Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors
Abstract
:1. Introduction
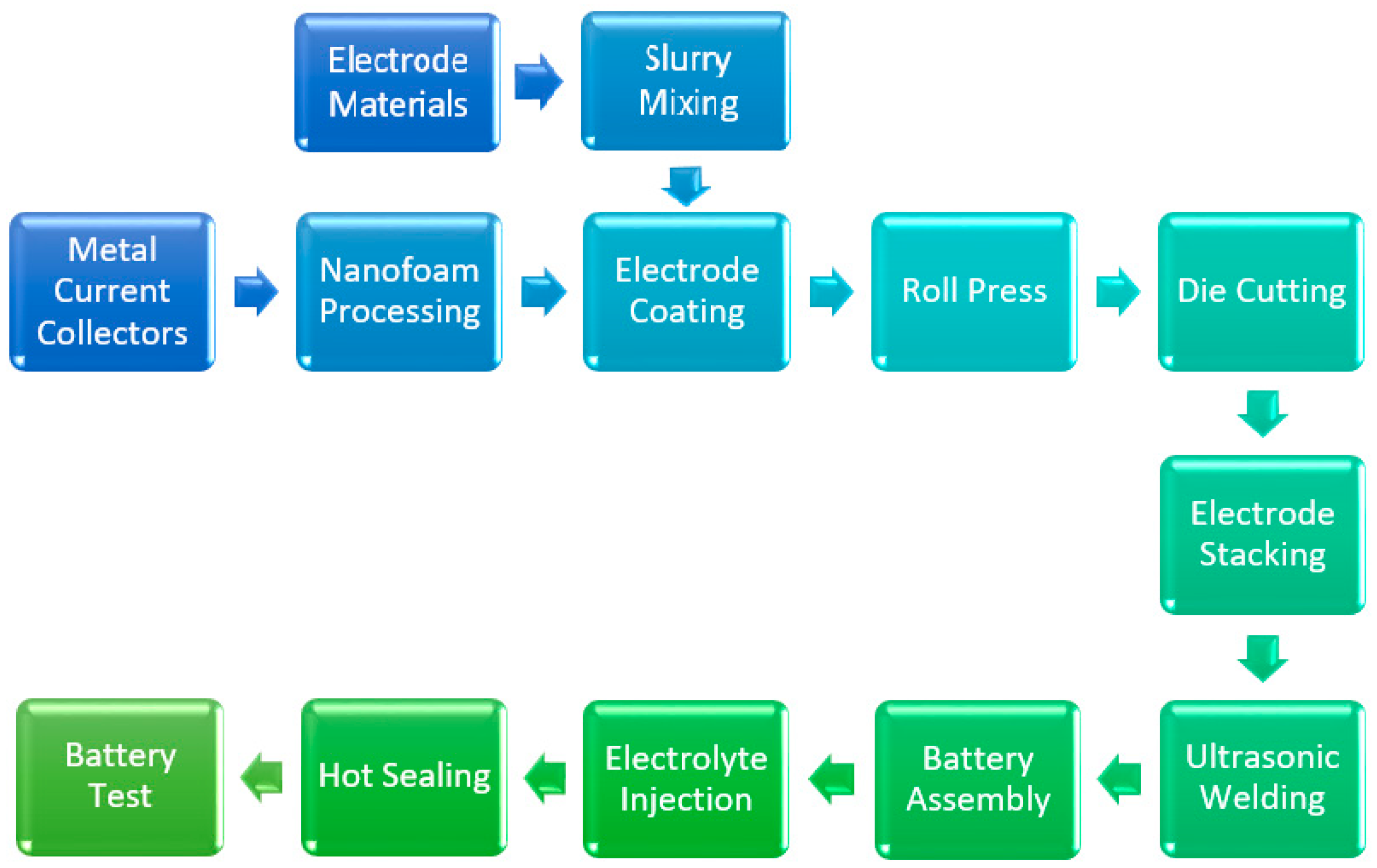
2. Experimental
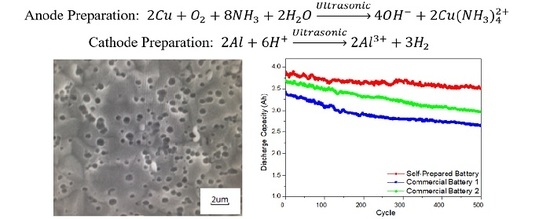
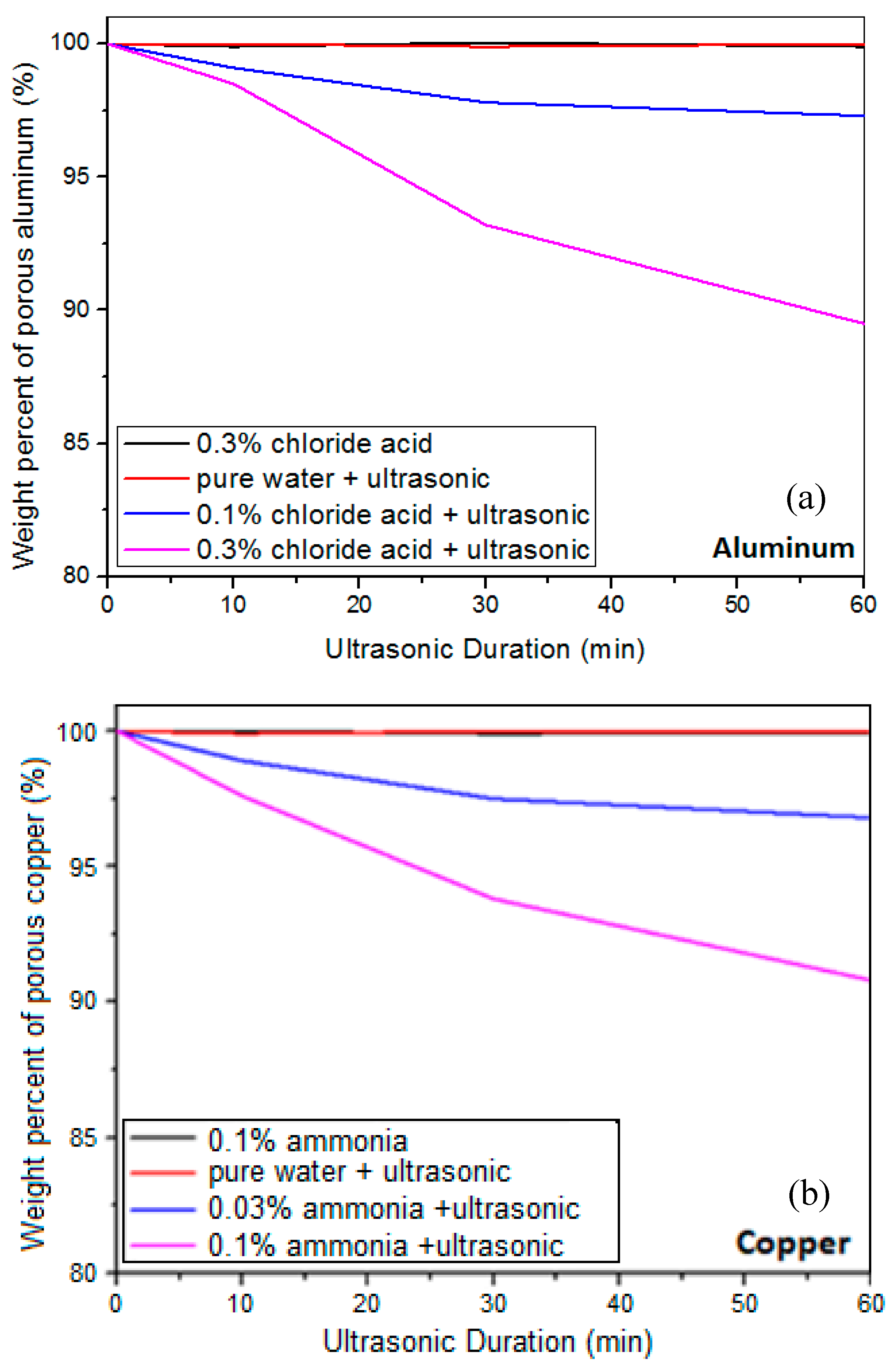
2.1. Preparation of Nanoporous Metal Current Collectors
2.2. Battery Assembly and Electrochemical Test of Lithium Ion Batteries with Nanoporous Current Collectors
3. Results and Discussion
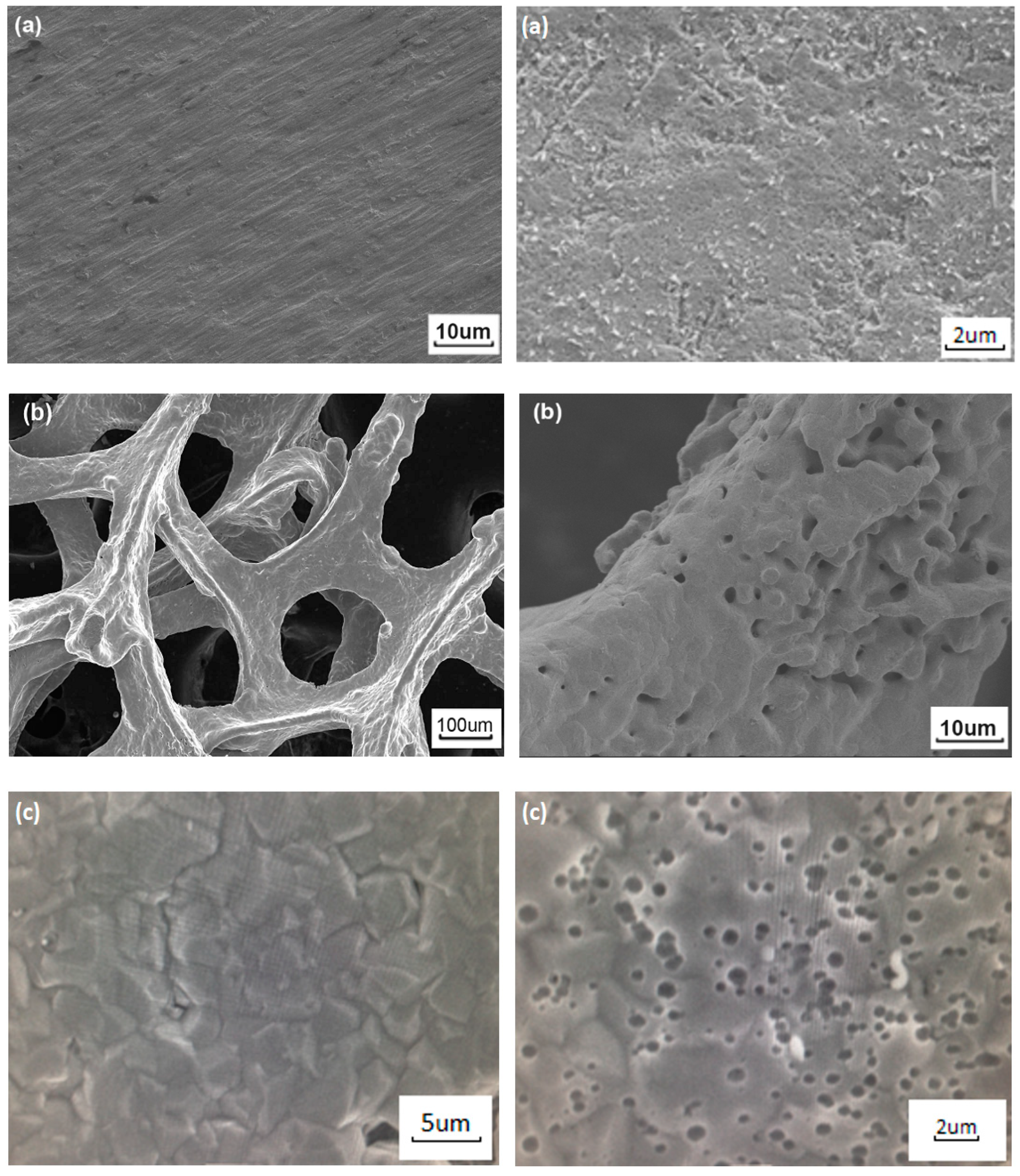
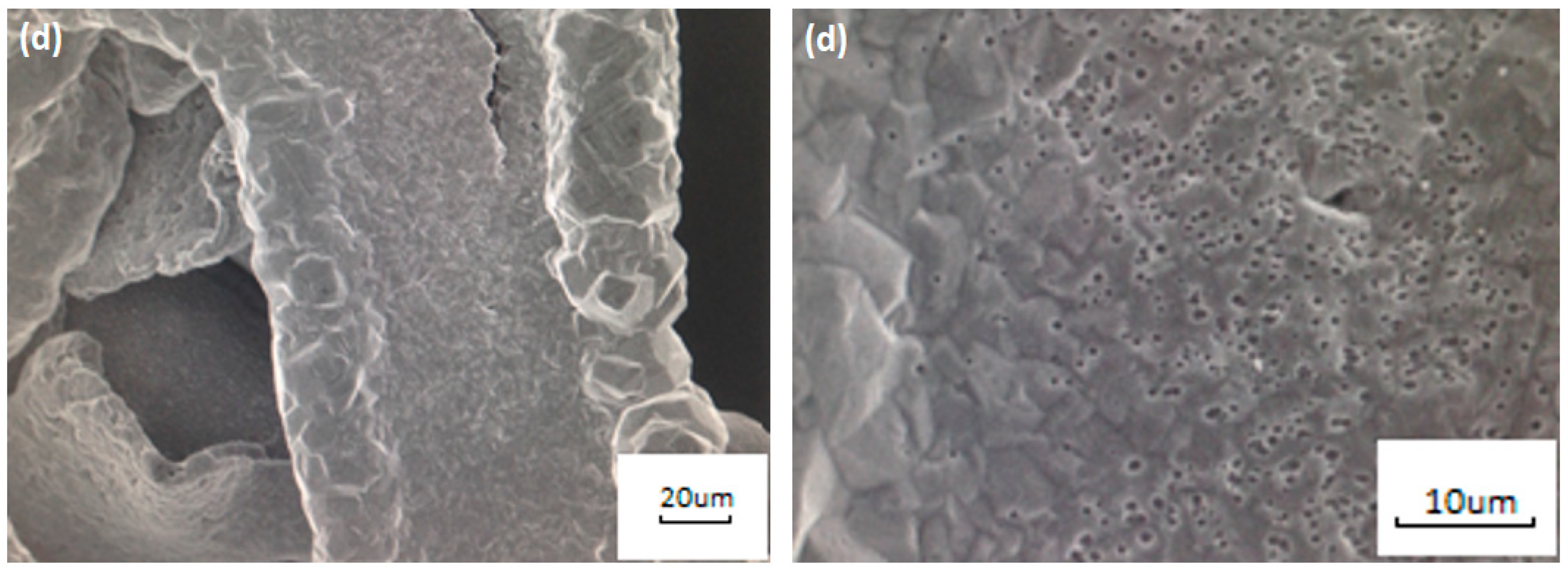
3.1. Formation of a Nanoporous Structure on the Surface of Metal Current Collectors
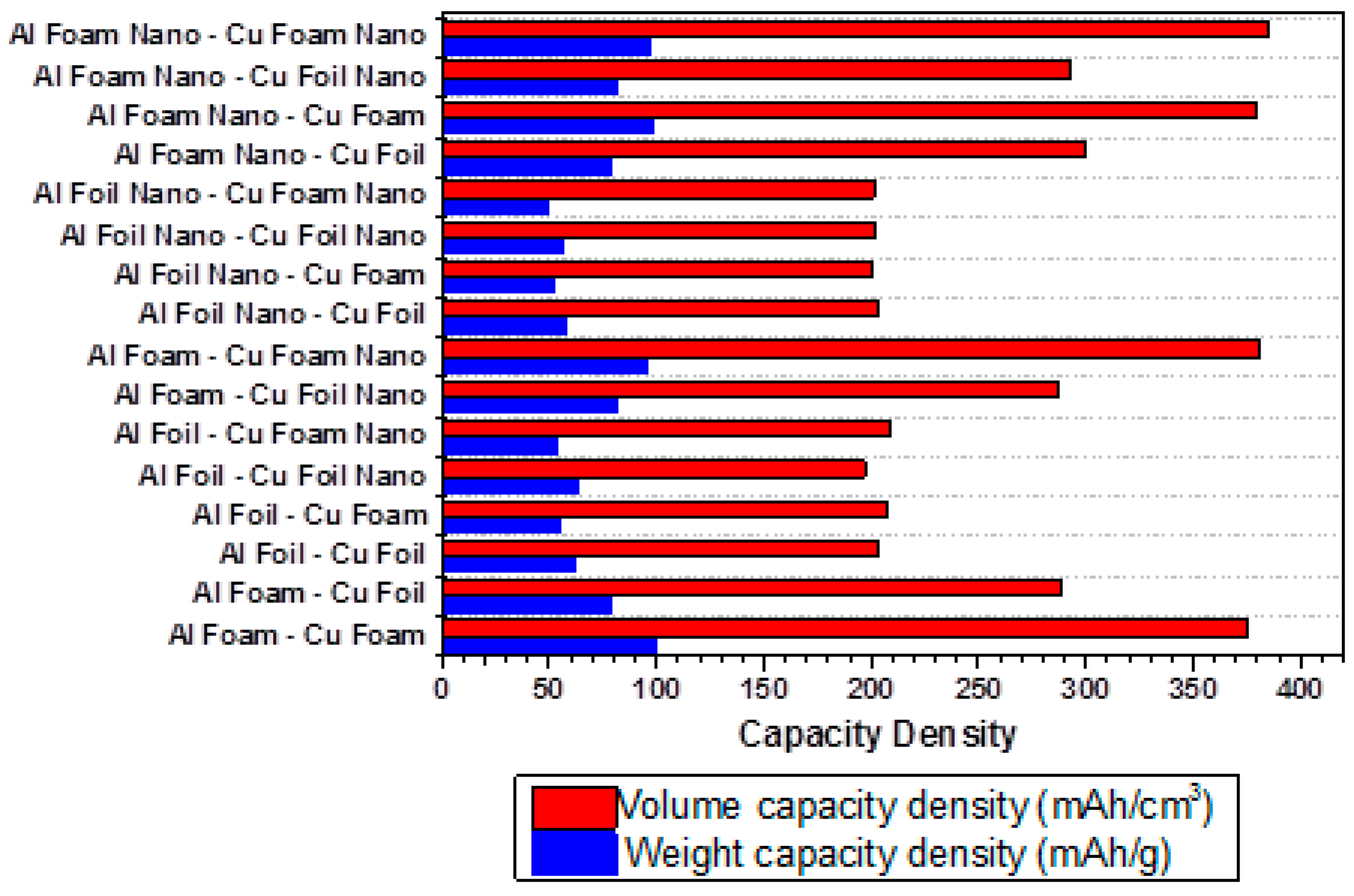
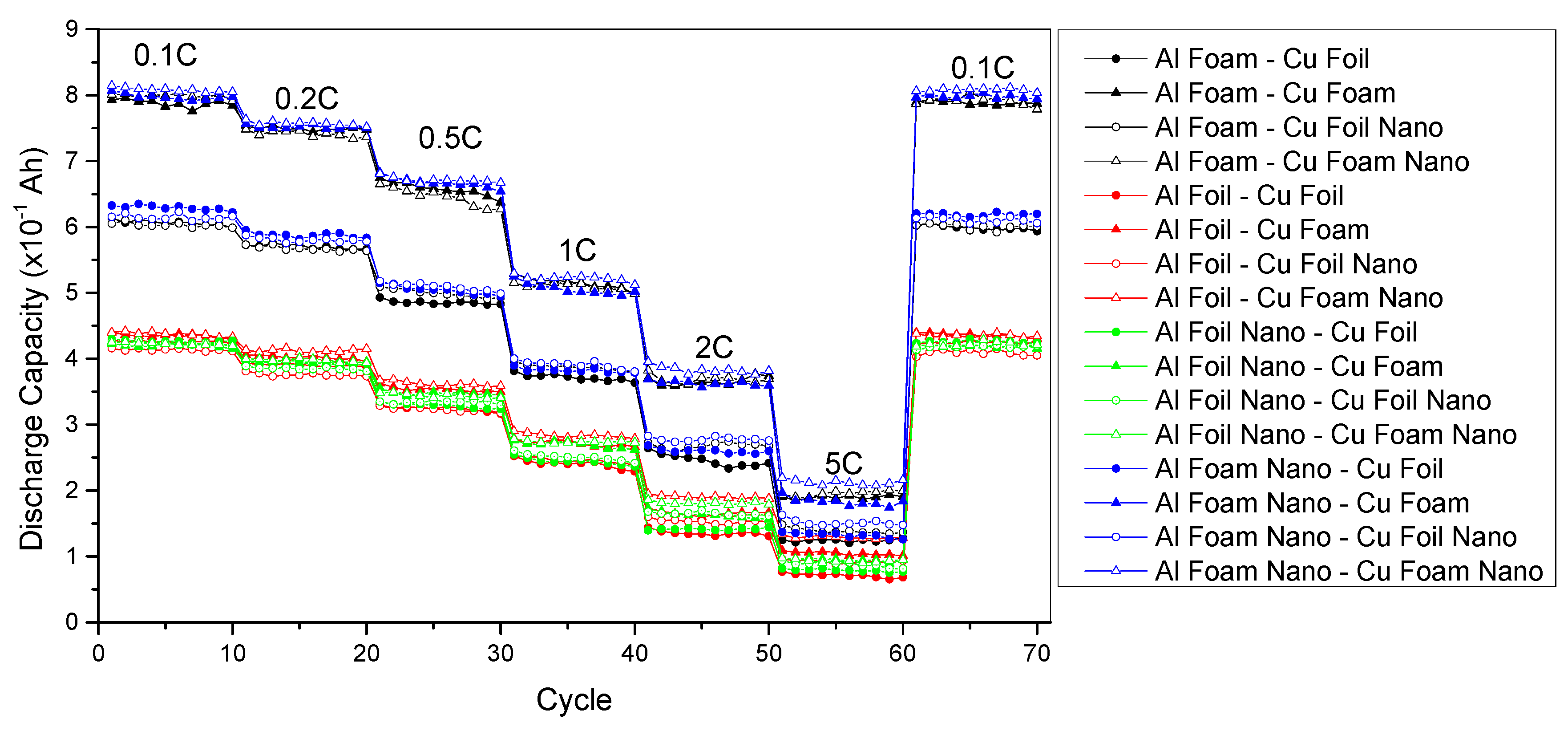
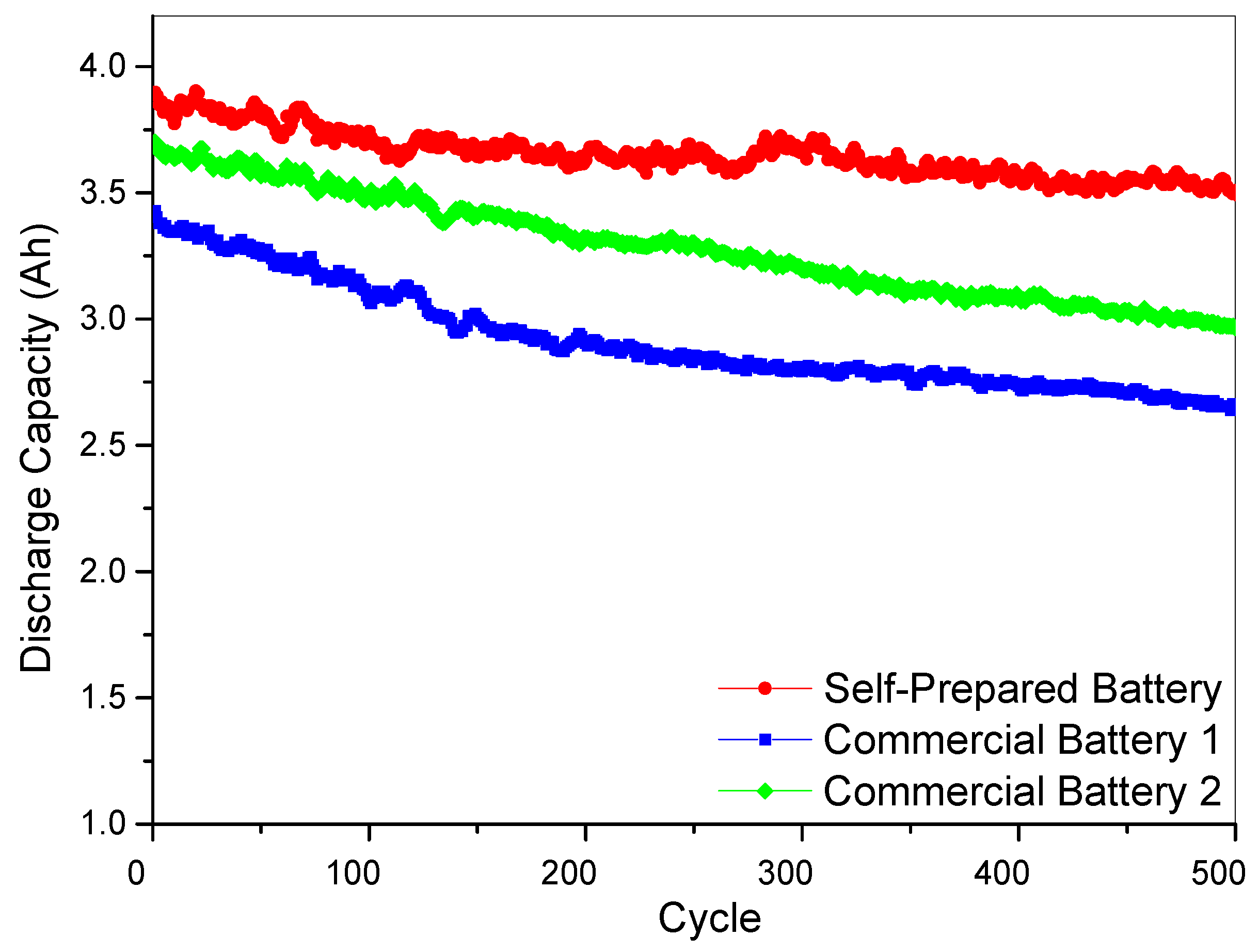
3.2. Electrochemical Performance of Lithium Ion Batteries with Different Nanoporous Metal Current Collectors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Soloveichik, G. Battery technologies for large-scale stationary energy storage. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, S.; Wan, L.; Xiao, F.; Wang, S. Electrodeposition of manganese oxide nanosheets on a continuous three dimensional nickel porous scaffold for high performance electrochemical capacitors. J. Power Sources 2014, 245, 1027–1034. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Shen, L.; Uchaker, E.; Zhang, X.; Cao, G. Hydrogenated Li4Ti5O12 nanowire arrays for high rate lithium ion batteries. Adv. Mater. 2012, 24, 6502–6506. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ou, C.; Huang, Y.; Shen, Y.; Li, N.; Zhang, H. Towards fast and ultralong-life Li-ion battery anodes: Embedding ultradispersed TiO2 quantum dots into three-dimensional porous graphene-like networks. Electrochim. Acta 2017, 246, 1183–1192. [Google Scholar] [CrossRef]
- Prada, E.; Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified electrochemical and thermal model of LiFePO4-graphite li-ion batteries for fast charge applications. J. Electrochem. Soc. 2012, 159, A1508–A1519. [Google Scholar] [CrossRef]
- Martens, J.; Jammaer, J.; Bajpe, S.; Aerts, A.; Lorgouilloux, Y.; Kirschhock, C. Simple synthesis recipes of porous materials. Microporous Mesoporous Mater. 2011, 140, 2–8. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Akagi, R.; Umemura, S. Enhancement of high-intensity focused ultrasound heating by short-pulse generated cavitation. Appl. Sci. 2017, 7, 7030288. [Google Scholar] [CrossRef]
- Skorb, E.; Fix, D.; Shchukin, D.; Möhwald, H.; Sviridov, D.; Mousa, R.; Wanderka, N.; Schäferhans, J.; Pazos-Pérez, N.; Fery, A.; et al. Sonochemical formation of metal sponges. Nanoscale 2011, 3, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.; Shchukin, D.G.; Möhwald, D.H.; Andreeva, D. Ultrasound-driven design of metal surface nanofoams. Nanoscale 2010, 2, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, X.; Braun, P. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 2011, 6, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Dunn, B. Hierarchical battery electrodes based on inverted opal structures. J. Mater. Chem. 2002, 12, 2859–2861. [Google Scholar] [CrossRef]
- Pikul, J.; Zhang, H.; Cho, J.; Braun, P.; King, W. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, I.; Brinke, G.; Loos, K. Block copolymer template-directed synthesis of well-ordered metallic nanostructures. Polymer 2013, 54, 2591–2605. [Google Scholar] [CrossRef]
- Pray, H.; Schweickert, C.; Minnich, B. Solubility of hydrogen, oxygen, nitrogen, and helium in water at elevated temperatures. Ind. Eng. Chem. 1952, 44, 1146–1151. [Google Scholar] [CrossRef]
- Carpenter, J. New measurements of oxygen solubility in pure and natural water. Limnol. Oceanogr. 1966, 11, 264–277. [Google Scholar] [CrossRef]
| Slurry | Material | Amount (g) |
|---|---|---|
| Cathode Slurry | NMC | 20 |
| NMP solvent | 38 | |
| PVDF | 1.2 | |
| Conductive ethylene black | 1.2 | |
| Anode Slurry | Graphite | 36 |
| NMP solvent | 30 | |
| PVDF | 1.8 |
| Battery | Capacity (mAh) | Weight (g) | Length (cm) | Width (cm) | Height (cm) | Weight Density (mAh g−1) | Volume Density (mAh cm−3) |
|---|---|---|---|---|---|---|---|
| Al Foil-Cu Foil Nano | 413.8 | 6.57 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 62.98 | 197.05 |
| Al Foil Nano-Cu Foam | 421.35 | 8.02 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 52.54 | 200.64 |
| Al Foil Nano-Cu Foam Nano | 423.12 | 8.64 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 48.97 | 201.49 |
| Al Foil Nano-Cu Foil Nano | 423.68 | 7.43 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 57.02 | 201.75 |
| Al Foil-Cu Foil | 426.14 | 6.85 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 62.21 | 202.92 |
| Al Foil Nano-Cu Foil | 426.15 | 7.41 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 57.51 | 202.93 |
| Al Foil-Cu Foam | 435.81 | 7.92 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 55.03 | 207.53 |
| Al Foil-Cu Foam Nano | 437.28 | 8.08 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 54.12 | 208.23 |
| Al Foam-Cu Foil Nano | 603.23 | 7.39 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 81.63 | 287.25 |
| Al Foam-Cu Foil | 607.19 | 7.72 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 78.65 | 289.14 |
| Al Foam Nano-Cu Foil Nano | 614.47 | 7.57 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 81.17 | 292.61 |
| Al Foam Nano-Cu Foil | 629.9 | 7.95 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 79.23 | 299.95 |
| Al Foam-Cu Foam | 787.97 | 7.86 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 100.25 | 375.22 |
| Al Foam Nano-Cu Foam | 797.4 | 8.16 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 97.72 | 379.71 |
| Al Foam-Cu Foam Nano | 799.78 | 8.33 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 96.01 | 380.85 |
| Al Foam Nano-Cu Foam Nano | 808.57 | 8.31 | 3.5 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.01 | 97.3 | 385.03 |
| Current Collector Match | Average Capacity (×10−1 Ah) under 5C Rate | Percent of Change with Nanoporous Structure |
|---|---|---|
| Al Foam-Cu Foam | 1.899537 | -- |
| Al Foam Nano-Cu Foam | 1.830201 | −3.65% |
| Al Foam-Cu Foam Nano | 1.958342 | 3.10% |
| Al Foam Nano-Cu Foam Nano | 2.124354 | 11.84% |
| Al Foam-Cu Foil | 1.244933 | -- |
| Al Foam-Cu Foil Nano | 1.387696 | 11.47% |
| Al Foam Nano-Cu Foil | 1.322247 | 6.21% |
| Al Foam Nano-Cu Foil Nano | 1.512555 | 21.50% |
| Al Foil-Cu Foil | 0.713199 | -- |
| Al Foil Nano-Cu Foil | 0.788985 | 10.63% |
| Al Foil-Cu Foil Nano | 0.921765 | 29.24% |
| Al Foil Nano-Cu Foil Nano | 0.878092 | 23.12% |
| Al Foil-Cu Foam | 1.046454 | -- |
| Al Foil-Cu Foam Nano | 1.292135 | 23.48% |
| Al Foil Nano-Cu Foam | 1.026151 | −1.94% |
| Al Foil Nano-Cu Foam Nano | 1.031377 | −1.44% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Chen, Y.; Wang, Y. Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors. Batteries 2019, 5, 21. https://doi.org/10.3390/batteries5010021
Feng H, Chen Y, Wang Y. Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors. Batteries. 2019; 5(1):21. https://doi.org/10.3390/batteries5010021
Chicago/Turabian StyleFeng, Huajun, Yuan Chen, and Yihua Wang. 2019. "Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors" Batteries 5, no. 1: 21. https://doi.org/10.3390/batteries5010021
APA StyleFeng, H., Chen, Y., & Wang, Y. (2019). Electrochemical Performance of a Lithium Ion Battery with Different Nanoporous Current Collectors. Batteries, 5(1), 21. https://doi.org/10.3390/batteries5010021