Gaseous Phase and Electrochemical Hydrogen Storage Properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for Nickel Metal Hydride Battery Applications
Abstract
:1. Introduction
2. Experimental Setup
3. Results
3.1. Alloy Preparation
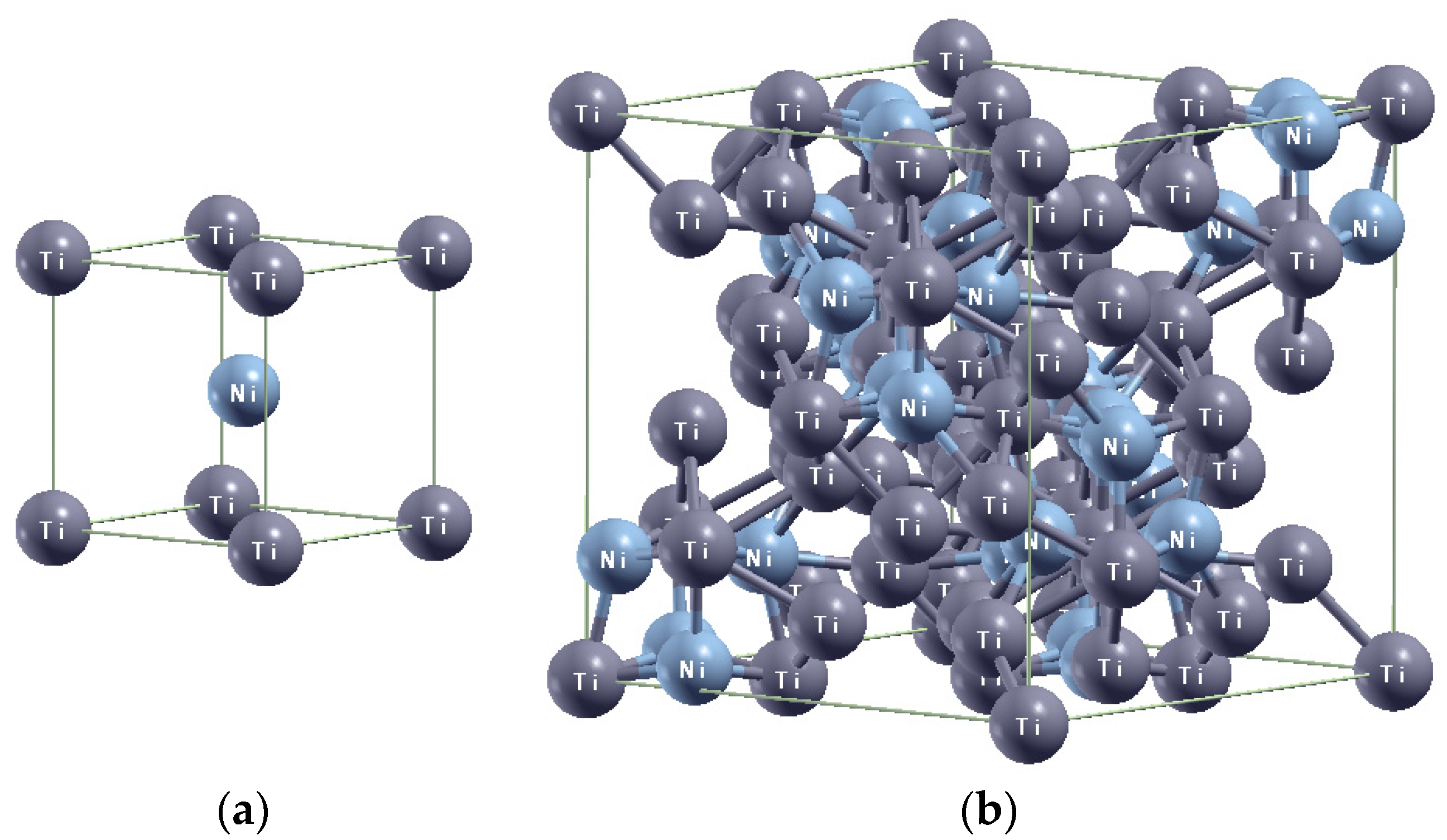
3.2. X-Ray Diffraction Analysis
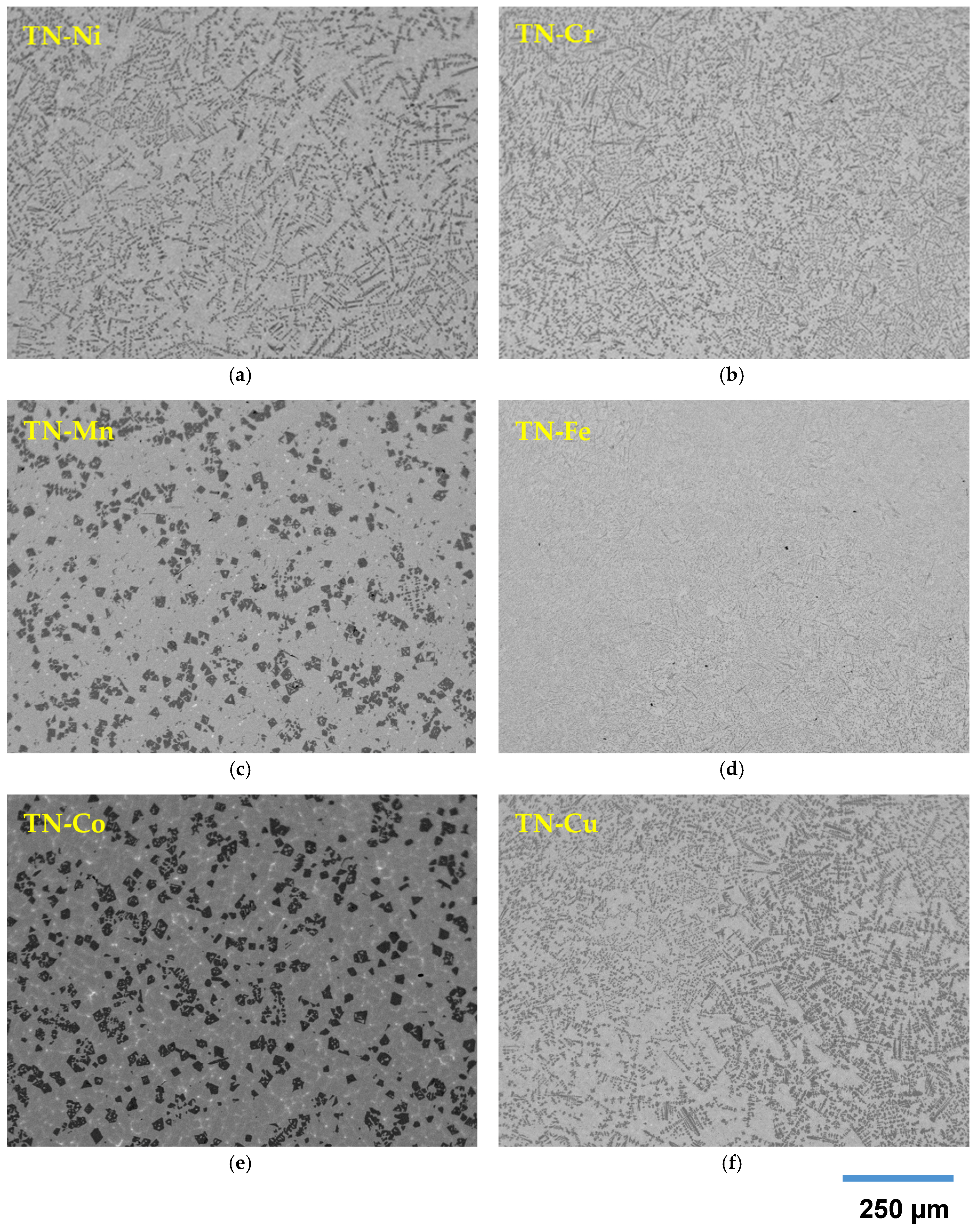
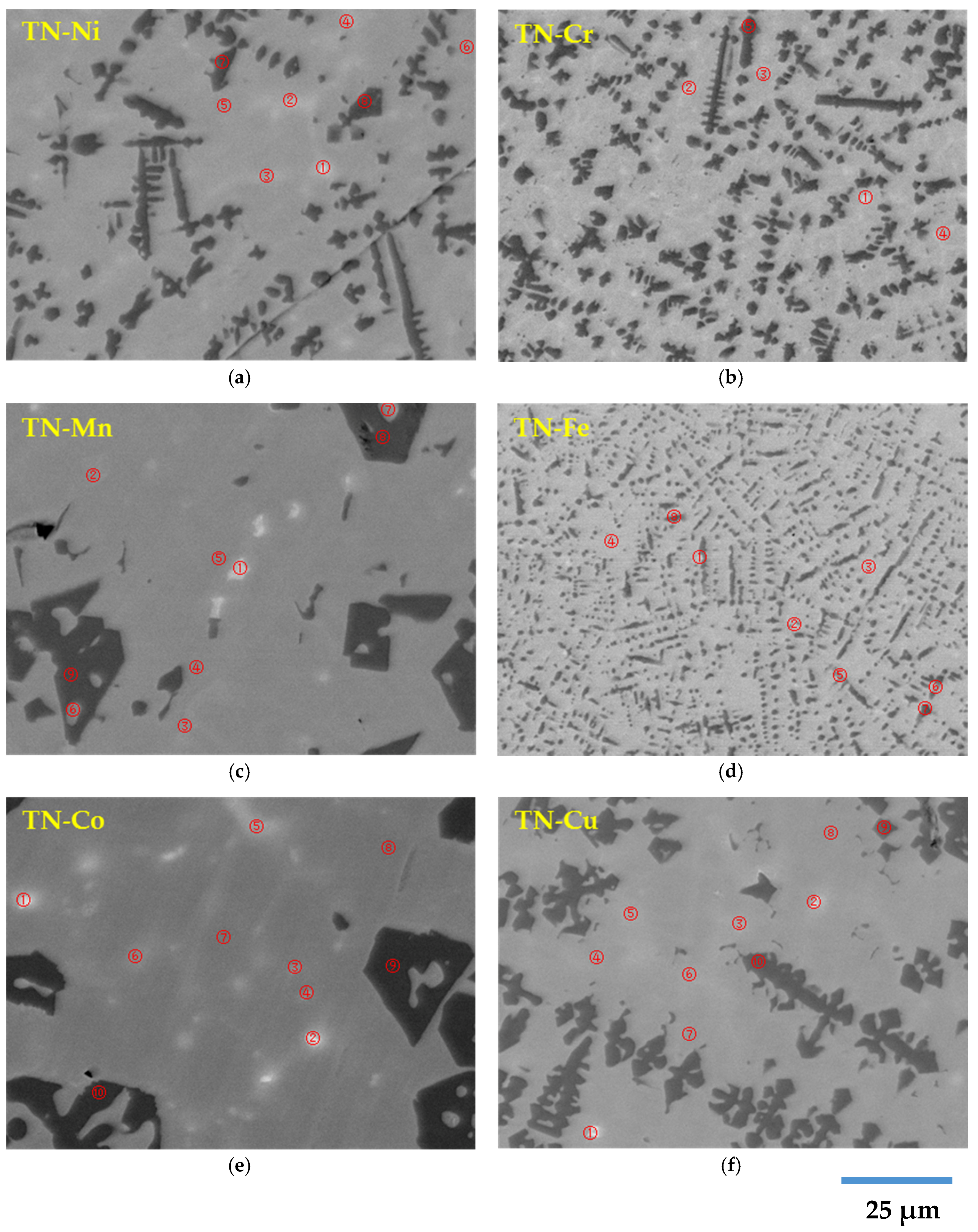
3.3. Scanning Electron Microscopy/Energy Dispersive Spectroscopy Study
- Matrix (TiNi-1): stoichiometric or slightly hyperstoichiometric TiNi with the Zr- and X-contents close to design.
- Minor phase (TiNi-2): this phase appears as bright spots in the micrographs and is distributed within the matrix. It is generally hyperstoichiometric, high-Zr, and high-X TiNi except for:
- ○
- Hypostoichiometric, high-Zr, and close to the design-X TiNi in alloy TN-Fe and
- ○
- Hyperstoichiometric, high-Zr, and low-X TiNi in alloy TN-Co.
- Secondary phase (Ti2Ni): this phase has the darkest contrast in the micrographs and appears next to the main TiNi-1 phase. It is stoichiometric or hyperstoichiometric, low-Zr Ti2Ni.
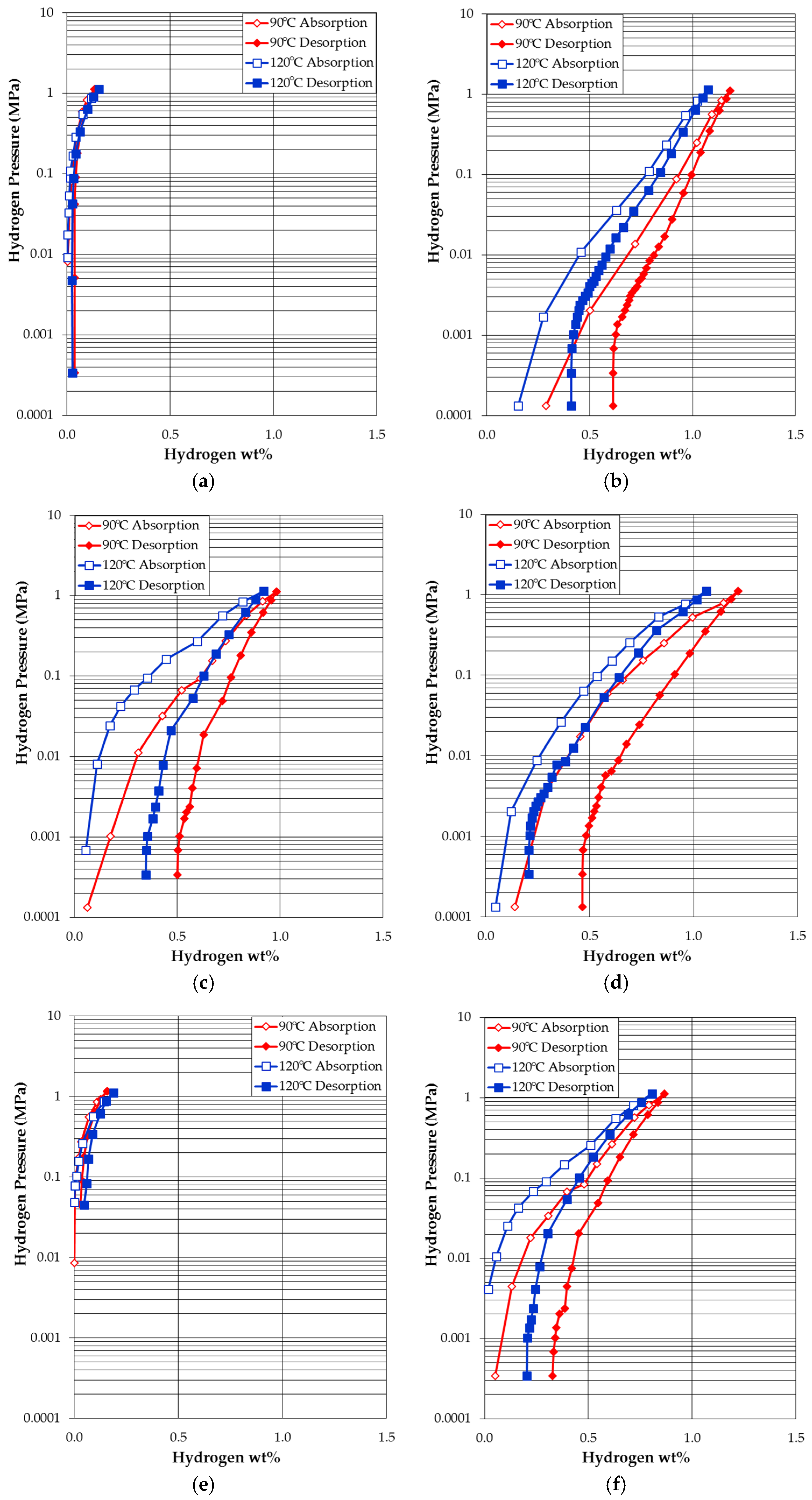
3.4. Pressure-Concentration-Temperature Measurement
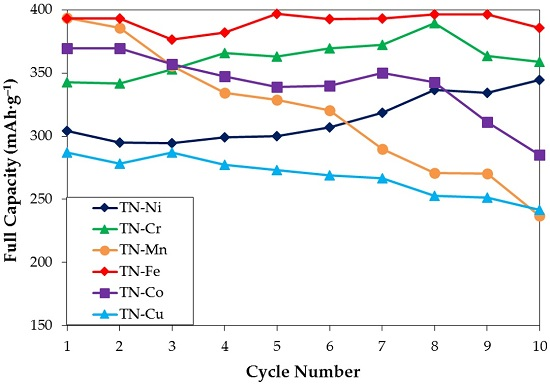
3.5. Electrochemical Measurement
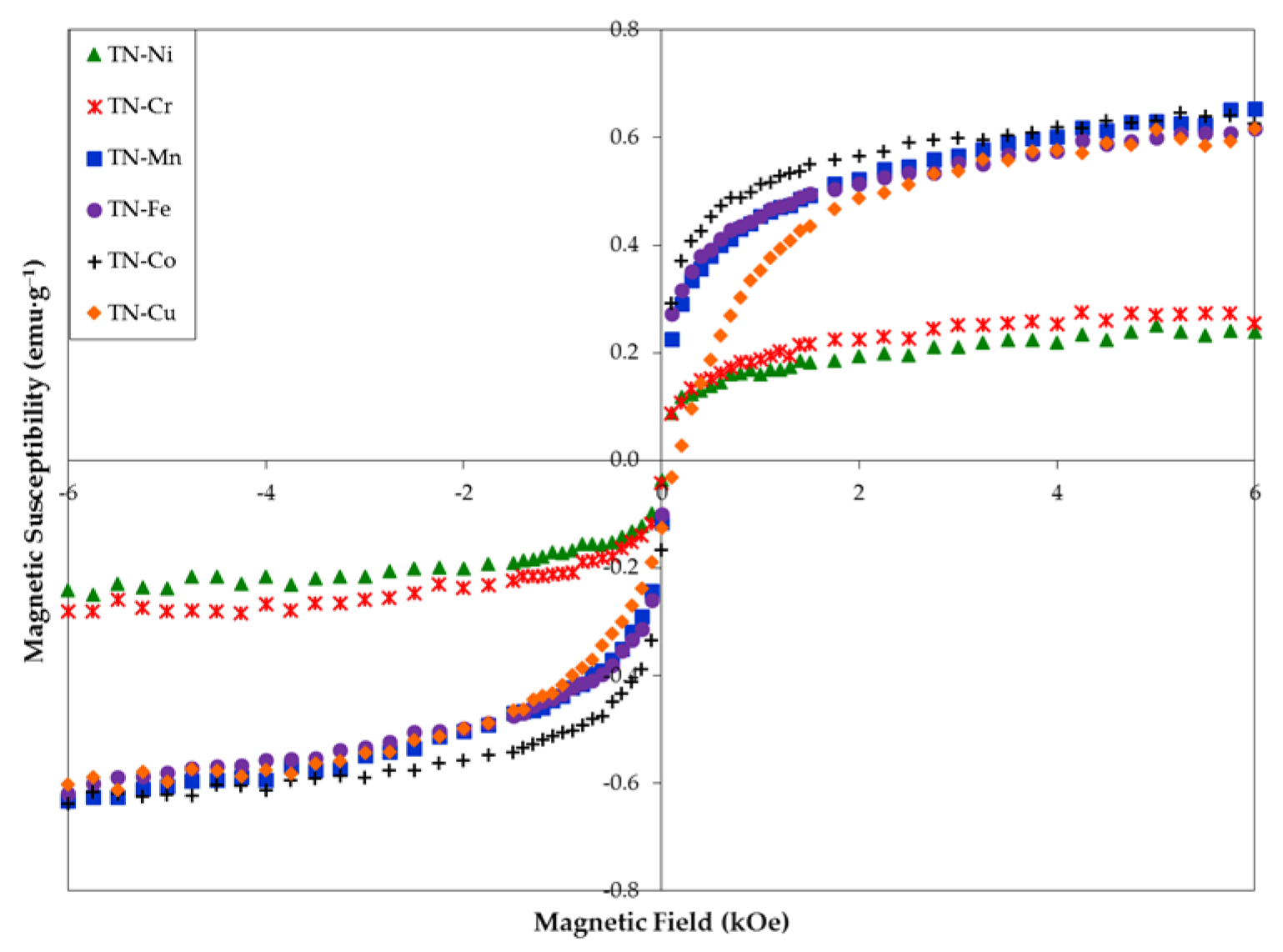
3.6. Magnetic Properties
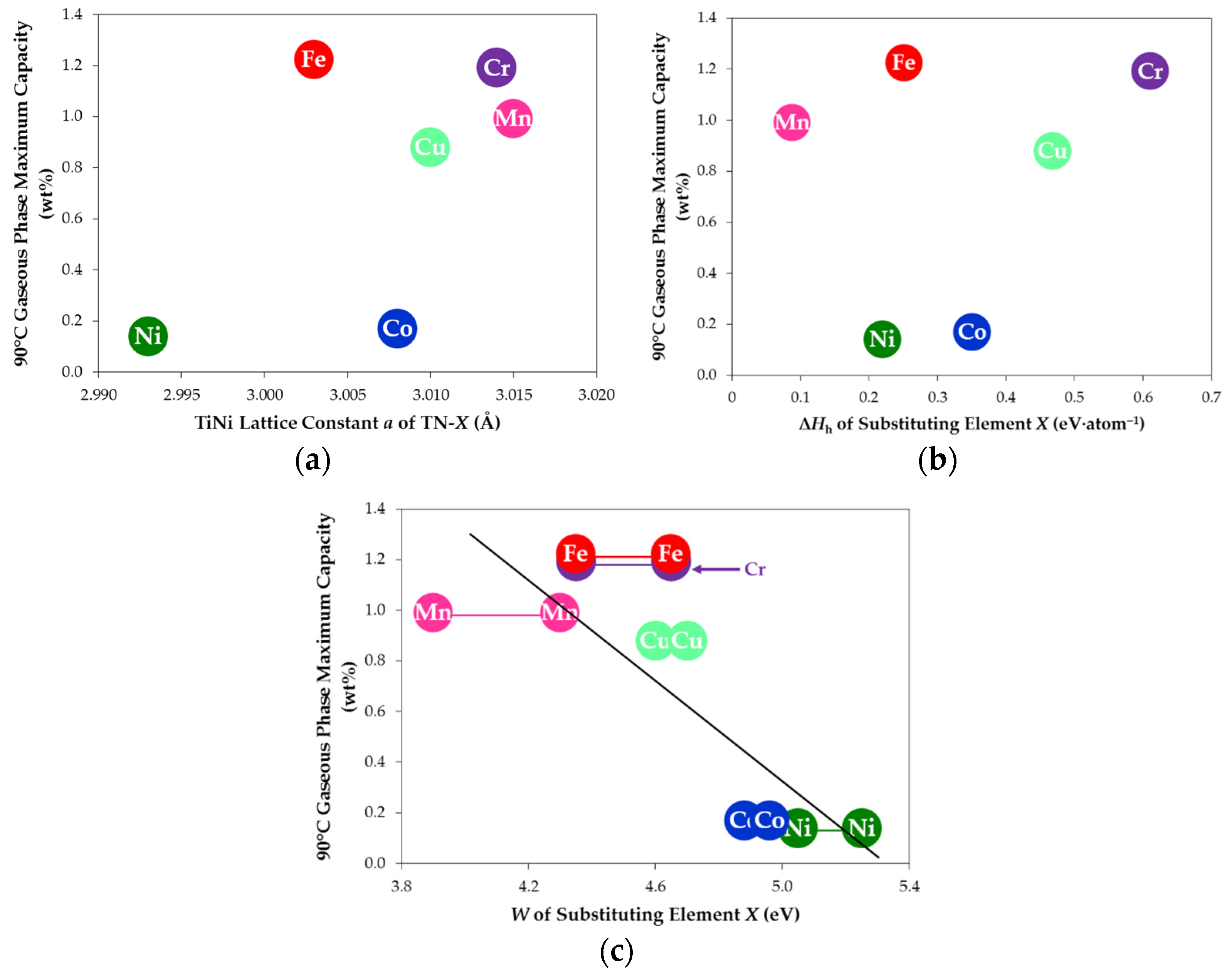
3.7. Comparison among Various Sbustitutions
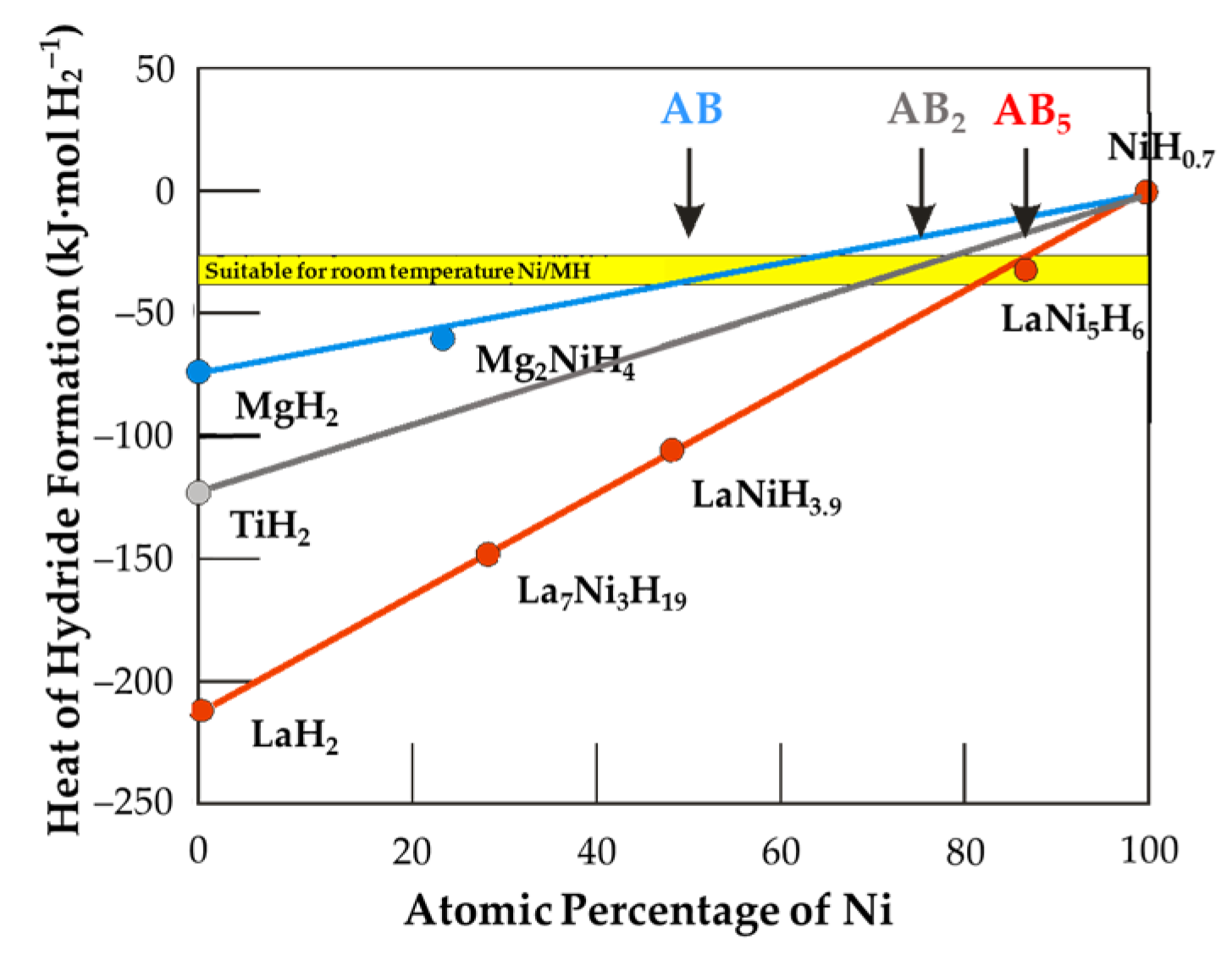
3.8. Property Comparison among Various Metal Hydride Alloy Systems
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ni/MH | Nickel/metal hydride |
| MH | Metal hydride |
| HRD | High-rate dischargeability |
| ΔHh | Heat of hydride formation |
| SN | Sintering |
| AM | Arc melting |
| PM | Melting in a plasma furnace |
| IM | Induction melting |
| CO | Co-precipitation |
| MC | Microencapsulation |
| MA | Mechanical alloying |
| ANN | Annealing |
| MS | Melt spinning |
| CHR | Calcium hydride reduction |
| MWCNT | Multiwall carbon nanotube |
| ICP | Inductively coupled plasma spectrometer/spectrometry |
| XRD | X-ray diffractometer/diffraction |
| SEM | Scanning electron microscope/microscopy |
| EDS | Energy dispersive spectroscopy |
| PCT | Pressure-concentration-temperature |
| W | Work function |
| EVAC | Electron potential in vacuum (EVAC) and the Fermi level (EF) |
| EF | Electron potential in the Fermi level |
| bcc | Body-centered-cubic |
| Io | Surface reaction exchange current |
| D | Bulk hydrogen diffusion coefficient |
| Ms | Saturated magnetic susceptibility |
| H1/2 | Magnetic field strength at one-half of the saturated magnetic susceptibility value |
References
- Zelinsky, M.; Koch, J.; Fetcenko, M. Heat Tolerant NiMH Batteries for Stationary Power. Available online: http://www.battcon.com/PapersFinal2010/ZelinskyPaper2010Final_12.pdf (accessed on 5 May 2016).
- Zelinsky, M.; Koch, J. Batteries and Heat—A Recipe for Success? Available online: http://www.battcon.com/PapersFinal2013/16-Mike%20Zelinsky%20-%20Batteries%20and%20Heat.pdf (accessed on 5 May 2016).
- Wikipedia, the Free Encyclopedia. General Motors EV1. Available online: https://en.wikipedia.org/wiki/General_Motors_EV1 (accessed on 20 June 2016).
- The Jaffes. EV1. Available online: http://thejaffes.org/content/ev1 (accessed on 20 June 2016).
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Paul-Boncour, V. Metal hydrides for hydrogen storage. J. Adv. Sci. 2007, 19, 16–21. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrog. Energy 2009, 34, 4788–4796. [Google Scholar] [CrossRef]
- Pukszhselvan, D.; Kumar, V.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Klebanoff, L.E.; Keller, J.O. 5 Years of hydrogen storage research in the U.S. DOE Metal Hydride Center of Excellence (MHCoE). Int. J. Hydrog. Energy 2013, 38, 4533–4576. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Young, K. Metal Hydrides. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier B.V.: Waltham, MA, USA, 2013. [Google Scholar]
- Liu, W.; Webb, C.J.; Gray, E.M. Review of hydrogen storage in AB3 alloys targeting stationary fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 3485–3507. [Google Scholar] [CrossRef]
- Gutjahr, M.A.; Buchner, H.; Beccu, K.D.; Säufferer, H. A New Type of Reversible Negative Electrode for Alkaline Storage Batteries Based on Metal Alloy Hydrides. In Power Sources; Collins, D.H., Wada, K., Hiraki, A., Eds.; Oriel Press: Newcastle upon Tyne, UK, 1973; Volume 4, pp. 79–91. [Google Scholar]
- Beccu, K. Negative Electrode of Titanium-Nickel Alloy Hydride Phases. U.S. Patent 3,824,131, 16 July 1974. [Google Scholar]
- Murray, J.L. Ni-Ti (Nickel-Titanium). In Binary Alloy Phase Diagram, 2nd ed.; Massalski, T.B., Okamoto, H., Subramanian, P.R., Kacprzak, L., Eds.; ASM International: Materials Park, OH, USA, 1990; Volume 3, pp. 2874–2876. [Google Scholar]
- Osumi, Y. Hydrogen Absorbing Alloy—The Physical Properties and Applications, 1st ed.; Agune Technology Center: Tokyo, Japan, 1993; p. 73. [Google Scholar]
- Young, K.; Ouchi, T.; Meng, T.; Wong, D.F. Studies on the synergetic effects in multi-phase metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Rotarov, D. Studies on MgNi-based metal hydride electrode with aqueous electrolytes composed of various hydroxides. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Justi, E.W.; Ewe, H.H.; Kalberlah, A.W.; Saridakis, N.M.; Schaefer, M.H. Electrocatalysis in the nickel-titanium system. Energy Convers. 1970, 10, 183–187. [Google Scholar] [CrossRef]
- Miles, M.H. Evaluation of electrocatalysts for water electrolysis in alkaline solutions. J. Electroanal. Chem. Interfacial Electrochem. 1975, 60, 89–96. [Google Scholar] [CrossRef]
- Wakao, S.; Yonemura, Y.; Nakano, H.; Shimada, H. Electrochemical capacities and corrosion of TiNix and its zirconium-substituted alloy hydride electrodes. J. Less Common Met. 1984, 104, 365–373. [Google Scholar] [CrossRef]
- Wakao, S.; Nakano, H.; Chubachi, S. Behaviour of hydrogen-absorbing metal alloys in an alkaline solution containing hydrazine. J. Less Common Met. 1984, 104, 385–393. [Google Scholar] [CrossRef]
- Wakao, S.; Sawa, H.; Nakano, H.; Chubachi, S.; Abe, M. Capacities and durabilities of Ti-Zr-Ni alloy hydride electrodes and effects of electroless plating on their performances. J. Less Common Met. 1987, 131, 311–319. [Google Scholar] [CrossRef]
- Song, D.; Gao, X.; Zhang, Y.; Lin, D.; Zhou, Z.; Wang, G.; Shen, P. Surface analysis of a Ti-Ni-B hydrogen storage electrode. J. Alloys Compd. 1993, 199, 161–163. [Google Scholar]
- Jordy, C.; Latroche, M.; Percheron-Guégan, A.; Achard, J.C. Effect of partial substitution in TiNi on its structural and electrochemical hydrogen storage properties. Z. Phys. Chem. 1994, 185, 119–130. [Google Scholar] [CrossRef]
- Yan, D.-Y. Catalytic effects of alloy surface on the oxygen consumption reaction in a sealed Ni/TiNiH battery. J. Alloys Compd. 1994, 209, 257–261. [Google Scholar] [CrossRef]
- Wang, C.; Lei, Y.; Yang, X.; Jiang, J.; Wu, J.; Wang, Q. Effects of phase structures of TiNi on the electrochemical properties. Acta Metall. Sin. 1995, 31, 440–444. [Google Scholar]
- Lei, Y.Q.; Wang, C.S.; Yang, X.G.; Pan, H.G.; Wu, J.; Wang, Q.D. A mathematical model for the cycle life of hydride electrodes. J. Alloys Compd. 1995, 231, 611–615. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Song, D.; Zhang, Y.; Ye, S. The characteristics of the microencapsulated Ti-Ni alloys and their electrodes. J. Alloys Compd. 1995, 231, 852–855. [Google Scholar]
- Jung, C.B.; Lee, K.S. Electrode characteristics of metal hydride electrodes prepared by mechanical alloying. J. Alloys Compd. 1997, 253–254, 605–608. [Google Scholar] [CrossRef]
- Jung, C.B.; Kim, J.H.; Lee, K.S. Electrode characteristics of nanostructured TiFe and ZrCr2 type metal hydride prepared by mechanical alloying. Nanostructured Mater. 1997, 8, 1093–1104. [Google Scholar] [CrossRef]
- Wang, C.S.; Lei, Y.Q.; Wang, Q.D. Effects of Nb and Pd on the electrochemical properties of a Ti-Ni hydrogen-storage electrode. J. Power Sources 1998, 70, 222–227. [Google Scholar] [CrossRef]
- Wang, C.S.; Lei, Y.Q.; Wang, Q.D. Studies of electrochemical properties of TiNi alloy used as an MH electrode—I. Discharge capacity. Electrochim. Acta 1998, 43, 3193–3207. [Google Scholar] [CrossRef]
- Wang, C.S.; Lei, Y.Q.; Wang, Q.D. Studies of electrochemical properties of TiNi alloy used as an MH electrode. II. Discharge kinetics. Electrochim. Acta 1998, 43, 3209–3216. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q.; Wang, C.S.; Wang, F.S.; Wang, Q.D. Structure of the secondary phase and its effects on hydrogen-storage properties in a Ti0.7Zr0.2V0.1Ni alloy. J. Power Sources 1998, 75, 288–291. [Google Scholar] [CrossRef]
- Han, S.S.; Goo, N.H.; Jeong, W.T.; Lee, K.S. Synthesis of composite metal hydride alloy of A2B and AB type by mechanical alloying. J. Power Sources 2001, 92, 157–162. [Google Scholar] [CrossRef]
- Cuevas, F.; Latroche, M.; Ochin, P.; Dezellus, A.; Fernández, J.F.; Sánchez, C.; Percheron-Guégan, A. Influence of the martensitic transformation on the hydrogenation properties of Ti50−xZrxNi50 alloys. J. Alloys Compd. 2002, 330–332, 250–255. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jankowska, E.; Nowak, M.; Jakubowicz, J. Nanocrystalline titanium-type metal hydride electrodes prepared by mechanical alloying. J. Alloys Compd. 2002, 336, 265–269. [Google Scholar] [CrossRef]
- Bobet, J.; Chevalier, B. Reactive mechanical grinding applied to a (Ti + Ni) mixture and to a TiNi compound. Intermetallics 2002, 10, 597–601. [Google Scholar] [CrossRef]
- Xu, Y.H.; Chen, C.P.; Wang, X.L.; Wang, Q.D. The analysis of the two discharge plateaus for Ti-Ni-based metal hydride electrode alloys. J. Power Sources 2002, 112, 105–108. [Google Scholar] [CrossRef]
- Szajek, A.; Jurczyk, M.; Jankowska, E. The electronic and electrochemical properties of the TiFe-based alloys. J. Alloys Compd. 2003, 348, 285–292. [Google Scholar] [CrossRef]
- Jurczyk, M.; Jankowska, E.; Makowiecka, M.; Wieczorek, I. Electrode characteristics of nanocrystalline TiFe-type alloys. J. Alloys Compd. 2003, 354, L1–L4. [Google Scholar] [CrossRef]
- Han, S.S.; Goo, N.H.; Lee, K.S. Effects of sintering on composite metal hydride alloy of Mg2Ni and TiNi synthesized by mechanical alloying. J. Alloys Compd. 2003, 360, 243–249. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Lei, Y.Q. Multi-component TiNi-based hydrogen storage alloys with the secondary Laves phase. J. Alloys Compd. 2004, 368, 362–366. [Google Scholar] [CrossRef]
- Jankowska, E.; Jurczyk, M. Electrochemical properties of sealed Ni-MH batteries using nanocrystalline TiFe-type anodes. J. Alloys Compd. 2004, 372, L9–L12. [Google Scholar] [CrossRef]
- Makowiecka, M.; Jankowska, E.; Okonska, I.; Jurczyk, M. Effect of Zr additions on the electrode characteristics of nanocrystalline TiNi-type hydrogen storage alloys. J. Alloys Compd. 2005, 388, 303–307. [Google Scholar] [CrossRef]
- Shcherbakova, L.G.; Solonin, S.M.; Kolomiets, L.L.; Katashinskii, V.P. Effect of phase composition and activation of a titanium nickelide surface by electrochemical cycling on its hydrogen sorption capacity. Powder Metall. Met. Ceram. 2005, 44, 389–395. [Google Scholar] [CrossRef]
- Szajek, A.; Makowiecka, M.; Jankowska, E.; Jurczyk, M. Electrochemical and electronic properties of nanocrystalline TiNi1−xMx (M = Mg, Mn, Zr, x = 0, 0.125, 0.25) ternary alloys. J. Alloys Compd. 2005, 403, 323–328. [Google Scholar] [CrossRef]
- Jankowska, E.; Makowiecka, M.; Jurczyk, M. Nickel-metal hydride battery using nanocrystalline TiFe-type hydrogen storage alloys. J. Alloys Compd. 2005, 404–406, 691–693. [Google Scholar] [CrossRef]
- Drenchev, B.; Spassov, T. Electrochemical hydriding of amorphous and nanocrystalline TiNi-based alloys. J. Alloys Compd. 2007, 441, 197–201. [Google Scholar] [CrossRef]
- Jankowska, E.; Makowiecka, M.; Jurczyk, M. Electrochemical performance of sealed Ni-MH batteries using nanocrystalline TiNi-type hydride electrodes. Renew. Energy 2008, 33, 211–215. [Google Scholar] [CrossRef]
- Drenchev, B.; Spassov, T.; Radev, D. Influence of alloying and microstructure on the electrochemical hydriding of TiNi-based ternary alloys. J. Appl. Electrochem. 2008, 38, 437–444. [Google Scholar] [CrossRef]
- Guiose, B.; Cuevas, F.; Décamps, B.; Percheron-Guégan, A. Solid-gas and electrochemical hydrogenation properties of pseudo-binary (Ti,Zr)Ni intermetallic compounds. Int. J. Hydrog. Energy 2008, 33, 5795–5800. [Google Scholar] [CrossRef]
- Drenchev, B.; Spassov, T. Influence of B substitution for Ti and Ni on the electrochemical hydriding of TiNi. J. Alloys Compd. 2009, 474, 527–530. [Google Scholar] [CrossRef]
- Guiose, B.; Cuevas, F.; Décamps, B.; Leroy, E.; Percheron-Guégan, A. Microstructural analysis of the ageing of pseudo-binary (Ti,Zr)Ni intermetallic compounds as negative electrodes of Ni-MH batteries. Electrochim. Acta 2009, 54, 2781–2789. [Google Scholar] [CrossRef]
- Qu, X.; Ma, L.; Yang, M.; Ding, Y. Effect of sintering temperature on electrochemical properties of TiNi hydrogen storage alloy. Chin. J. Rare Met. 2010, 34, 331–335. [Google Scholar]
- Yang, M.; Zhao, X.; Ding, Y.; Ma, L.; Qu, X.; Gao, Y. Electrochemical properties of titanium-based hydrogen storage alloy prepared by solid phase sintering. Int. J. Hydrog. Energy 2010, 35, 2717–2721. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L.; Yang, M.; Ding, Y.; Shen, X. Electrochemical properties of Ti-Ni-H powders prepared by milling titanium hydride and nickel. Int. J. Hydrog. Energy 2010, 35, 3076–3079. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Q.; Zhang, L. Electrochemical hydrogen storage property of NiTi alloys with different Ti content prepared by mechanical alloying. Rare Met. 2011, 30, 63–67. [Google Scholar] [CrossRef]
- Zlatanova, Z.; Spassov, T.; Eggeler, G.; Spassova, M. Synthesis and hydriding/dehydriding properties of Mg2Ni-AB (AB = TiNi or TiFe) nanocomposites. Int. J. Hydrog. Energy 2011, 36, 7559–7566. [Google Scholar] [CrossRef]
- Emami, H.; Cuevas, F. Hydrogenation properties of shape memory Ti(Ni,Pd) compounds. Intermetallics 2011, 19, 876–886. [Google Scholar] [CrossRef]
- Hu, R.; Liu, H.; Zeng, M.; Liu, J.; Zhu, M. Influence of Sn content on microstructure and electrochemical properties of Sn-NiTi film anodes in lithium ion batteries. J. Power Sources 2012, 244, 456–462. [Google Scholar] [CrossRef]
- Bououdina, M.; Oumellal, Y.; Dupont, L.; Aymard, L.; Al-Gharni, H.; Al-Hajry, A.; Maark, T.A.; De Sarkar, A.; Ahuja, R.; Deshpande, M.D.; et al. Lithium storage in amorphous TiNi hydride: Electrode for rechargeable lithium-ion batteries. Mater. Chem. Phys. 2013, 141, 348–354. [Google Scholar] [CrossRef]
- Balcerzak, M.; Nowak, M.; Jakubowicz, J.; Jurczyk, M. Electrochemical behavior of nanocrystalline TiNi doped by MWCNTs and Pd. Renew. Energy 2014, 62, 432–438. [Google Scholar] [CrossRef]
- Li, X.D.; Elkedim, O.; Nowak, M.; Jurczyk, M. Characterization and first principle study of ball milled Ti-Ni with Mg doping as hydrogen storage alloy. Int. J. Hydrog. Energy 2014, 39, 9735–9743. [Google Scholar] [CrossRef]
- Emami, H.; Cuevas, F.; Latroche, M. Ti(Ni,Cu) pseudobinary compounds as efficient negative electrodes for Ni-MH batteries. J. Power Sources 2014, 265, 182–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Elkedim, O.; Balcerzak, M.; Jurczyk, M. Structural and electrochemical hydrogen storage properties of MgTiNx (x = 0.1, 0.5, 1, 2) alloys prepared by ball milling. Int. J. Hydrog. Energy 2016, in press. [Google Scholar]
- Balcerzak, M. Electrochemical and structural studies on Ti-Zr-Ni and Ti-Zr-Ni-Pd alloys and composites. J. Alloys Compd. 2016, 658, 576–587. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K.Y.S. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La, and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrog. Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Hasson, D.F.; Arsenault, R.J. Substitutional-Interstitial Interactions in bcc Alloys. In Treatise on Materials Science and Technology: Materials Science Series; Herman, H., Ed.; Academic Press: New York, NY, USA, 1972; Volume 1, p. 218. [Google Scholar]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Dwight, A.E. CsCl-type equiatomic phases in binary alloys of transition elements. Trans. Am. Inst. Min. Metall. Pet. Eng. 1959, 215, 283–286. [Google Scholar]
- The Japan Institute of Metals and Materials. Non-Stoichiometric Metal Compounds; Maruzen: Tokyo, Japan, 1975; p. 296. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1974; p. 656. [Google Scholar]
- Bohnenstiehl, S.D.; Susner, M.A.; Dregia, S.A.; Sumption, M.D.; Donovan, J.; Collings, E.W. Experimental determination of the peritectic transition temperature of MgB2 in the Mg-B phase diagram. Thermochim. Acta 2014, 576, 27–35. [Google Scholar] [CrossRef]
- Yen, F.; Hwang, K. Shape memory characteristics and mechanical properties of high-density powder metal TiNi with post-sintering heat treatment. Mater. Sci. Eng. A 2011, 528, 5296–5305. [Google Scholar] [CrossRef]
- Gupta, K.P. The Ni-Ti-Zr system (nickel-titanium-zirconium). J. Phase Equilib. 1999, 20, 441–448. [Google Scholar] [CrossRef]
- Osumi, Y. Hydrogen Absorbing Alloy—The Physical Properties and Applications, 1st ed.; Agune Technology Center: Tokyo, Japan, 1993; p. 57. [Google Scholar]
- Liu, Y.; Young, K. Microstructure investigation on metal hydride alloys by electron backscatter diffraction technique. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Drummond, T.J. Work Functions of the Transition Metals and Metal Silicides; SAND99-0391J; Sandia National Labs.: Albuquerque, NM, USA; Livermore, CA, USA, 1999.
- Sun, D.; Jiang, J.; Lei, Y.; Liu, W.; Wu, J.; Wang, Q.; Yang, G. Effects of measurement factor on electrochemical capacity of some hydrogen storage alloys. Mater. Sci. Eng. B 1995, 30, 19–22. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamic, and electrochemical properties of TixZr1−x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloys Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Huang, B.; Ouchi, T.; Fetcenko, M.A. Studies of Ti1.5Zr5.5V0.5(MxNi1−x)9.5 (M = Cr, Mn, Fe, Co, Cu, Al): Part 2. Hydrogen storage and electrochemical properties. J. Alloys Compd. 2010, 501, 245–254. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Huang, B.; Nei, J. Studies on the hydrogen storage characteristic of La1−xCex(NiCoMnAlCuSiZr)5.7 with a B2 secondary phase. J. Alloys Compd. 2014, 585, 760–770. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L. Effect of Ti/Cr content on the microstructures and hydrogen storage properties of Laves phase-related body-centered-cubic solid solution alloys. J. Alloys Compd. 2015, 622, 885–893. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Meng, T. Effects of Cr, Zr, V, Mn, Fe, and Co to the hydride properties of Laves phase-related body-centered-cubic solid solution alloys. J. Power Sources 2015, 281, 164–172. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Wang, L. Annealing effects on Laves phase-related body-centered-cubic solid solution metal hydride alloys. J. Alloys Compd. 2016, 654, 216–225. [Google Scholar] [CrossRef]
- Young, K. Stoichiometry in Inter-Metallic Compounds for Hydrogen Storage Applications. In Stoichiometry and Materials Science—When Numbers Matter; Innocenti, A., Kamarulzaman, N., Eds.; Intech: Rijeka, Crotia, 2012; p. 150. [Google Scholar]
- Young, K.; Young, M.; Chang, S.; Huang, B. Synergetic effects in electrochemical properties of ZrVxNi4.5−x (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) metal hydride alloys. J. Alloys Compd. 2013, 560, 33–41. [Google Scholar] [CrossRef]
- Mosavati, N.; Young, K.; Meng, T.; Ng, K.Y.S. Electrochemical open-circuit voltage and pressure-concentration-temperature isotherm comparison for metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Lin, X.; Reichman, B. Effects of Zn-addition to C14 metal hydride alloys and comparisons to Si, Fe, Cu, Y, and Mo-additives. J. Alloys Compd. 2016, 655, 50–59. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Wong, D.F.; Wang, L. Structural, hydrogen storage, and electrochemical properties of Laves phase-related body-centered-cubic solid solution metal hydride alloys. Int. J. Hydrog. Energy 2014, 39, 21489–21499. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J. Effects of vanadium/nickel contents in Laves phase-related body-centered-cubic solid solution metal hydride alloys. Batteries 2015, 1, 34–53. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Regmi, R.; Lawes, G.; Salley, S.O.; Ng, K.Y.S. Gaseous phase hydrogen storage and electrochemical properties of Zr8Ni21, Zr7Ni10, Zr9Ni11, and ZrNi metal hydride alloys. Int. J. Hydrog. Energy 2012, 37, 16042–16055. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Reichman, B.; Koch, J.; Fetcenko, M.A. Improvement in the low-temperature performance of AB5 metal hydride alloys by Fe-addition. J. Alloys Compd. 2011, 509, 7611–7617. [Google Scholar] [CrossRef]
- Young, K.; Yasuoka, S. Capacity degradation mechanisms in nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. The role of Mn in C14 Laves phase multi-component alloys for NiMH battery application. J. Alloys Compd. 2009, 477, 749–758. [Google Scholar] [CrossRef]
- Chang, S.; Young, K.; Ouchi, T.; Meng, T.; Nei, J.; Wu, X. Studies on incorporation of Mg in Zr-based AB2 metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Moghe, D. Importance of rare-earth additions in Zr-based AB2 metal hydride alloys. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Ouchi, T.; Huang, B.; Reichman, B. Effects of La-addition to the structure, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. Electrochim. Acta 2015, 174, 815–825. [Google Scholar] [CrossRef]
- Ouchi, T.; Young, K.; Moghe, D. Reviews on the Japanese patent applications regarding nickel/metal hydride batteries. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Young, K.; Koch, J.; Yasuoka, S.; Shen, H.; Bendersky, L.A. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sources 2015, 277, 433–442. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; Ouchi, T.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 1 Structural, hydrogen storage and electrochemical properties. J. Alloys Compd. 2016, 660, 407–415. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 Battery performance and failure mechanism. J. Alloys Compd. 2016, 664, 417–427. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Koch, J.; Ouchi, T.; Yasuoka, S. Failure mechanisms of nickel/metal hydride batteries with cobalt-substituted superlattice hydrogen-absorbing alloy anodes at 50 °C. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Nei, J. Structure, hydrogen storage, and electrochemical properties of body-centered-cubic Ti40V30Cr15Mn13X2 alloys (X = B, Si, Mn, Ni, Zr, Nb, Mo, and La). Batteries 2015, 1, 74–90. [Google Scholar] [CrossRef]
- Young, K.; Ng, K.Y.S.; Bendersky, L.A. A technical report of the robust affordable next generation energy storage system-BASF program. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Yan, S.; Young, K.; Ng, K.Y.S. Effects of salt additives to the KOH electrolyte used in Ni/MH batteries. Batteries 2015, 1, 54–73. [Google Scholar] [CrossRef]

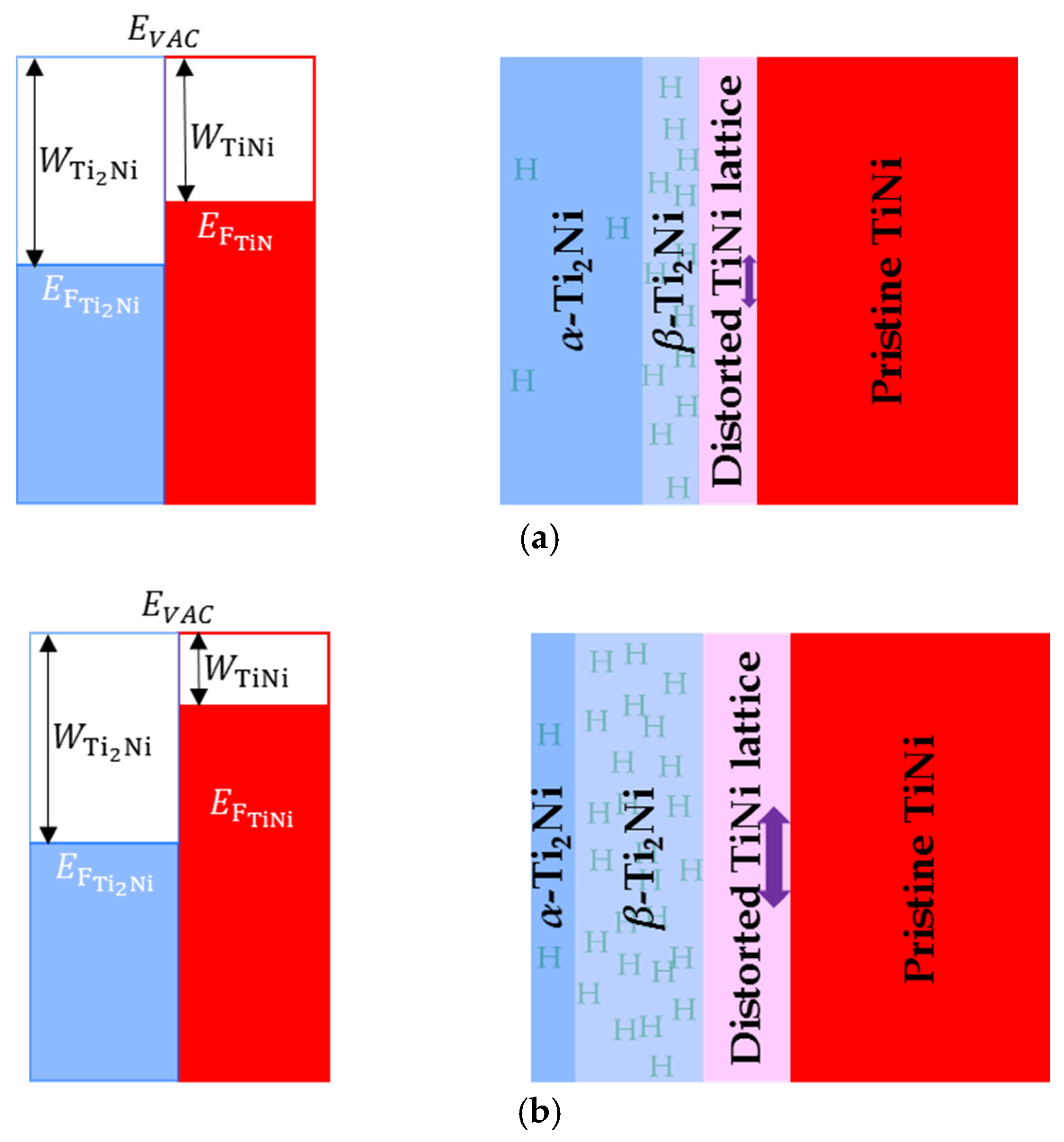


| Alloy Formula | Preparation Method | TiNi Phase Structure | Main Discoveries | Reference |
|---|---|---|---|---|
| Ti53.6Ni46.4 | - | - |
| [19] |
| TiNi | SN | B2 |
| [13] |
| TiNi + Ti2Ni | SN | B2 |
| [13] |
| TiNi | SN | B2 |
| [14] |
| TiNi | AM | - |
| [20] |
| TiNix (x = 0.5–1.0) | SN vs. PM | - |
| [21] |
| Ti0.8Zr0.2Nix (x = 0.5–1.0) | SN vs. PM | - |
| [21] |
| TiNi | SN | - |
| [22] |
| Ti1−yZryNix (x = 0.50–1.45, y = 0–1.0) | SN | B2 |
| [23] |
| Ti0.5Zr0.5Ni0.95–xCu0.05+x (x = 0–0.05) | SN | B2 |
| [23] |
| TiNi0.9B0.1 | SN | - |
| [24] |
| Ti0.7Zr0.2V0.1Ni | IM | B2 |
| [25] |
| TiNi | CO + SN | - |
| [26] |
| TiNi | - | B2 vs. B19’ |
| [27] |
| TiNi | AM | - |
| [28] |
| TiNi + 5% M (M = Ni, Cu) | SN + MC | - |
| [29] |
| TiNi0.5Fe0.5 | AM vs. MA | - |
| [30] |
| TiNi0.6Fe0.4 | AM vs. AM + MA vs. MA | B2 |
| [31] |
| Ti50Ni41Nb9 | AM | B2 |
| [32] |
| TiNi | AM | - |
| [33] |
| TiNi | AM | - |
| [34] |
| Ti0.7Zr0.2V0.1Ni | AM + ANN | B2 |
| [35] |
| Mg2Ni + TiNi | MA | B2 |
| [36] |
| Ti50−xZrxNi50 (x = 0–24) | IM vs. MS | B19’ for IM alloy vs. B2 for MS alloy |
| [37] |
| TiNixFe1−x (x = 0–1.0) | MA + ANN | B2 |
| [38] |
| (Ti + Ni) | MA under H2 | - |
| [39] |
| Ti0.5Ni0.25Al0.25 | IM | B2 |
| [40] |
| Ti(Ni,Fe,Mo,Cr,Co) | MA + ANN | B2 |
| [41] |
| Ti(Ni,Fe,Mo,Cr,Co) | AM + ANN vs. MA + ANN | B2 |
| [42] |
| Mg2Ni + TiNi | MA vs. MA + ANN | B2 |
| [43] |
| (Ti,Zr,V,Cr,Mn)Ni | AM | B2 |
| [44] |
| TiNi0.75Fe0.25 | MA + ANN | B2 |
| [45] |
| Ti(Ni,Fe,Zr) | MA + ANN | B2 |
| [46] |
| TiNi | CHR vs. CHR + ANN | B2 + B19’ for CHR alloy vs. B2 for CHR + ANN alloy |
| [47] |
| TiNi1−xMx (M = Mg, Mn, Zr, x = 0–0.25) | MA + ANN | B2 |
| [48] |
| Ti (Ni, Fe, Zr, Mo, Cr, Co, Al) | MA + ANN | B2 |
| [49] |
| TiNi1−xMx (M = Co, Fe, Sn, x = 0–0.2) | MA vs. MA + ANN | - |
| [50] |
| Ti (Ni, Fe, Zr, Mo, Cr, Co) | MA | B2 |
| [51] |
| Ti0.8M0.2Ni (M = Zr, V) | MA vs. MA + ANN | B2 |
| [52] |
| TiNi0.8M0.2 (M = Cu, Mn) | MA vs. MA + ANN | - |
| [52] |
| TiNi1−xMnx (x = 0.2–1.0) | MA vs. MA + ANN | - |
| [52] |
| Ti1.02−xZrxNi0.98 (x = 0–0.48) | IM + ANN | B19’ |
| [53] |
| TiNi0.8B0.2, Ti0.8B0.2Ni, | MA vs. MA + ANN | B2 |
| [54] |
| Ti1.02−xZrxNi0.98 (x = 0–0.48) | IM + ANN | B19’ |
| [55] |
| TiNi | SN (750–950 °C) | B2 |
| [56] |
| TiNi | SN | B2 |
| [57] |
| TiNi | MA (20–60 h) | - |
| [58] |
| TiNi | MA vs. MA + ANN | B2 |
| [59] |
| Mg2Ni + TiNi | MA | B2 |
| [60] |
| Ti1.04Ni0.96−xPdx (x = 0–0.5) | IM + ANN | As x increases, B2 + B19’ → B2 + B19’ + R → B2 + B19 |
| [61] |
| Sn-doped TiNi | Thin film sputtering | - |
| [62] |
| TiNi | MA | - |
| [63] |
| TiNi-5 wt% Pd + 5 wt% MWCNT | MA + ANN + MA with MWCNT | B2 |
| [64] |
| (TiNi)1−xMgx (x = 0–0.3) | MA (10 to 40 h) | B2 |
| [65] |
| Ti1.01Ni0.99−xCux (x = 0–0.5) | IM + ANN | As x increases, B19’ → B19 |
| [66] |
| MgTiNi2 | MA | - |
| [67] |
| Ti1−xZrxNi (x = 0–0.5) | MA + ANN | B2 |
| [68] |
| Ti0.75Zr0.25Ni-5 wt% Pd vs. Ti0.75Zr0.25Ni + 5 wt% Pd | MA + ANN vs. MA + ANN + MA with Pd | B2 |
| [68] |
| Alloy TN-X | Source | Ti | Zr | Ni | X | B/A |
|---|---|---|---|---|---|---|
| TN-Ni | Design | 50.0 | 1.0 | 49.0 | - | 0.96 |
| ICP | 50.0 | 0.6 | 49.4 | 0.0 | 0.98 | |
| TN-Cr | Design | 50.0 | 1.0 | 44.0 | 5.0 | 0.96 |
| ICP | 49.7 | 1.1 | 44.4 | 4.7 | 0.97 | |
| TN-Mn | Design | 50.0 | 1.0 | 44.0 | 5.0 | 0.96 |
| ICP | 49.2 | 1.0 | 44.9 | 4.9 | 0.99 | |
| TN-Fe | Design | 50.0 | 1.0 | 44.0 | 5.0 | 0.96 |
| ICP | 49.9 | 1.0 | 44.3 | 4.8 | 0.96 | |
| TN-Co | Design | 50.0 | 1.0 | 44.0 | 5.0 | 0.96 |
| ICP | 49.8 | 0.9 | 44.5 | 4.8 | 0.97 | |
| TN-Cu | Design | 50.0 | 1.0 | 44.0 | 5.0 | 0.96 |
| ICP | 49.5 | 1.3 | 44.5 | 4.7 | 0.97 |
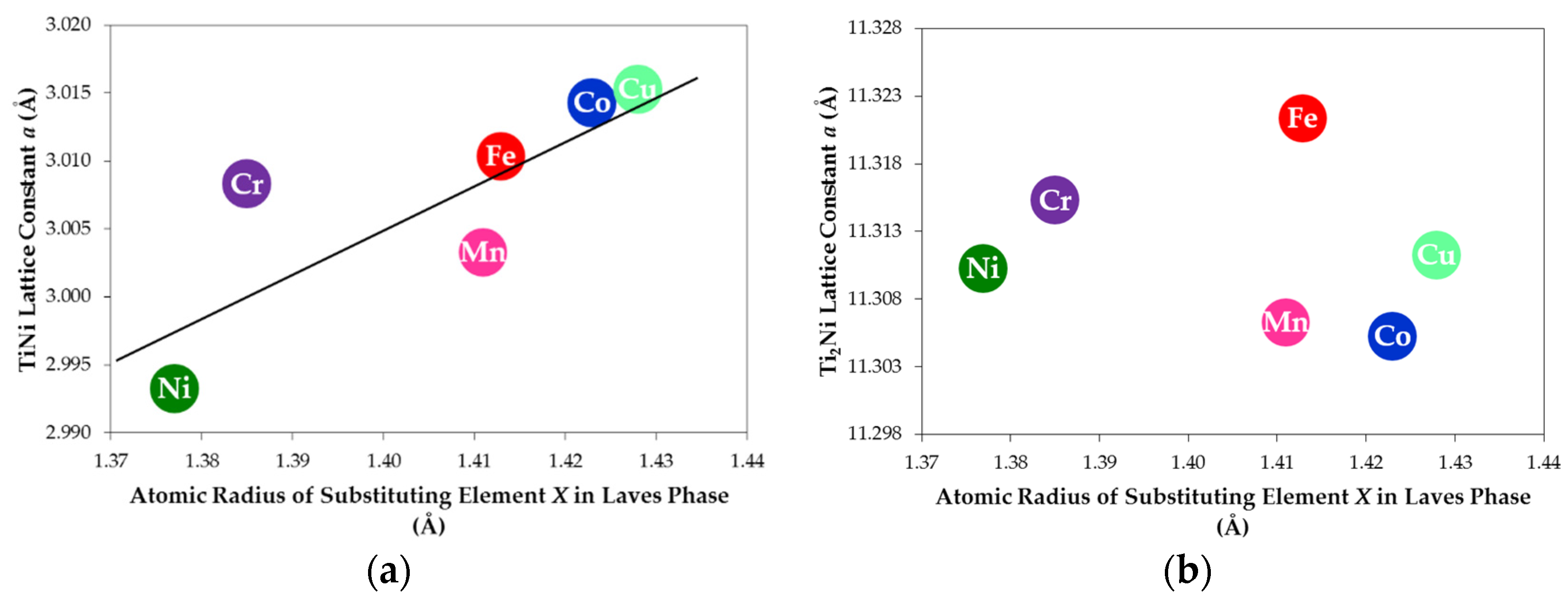
| Alloy TN-X | a of TiNi (Å) | a of Ti2Ni (Å) | TiNi Abundance (wt%) | Ti2Ni Abundance (wt%) | TiNi Crystallite Size (Å) | Ti2Ni Crystallite Size (Å) |
|---|---|---|---|---|---|---|
| TN-Ni | 2.993 | 11.310 | 68.7 | 31.3 | 139 | 448 |
| TN-Cr | 3.014 | 11.305 | 75.5 | 24.5 | 368 | 804 |
| TN-Mn | 3.015 | 11.311 | 78.4 | 21.6 | 251 | >1000 |
| TN-Fe | 3.003 | 11.306 | 80.7 | 19.3 | 346 | 812 |
| TN-Co | 3.008 | 11.315 | 80.6 | 19.4 | 212 | 655 |
| TN-Cu | 3.010 | 11.321 | 71.6 | 28.4 | 206 | 727 |
| Alloy TN-X | Area | Ti | Zr | Ni | X | B/A | Phase(s) |
|---|---|---|---|---|---|---|---|
| TN-Ni | 1 | 36.3 | 8.4 | 55.3 | 0.0 | 1.24 | TiNi-2 |
| 2 | 40.9 | 4.7 | 54.3 | 0.0 | 1.19 | TiNi-2 | |
| 3 | 44.2 | 2.3 | 53.5 | 0.0 | 1.15 | TiNi-2 | |
| 4 | 42.4 | 3.4 | 54.2 | 0.0 | 1.18 | TiNi-2 | |
| 5 | 46.9 | 0.6 | 52.5 | 0.0 | 1.11 | TiNi-1 | |
| 6 | 47.5 | 0.5 | 52.0 | 0.0 | 1.08 | TiNi-1 | |
| 7 | 64.2 | 0.5 | 35.3 | 0.0 | 0.55 | Ti2Ni | |
| 8 | 63.6 | 0.4 | 36.0 | 0.0 | 0.56 | Ti2Ni | |
| TN-Cr | 1 | 41.0 | 4.6 | 44.4 | 10.0 | 1.19 | TiNi-2 |
| 2 | 42.0 | 2.7 | 43.9 | 11.3 | 1.24 | TiNi-2 | |
| 3 | 47.5 | 0.9 | 47.6 | 4.0 | 1.07 | TiNi-1 | |
| 4 | 46.3 | 1.4 | 46.9 | 5.4 | 1.10 | TiNi-1 | |
| 5 | 62.2 | 0.8 | 33.3 | 3.7 | 0.59 | Ti2Ni | |
| TN-Mn | 1 | 33.8 | 12.0 | 32.5 | 21.7 | 1.18 | TiNi-2 |
| 2 | 44.1 | 2.7 | 43.0 | 10.1 | 1.13 | TiNi-2 | |
| 3 | 43.7 | 3.1 | 42.5 | 10.6 | 1.13 | TiNi-2 | |
| 4 | 46.6 | 1.0 | 46.2 | 6.1 | 1.10 | TiNi-1 | |
| 5 | 47.0 | 0.8 | 46.7 | 5.5 | 1.09 | TiNi-1 | |
| 6 | 49.0 | 0.6 | 45.3 | 5.0 | 1.01 | TiNi-1 | |
| 7 | 46.5 | 1.0 | 47.0 | 5.5 | 1.11 | TiNi-1 | |
| 8 | 65.3 | 0.6 | 32.4 | 1.7 | 0.52 | Ti2Ni | |
| 9 | 65.0 | 0.6 | 32.5 | 1.9 | 0.52 | Ti2Ni | |
| TN-Fe | 1 | 46.4 | 7.0 | 42.1 | 4.5 | 0.87 | TiNi-2 |
| 2 | 46.1 | 6.2 | 42.9 | 4.8 | 0.91 | TiNi-2 | |
| 3 | 47.2 | 1.3 | 46.3 | 5.2 | 1.06 | TiNi-1 | |
| 4 | 46.8 | 2.2 | 45.9 | 5.2 | 1.04 | TiNi-1 | |
| 5 | 52.3 | 1.1 | 42.0 | 4.6 | 0.87 | TiNi + Ti2Ni | |
| 6 | 52.4 | 1.2 | 41.6 | 4.8 | 0.87 | TiNi + Ti2Ni | |
| 7 | 62.8 | 0.9 | 32.6 | 3.7 | 0.57 | Ti2Ni | |
| 8 | 57.8 | 1.0 | 37.1 | 4.1 | 0.70 | Ti2Ni | |
| TN-Co | 1 | 29.4 | 13.7 | 55.6 | 1.3 | 1.32 | TiNi-2 |
| 2 | 29.4 | 13.3 | 55.8 | 1.4 | 1.34 | TiNi-2 | |
| 3 | 37.2 | 26.6 | 33.1 | 3.2 | 0.57 | (TiZr)2Ni | |
| 4 | 41.0 | 15.1 | 39.7 | 4.1 | 0.78 | (TiZr)2Ni | |
| 5 | 40.6 | 5.0 | 51.7 | 2.7 | 1.19 | TiNi-2 | |
| 6 | 43.9 | 2.8 | 49.7 | 3.5 | 1.14 | TiNi-2 | |
| 7 | 47.6 | 0.5 | 45.1 | 6.7 | 1.08 | TiNi-1 | |
| 8 | 47.9 | 0.6 | 44.9 | 6.6 | 1.06 | TiNi-1 | |
| 9 | 65.5 | 0.7 | 29.9 | 3.9 | 0.51 | Ti2Ni | |
| 10 | 65.4 | 0.6 | 30.3 | 3.7 | 0.51 | Ti2Ni | |
| TN-Cu | 1 | 29.1 | 10.7 | 41.0 | 19.1 | 1.51 | TiNi-2 |
| 2 | 34.7 | 9.1 | 40.1 | 16.0 | 1.28 | TiNi-2 | |
| 3 | 34.0 | 30.5 | 31.0 | 4.5 | 0.55 | (TiZr)2Ni | |
| 4 | 42.1 | 14.2 | 39.4 | 4.4 | 0.78 | (TiZr)2Ni | |
| 5 | 43.7 | 2.9 | 46.5 | 6.8 | 1.14 | TiNi | |
| 6 | 44.5 | 2.4 | 47.1 | 6.0 | 1.13 | TiNi | |
| 7 | 47.2 | 1.0 | 47.3 | 4.4 | 1.07 | TiNi-1 | |
| 8 | 46.8 | 1.0 | 47.6 | 4.6 | 1.09 | TiNi-1 | |
| 9 | 63.5 | 0.8 | 34.0 | 1.7 | 0.55 | Ti2Ni | |
| 10 | 65.6 | 0.8 | 32.4 | 1.2 | 0.51 | Ti2Ni |
| Alloy TN-X | Maximum Capacity at 90 °C (wt%) | Reversible Capacity at 90 °C (wt%) | Maximum Capacity at 120 °C (wt%) | Reversible Capacity at 120 °C (wt%) |
|---|---|---|---|---|
| TN-Ni | 0.13 | 0.09 | 0.15 | 0.13 |
| TN-Cr | 1.18 | 0.57 | 1.08 | 0.67 |
| TN-Mn | 0.98 | 0.48 | 0.92 | 0.57 |
| TN-Fe | 1.21 | 0.75 | 1.06 | 0.85 |
| TN-Co | 0.16 | 0.13 | 0.19 | 0.14 |
| TN-Cu | 0.87 | 0.54 | 0.81 | 0.60 |
| Alloy TN-X | TN-Ni | TN-Cr | TN-Mn | TN-Fe | TN-Co | TN-Cu |
| Maximum Full Capacity @4 mA·g−1 (mAh·g−1) | 345 | 389 | 394 | 397 | 370 | 308 |
| HRD @2nd or 3rd cycle (%) | 63 | 66 | 71 | 66 | 63 | 79 |
| Number of Cycles Needed to Reach 95% of Maximum Full Capacity | 10 | 6 | 1 | 1 | 1 | 1 |
| Degradation Performance (%) | 0 | 8 | 40 | 3 | 23 | 21 |
| D (10−10 cm2·s−1) | 3.15 | 2.71 | 2.68 | 1.87 | 2.37 | 1.94 |
| Io (mA·g−1) | 22.15 | 24.77 | 34.08 | 37.47 | 26.19 | 36.43 |
| Ms (emu·g−1) | 0.187 | 0.219 | 0.509 | 0.511 | 0.586 | 0.542 |
| H1/2 (kOe) | 0.172 | 0.221 | 0.170 | 0.159 | 0.151 | 0.484 |
| Alloy TN-X | Gaseous Phase Capacity | Electrochemical Full Capacity | HRD | Activation | Degradation | D | Io | Ms |
|---|---|---|---|---|---|---|---|---|
| TN-Cr | + + + + | + + + + | + | + + | – | – – | + | + |
| TN-Mn | + + + | + + + + + | + + | + + + + + | – – – – – | – – | + + + + | + + + + |
| TN-Fe | + + + + + | + + + + + | + | + + + + + | ≈ | – – – – – | + + + + + | + + + + |
| TN-Co | ≈ | + + | ≈ | + + + + + | – – – | – – – | + | + + + + + |
| TN-Cu | + + + | – – – | + + + + + | + + + + + | – – | – – – – | + + + + + | + + + + + |
| Alloy | Cost | Capacity | HRD | Activation | Low Temp. | High Temp. | Charge Retention | Cycle Life |
|---|---|---|---|---|---|---|---|---|
| AB5 | ⋆⋆⋆⋆ | ⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ |
| AB2 | ⋆⋆⋆ | ⋆⋆⋆⋆ | ⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆⋆⋆⋆ |
| A2B7 | ⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆ |
| bcc | ⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆⋆⋆ | TBD | TBD | ⋆ | ⋆ |
| Laves-bcc | ⋆ | ⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆⋆ | ⋆ | ⋆⋆⋆ |
| MgNi | ⋆⋆⋆⋆⋆ | ⋆⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆⋆⋆ | TBD | TBD | TBD | ⋆ |
| TiNi | ⋆⋆⋆⋆⋆ | ⋆⋆⋆ | ⋆ | ⋆⋆ | TBD | TBD | TBD | ⋆⋆⋆⋆⋆ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nei, J.; Young, K.-H. Gaseous Phase and Electrochemical Hydrogen Storage Properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for Nickel Metal Hydride Battery Applications. Batteries 2016, 2, 24. https://doi.org/10.3390/batteries2030024
Nei J, Young K-H. Gaseous Phase and Electrochemical Hydrogen Storage Properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for Nickel Metal Hydride Battery Applications. Batteries. 2016; 2(3):24. https://doi.org/10.3390/batteries2030024
Chicago/Turabian StyleNei, Jean, and Kwo-Hsiung Young. 2016. "Gaseous Phase and Electrochemical Hydrogen Storage Properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for Nickel Metal Hydride Battery Applications" Batteries 2, no. 3: 24. https://doi.org/10.3390/batteries2030024
APA StyleNei, J., & Young, K.-H. (2016). Gaseous Phase and Electrochemical Hydrogen Storage Properties of Ti50Zr1Ni44X5 (X = Ni, Cr, Mn, Fe, Co, or Cu) for Nickel Metal Hydride Battery Applications. Batteries, 2(3), 24. https://doi.org/10.3390/batteries2030024