Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA)
Abstract
:1. Introduction
2. Experimental Section
- Stage 1
- 12 a.m.–9 a.m.: 45 °C
- Stage 2
- 9 a.m.–12 p.m.: 45–65 °C
- Stage 3
- 12 p.m.–9 p.m.: 65 °C
- Stage 4
- 9 p.m.–12 a.m.: 65–45 °C
3. Results and Discussion
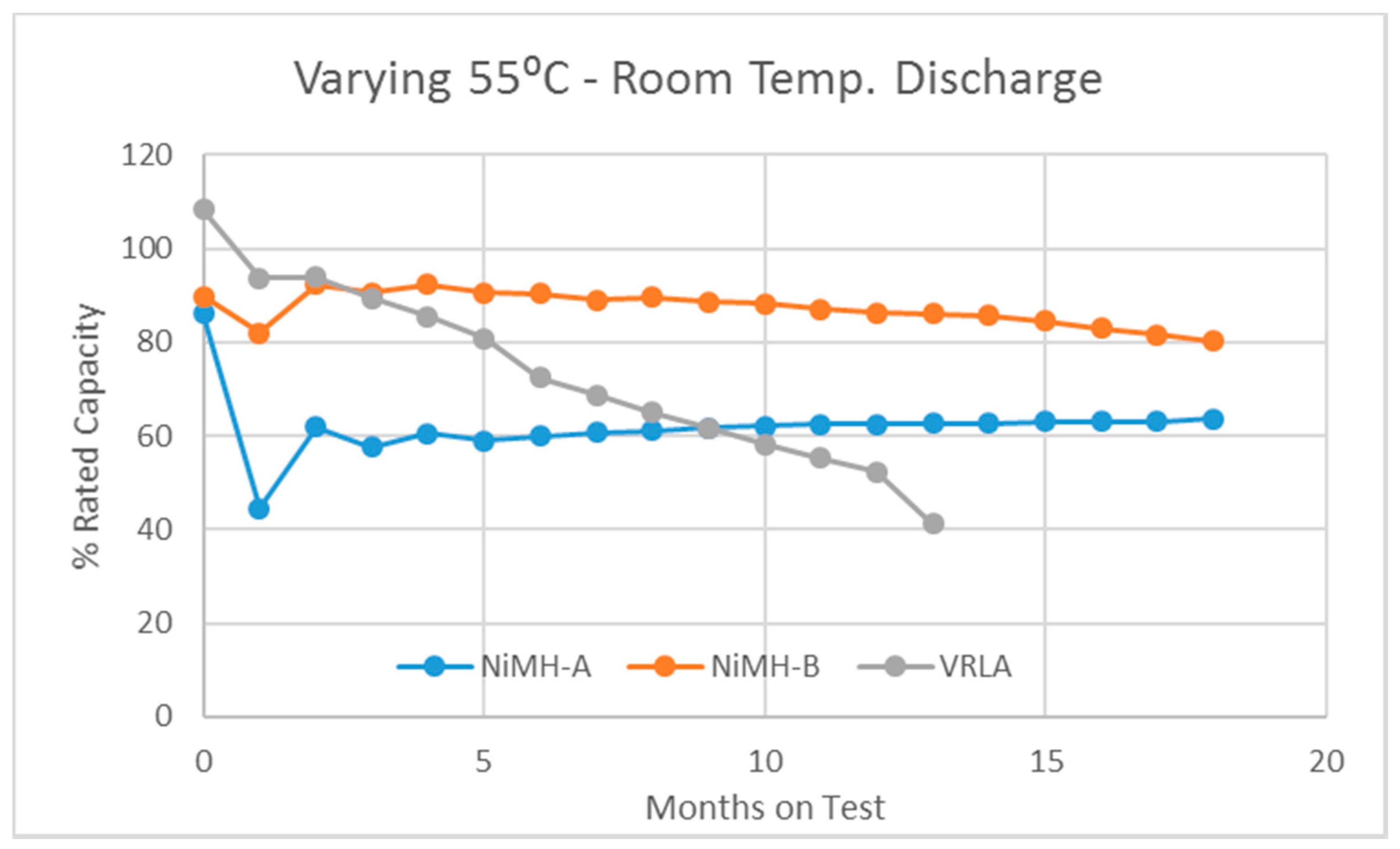
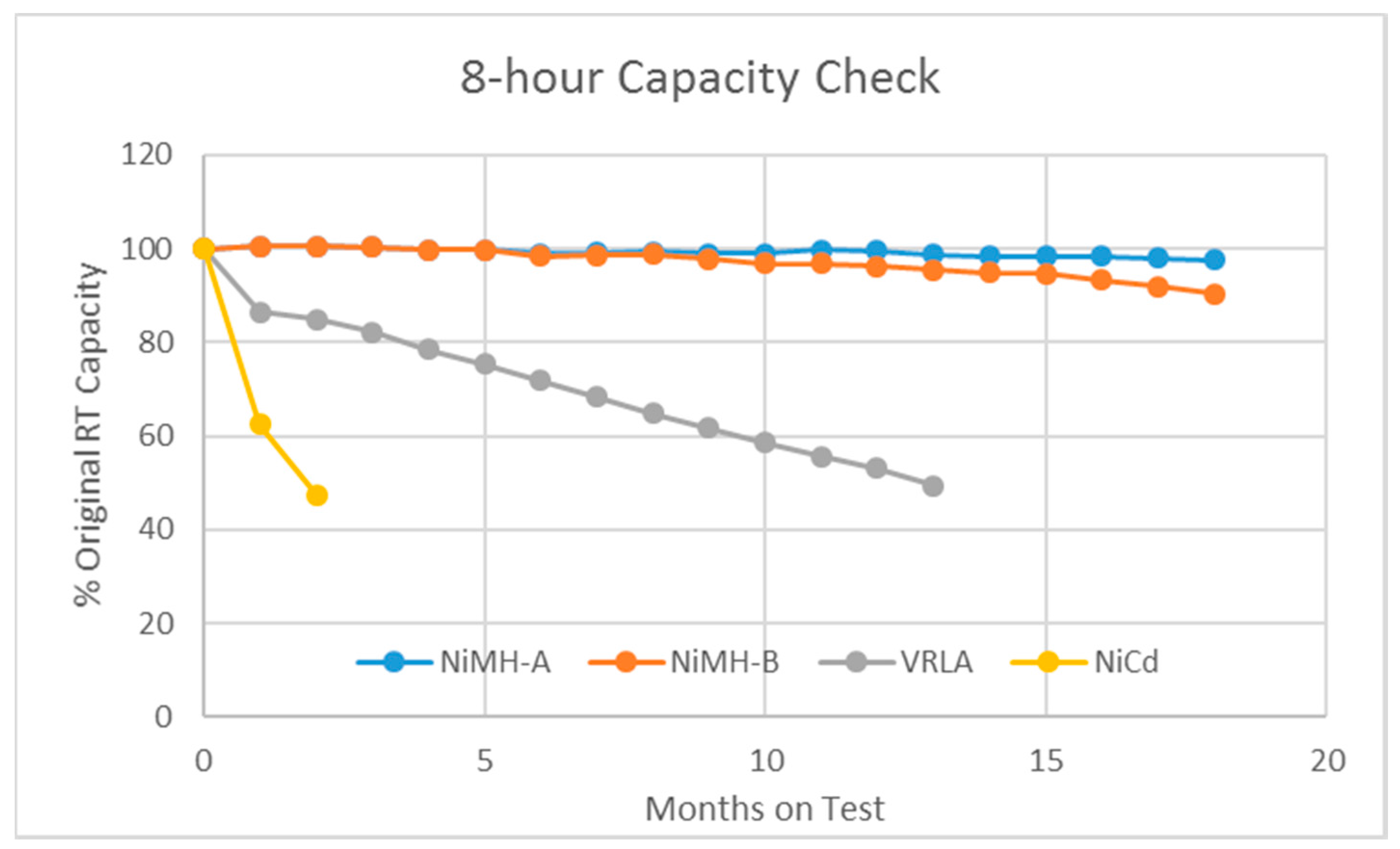
3.1. The Varying-55 °C Test
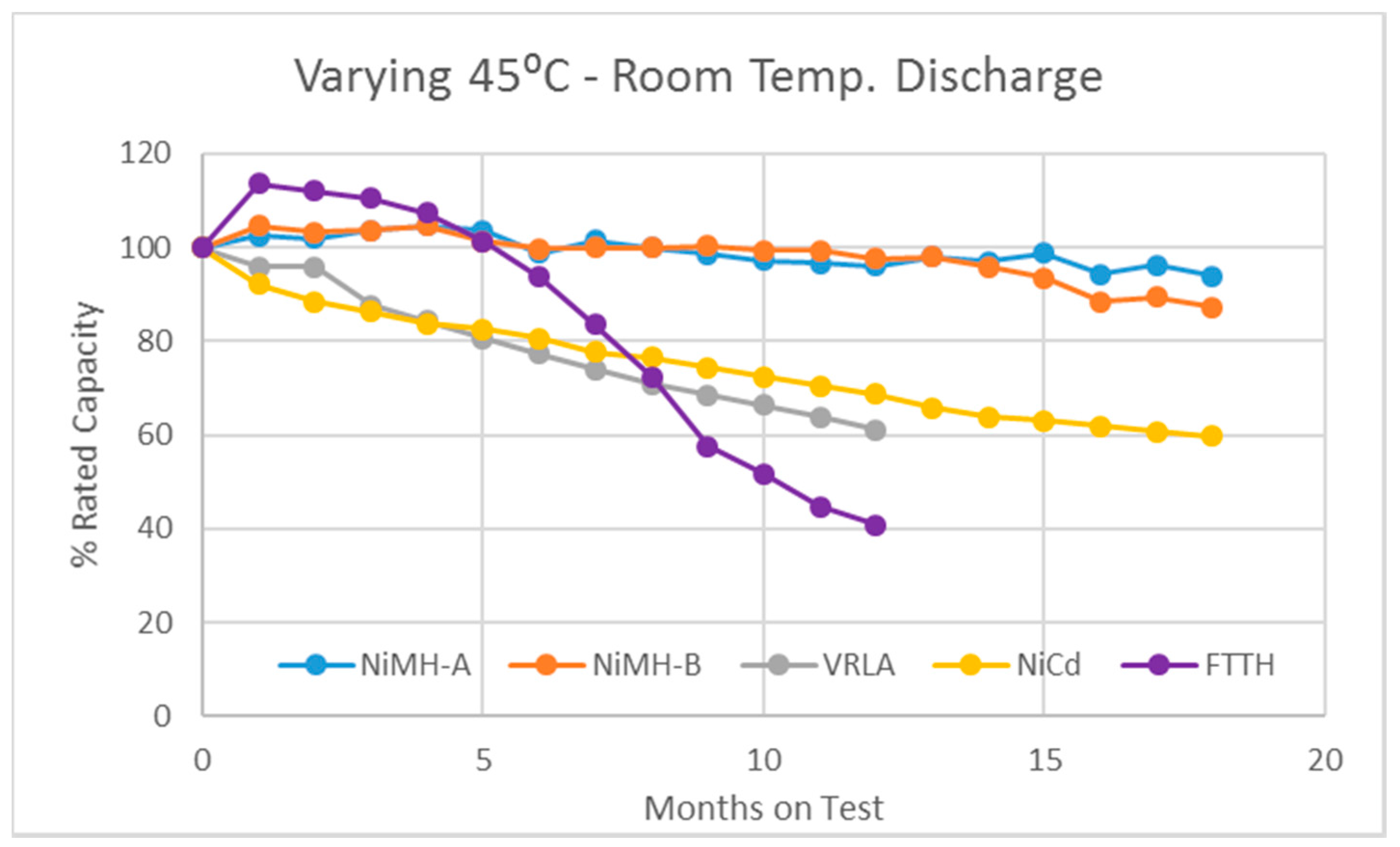
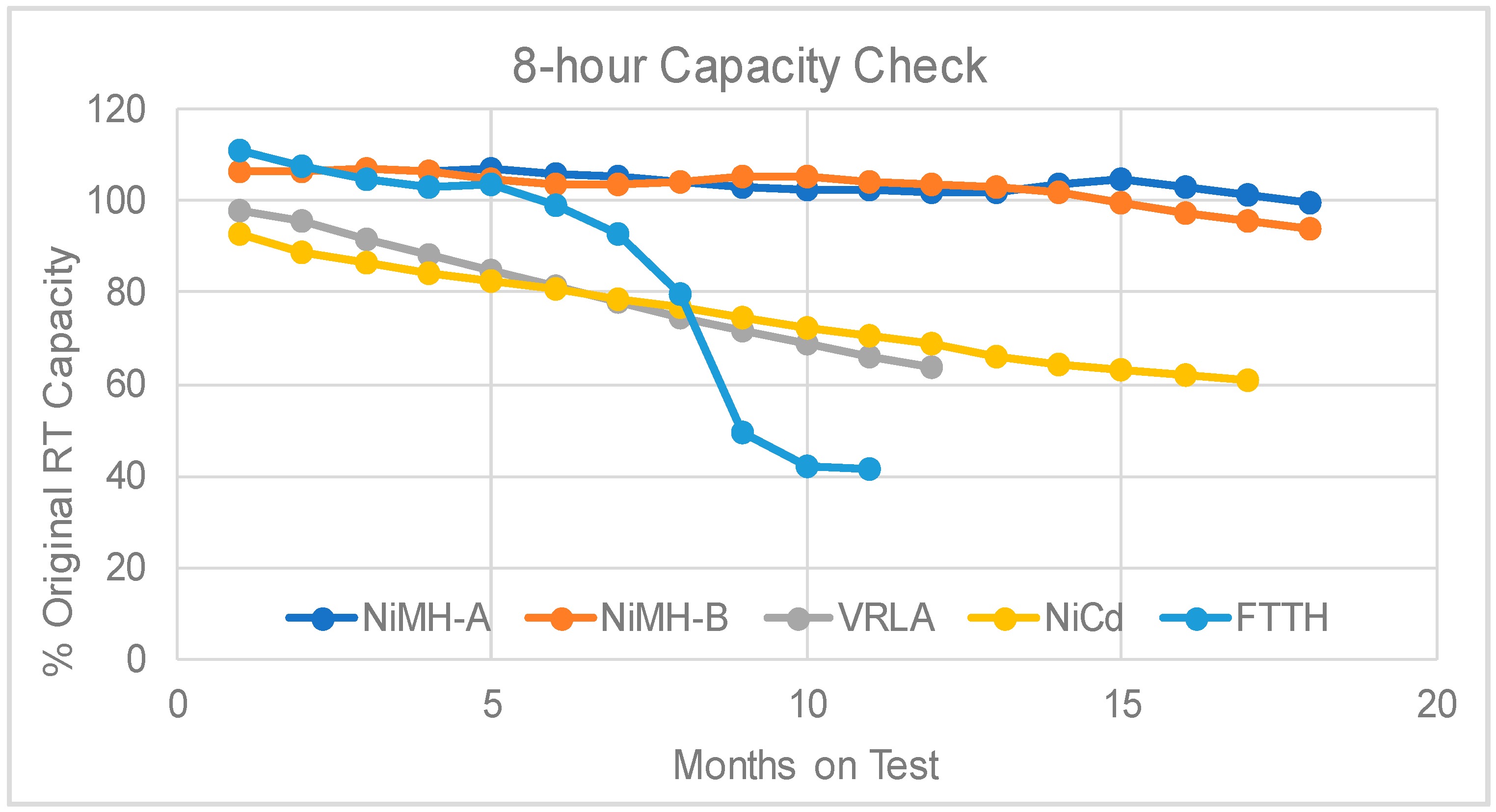
3.2. The Varying-45 °C Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Telecom | telecommunications |
| VRLA | valve-regulated lead acid |
| Ni–Cd | nickel cadmium |
| Ni/MH | nickel/metal hydride |
| HEV | hybrid electric vehicle |
| MH | metal hydride |
| RT | room temperature |
| FTTH | fiber-to-the-home |
| BPS | battery power system |
References
- Zelinsky, M. Heat Tolerant Ni/MH Batteries for Stationary Power. In Proceedings of the Battcon 2010 International Stationary Battery Conference, Hollywood, FL, USA, 17–19 May 2017. [Google Scholar]
- Fetcenko, M. Battery Materials for E-Mobility. In Proceedings of the 33rd International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21–24 March 2016. [Google Scholar]
- Zelinsky, M. Batteries and Heat—A Recipe for Success? In Proceedings of the Battcon 2013 International Stationary Battery Conference, Orlando, FL, USA, 6–8 May 2013. [Google Scholar]
- Alpha Technologies Ltd. Cellect™ 600 Product Data Sheet. Available online: https://atl.app.box.com/v/cellect-600-48v-dc (accessed on 31 July 2017).
- Palu, J. Design considerations for data-storage memory back-up. Available online: https://www.electronicproducts.com/Power_Products/Batteries_and_Fuel_Cells/Design_considerations_for_data-storage_memory_back-up.aspx (accessed on 22 December 2017).
- WMATA Energy Storage Demonstration Project, Federal Transit Administration Final Report. June 2015. Available online: https://www.transit.dot.gov/sites/fta.dot.gov/files/docs/FTA_Report_No._0086.pdf (accessed on 31 July 2017).
- Nishimura, K.; Takasaki, T.; Sakai, T. Introduction of large-sized nickel-metal hydride battery GIGACELL for industrial applications. J. Alloy. Compd. 2013, 580, S353–S358. [Google Scholar] [CrossRef]
- Bai, W. The Current Status and Future Trends of Domestic and Foreign NiMH Battery Market. Available online: http://cbea.com/u/cms/www/201406/06163842rc0l.pdf (accessed on 31 August 2017).
- Available online: http://telecom-info.telcordia.com/site-cgi/ido/docs.cgi?ID=SEARCH&DOCUMENT=GR-3168& (accessed on 31 August 2017).
- EnerSys PowerSafe SBS 100 Technical Specifications. Available online: http://www.enersys.com/Asia/PowerSafe_SBS_Batteries.aspx?langType=1033 (accessed on 31 July 2017).
- Saft Tel.X Ni–Cd Batteries for Telecom Networks Technical Manual. Available online: http://www.npstelecom.com/resources/products/fcm/_10q11wyx4nw9122mr4nbh87x1066kfv8z6smqm9djyh8pn584.pdf (accessed on 31 July 2017).
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 9 April 2016).
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium, Osaka, Japan, 7–9 October 2013; p. 210. [Google Scholar]
- Teraoka, H. Development of Ni-MH EThSS with Lifetime and Performance Estimation Technology. In Proceedings of the 34th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 20‒23 March 2017. [Google Scholar]
- Teraoka, H. Ni-MH Stationary Energy Storage: Extreme Temperature & Long Life Developments. In Proceedings of the 33th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 21‒24 March 2016. [Google Scholar]
- Teraoka, H. Development of Highly Durable and Long Life Ni-MH Batteries for Energy Storage Systems. In Proceedings of the 32th International Battery Seminar & Exhibit, Fort Lauderdale, FL, USA, 9‒12 March 2015. [Google Scholar]
- Young, K.; Yasuoka, S. Past, present, and future of metal hydride alloys in nickel-metal hydride batteries. In Proceedings of the 14th International Symposium on Metal-Hydrogen Systems, Manchester, UK, 21–25 July 2014. [Google Scholar]
- Young, K.; Chang, S.; Lin, X. C14 Laves phase metal hydride alloys for Ni/MH batteries applications. Batteries 2017, 3, 27. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sources 2015, 277, 433–442. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Alloy. Compd. 2016, 660, 407–415. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 Battery performance and failure mechanism. J. Alloy. Compd. 2016, 664, 417–427. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The influence of nickel-hydroxide composition and microstructure on the high-temperature performance of nickel metal hydride batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Koch, J.; Ouchi, T.; Yasuoka, S. Batteries with cobalt-substituted superlattice hydrogen-absorbing alloy anodes at 50 °C. Batteries 2016, 2, 20. [Google Scholar] [CrossRef]
- GS Yuasa GoldTop Technical Specifications. Available online: http://www.gsbattery.com/fiber-home-batteries-ftth (accessed on 31 July 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelinsky, M.A.; Koch, J.M.; Young, K.-H. Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA). Batteries 2018, 4, 1. https://doi.org/10.3390/batteries4010001
Zelinsky MA, Koch JM, Young K-H. Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA). Batteries. 2018; 4(1):1. https://doi.org/10.3390/batteries4010001
Chicago/Turabian StyleZelinsky, Michael A., John M. Koch, and Kwo-Hsiung Young. 2018. "Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA)" Batteries 4, no. 1: 1. https://doi.org/10.3390/batteries4010001
APA StyleZelinsky, M. A., Koch, J. M., & Young, K.-H. (2018). Performance Comparison of Rechargeable Batteries for Stationary Applications (Ni/MH vs. Ni–Cd and VRLA). Batteries, 4(1), 1. https://doi.org/10.3390/batteries4010001





