Effect of Prelithiation Process for Hard Carbon Negative Electrode on the Rate and Cycling Behaviors of Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Electrodes Preparation
2.2. Lithium Nickel Cobalt Manganese Oxide (NCM)/Li and Hard Carbon (HC)/Li Half-Cells Assembly
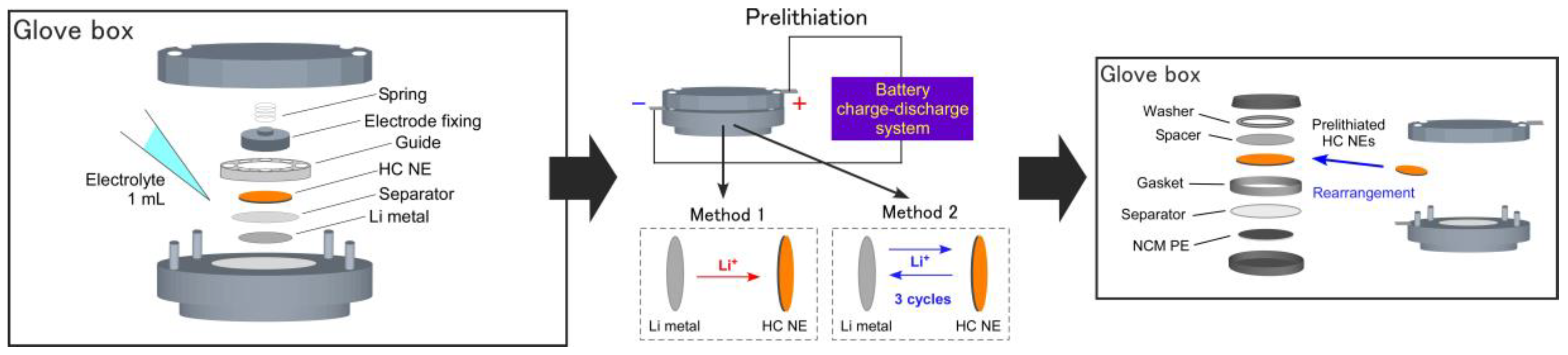
2.3. Prelithiation Processes and the NCM/HC Full-Cell Assembly
2.4. Electrochemical Measurements
2.5. X-Ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
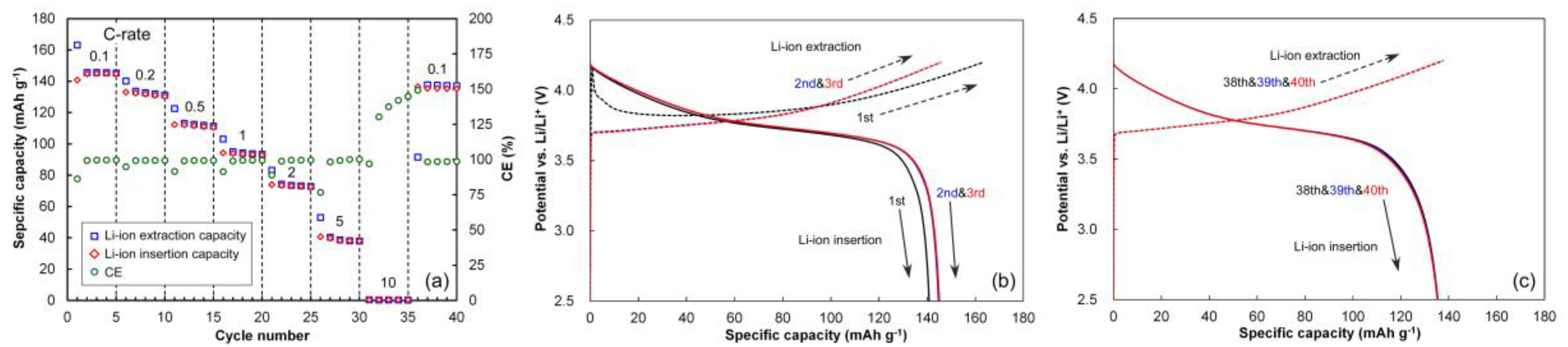
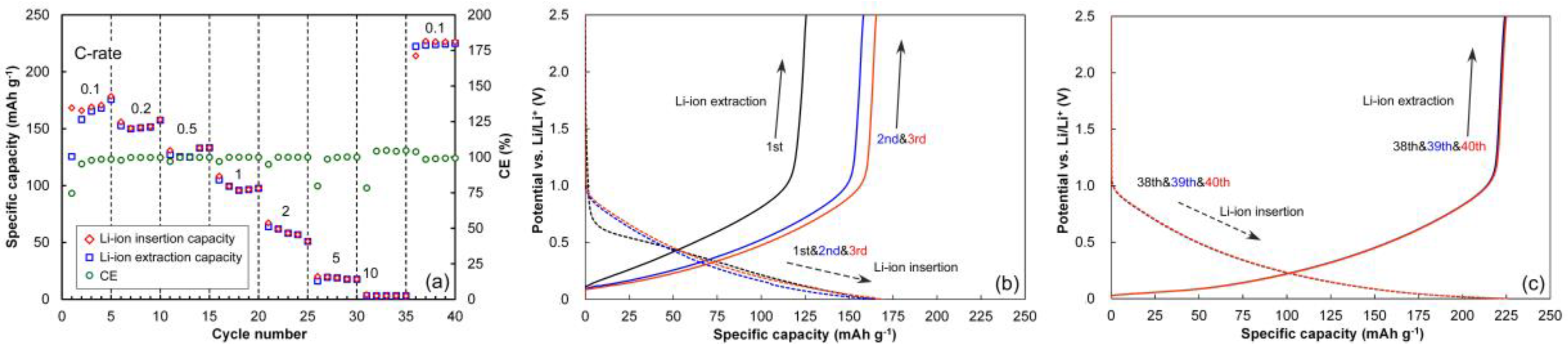
3.1. Half-Cell Performances of NCM and HC Electrodes
3.2. Prelithiation of the HC Negative Electrodes (NEs) and the NCM/HC Full-Cell Design
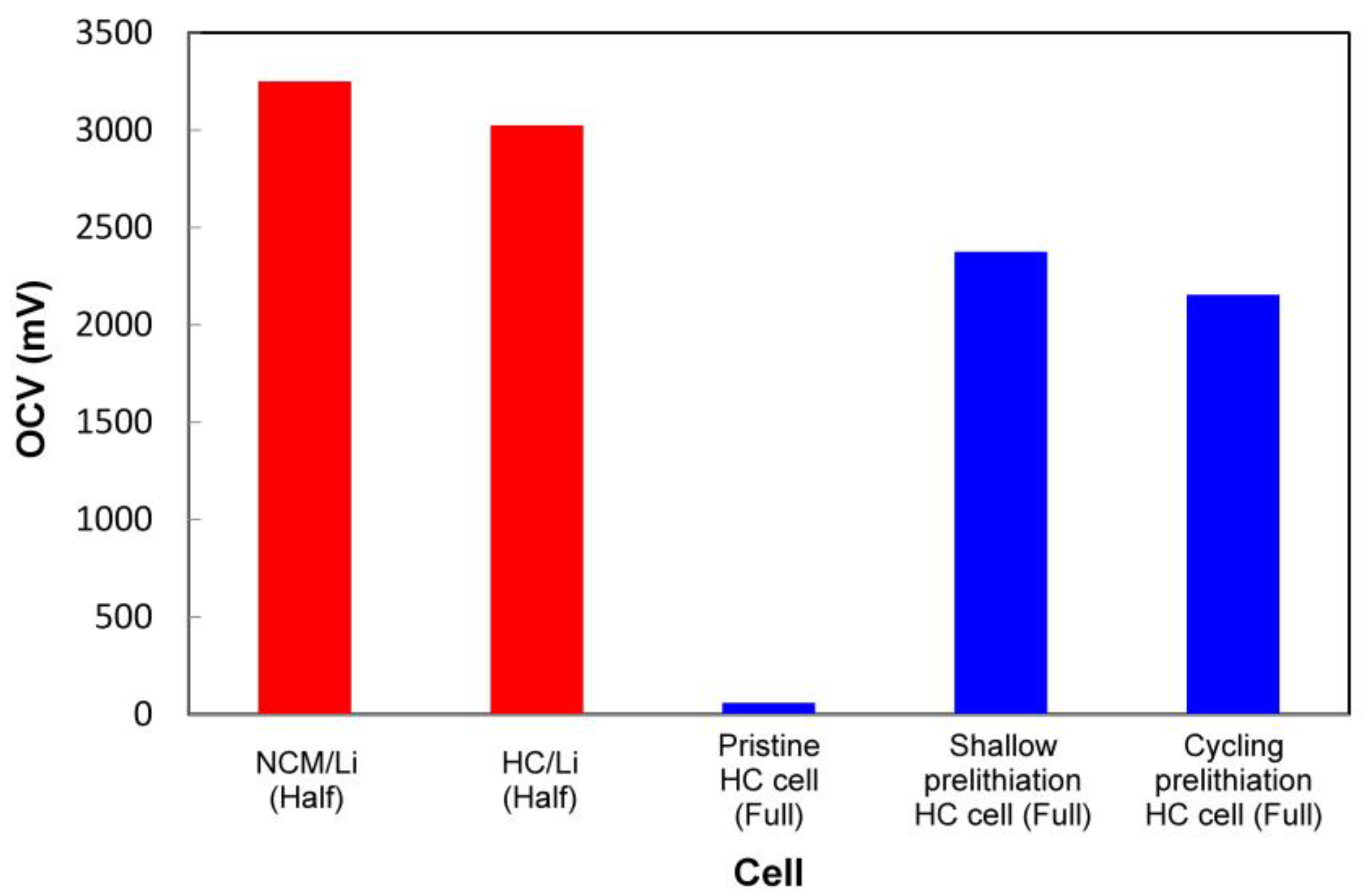
3.3. Open Circuit Voltage (OCV) Measurement after Cell Assembly
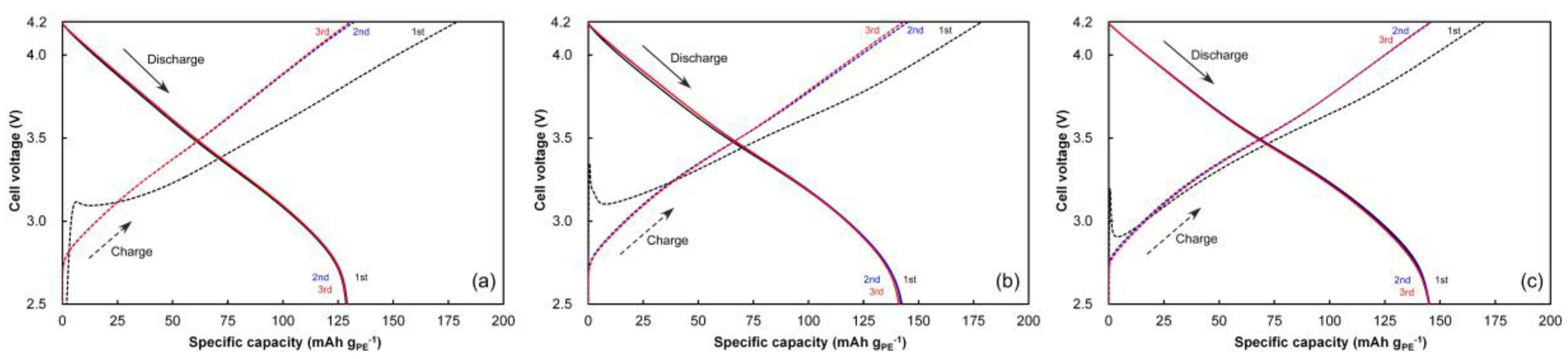
3.4. Pre-Cycling for Cell Formation
3.5. Cycling Behavior
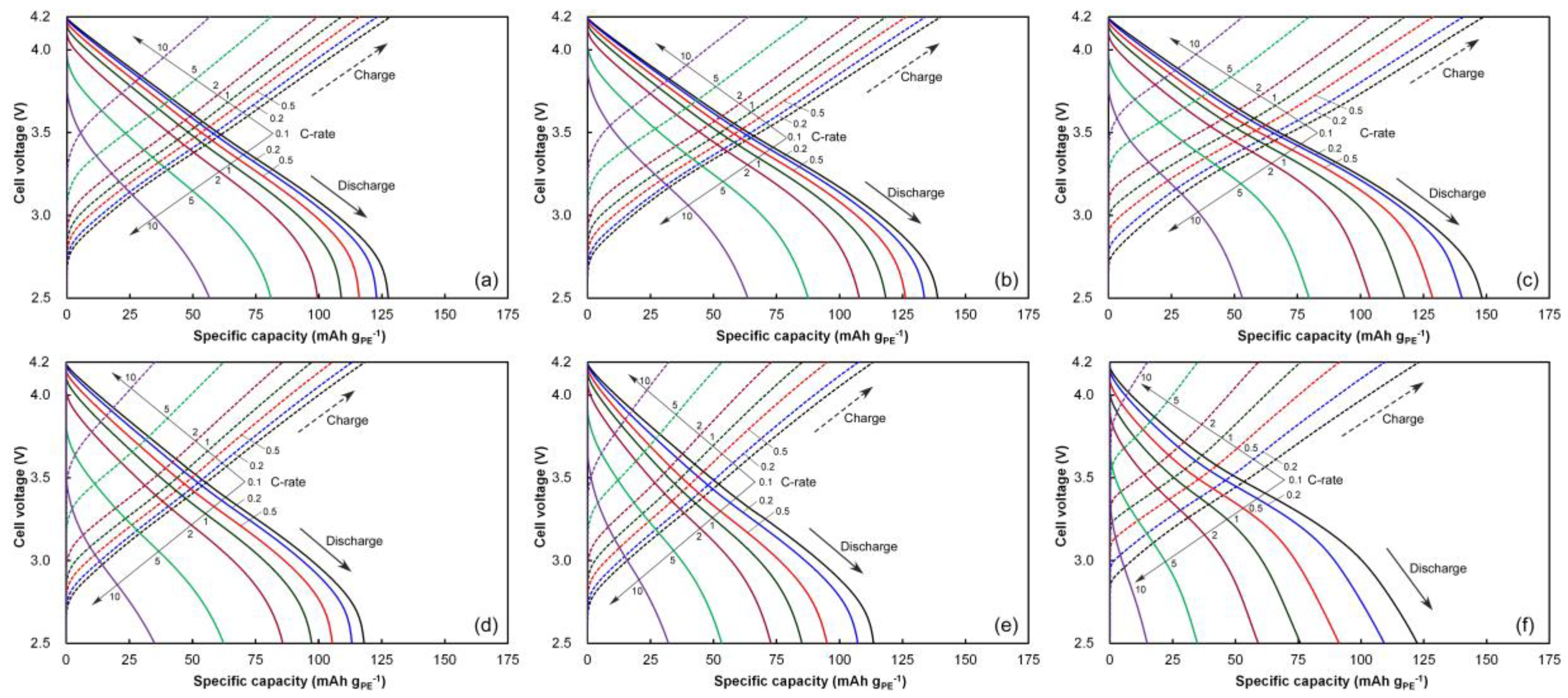
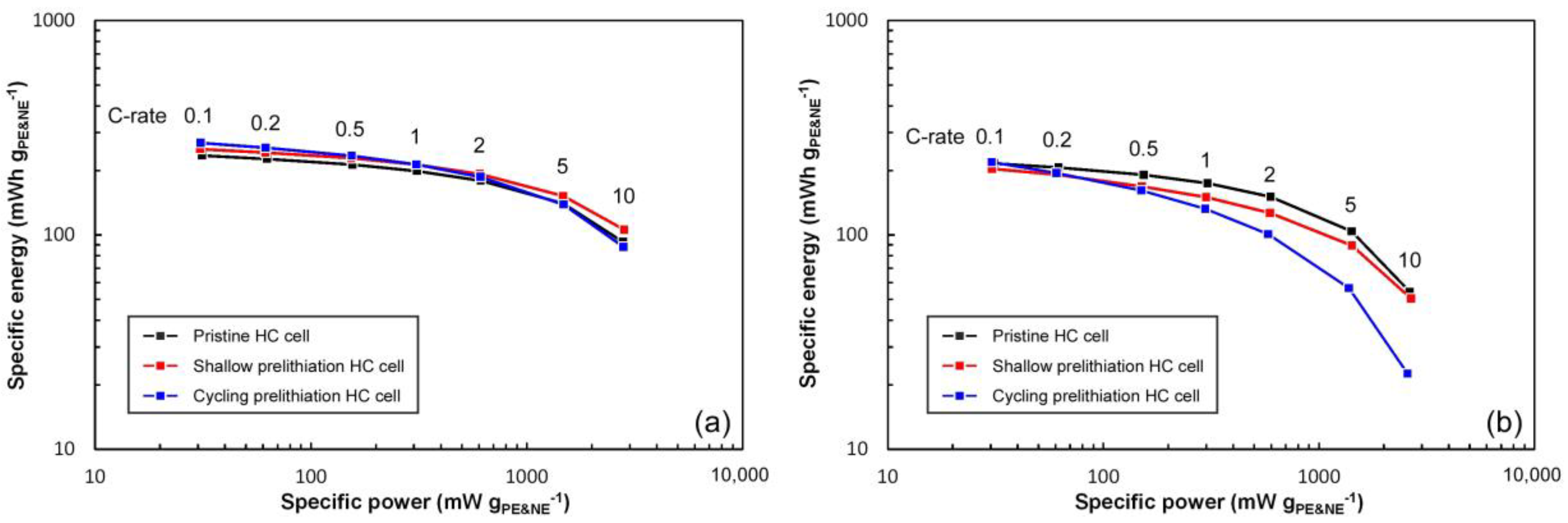
3.6. Rate Behaviors
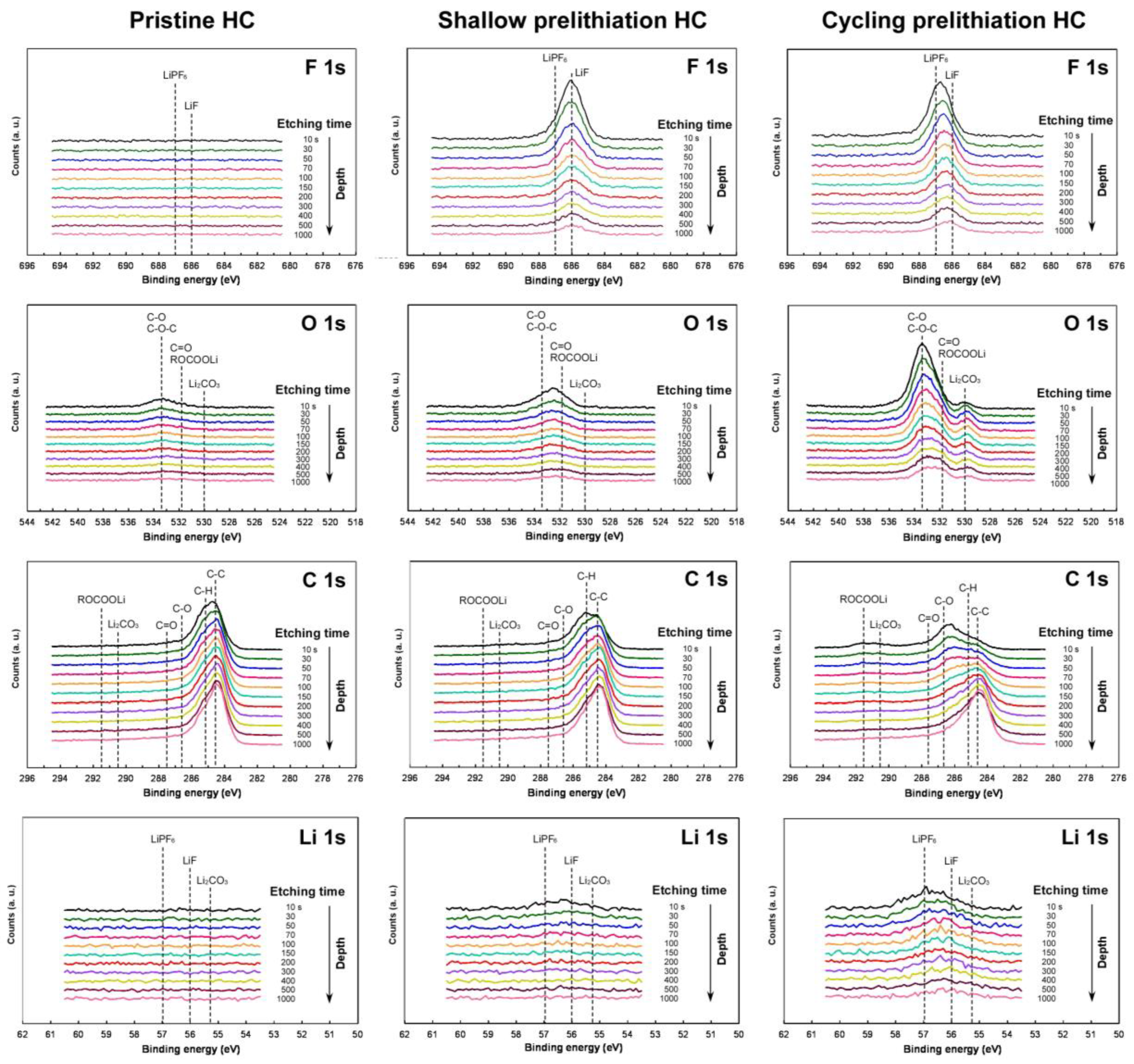
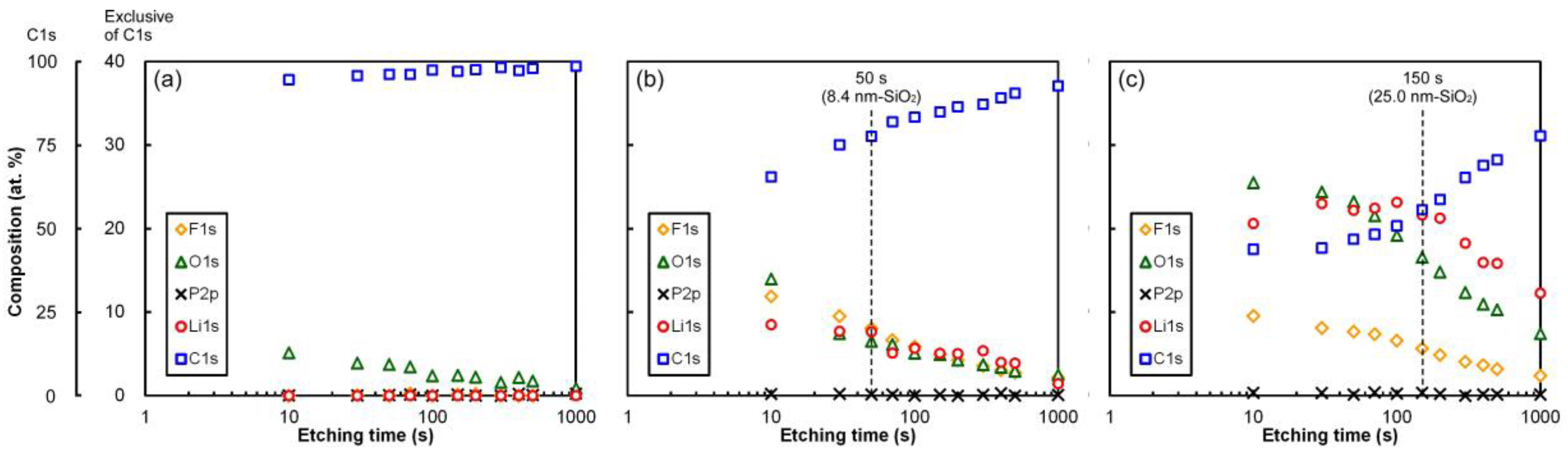
3.7. XPS for Pristine and Prelithiated HC Electrodes
3.8. Effect of Prelithiation Process on Cell Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspective. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Bresser, D.; Hosoi, K.; Howell, D.; Li, H.; Zeisel, H.; Amine, K.; Passerini, S. Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Sources 2018, 382, 176–178. [Google Scholar]
- Andwari, A.M.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A review of battery electric vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Andre, D.; Hain, H.; Lamp, P.; Maglia, F.; Stiaszny, B. Future high-energy density anode materials from an automotive application perspective. J. Mater. Chem. A 2017, 5, 17174–17198. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Yang, X.; Zuo, Z.; Wang, H.; Chen, Q.; Zhang, H.; Huang, Z.; Wu, B.; Zhou, H. The contradiction between the half-cell and full-battery evaluations on the tungsten-coating LiNi0.5Co0.2Mn0.3O2 cathode. Electrochim. Acta 2015, 180, 604–609. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Z.; Sun, Z.; Wu, T.; Gao, Y.; Li, H.; Li, F.; Niu, L. An advanced lithium ion battery based on a high quality graphitic graphene anode and a Li[Ni0.6Co0.2Mn0.2]O2 cathode. Electrochim. Acta 2018, 259, 48–55. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, R.; Wang, L.; Cheng, K.; Zhao, Z.; Mu, D.B.; Wu, B. A short review on layered LiNi0.8Co0.1Mn0.1O2 positive electrode material for lithium-ion batteries. Energy Procedia 2017, 105, 2941–2952. [Google Scholar]
- Azuma, H.; Imoto, H.; Yamada, S.; Sekai, K. Advanced carbon anode materials for lithium ion cells. J. Power Sources 1999, 81–82, 1–7. [Google Scholar] [CrossRef]
- Eftekhari, A. Low voltage anode materials for lithium-ion batteries. Energy Storage Mater. 2017, 7, 157–180. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Han, S. Improving the initial Coulombic efficiency of hard carbon-based anode for rechargeable batteries with high energy density. J. Mater. Sci. 2017, 52, 10418–10430. [Google Scholar] [CrossRef]
- Ni, J.; Huang, Y.; Gao, L. A high-performance hard carbon for Li-ion batteries and supercapacitors application. J. Power Sources 2013, 223, 306–311. [Google Scholar] [CrossRef]
- Yu, H.; Dong, X.; Pang, Y.; Wang, Y.; Xia, Y. High power lithium-ion battery based on spinel cathode and hard carbon anode. Electrochim. Acta 2017, 228, 251–258. [Google Scholar] [CrossRef]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-Lithiation strategies for rechargeble energy storage technologies: Concepts, promises and challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
- Libich, J.; Máca, J.; Vondrák, J.; Cech, O.; Sedlaríková, M. Irreversible capacity and rate-capability properties of lithium-ion negative electrode based on natural graphite. J. Energy Storage 2017, 14, 383–390. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Zhang, Z.; Yuan, S.; Amine, K.; Battaglia, V.; Liu, G. Application of Stabilized Lithium Metal Powder (SLMP®) in graphite anode—A high efficient prelithiation method for lithium-ion batteries. J. Power Sources 2014, 260, 57–61. [Google Scholar] [CrossRef]
- Jarvis, C.R.; Lain, M.J.; Yakovleva, M.V.; Gao, Y. A prelithiated carbon anode for lithium-ion battery applications. J. Power Sources 2006, 162, 800–802. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene balls for lithium rechargeable batteries with fast charging and high volumetric energy densities. Nat. Commun. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Sun, H.; He, X.; Ren, J.; Li, J.; Jiang, C.; Wan, C. Hard carbon/lithium composite anode materials for Li-ion batteries. Electrochim. Acta 2007, 52, 4312–4316. [Google Scholar] [CrossRef]
- Wang, H.; Lai, C.; Xiao, Y.; Ai, X. A new lithium-ion battery with LiNi0.80Co0.15Al0.05O2 cathode and lithium pre-doping hard carbon anode. Mater. Lett. 2015, 160, 250–254. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, J.; Shi, J.; Shi, Z. Different types of pre-lithiated hard carbon as negative electrode material for lithium-ion capacitors. Electrochim. Acta 2016, 187, 134–142. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Dong, X.; Wang, Y.; Xia, Y. A simple prelithiation strategy to build a high-rate and long-life lithium-ion battery with improved low-temperature performance. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar] [CrossRef]
- Hori, H.; Shikano, M.; Kobayashi, H.; Koike, S.; Sakaebe, H.; Saito, Y.; Tatsumi, K.; Yoshikawa, H.; Ikenaga, E. Analysis of hard carbon for lithium-ion batteries by hard X-ray photoelectron spectroscopy. J. Power Sources 2013, 242, 844–847. [Google Scholar] [CrossRef]
- Guan, T.; Sun, S.; Yu, F.; Gao, Y.; Fan, P.; Zuo, P.; Du, C.; Yin, G. The degradation of LiCoO2/graphite batteries at different rates. Electrochim. Acta 2018, 279, 204–212. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Placke, T.; Streipert, B.; Rothermel, S.; Wagner, R.; Meister, P.; Laskovic, I.C.; Winter, M. A tutorial into practical capacity and mass balancing of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A2479–A2486. [Google Scholar] [CrossRef]
- Loeffler, N.; Zamory, J.V.; Laszczynski, N.; Doberdo, I.; Kim, G.T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder. J. Power Sources 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Pasqualini, M.; Witkowska, A.; Gunnella, R.; Birrozzi, A.; Minicucci, M.; Rajantie, H.; Copley, M.; Nobili, F.; Cicco, A.D. Binder-induced surface structure evoluation effects on Li-ion battery performance. Appl. Surf. Sci. 2018, 435, 1029–1036. [Google Scholar] [CrossRef]
- Oswald, S. Binding energy referencing for XPS in alkali metal-based battery materials research (I): Basic model investigations. Appl. Surf. Sic. 2015, 351, 492–503. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chao, S.C.; Wu, H.C.; Wu, N.L. Study on solid-electrolyte-interphase of Si and C-coated Si electrodes in lithium cells. J. Electrochem. Soc. 2009, 156, A95–A102. [Google Scholar] [CrossRef]
- Nie, M.; Demeaux, J.; Young, B.T.; Heskett, D.R.; Chen, Y.; Bose, A.; Woicik, J.C.; Lucht, B.L. Effect of vinylene carbonates and fluoroethylene carbonates on SEI formation on graphite anodes in Li-ion batteries. J. Electrochem. Soc. 2015, 162, A7008–A7014. [Google Scholar] [CrossRef]
- Dedryvere, R.; Martinez, H.; Leroy, S.; Lemordant, D.; Bonhomme, F.; Biensan, P.; Gonbeau, D. Surface film formation on electrode in a LiCoO2/graphite cell: A step by step XPS study. J. Power Sources 2007, 174, 462–468. [Google Scholar] [CrossRef]
- Andersson, A.M.; Henningson, A.; Siegbahn, H.; Jansson, U.; Edstrom, K. Electrochemically lithiated graphite characterised by photoelectron spectroscopy. J. Power Sources 2003, 119–121, 522–527. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review—SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]

| Prelithiation | First Cycle | Second Cycle | Third Cycle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CapLi-in 1 (mAh g−1) | CapLi-ex 2 (mAh g−1) | CE 3 (%) | CapLi-in 1 (mAh g−1) | CapLi-ex 2 (mAh g−1) | CE 3 (%) | CapLi-in 1 (mAh g−1) | CapLi-ex 2 (mAh g−1) | CE 3 (%) | |
| Shallow Li-ion insertion | 52.9 | - | - | - | - | - | - | - | - |
| Thrice-repeated deep Li-ion insertion and extraction | 497 | 361 | 72.6 | 383 | 357 | 93.3 | 375 | 357 | 95.0 |
| Cell | First Cycle | Second Cycle | Third Cycle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CapC 1 (mAh gPE−1) | CapD 2 (mAh gPE−1) | CE 3 (%) | CapC 1 (mAh gPE−1) | CapD 2 (mAh gPE−1) | CE 3 (%) | CapC 1 (mAh gPE−1) | CapD 2 (mAh gPE−1) | CE 3 (%) | |
| Pristine HC cell | 179 | 129 | 71.9 | 132 | 129 | 97.6 | 131 | 129 | 98.6 |
| Shallow prelithiation HC cell | 179 | 142 | 79.6 | 145 | 142 | 97.6 | 143 | 141 | 98.4 |
| Cycling prelithiation HC cell | 176 | 150 | 85.4 | 151 | 150 | 99.4 | 151 | 150 | 99.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Saito, T.; Kumagai, S. Effect of Prelithiation Process for Hard Carbon Negative Electrode on the Rate and Cycling Behaviors of Lithium-Ion Batteries. Batteries 2018, 4, 71. https://doi.org/10.3390/batteries4040071
Abe Y, Saito T, Kumagai S. Effect of Prelithiation Process for Hard Carbon Negative Electrode on the Rate and Cycling Behaviors of Lithium-Ion Batteries. Batteries. 2018; 4(4):71. https://doi.org/10.3390/batteries4040071
Chicago/Turabian StyleAbe, Yusuke, Tomoaki Saito, and Seiji Kumagai. 2018. "Effect of Prelithiation Process for Hard Carbon Negative Electrode on the Rate and Cycling Behaviors of Lithium-Ion Batteries" Batteries 4, no. 4: 71. https://doi.org/10.3390/batteries4040071
APA StyleAbe, Y., Saito, T., & Kumagai, S. (2018). Effect of Prelithiation Process for Hard Carbon Negative Electrode on the Rate and Cycling Behaviors of Lithium-Ion Batteries. Batteries, 4(4), 71. https://doi.org/10.3390/batteries4040071





