Developing Electrolyte for a Soluble Lead Redox Flow Battery by Reprocessing Spent Lead Acid Battery Electrodes
Abstract
:1. Introduction
2. Experimental
2.1. Chemical Reagents
2.2. Sample
2.3. Equipment
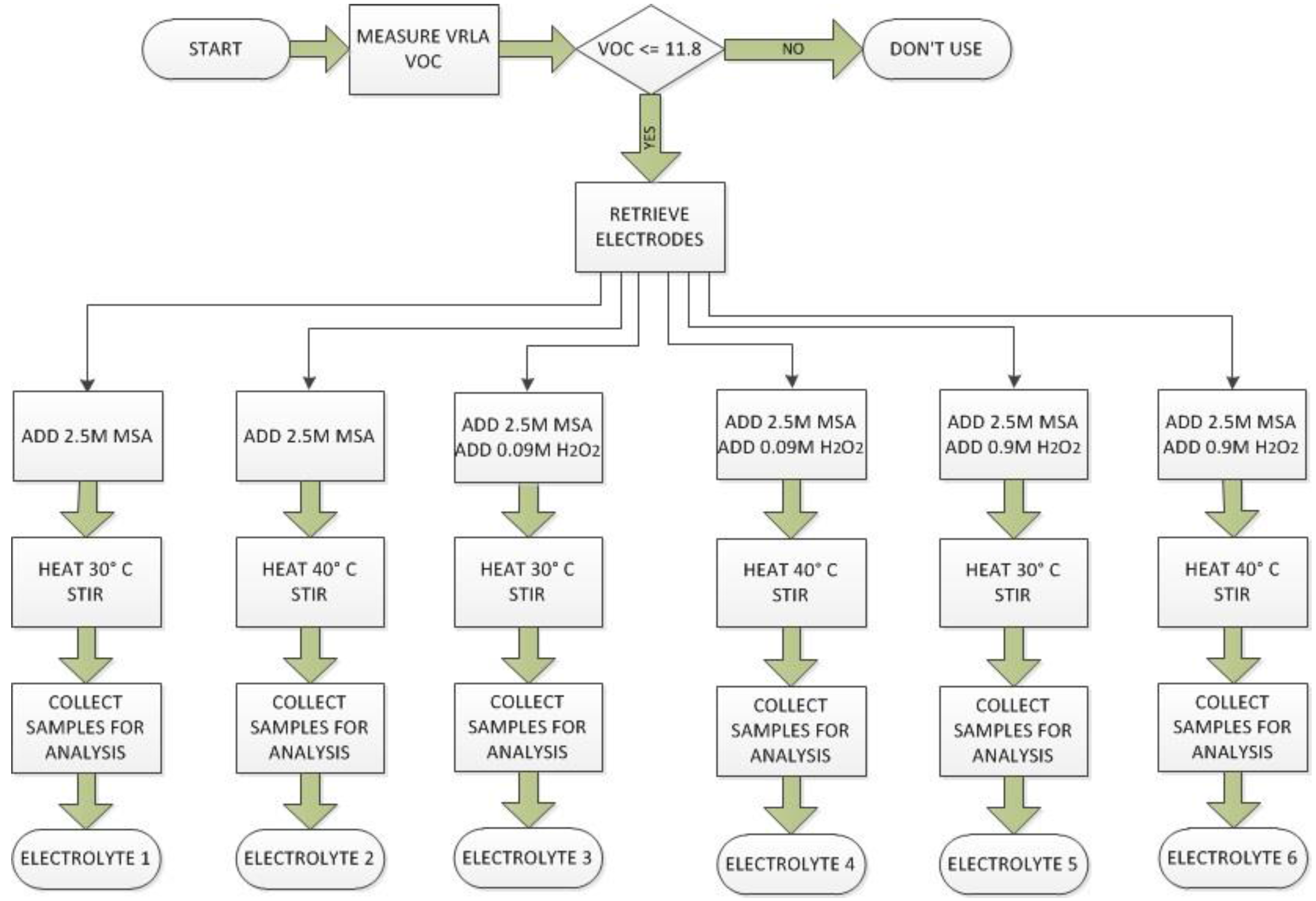
2.4. Development of Lead Recovery Method
2.4.1. Use of Methanesulfonic Acid
2.4.2. Heating
2.4.3. Use of Hydrogen Peroxide
2.5. Lead Recovery Method
2.5.1. Determining Battery State of Health
2.5.2. Disassembling the Battery
- (1)
- Sulfuric acid was emptied from the battery.
- (2)
- The battery was rinsed with distilled water three times.
- (3)
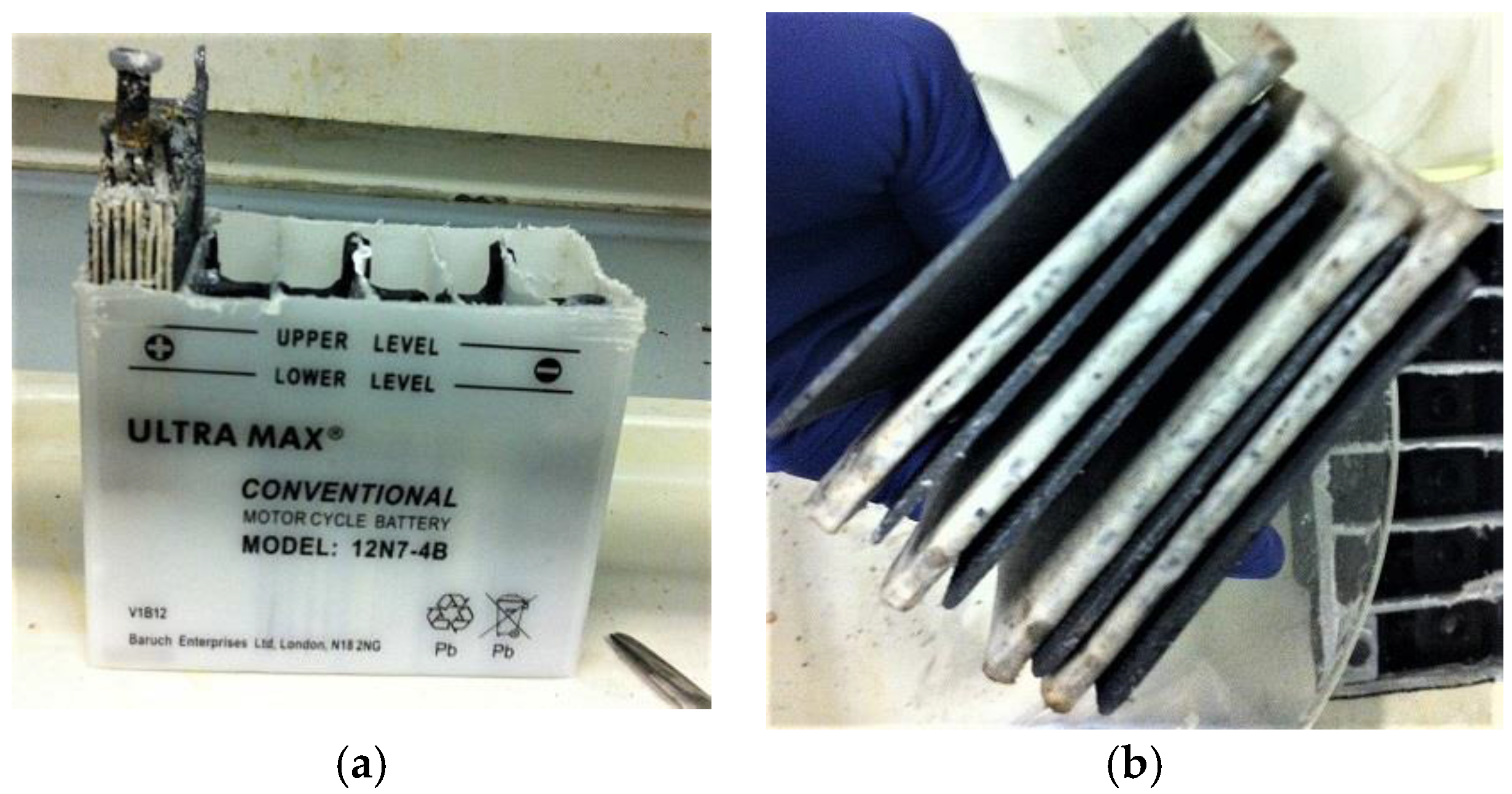
- The top cover of the battery was sawn off, and electrodes were retrieved out of each cell chamber as shown in Figure 2a,b.
2.5.3. Dissolving Lead from Battery Electrodes
2.5.4. Electrolyte Preparation
2.6. Measurements
2.6.1. Inductively Coupled Plasma Mass Spectroscopy
2.6.2. Change in Mass of Solution
2.6.3. Titration
- (1)
- An aliquot of the Pb2+ solution collected after centrifuging was diluted a 100 times. The solution was made to 25 cm³.
- (2)
- To make the solution basic, 5 mm of pH 10 ammonium hydroxide was added to the solution. This resulted in formation of solid Pb(OH)2.
- (3)
- To keep the Pb2+ ions in solution, a spatula tip of powder tartaric acid was added. Tartaric acid complexes weakly with Pb²+ ions, keeping the Pb²+ ions aqueous.
- (4)
- Before titration, a colour indicator was added to the solution. The indicator was prepared by grinding 100 mg of Erio Black T with 5 g of KCl. The indicator attaches to Pb2+ ions to form a reddish violet coloured complex, as indicated by Equation (13):where In is the indicator.
- (5)
- A standard solution of 0.01 mol·dm−³ EDTA was titrated out of a burette into the solution under investigation.
- (6)
- The EDTA replaced the indicator from the complex to form the strongly bonding until the indicator existed as a free protonated dye with a blue colour. Replacement of the indicator by EDTA is shown in Equation (14):At the end-point, the colour of the solution changed from violet to blue.
- (7)
- The concentration of lead in the solution was related to the concentration of EDTA by the equation:
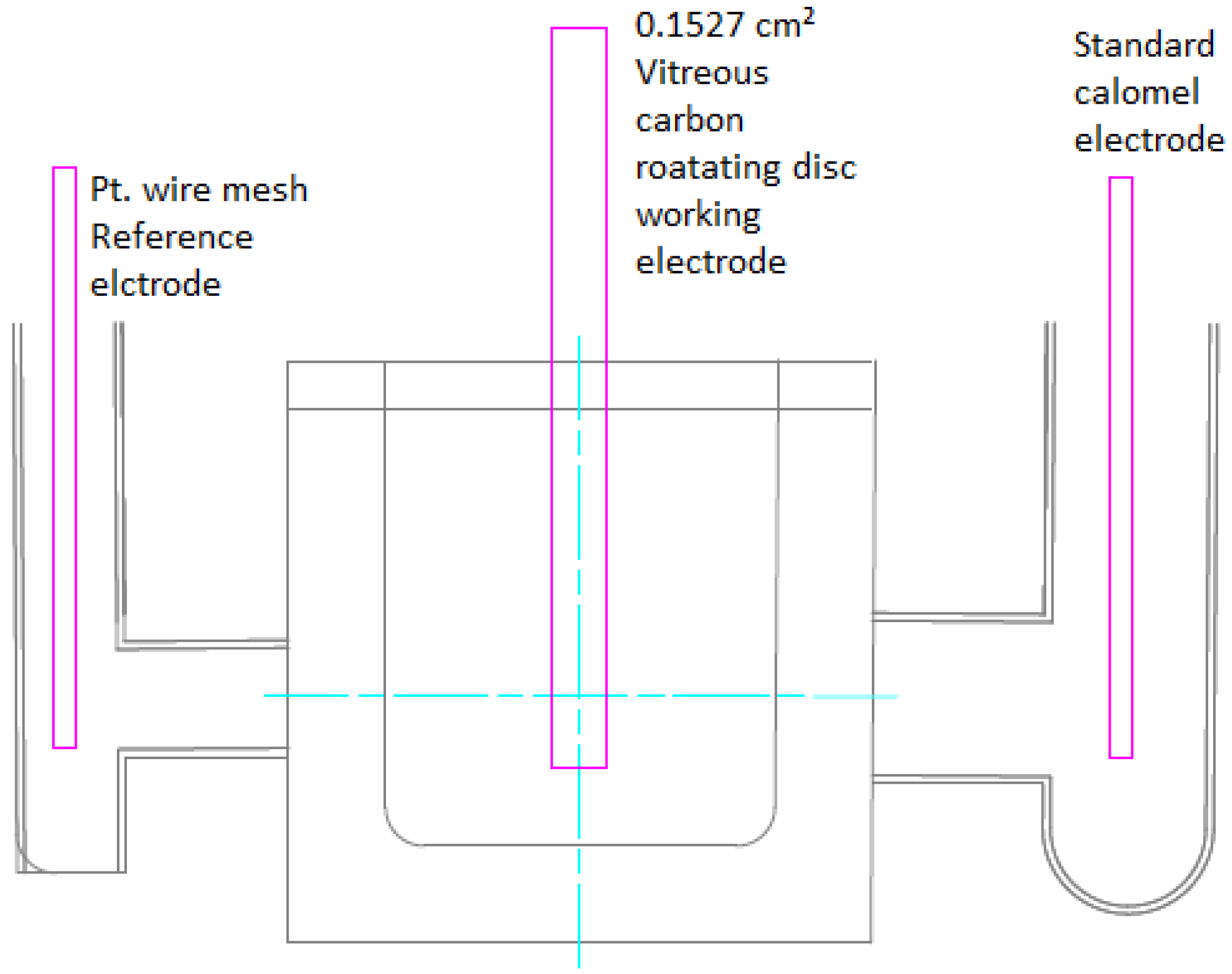
2.6.4. Cyclic Voltammetry
2.6.5. Flow Cell Testing
3. Results and Discussion
3.1. State of Health of Lead Acid Battery
3.2. Lead Recovered
3.2.1. Recovered Electrolyte Qualitative Analysis
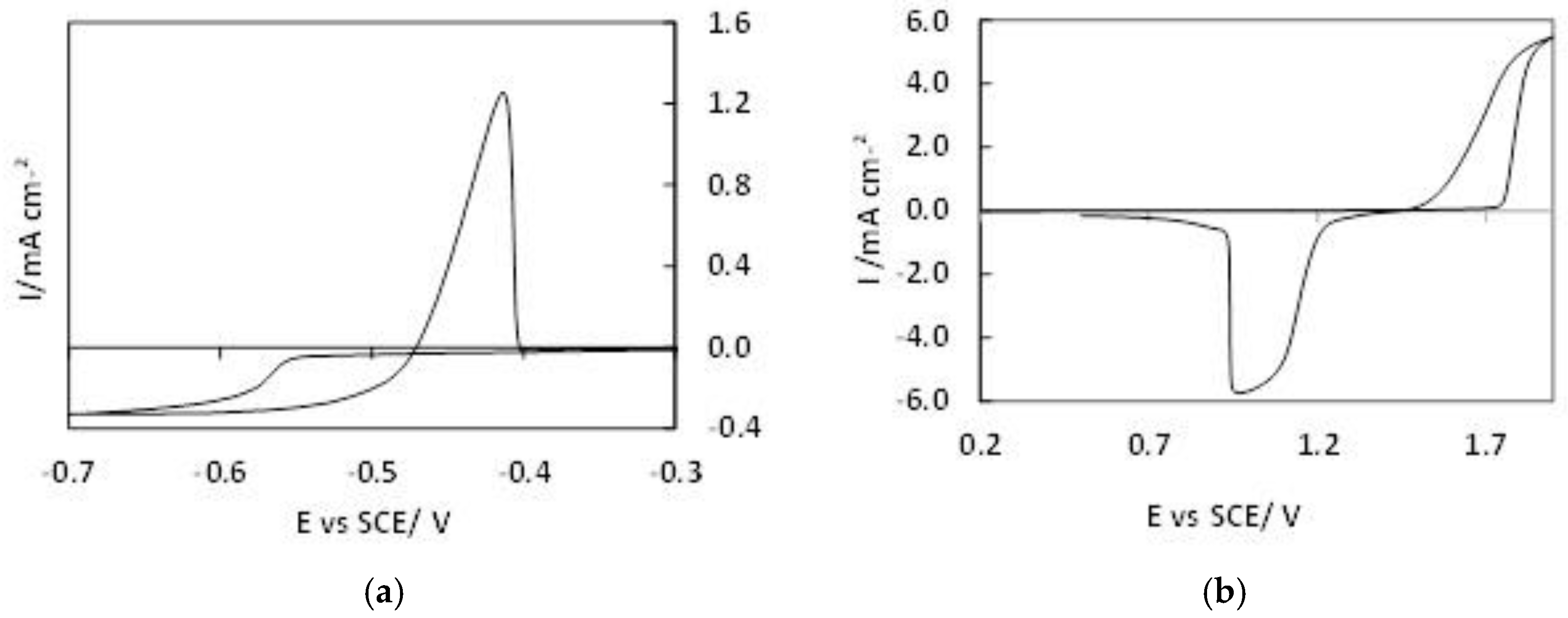
3.2.2. Cyclic Voltammetry
3.2.3. Comparison of Methods Used to Quantify Lead (II) Ions Recovered
3.2.4. Quantity of Lead (II) Ions Recovered
3.3. Flow Cell
Comparison of Recovered and Standard Electrolytes
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Teller, O.; Nicolai, J.-P.; Lafoz, M.; Laing, D.; Tamme, R.; Pedersen, A.S.; Andersson, M.; Folke, C.; Bourdil, C.; Conte, M.; et al. Joint EASE/EERA Recommendations for a European Energy Storage Technology Development Roadmap Towards 2030; Martens, D., Ed.; European Association for Storage of Energy (EASE) and European Energy Research Alliance (EERA): Brussels, Belgium, 2013; pp. 1–226. [Google Scholar]
- Spiers, D.J.; Rasinkoski, A.D. Predicting the service lifetime of lead/acid batteries in photovoltaic systems. J. Power Sources 1995, 53, 245–253. [Google Scholar] [CrossRef]
- Jossen, A.; Garche, J.; Sauer, D.U. Operation conditions of batteries in PV applications. Sol. Energy 2004, 76, 759–769. [Google Scholar] [CrossRef]
- Garche, J.; Jossen, A.; Döring, H. The influence of different operating conditions, especially over-discharge, on the lifetime and performance of lead/acid batteries for photovoltaic systems. J. Power Sources 1997, 67, 201–212. [Google Scholar] [CrossRef]
- Salkind, A.J.; Cannone, A.G.; Trumbure, F.A. Lead-acid batteries. In Handbook of Batteries; Linden, D., Reddy, T., Eds.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Ponce de León, C.; Frías-Ferrer, A.; González-García, J.; Szánto, D.A.; Walsh, F.C. Redox flow cells for energy conversion. J. Power Sources 2006, 160, 716–732. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Weber, A.; Mench, M.; Meyers, J.; Ross, P.; Gostick, J.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Akhil, A.A.; Huff, G.; Currier, A.B.; Kaun, B.C.; Rastler, D.M.; Chen, M.C.; Cotter, A.L.; Bradshaw, D.T.; Gauntlett, W.D. DOE/EPRI 2013 Electricity Storage Handbook in Collaboration with NRECA; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2013. [Google Scholar]
- Pletcher, D.; Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead(II) Part II. Flow cell studies. Phys. Chem. Chem. Phys. 2004, 6, 1779–1785. [Google Scholar] [CrossRef]
- Cunha, Á.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Verde, M.G.; Carroll, K.J.; Wang, Z.; Sathrum, A.; Meng, Y.S. Achieving high efficiency and cyclability in inexpensive soluble lead flow batteries. Energy Environ. Sci. 2013, 6, 1573–1581. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, G.; Poon, G.; Verseema, H. Recent advances with UNSW vanadium-based redox flow batteries. Int. J. Energy Res. 2010, 34, 182–189. [Google Scholar] [CrossRef]
- Perrin, M.; Saint-Drenan, Y.M.; Mattera, F.; Malbranche, P. Lead-acid batteries in stationary applications: Competitors and new markets for large penetration of renewable energies. J. Power Sources 2005, 144, 402–410. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Chakrabarti, M.H.; Hajimolana, S.A.; Mjalli, F.S.; Saleem, M. Progress in flow battery research and development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Hazza, A.; Pletcher, D.; Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead(II) Part I. Preliminary studies. Phys. Chem. Chem. Phys. 2004, 6, 1773–1778. [Google Scholar] [CrossRef]
- Collins, J.; Kear, G.; Li, X.; Low, C.T.J.; Pletcher, D.; Tangirala, R.; Stratton-Campbell, D.; Walsh, F.C.; Zhang, C. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead(II) Part VIII. The cycling of a 10 cm × 10 cm flow cell. J. Power Sources 2010, 195, 1731–1738. [Google Scholar] [CrossRef]
- Collins, J.; Li, X.; Pletcher, D.; Tangirala, R.; Stratton-Campbell, D.; Walsh, F.C.; Zhang, C. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead(II). Part IX: Electrode and electrolyte conditioning with hydrogen peroxide. J. Power Sources 2010, 195, 2975–2978. [Google Scholar] [CrossRef]
- Wills, G.A.R. A Lead Acid Flow Battery for Utility Scale Energy Storage and Load Levelling; Soton eprints; Faculty of Chemistry, University of Southampton: Southampton, UK, 2004; p. 153. [Google Scholar]
- Pletcher, D.; Zhou, H.; Kear, G.; Low, C.T.J.; Walsh, F.C.; Wills, R.G.A. A novel flow battery—A lead-acid battery based on an electrolyte with soluble lead(II) V. Studies of the lead negative electrode. J. Power Sources 2008, 180, 621–629. [Google Scholar] [CrossRef]
- Battery Council International. National Recycling Rate Study; SmithBucklin Statistics Group: Chicago, IL, USA, 2014. [Google Scholar]
- Ellis, T.W.; Mirza, A.H. The refining of secondary lead for use in advanced lead-acid batteries. J. Power Sources 2010, 195, 4525–4529. [Google Scholar] [CrossRef]
- Yuasa Battery Inc. NP Series: NP7-12; Yuasa Battery Inc.: Laureldale, PA, USA, 2008. [Google Scholar]
- MacFarlane, D.R.; Pringle, J.M.; Howlett, P.C.; Forsyth, M. Ionic liquids and reactions at the electrochemical interface. Phys. Chem. Chem. Phys. 2010, 12, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Gernon, M.; Wu, M.; Buszta, T.; Janney, P. Environmental benefits of methanesulfonic acid. Comparative properties and advantages. Green Chem. 1999, 1, 127–140. [Google Scholar] [CrossRef]
- Pletcher, D.; Zhou, H.; Kear, G.; Low, C.T.J.; Walsh, F.C.; Wills, R.G.A. A novel flow battery—A lead-acid battery based on an electrolyte with soluble lead(II) Part VI. Studies of the lead dioxide positive electrode. J. Power Sources 2008, 180, 630–634. [Google Scholar] [CrossRef]
- Ropp, R.C. Chapter 5—Group 14 (C, Si, Ge, Sn, and Pb) alkaline earth compounds. In Encyclopedia of the Alkaline Earth Compounds; Elsevier: Amsterdam, The Netherlands, 2013; pp. 351–480. [Google Scholar]
- Pavlov, D. Chapter 14—Methods to restore the water decomposed during charge and overcharge of lead-acid batteries. VRLA Batteries. In Lead-Acid Batteries: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 567–603. [Google Scholar]
- IEEE-SA. IEEE recommended practice for maintenance, testing, and replacement of valve-regulated lead-acid (VRLA) batteries for stationary applications. In IEEE Std 1188-2005 (Revision of IEEE Std 1188-1996); American National Standards Institute: New York, NY, USA, 2006; pp. 1–44. [Google Scholar]
- Yuasa Technical Manual. Available online: http://ktm950.info/library/assets/pdfs/Yuasa%20Batteries.pdf (accessed on 4 December 2015).
- Finger, P.E.; Boden, D. Electrode materials. In Battery Book 2; Curtis Instruments Inc.: Mt Kisco, NY, USA, 2007. [Google Scholar]
- Moseley, P.T.; Rand, D.A.J. Chapter 1—The valve-regulated battery—A paradigm shift in lead-acid technology. In Valve-Regulated Lead-Acid Batteries; Rand, D.A.J., Garche, J., Moseley, P.T., Parker, C.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–14. [Google Scholar]
- Prengaman, R.D.; Rand, D.A.J. Chapter 2—Lead alloys for valve-regulated lead-acid batteries A2. In Valve-Regulated Lead-Acid Batteries; Rand, D.A.J., Garche, J., Moseley, P.T., Parker, C.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 15–35. [Google Scholar]
- Fitch, A. Lead analysis: Past and present. Crit. Rev. Anal. Chem. 1998, 28, 267–345. [Google Scholar] [CrossRef]
- Vogel, A.I.; Jeffery, G.H. Vogel’s Textbook of Quantitative Chemical Analysis; Longman Scientific & Technical: London, UK, 1989. [Google Scholar]

| Technology | Lead Acid | Soluble Lead Flow Battery | All Vanadium/ Vanadium–Bromine | Zinc–Bromine |
|---|---|---|---|---|
| Cell voltage, V | 2.0 [5] | 1.78 | 1.4/1.0 [1] | 1.8 |
| Energy efficiency, % | 75–85 [9] | 65% [10] | (80–85)/(60–70) [11] | 65–75 |
| Cycle life | 200–2000 | 2000 [12] | 12,000 [13] | 10,000 |
| @ Depth of Discharge | 70%–30% | 100% | 100% | 100% |
| Recommended operating temperature range, °C | 20–25 | 35–55 | (5–40)/(0–50) [11] | 20–50 |
| Cost, €/kWh | 50–150 [14] | - | 140–400 [15] | 800 |
| Chemical | Molecular Formula | Purity/Concentration | Supplier |
|---|---|---|---|
| Methanesulfonic acid (MSA) | CH3SO3H | 99% | Sigma Aldrich, London, UK |
| Lead methanesulfonate | Pb(CH3SO3)2 | 50% w/w | Sigma Aldrich |
| Hydrogen peroxide | H2O2 | 30% w/w | Sigma Aldrich |
| EDTA | C10H16N2O8 | 99.4–100.6 | Sigma Aldrich |
| Eriochrome Black T | C20H12N3NaO7S | - | Sigma Aldrich |
| Ammonium hydroxide | NH4OH | 28.0–30% NH3 | Sigma Aldrich |
| Ammonium chloride | NH4Cl | 99.5% | Sigma Aldrich |
| Potassium chloride | KCl | 99.5% | Fisher Scientific, Loughborough, UK |
| Tartaric acid | C4H6O6 | 99.5% | Sigma Aldrich |
| [Pb²+] in mol·dm−³ by | %Variance | |
|---|---|---|
| Mass Measurements | Titration | |
| 0.65 | 0.656 | 0.9 |
| 0.68 | 0.670 | 1.5 |
| 0.73 | 0.716 | 1.9 |
| 0.87 | 0.860 | 1.2 |
| 0.95 | 0.890 | 6.3 |
| 0.96 | 0.900 | 6.3 |
| 1.06 | 0.930 | 9.7 |
| Method | Electrode Material (g) | Average Temperature (°C) | [H2O2] (mol·dm−³) | [Pb2+] (mol·dm−³) |
|---|---|---|---|---|
| 1 | 250 | 30 | - | 0.16 |
| 2 | 250 | 45 | - | 0.07 |
| 3 | 250 | 30 | 0.09 | 0.51 |
| 4 | 250 | 40 | 0.09 | 0.75 |
| 5 | 250 | 30 | 0.90 | 1.00 |
| 6 | 250 | 40 | 0.90 | 0.91 |
| Electrolyte | Charge Efficiency/% | Energy Efficiency/% | Voltage Efficiency/% |
|---|---|---|---|
| Recovered | 81 | 52 | 64 |
| Standard | 85 | 54 | 64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orapeleng, K.; Wills, R.G.A.; Cruden, A. Developing Electrolyte for a Soluble Lead Redox Flow Battery by Reprocessing Spent Lead Acid Battery Electrodes. Batteries 2017, 3, 15. https://doi.org/10.3390/batteries3020015
Orapeleng K, Wills RGA, Cruden A. Developing Electrolyte for a Soluble Lead Redox Flow Battery by Reprocessing Spent Lead Acid Battery Electrodes. Batteries. 2017; 3(2):15. https://doi.org/10.3390/batteries3020015
Chicago/Turabian StyleOrapeleng, Keletso, Richard G. A. Wills, and Andrew Cruden. 2017. "Developing Electrolyte for a Soluble Lead Redox Flow Battery by Reprocessing Spent Lead Acid Battery Electrodes" Batteries 3, no. 2: 15. https://doi.org/10.3390/batteries3020015
APA StyleOrapeleng, K., Wills, R. G. A., & Cruden, A. (2017). Developing Electrolyte for a Soluble Lead Redox Flow Battery by Reprocessing Spent Lead Acid Battery Electrodes. Batteries, 3(2), 15. https://doi.org/10.3390/batteries3020015








