Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs
Abstract
1. Introduction
2. Materials and Methods
2.1. Goal and Scope
2.2. Inventory
2.2.1. Positive Electrode Paste
2.2.2. Negative Electrode Paste
2.2.3. Electrolyte
2.2.4. Electrode Substrates
2.2.5. Separator
2.2.6. Cell Container
2.2.7. Module and Pack Housing
2.3. Cell Cost
- : baseline cost;
- : mass of material i necessary to produce one kg of active material; and
- : cost of material i.
2.4. Pack Cost
3. Results and Discussion
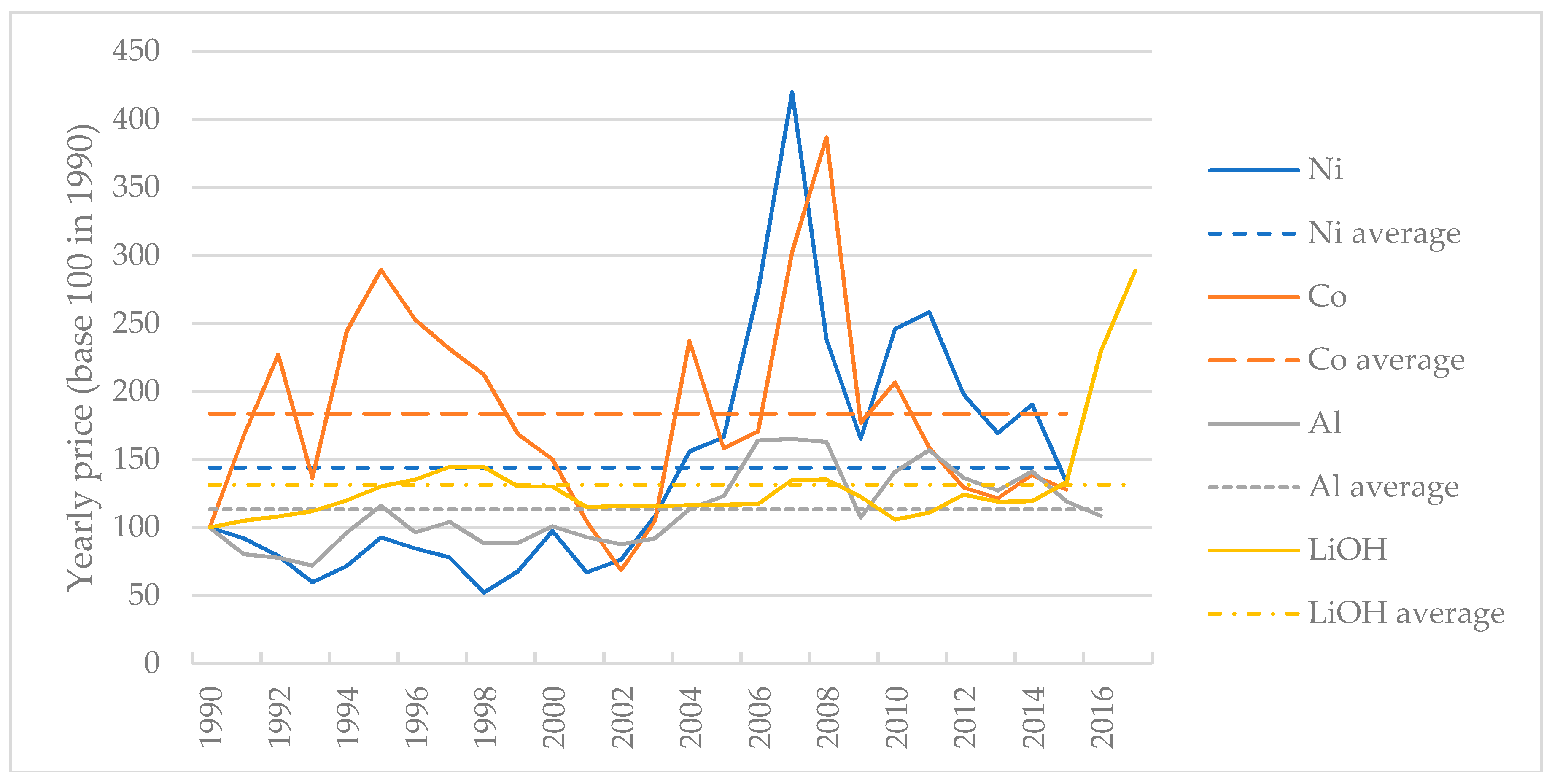
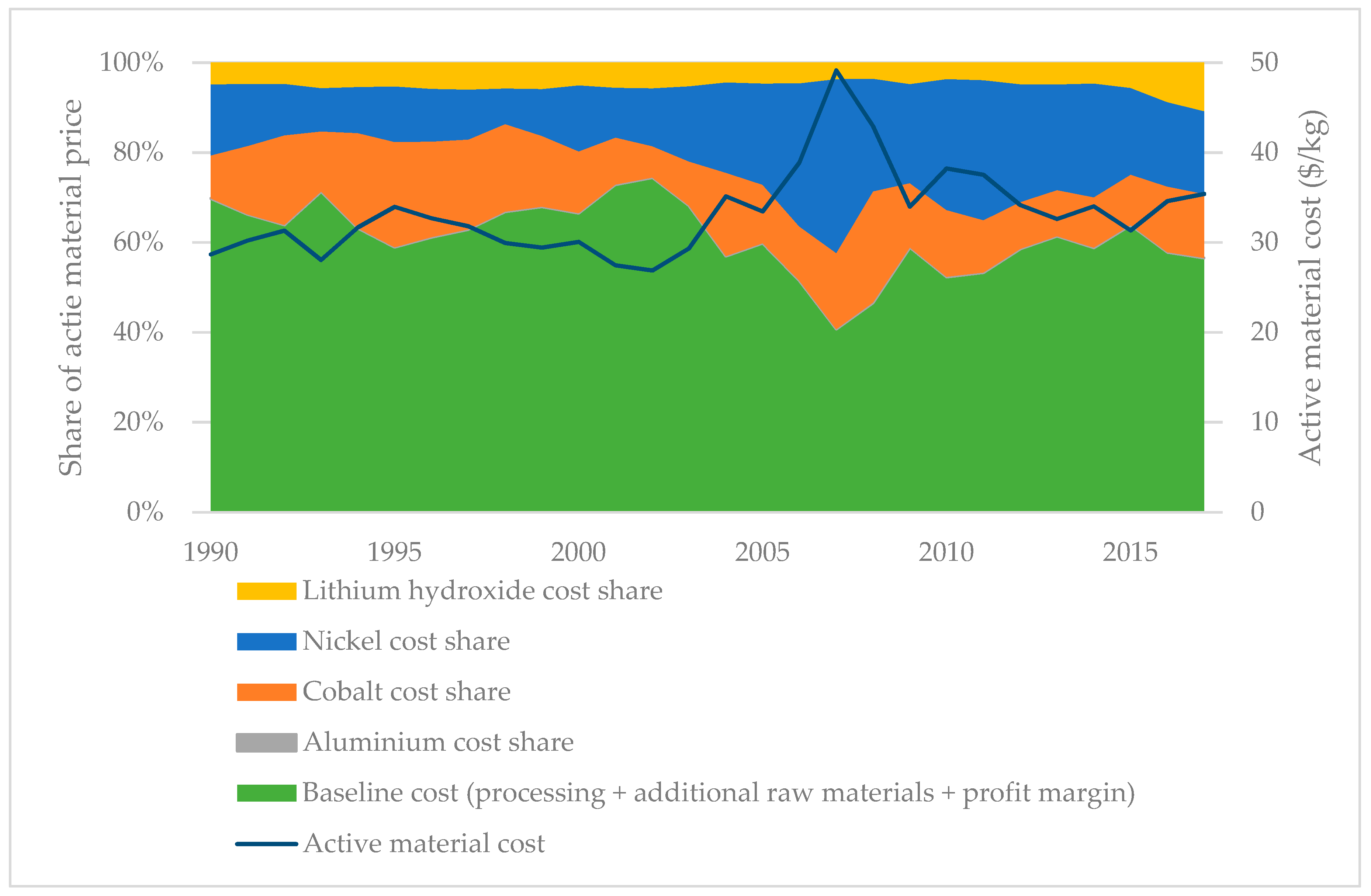
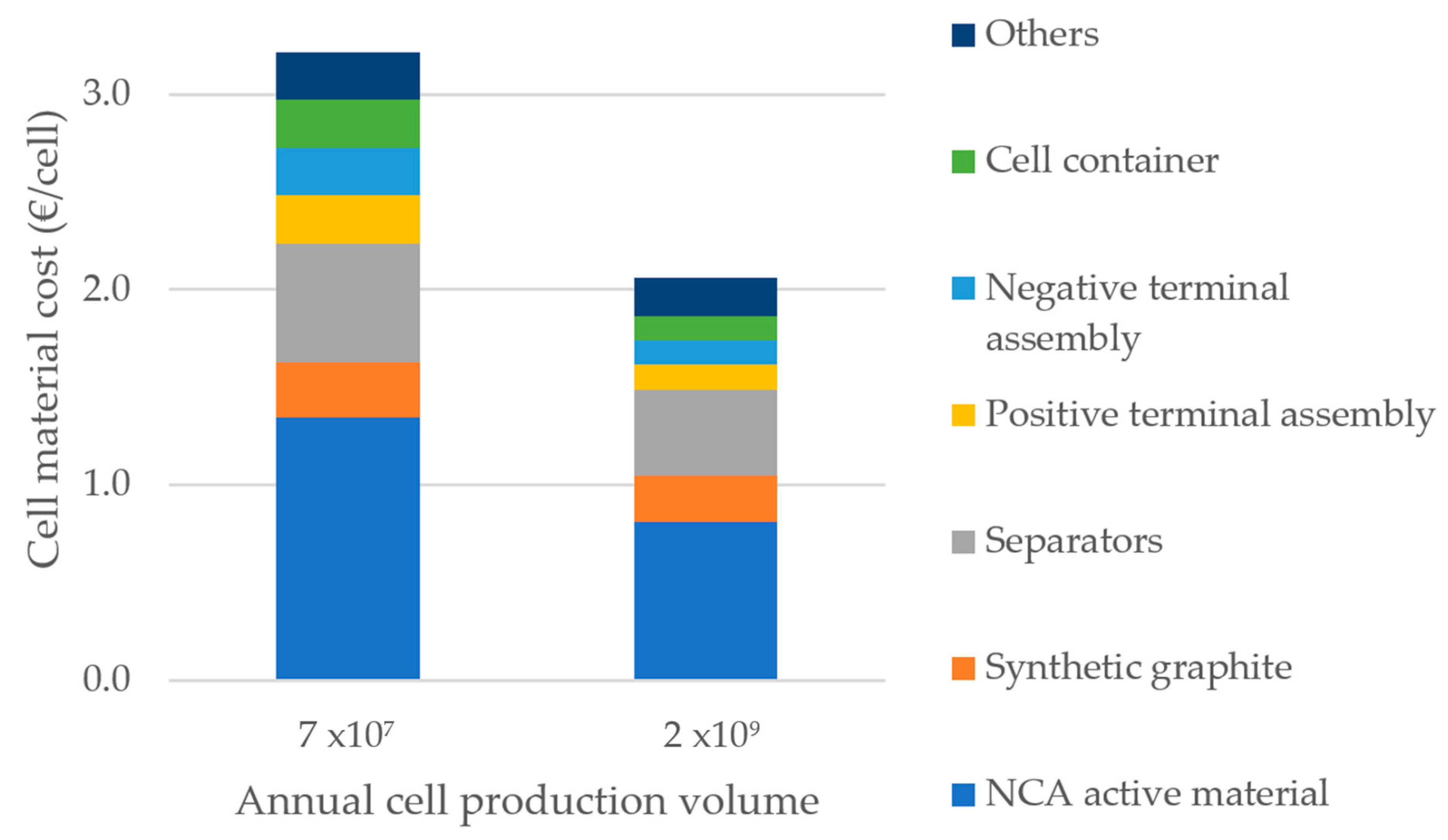
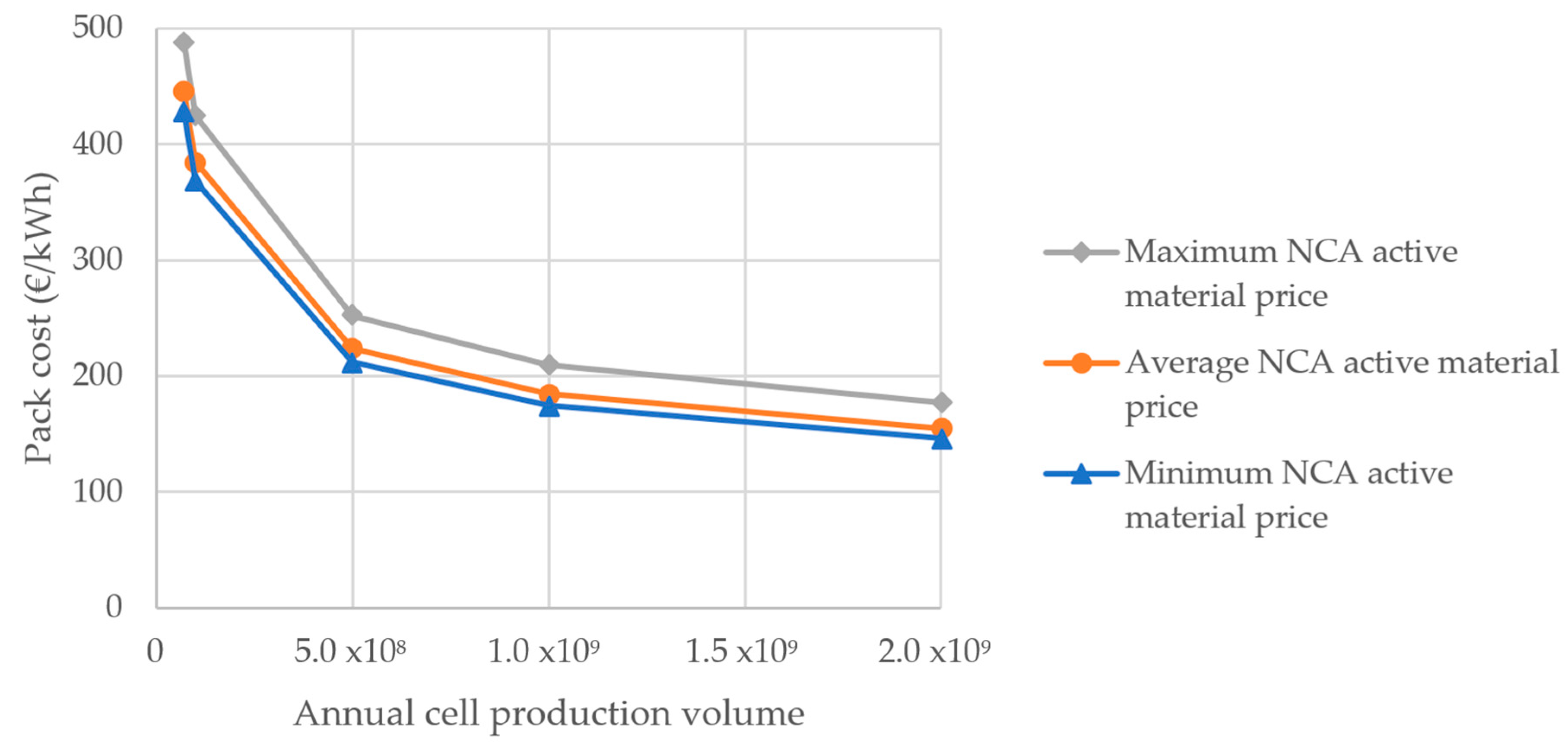
3.1. Cost: Influence of Active Material Price and Production Volume
3.1.1. Material Cost
3.1.2. Pack Cost
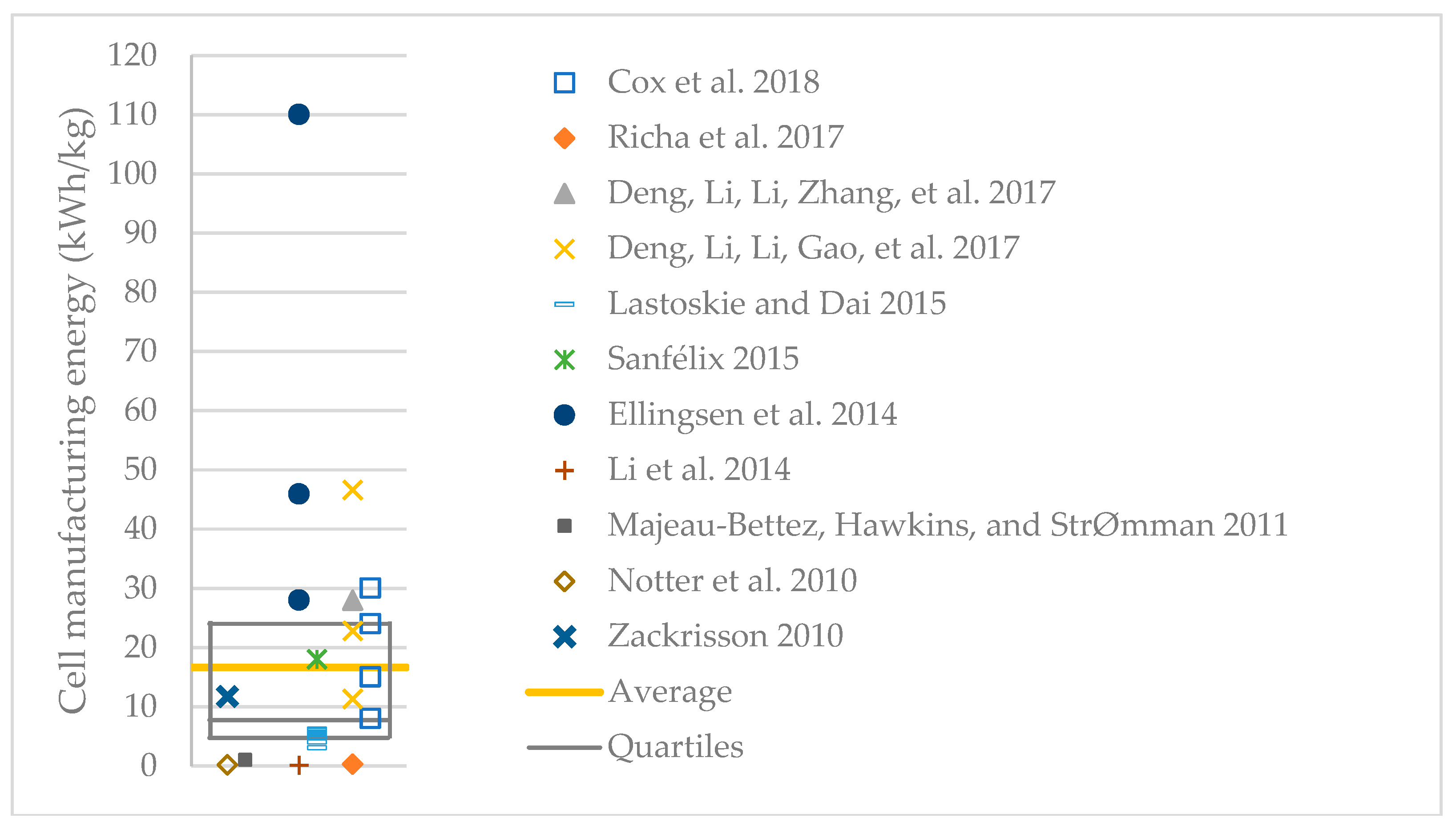
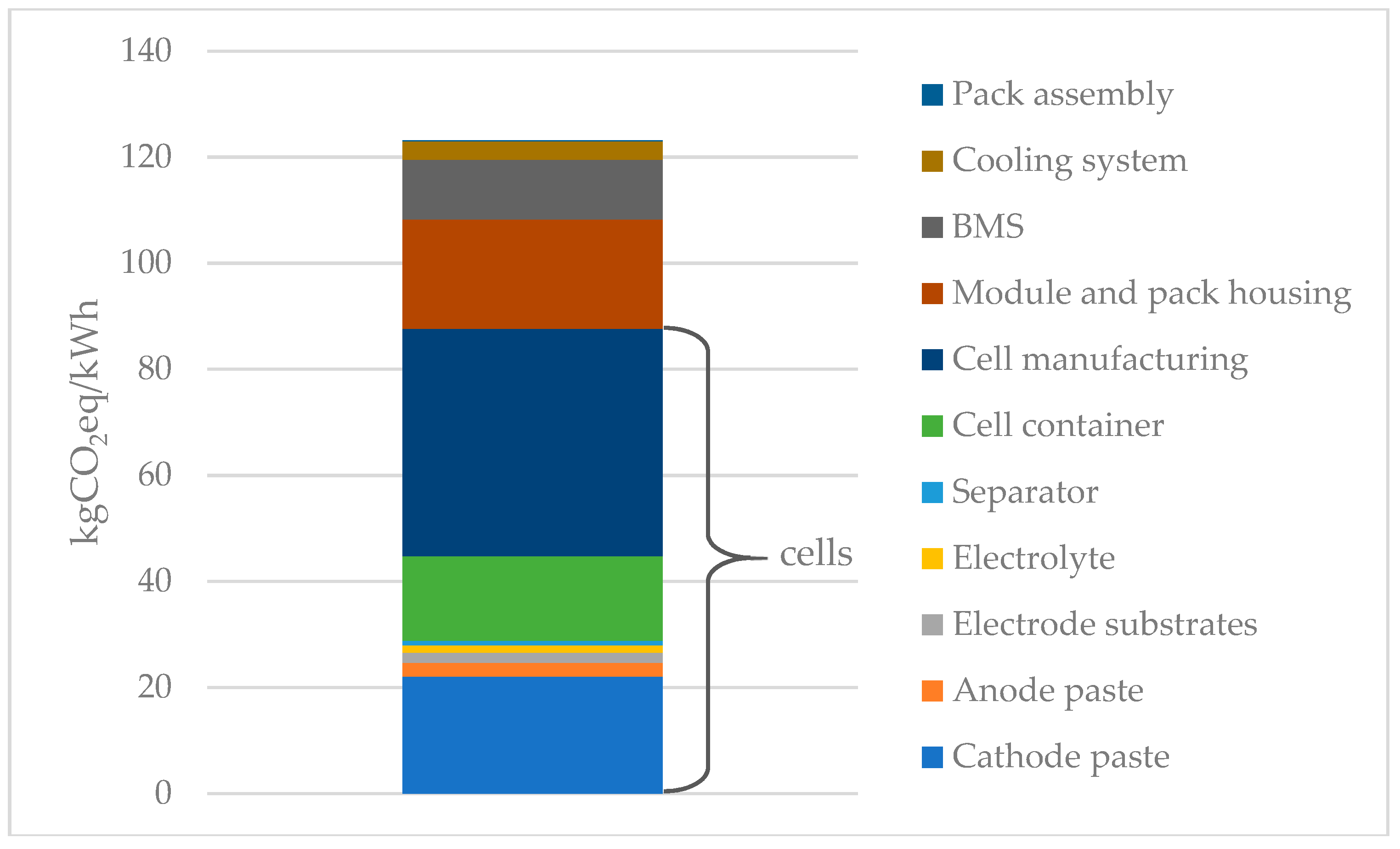
3.2. Environmental Impact
3.2.1. Contribution Analysis
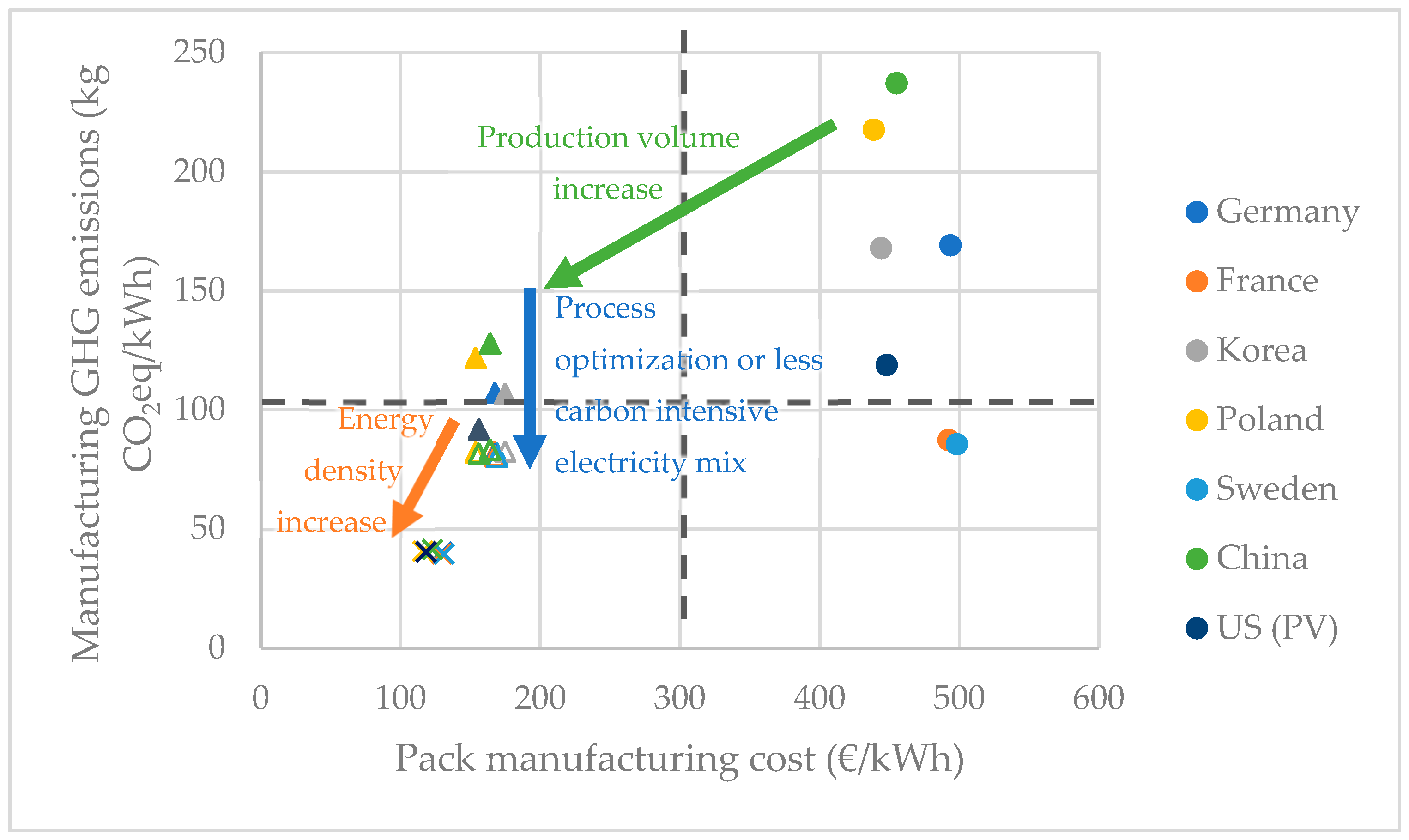
3.2.2. Influence of the Manufacturing Country
3.2.3. Influence of the Production Volume
3.3. Eco-Efficiency
Supplementary Materials
Author Contributions
Funding
 innovation program under grant agreements no 769935.
innovation program under grant agreements no 769935. Acknowledgments
Conflicts of Interest
References
- Bloomberg NEF. Cumulative Global EV Sales Hit 4 Million. Available online: https://about.bnef.com/blog/cumulative-global-ev-sales-hit-4-million/ (accessed on 14 November 2018).
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Bloomberg NEF. Why Battery Cost Could Put the Brakes on Electric Car Sales. Available online: https://about.bnef.com/blog/why-battery-cost-could-put-the-brakes-on-electric-car-sales/ (accessed on 14 November 2018).
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters—A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Curry, C. Lithium-Ion Battery Costs and Market. Available online: https://data.bloomberglp.com/bnef/sites/14/2017/07/BNEF-Lithium-ion-battery-costs-and-market.pdf (accessed on 18 October 2018).
- Chung, D.; Elgqvist, E.; Santhanagopalan, S. Automotive Lithium-Ion Cell Manufacturing: Regional Cost Structures and Supply Chain Considerations. Available online: www.nrel.gov/publications (accessed on 24 October 2018).
- Rolander, N.; Starn, J.; Behrmann, E. The Dirt on Clean Electric Cars. Bloomberg. Available online: https://www.bloomberg.com/news/articles/2018-10-16/the-dirt-on-clean-electric-cars?srnd=hyperdrive (accessed on 29 October 2018).
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Mierlo, J.V. Cost projection of state of the art lithium-ion batteries for electric vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Cluzel, C.; Douglas, C. Cost and Performance of EV Batteries. Available online: https://www.theccc.org.uk/archive/aws/IA&S/CCC battery cost_ Element Energy report_March2012_Public.pdf (accessed on 12 June 2018).
- Brodd, R.J.; Helou, C. Cost comparison of producing high-performance Li-ion batteries in the U.S. and in China. J. Power Sources 2013, 231, 293–300. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Effects of battery chemistry and performance on the life cycle greenhouse gas intensity of electric mobility. Transp. Res. Part D Transp. Environ. 2016, 47, 182–194. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Comparison between cylindrical and prismatic lithium-ion cell costs using a process based cost model. J. Power Sources 2017, 340, 273–281. [Google Scholar] [CrossRef]
- Giordano, A.; Fischbeck, P.; Matthews, H.S. Environmental and economic comparison of diesel and battery electric delivery vans to inform city logistics fleet replacement strategies. Transp. Res. Part D Transp. Environ. 2017, 64, 1–14. [Google Scholar] [CrossRef]
- Lastoskie, C.M.; Dai, Q. Comparative life cycle assessment of laminated and vacuum vapor-deposited thin film solid-state batteries. J. Clean Prod. 2015, 91, 158–169. [Google Scholar] [CrossRef]
- Rempel, J.; Barnett, B.; Hyung, Y. PHEV Battery Cost Assessment. Available online: https://www.energy.gov/sites/prod/files/2014/03/f13/es001_barnett_2013_o.pdf (accessed on 28 May 2018).
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- Helbig, C.; Bradshaw, A.M.; Thorenz, A.; Tuma, A. Supply risks associated with lithium-ion battery materials. J. Clean Prod. 2017, 172, 274–286. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials. Available online: https://publications.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1/language-en (accessed on 12 June 2018).
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Rivas, M.H.; Van Mierlo, J. Key issues of lithium-ion batteries—From resource depletion to environmental performance indicators. J. Clean Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. The cost of lithium is unlikely to upend the price of Li-ion storage systems. J. Power Sources 2016, 320, 310–313. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Factsheets. Available online: https://publications.europa.eu/en/publication-detail/-/publication/7345e3e8-98fc-11e7-b92d-01aa75ed71a1/language-en (accessed on 12 June 2018).
- Gopalakrishnan, R.; Goutam, S.; Oliveira, M.L.; Timmermans, J.M.; Omar, N.; Messagie, M.; Van den Bossche, P.; van Mierlo, J. A comprehensive study on rechargeable energy storage technologies. J. Electrochem. Energy Convers. Storage 2017, 13, 040801. [Google Scholar] [CrossRef]
- ISO. Environmental Management—Life Cycle Assessment—Principles and Framework, ISO 14040:2006. Available online: https://www.iso.org/standard/37456.html (accessed on 28 May 2018).
- Nelson, P.A.; Gallagher, K.G.; Bloom, I.; Dees, D.; Ahmed, S. BatPac version 3.1. Available online: http://www.cse.anl.gov/batpac/about.html (accessed on 28 May 2018).
- Cox, B.; Mutel, C.L.; Bauer, C.; Beltran, A.M.; van Vuuren, D.P. Uncertain environmental footprint of current and future battery electric vehicles. Environ. Sci. Technol. 2018, 52, 4989–4995. [Google Scholar] [CrossRef]
- Richa, K.; Babbitt, C.W.; Nenadic, N.G.; Gaustad, G. Environmental trade-offs across cascading lithium-ion battery life cycles. Int. J. Life Cycle Assess 2017, 22, 66–81. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, T.; Zhang, J.; Yang, F.; Yuan, C. Life cycle assessment of high capacity molybdenum disulfide lithium-ion battery for electric vehicles. Energy 2017, 123, 77–88. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Li, T.; Gao, X.; Yuan, C. Life cycle assessment of lithium sulfur battery for electric vehicles. J. Power Sources 2017, 343, 284–295. [Google Scholar] [CrossRef]
- Sanfélix, J. Multiregional Input-Output Life Cycle Analysis of a Battery Pack for Electric Vehicle Applications; VUB PRESS: Brussel, Belgium, 2015; ISBN 978-9-4619734-7-4. [Google Scholar]
- Ellingsen, L.A.W.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life cycle assessment of a lithium-ion battery vehicle pack. J. Ind. Ecol. 2014, 18, 113–124. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Li, J.; Yuan, C. Life cycle environmental impact of high-capacity lithium ion battery with silicon nanowires anode for electric vehicles. Environ. Sci. Technol. 2014, 48, 3047–3055. [Google Scholar] [CrossRef]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef]
- Notter, D.A.; Gauch, M.; Widmer, R.; Wäger, P.; Stamp, A.; Zah, R.; Althaus, H.J. Contribution of Li-ion batteries to the environmental impact of electric vehicles. Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean Prod. 2010, 18, 1517–1527. [Google Scholar] [CrossRef]
- Benavides, P.T.; Dai, Q.; Kelly, J.; Dunn, J.B. Addition of Nickel Cobalt Aluminum (NCA) Cathode Material to GREET2. Available online: https://greet.es.anl.gov/publication-NCA-Cathode-2016 (accessed on 28 May 2018).
- Gediga, J.; Sandilands, J.; Roomanay, N.; Boonzaier, S. Life Cycle Assessment of Nickel Products. Study Commissioned by the Nickel Institute. Available online: https://www.nickelinstitute.org/policy/nickel-life-cycle-management/nickel-life-cycle-data/ (accessed on 28 May 2018).
- Schmidt, T.; Buchert, M.; Schebek, L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour. Conserv. Recycl. 2016, 112, 107–122. [Google Scholar] [CrossRef]
- Wood, W.; Lee, D.; Frenzel, J.; Wandera, D.; Spitz, D.; Wimer, A.; Waterhouse, R.; Pekala, R.W. Structure-Property Relationships of Ceramic-Modified Separators. Available online: http://static.entek.com/160323CeramicModifiedSeparators-Final.pdf (accessed on 28 May 2018).
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- U.S. Department of the Interior, U.S. Geological Survey. Mineral Commodity Summaries 2017: U.S. Geological Survey. Available online: https://minerals.usgs.gov/minerals/pubs/mcs/2017/mcs2017.pdf (accessed on 28 May 2018).
- The London Metal Exchange. LME EV Battery Materials. Available online: https://www.lme.com/Trading/New-initiatives/Electric-Vehicle-Battery-Materials (accessed on 28 May 2018).
- Nelson, P.A.; Gallagher, K.G.; Bloom, I.; Dees, D.W. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles. Available online: http://www.cse.anl.gov/batpac/files/BatPaC ANL-12_55.pdf (accessed on 24 May 2018).
- The Conference Board. International Comparisons of Hourly Compensation Costs in Manufacturing, 2016 —Summary Tables. Available online: https://www.conference-board.org/ilcprogram/index.cfm?id=38269 (accessed on 23 August 2018).
- Eurostat. Hourly Labour Costs. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Hourly_labour_costs (accessed on 21 August 2018).
- Neal, T.; Rawlinson, S. International Construction Cost Report. Available online: https://www.arcadis.com/media/3/2/5/%7B325E15B1-332B-45FB-A70A-6115404D36B9%7D9110R_International Cost Construction Report.pdf (accessed on 30 October 2018).
- Eurostat. Harmonised Index of Consumer Prices. Available online: http://appsso.eurostat.ec.europa.eu/nui/show.do?query=BOOKMARK_DS-055100_QID_-6581D607_UID_-3F171EB0&layout=TIME,C,X,0;GEO,L,Y,0;UNIT,L,Z,0;COICOP,L,Z,1;INDICATORS,C,Z,2;&zSelection=DS-055100UNIT,INX_A_AVG;DS-055100COICOP,CP00;DS-055100INDICATORS,OBS_FLAG (accessed on 23 August 2018).
- National Bureau of Statistics of China. National Data. Available online: http://data.stats.gov.cn/english/easyquery.htm?cn=C01 (accessed on 23 August 2018).
- Bureau of Labor Statistics. Databases, Tables and Calculators by Subject. Available online: https://data.bls.gov/pdq/SurveyOutputServlet (accessed on 24 August 2018).
- Statistics Korea. Prices. Available online: http://kostat.go.kr/portal/eng/pressReleases/9/1/index.board (accessed on 23 August 2018).
- Frankel, D.; Wagner, A. Battery Storage: The Next Disruptive Technology in the Power Sector. Available online: https://www.mckinsey.com/business-functions/sustainability/our-insights/battery-storage-the-next-disruptive-technology-in-the-power-sector (accessed on 31 October 2018).
- The Boston Consulting Group. Batteries for Electric Cars. Challenges, Opportunities, and the Outlook to 2020. Available online: https://www.bcg.com/documents/file36615.pdf (accessed on 18 June 2018).
- Philippot, M.; Smekens, J.; Van Mierlo, J.; Messagie, M. Life cycle assessment of silicon alloy based lithium-ion battery for electric vehicles. In WIT Transactions on The Built Environment; WIT Press: Southampton, UK, 2018. [Google Scholar]
- Kim, H.C.; Wallington, T.J.; Arsenault, R.; Bae, C.; Ahn, S.; Lee, J. Cradle-to-gate emissions from a commercial electric vehicle li-ion battery: A comparative analysis. Environ. Sci. Technol. 2016, 50, 7715–7722. [Google Scholar] [CrossRef]
- US Energy Information Administration. Levelized Cost and Levelized Avoided Cost of New Generation Resources in the Annual Energy Outlook 2018. Available online: http://www.eia.gov/outlooks/aeo/assumptions/ (accessed on 3 September 2018).
- Peters, J.F.; Weil, M. Providing a common base for life cycle assessments of Li-Ion batteries. J. Clean Prod. 2017, 171, 704–713. [Google Scholar] [CrossRef]
- Gaines, L.; Burnham, A.; Gaines, L.; Belharouak, I. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling Paper. Available online: https://www.researchgate.net/publication/265158823_Paper_No_11-3891_Life-Cycle_Analysis_for_Lithium-Ion_Battery_Production_and_Recycling (accessed on 28 May 2018).
- Richa, K.; Babbitt, C.W.; Gaustad, G. Eco-efficiency analysis of a lithium-ion battery waste hierarchy inspired by circular economy. J. Ind. Ecol. 2017, 21, 715–730. [Google Scholar] [CrossRef]
- Sanfélix, J.; de la Rúa, C.; Schmidt, J.H.; Messagie, M.; Van Mierlo, J. Environmental and economic performance of an li-ion battery pack: A multiregional input-output approach. Energies 2016, 9, 584. [Google Scholar] [CrossRef]
- Marmiroli, B.; Messagie, M.; Dotelli, G.; Van Mierlo, J. Electricity generation in lca of electric vehicles: A review. Appl. Sci. 2018, 8, 1384. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Projected Costs of Generating Electricity 2015 Edition. Available online: http://www.iea.org/publications/freepublications/publication/ElecCost2015.pdf (accessed on 3 September 2018).

| Component | Weight | Mass Share |
|---|---|---|
| Positive electrode paste | 25.19 g | 37.58% |
| Negative electrode paste | 15.66 g | 23.37% |
| Separator | 1.31 g | 1.95% |
| Substrate, positive electrode | 1.75 g | 2.61% |
| Substrate, negative electrode | 4.79 g | 7.15% |
| Electrolyte | 6.47 g | 9.65% |
| Cell container, tab, and terminals | 11.85 g | 17.68% |
| Country | Cost of Building (€/m2) | Labor Cost (€/h) |
|---|---|---|
| China | 1950 | 3.1 |
| France | 4390 | 38.1 |
| Germany | 4020 | 39.4 |
| Korea | 2810 | 17 |
| Poland | 2060 | 9.1 |
| Sweden | 4750 | 41.1 |
| USA | 3636 | 13.1 |
| Cost Item | Cost Contribution | ||||
|---|---|---|---|---|---|
| Annual cell production volume (cells) | 7 × 107 | 1 × 108 | 5 × 108 | 1 × 109 | 2 × 109 |
| Factory capacity (GWh) | 1.2 | 1.7 | 8.7 | 17.4 | 34.8 |
| Materials and purchased items | 64% | 66% | 72% | 74% | 77% |
| Direct labor | 4% | 4% | 2% | 2% | 2% |
| Variable overhead | 3% | 3% | 2% | 2% | 2% |
| General, sales, administration | 4% | 4% | 3% | 3% | 2% |
| Research and development | 4% | 3% | 3% | 3% | 2% |
| Depreciation | 9% | 9% | 7% | 6% | 6% |
| Profit | 6% | 6% | 5% | 5% | 4% |
| Warranty | 5% | 5% | 5% | 5% | 5% |
| Annual Cell Production Volume (cells) | 7 × 107 | 1 × 108 | 5 × 108 | 1 × 109 | 2 × 109 |
|---|---|---|---|---|---|
| Impact on CC (kg CO2 eq/kWh) | 168 | 157 | 123 | 114 | 107 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippot, M.; Alvarez, G.; Ayerbe, E.; Van Mierlo, J.; Messagie, M. Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs. Batteries 2019, 5, 23. https://doi.org/10.3390/batteries5010023
Philippot M, Alvarez G, Ayerbe E, Van Mierlo J, Messagie M. Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs. Batteries. 2019; 5(1):23. https://doi.org/10.3390/batteries5010023
Chicago/Turabian StylePhilippot, Maeva, Garbiñe Alvarez, Elixabete Ayerbe, Joeri Van Mierlo, and Maarten Messagie. 2019. "Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs" Batteries 5, no. 1: 23. https://doi.org/10.3390/batteries5010023
APA StylePhilippot, M., Alvarez, G., Ayerbe, E., Van Mierlo, J., & Messagie, M. (2019). Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs. Batteries, 5(1), 23. https://doi.org/10.3390/batteries5010023







