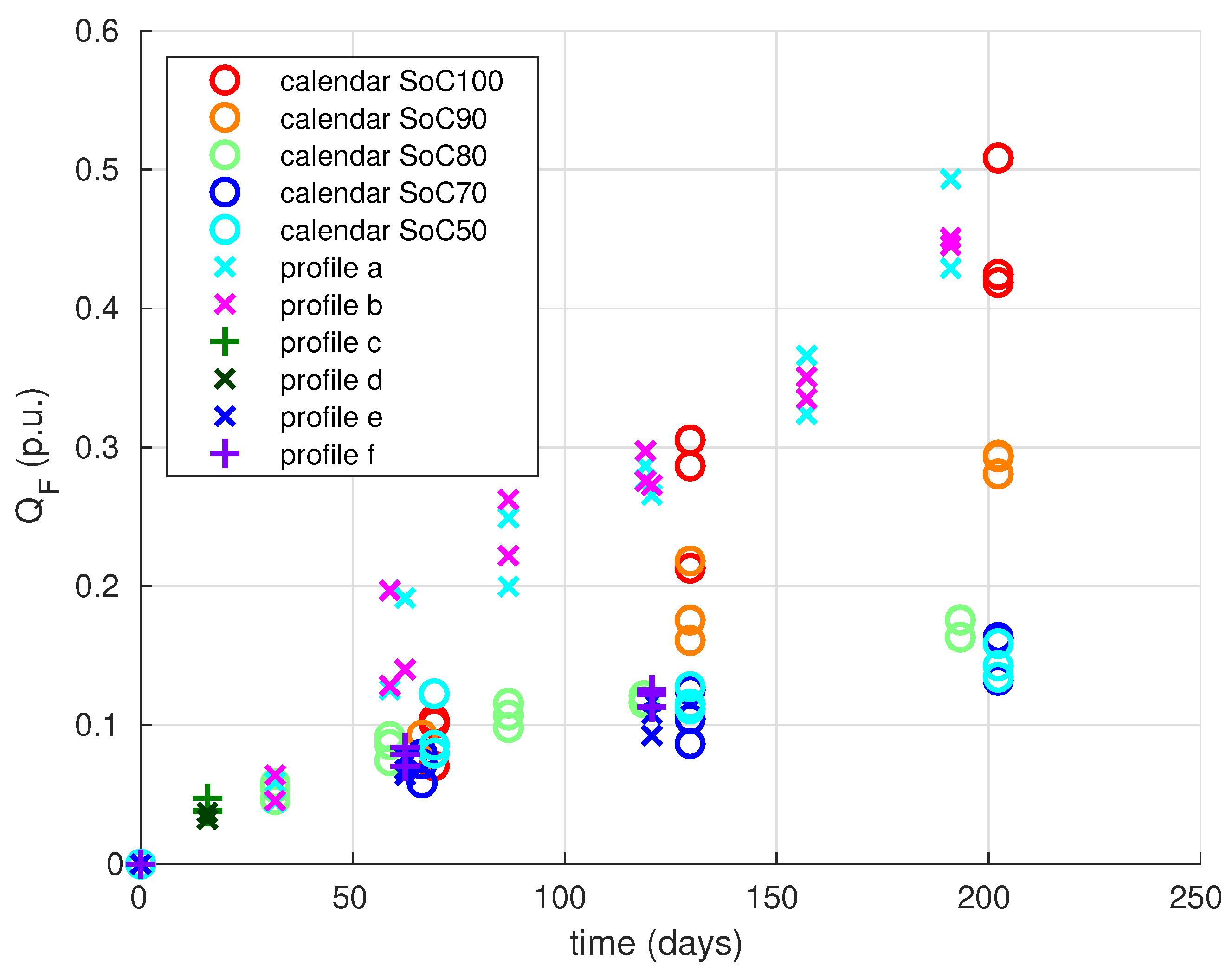
The simulation results for all ageing conditions are shown in
Figure 5. First, we can see that calendar ageing simulations (dashed lines) fit very well to measurements (circles).
For the cycling profiles, the results are not very good for profiles b and e: capacity fade is underestimated in profile b and it is overestimated in profile e. Nevertheless, the model reproduces quite well the capacity fade found by experiments for all other profiles (a, c, d, f), especially capacity fade for profile a that has not been used for identification (profile a).
6. Discussion
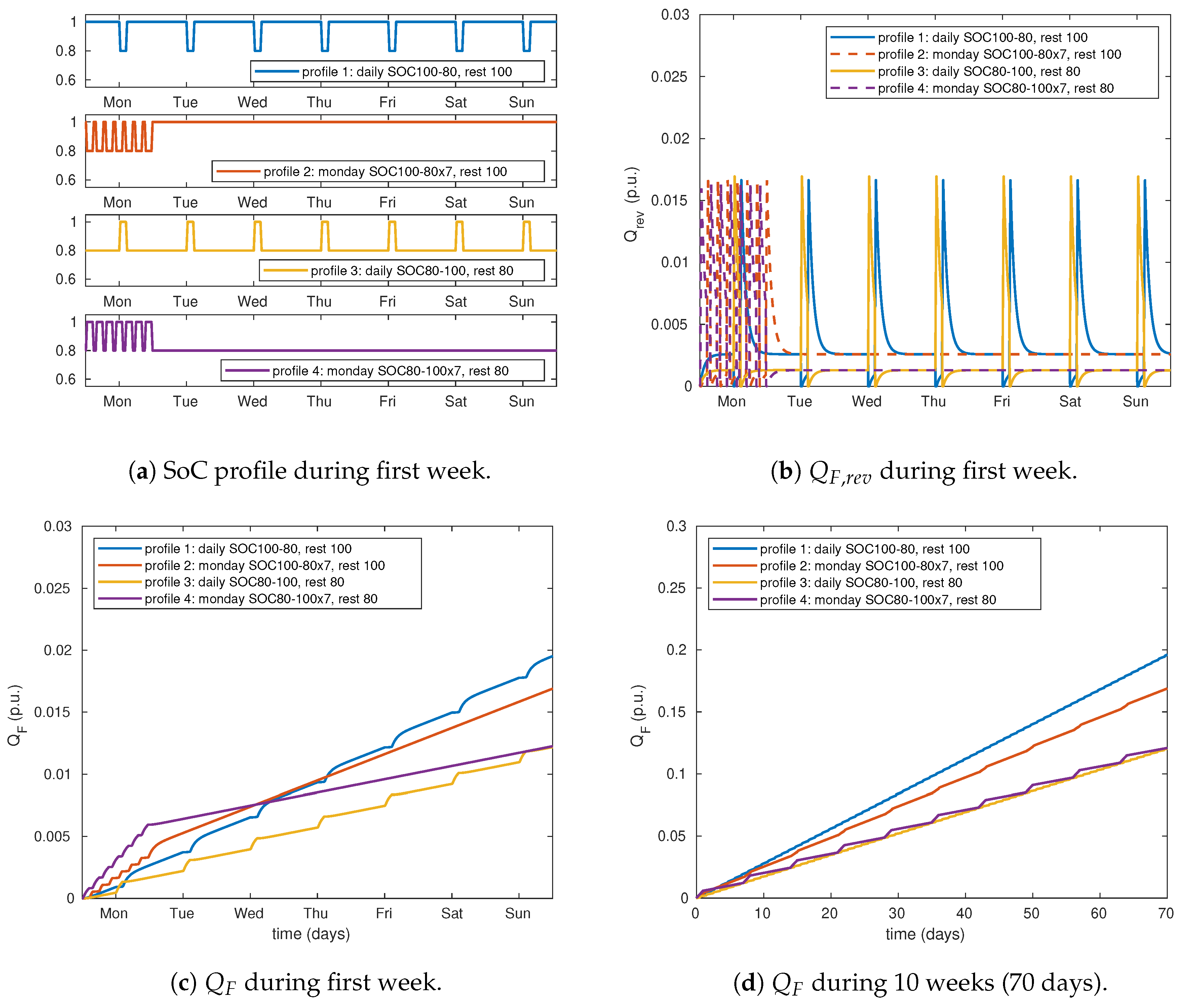
In this section some application examples allow to illustrate the model behaviour particularities. The first example is composed of four different use profiles with the same amount of charge throughput and current rates (
Figure 6). The first profile consists in a daily discharge of 20%
at
C/2 followed by a 2 h rest and a full charge at
C/2 (
Figure 6a). In this use profile battery is at 100%
most of time: 21.2 h per day (blue line). The second profile consists in repeating once a week (on Monday) seven times the following pattern: 20%
discharge at
C/2, 2 hour rest, full charge at
C/2 and 42 min rest. The battery is left at 100%
the rest of the week (red line). Rest times have been adjusted to make
levels (minimum, maximum and average) equal in profiles 1 and 2: the only difference is the distribution of charges and discharges within a week. The third and fourth profiles (yellow and violet lines respectively) are complementary two the preceding ones but the battery is kept at 80%
when it is not used. As for profiles 1 and 2,
levels of profile 3 are equal than those of profile 4.
The simulation results for one week (7 days) are illustrated by
Figure 6b,c showing respectively
and
. As a consequence of ODE system formed by Equations (
18)–(20),
is first produced from
Q. Then,
is consumed: a part of
is reversibly transformed to
Q and the other part is irreversibly lost (
).
During each charge
grows to about 0.016
p.u. Inversely, during each discharge,
decreases rapidly and reaches the constraint value of 0
p.u. (Equation (
25), non negativity of mass). During rest times, after each charge (discharge),
will decrease (increase) to converge to a value depending of the
level:
. In other words, each use phase (charge or discharge) makes the cell move from an equilibrium (
); when the battery is at rest, a new equilibrium is found between
generation and consumption and it converges to a value depending of
(
). In
Figure 6b we can see that
is about 0.0025
p.u. for 100 %
(blue and red lines, during long rest periods), while it is about 0.0015
p.u. for 80%
(yellow and violet lines, during long rest periods).
The evolution of
is shown in
Figure 6c. As we can see, after each charge there is an acceleration of
and inversely, after each discharge,
evolution decelerates. This behaviour is explained by Equation (20):
is proportional to the integral of
. The same pattern will be repeated during ten weeks as we can see in
Figure 6d.
An important consequence of this model appears by comparing
of profiles 1 and 2 (
Figure 6d, blue and red lines respectively). Each Monday, profile 2 makes the battery degrade faster than profile 1. This is because profile 2 contains seven partial cycles on Monday, while profile 1 represents only one. From Tuesday to Sunday, degradation rate is lower in profile 2 than in profile 1. After several weeks, it clearly appears that, despite of similar
levels, current rates and charge throughputs, profile 2 induces a lower average degradation rate than profile 1. Similarly, when comparing profiles 3 and 4, degradation is faster on Mondays for profile 3 respect to profile 4, but it is slower from Tuesday to Sunday. In a different way than for profiles 1 and 2, average degradation rates are similar in profiles 3 and 4 after a whole number of weeks (same cycle number): the acceleration that occurs every Monday on profile 3 is compensated by the deceleration of the rest of the week. As a conclusion, the degradation produced by combination of cycling and calendar periods can be different depending of the time distribution of charges and discharges (as for profile 2 respect to profile 1) or not (as for profile 3 respect to profile 4). The proposed model is able to reproduce a such particular behaviour inspired by experimental results, what could not be easily modelled by other approaches described in
Section 2.
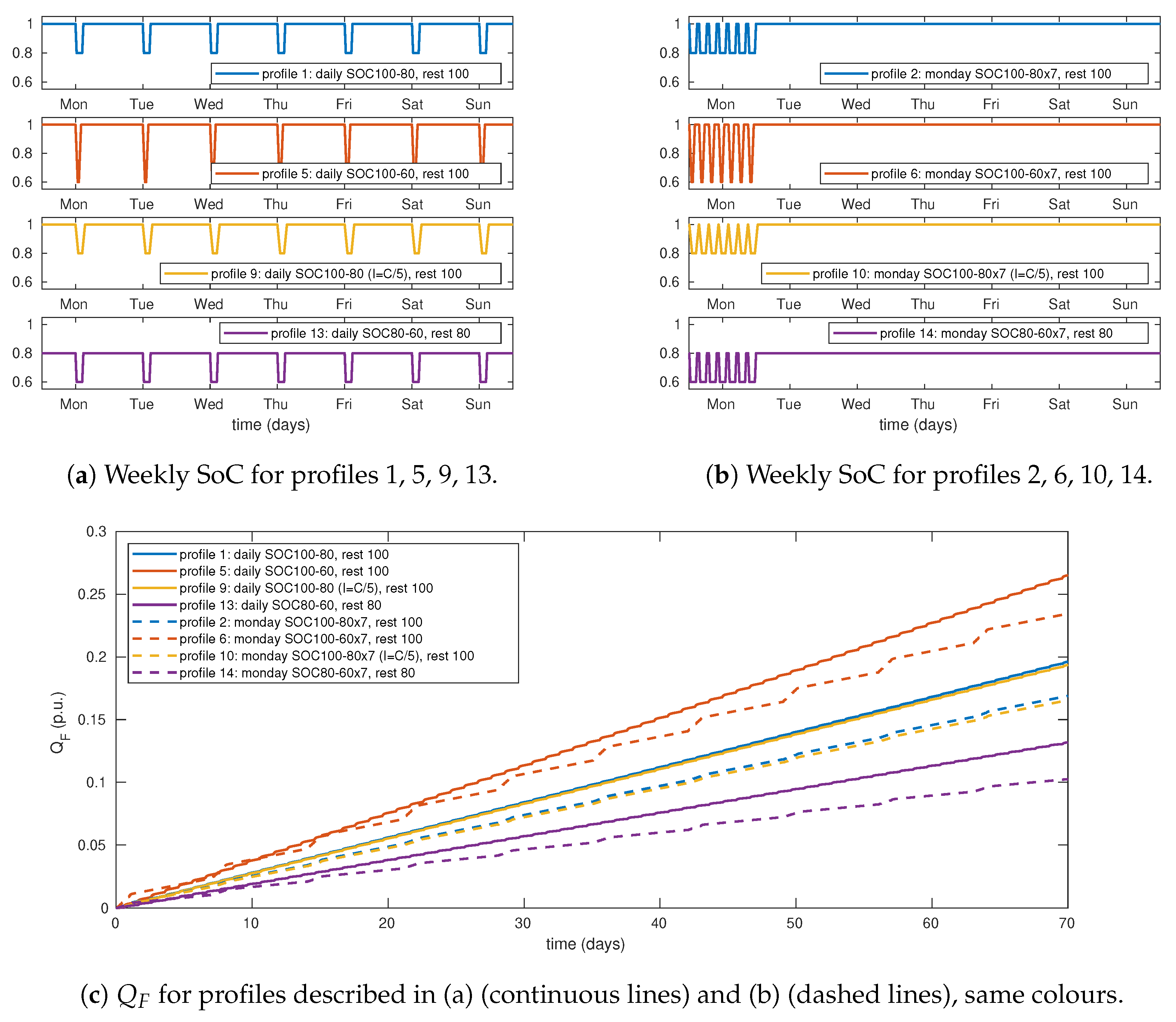
Other factors such as
range, mean
or current rate and may influence
. To explore how these factors can affect
, other profiles were simulated (
Table 4). From profiles in example 1, twelve supplementary profiles were generated by changing
,
or current rate. Profiles 5 to 8 are designed to test the influence of
range:
is fixed to 0.6, then the weekly charge throughput is 2.8
p.u.With profiles 9 to 12 we can test the influence of current rate by comparing the results from these profiles to those of profiles 1 to 4. Finally, profiles 13 to 16 are identical to profiles 1 to 4 but moving levels (max, min, avg) 0.2 p.u. to the bottom. Rest times where adjusted to make average SoC () correspond to 0.98, 0.82, 0.78 or 0.62, that is, or .
In
Figure 7 a selection of these results are shown. To explore how
range influences
, we can compare profiles 1 and 2 to profiles 5 and 6 respectively.
after 10 weeks under profile 5 was 26.51% of initial capacity compared to 19.62% under profile 1, that is, degradation rate was 35% faster with
equal to 0.4
p.u. When comparing the weekly profiles (profile 6 versus profile 2), the relative increase of the degradation rate was 39%. The influence of current rate is negligible, the difference between
under profiles 9 to 12 compared respectively to profiles 1 to 4 is lower than 1%. Finally, as shown by the results of simulations made with profiles 13 to 16, the influence of
level is very important, with degradation rates 15 to 40% slower than those of profiles 1 to 4.
These simulation results, which need to be validated by experiments, show that battery use management can have a significant influence on ageing. This model allows the evaluation of complex use scenarios, such as a fleet of vehicles: in some situations, it may be beneficial to use a vehicle intensively one day a week rather than using it regularly every day.
7. Conclusions
Battery ageing in electric vehicles is composed of calendar and cycling ageing. When cycling is performed at low current rates, typical cycling ageing mechanisms such as lithium plating or particle cracking can be neglected compared to the main calendar ageing mechanism in graphite based lithium-ion batteries: SEI growth. However, it has been found that cycling can influence subsequent calendar ageing. The combination between cycling and calendar ageing has a very non linear behaviour.
In this work we have modelled the capacity fade of NMC/C cells subjected to combined calendar and cycling ageing. The accelerated ageing test campaign was designed to investigate battery ageing at levels and current profiles representative of electric vehicle’s use.
The model identification consisted in two steps. In the first one, a calendar ageing model based on the Eyring law is proposed and a parameter identification is performed on calendar ageing experiment results. In the second step, cycling ageing experimental results are combined to the firstly identified calendar ageing model to obtain the combined ageing model parameters.
The proposed combined ageing model lies on the formulation of a two-step reaction rate. With the analogy between reaction rate equations and capacity fade, this ageing model is simple but effective: based on only two differential equations and seven parameters, it can reproduce the capacity evolution of lithium-ion cells subjected to cycling profiles similar to those found in electric vehicle applications. Moreover, the strong non-linearity of the cycling-calendar combination on ageing can be simulated with this model whereas it cannot be explained with models based on weighted Ah or event-oriented modelling approaches.
The obtained model opens the perspective of a wide range of applications, for example: battery use assessment, optimal electric vehicle charge scheduling or plug-in hybrid electric vehicle energy management strategy design.
Further work will consist in expand the domain of study. Especially, it will be important to study the combination of cycling and calendar ageing at levels different than 20%, at levels below 50% and at colder temperatures: this would allow to estimate other model parameters as, for instance, the activation energy ().
Other studies could consist in thermal cycling, which may cause different ageing mechanisms to coexist, particularly lithium plating and SEI growth. The succession of ageing mechanisms of different nature may lead to multiple interactions between mechanisms. Analysing such interactions will be helpful to better understand battery degradation in real operation conditions.