Microstructures of the Activated Si-Containing AB2 Metal Hydride Alloy Surface by Transmission Electron Microscope
Abstract
:1. Introduction
2. Experimental Section
- (1)
- Mixing of the powders with M-Bond 610 glue, followed by placement of the mixture into a 3 mm diameter copper pipe. This was then cured for 8 h at 150 °C;
- (2)
- Slicing the copper pipe containing the glued powders into 0.5 mm thick disks using a low-speed diamond saw;
- (3)
- Polishing of the 3-mm-diameter disk down to approximately 100 µm using SiC media from 320 grit down to 1200 grit, then dimpling grinding;
- (4)
- Finishing of thinning with 5 kV ion milling (on a liquid N2 cooled stage);
- (5)
- Finally, insertion of the sample into the microscope to examine for electron beam transparency areas. If none are detected, additional ion milling time, followed by TEM examination, is required. This step was repeated as many times as necessary to provide high quality thin areas for detailed TEM studies.
3. Results and Discussion
3.1. Summary of Alloy Properties
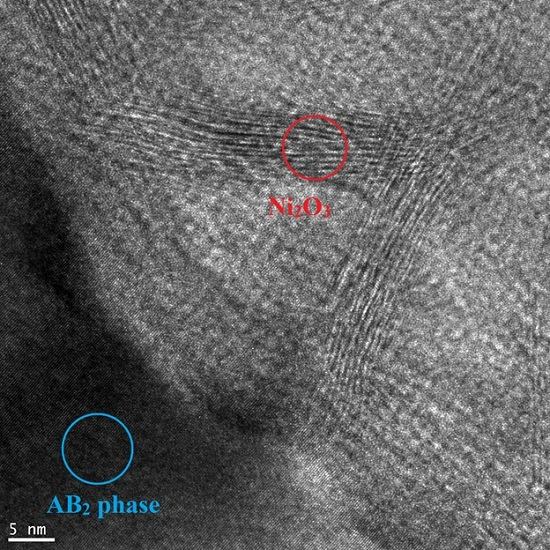
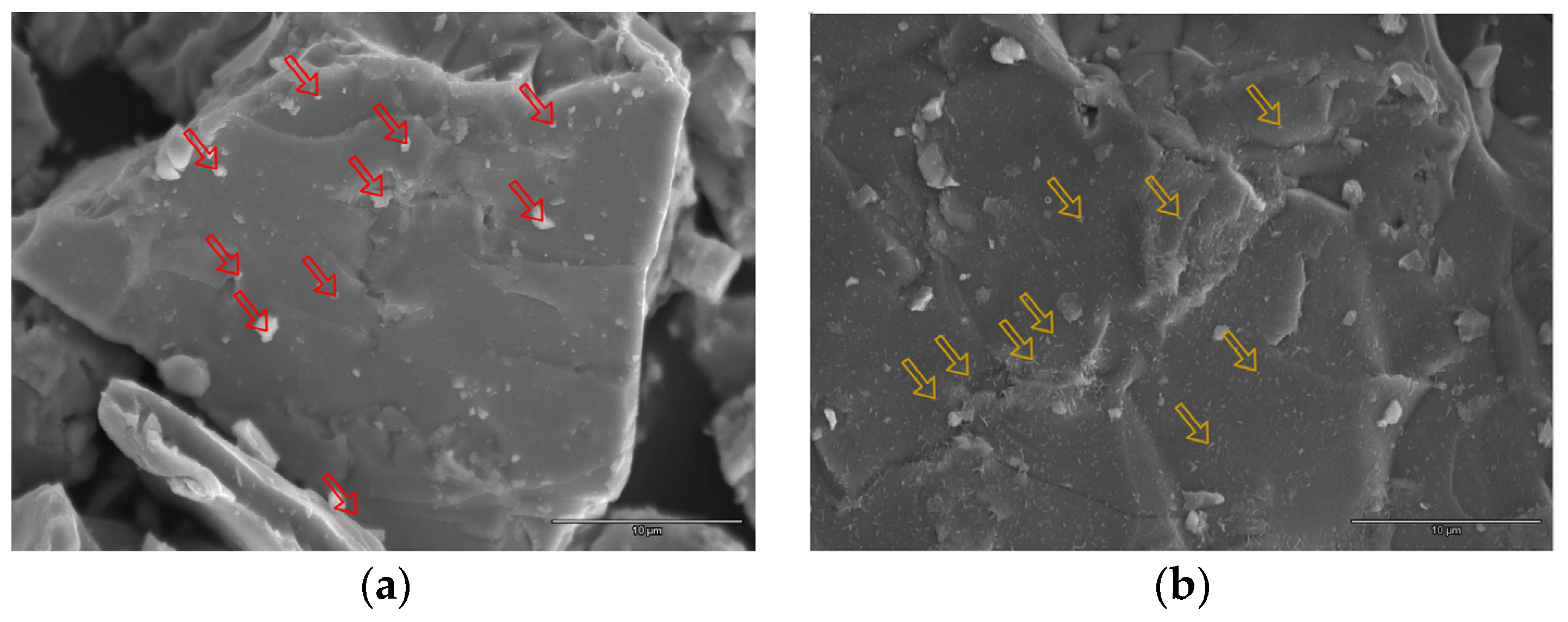
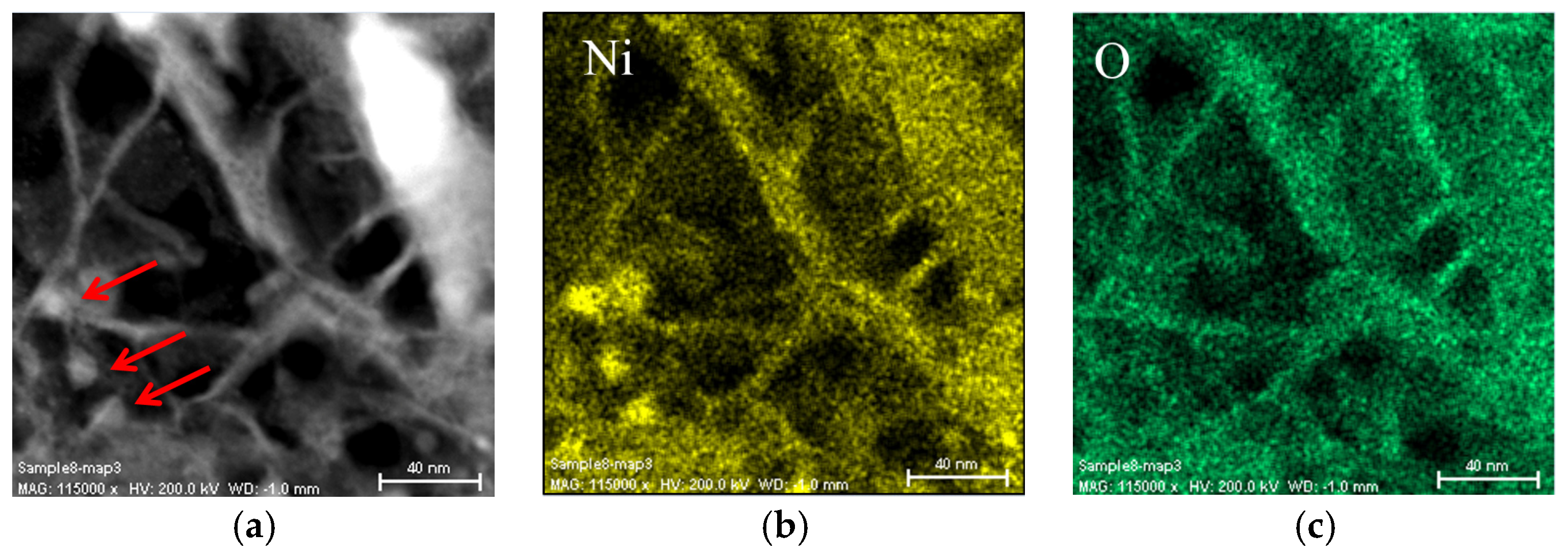
3.2. Scanning Electron Microscope and X-Ray Energy Dispersive Spectroscopy Analyses on the Activated Alloy Surface
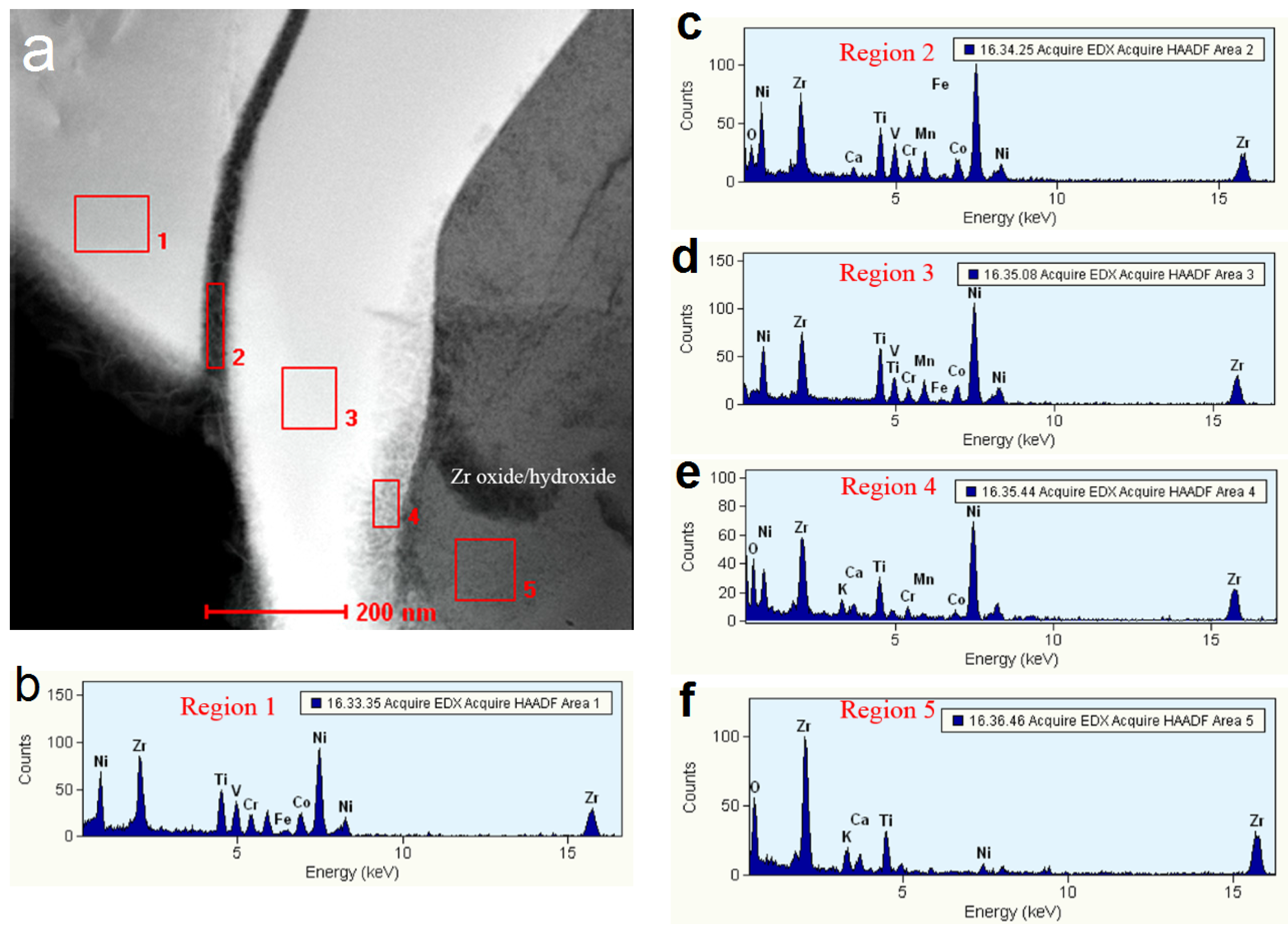
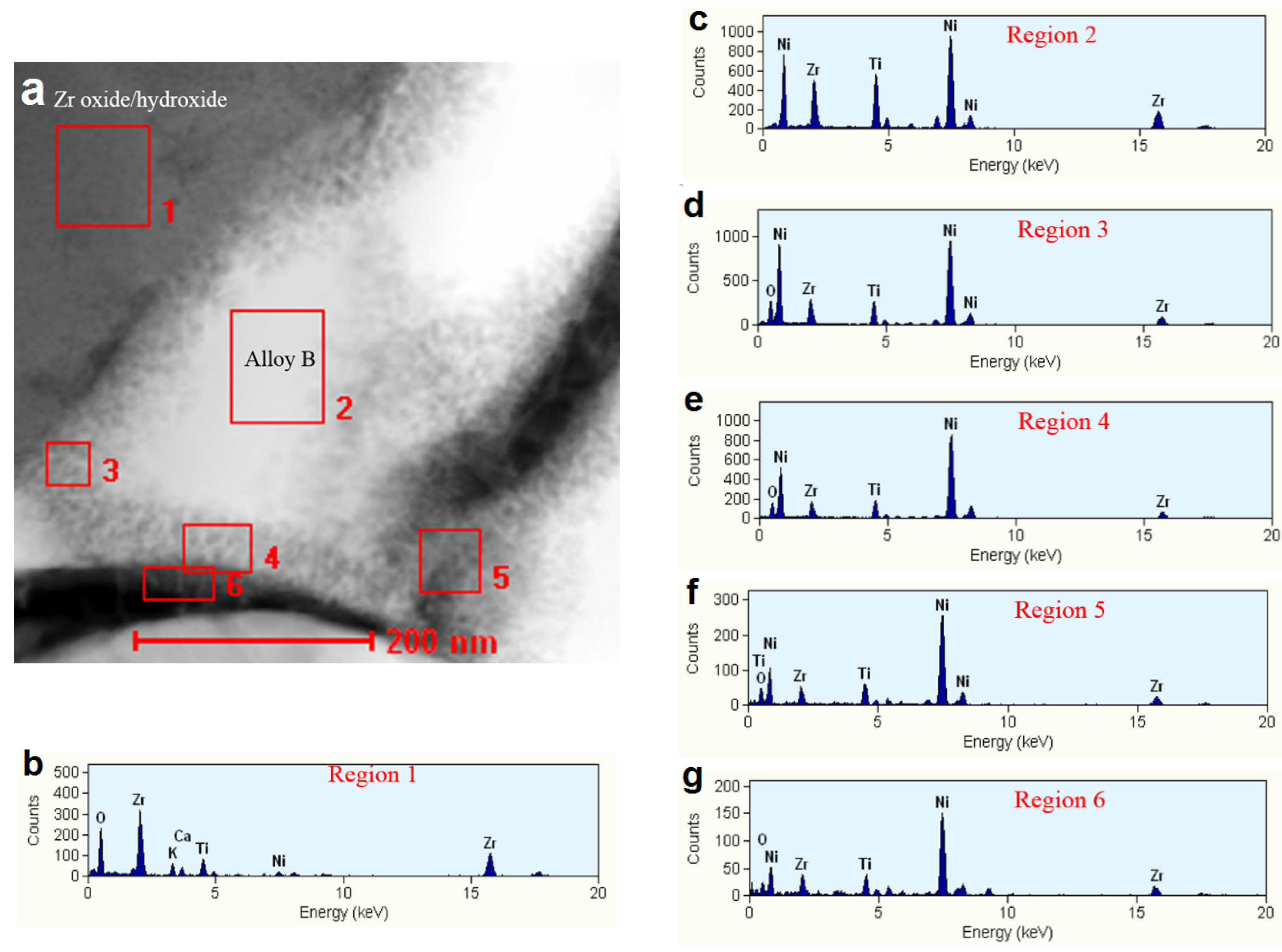
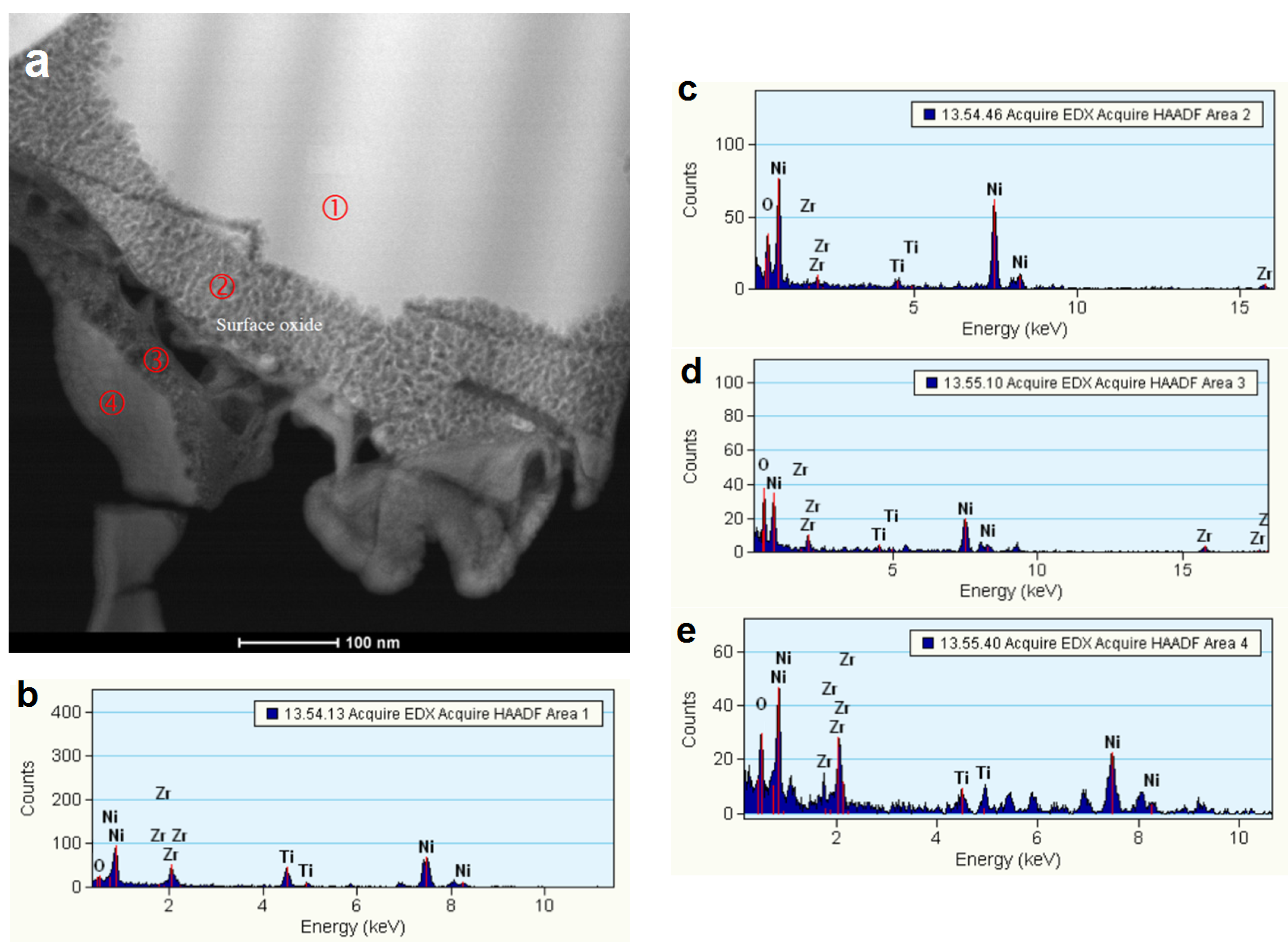
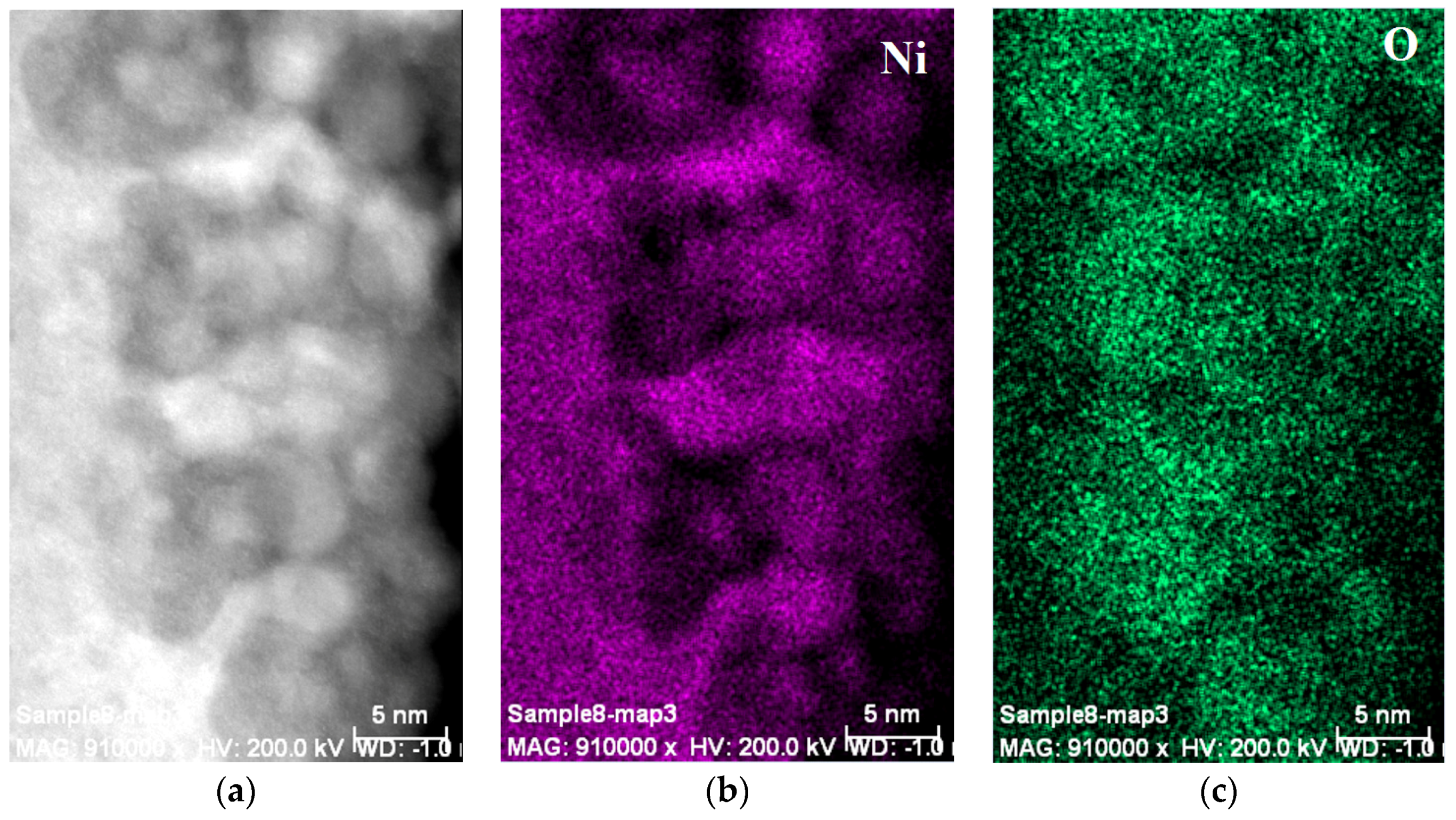
3.3. Regions under the Zr Oxide/Hydroxide Layer
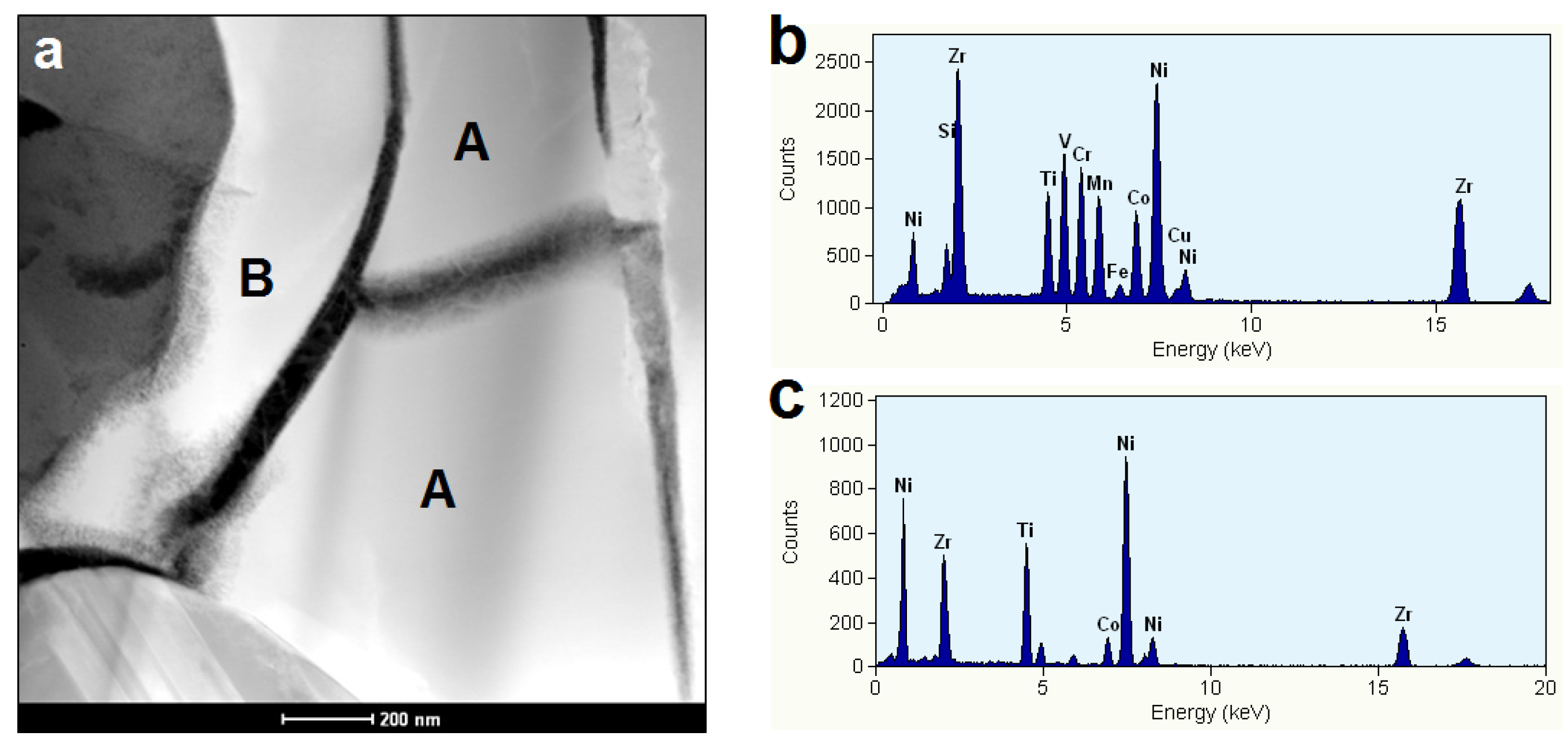
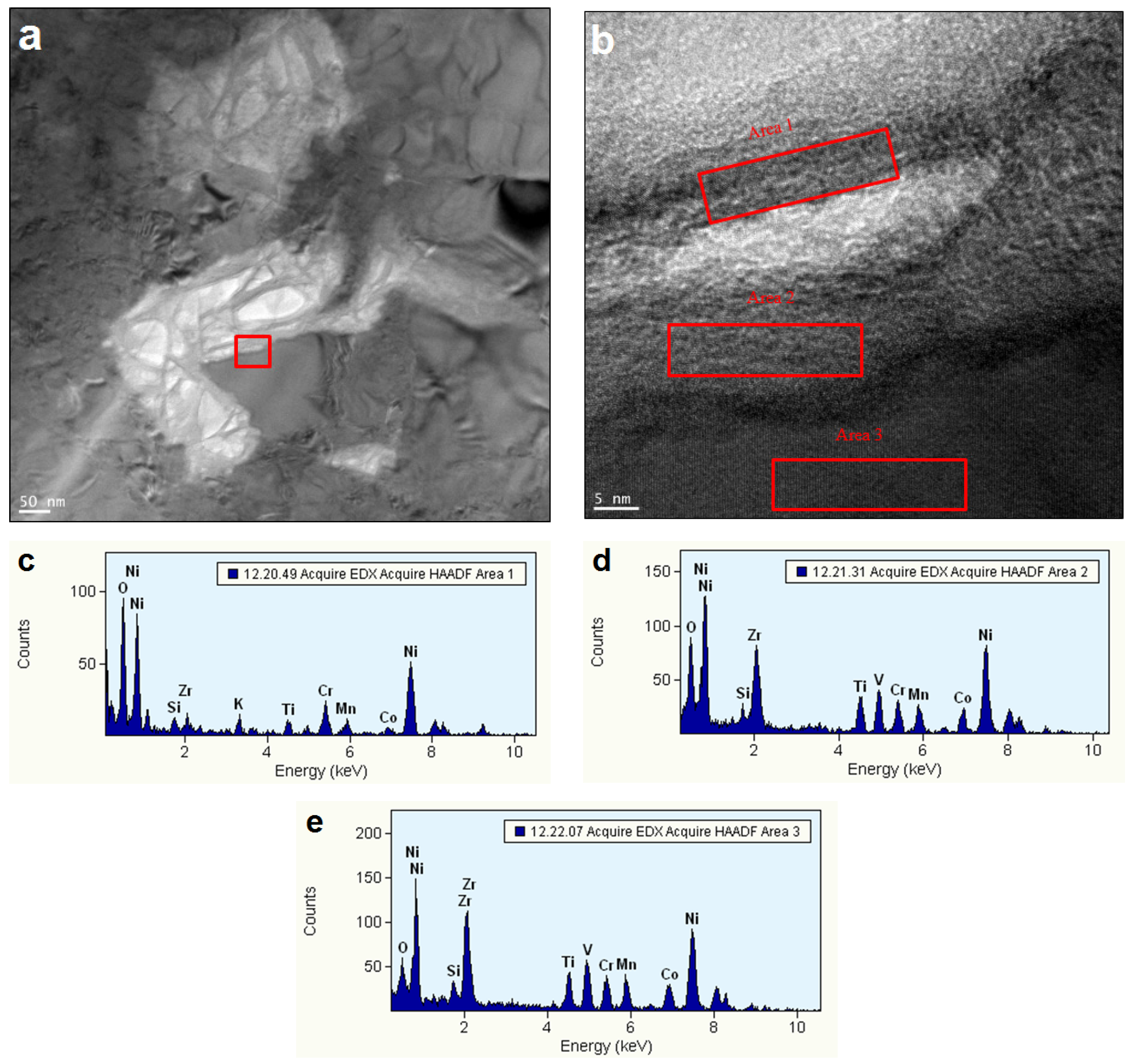
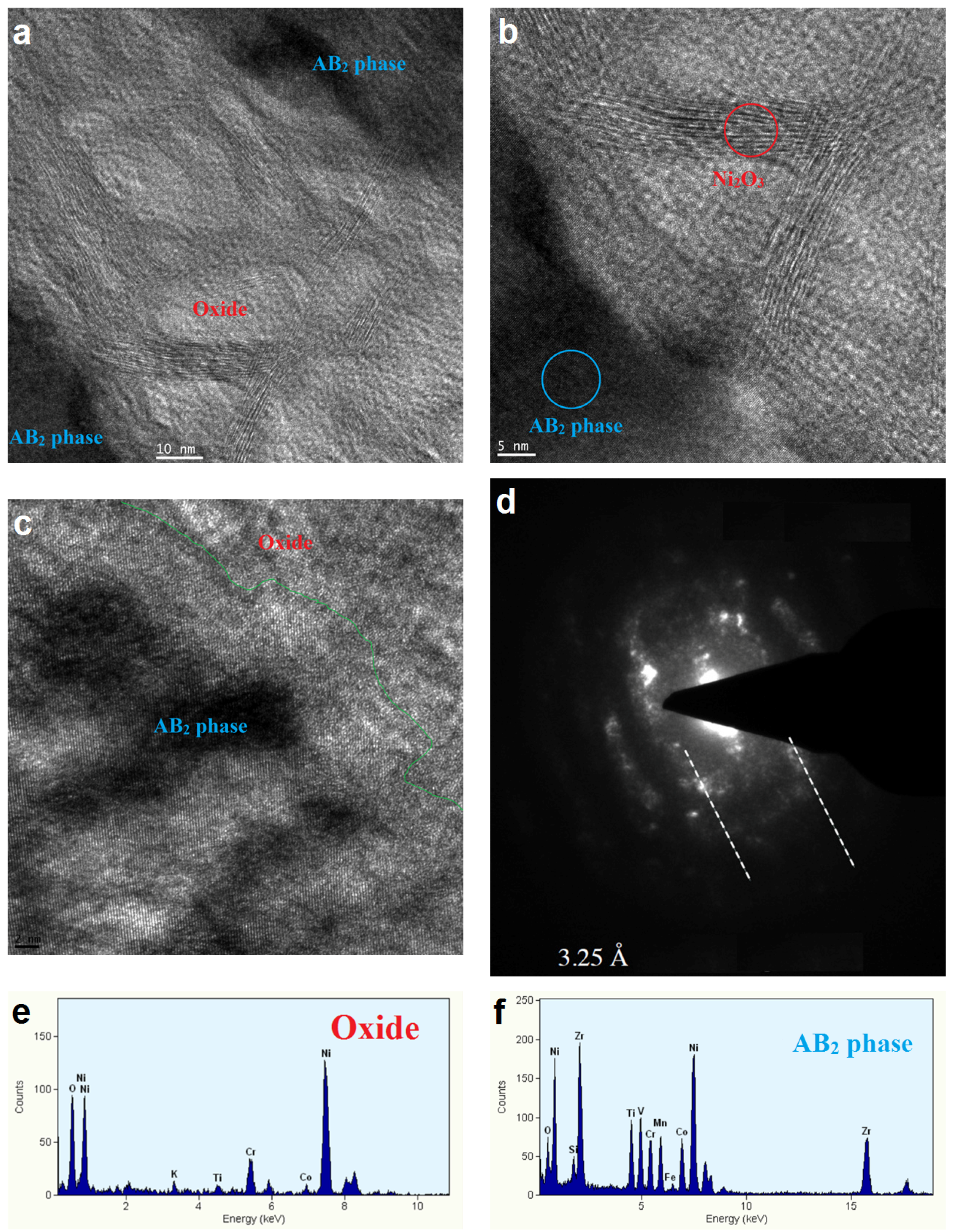
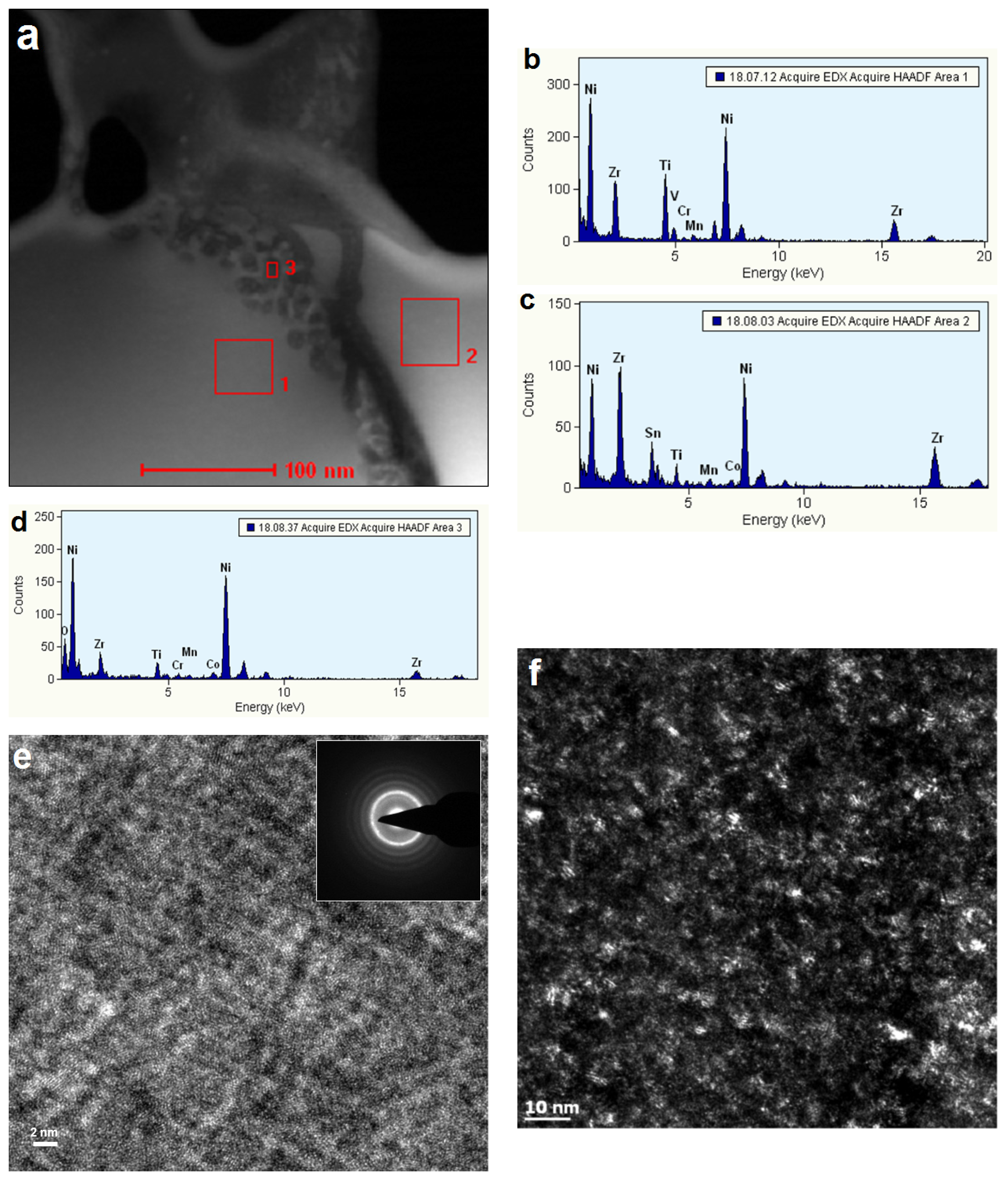
3.4. Electrochemically Active Surfaces on the AB2 Phase
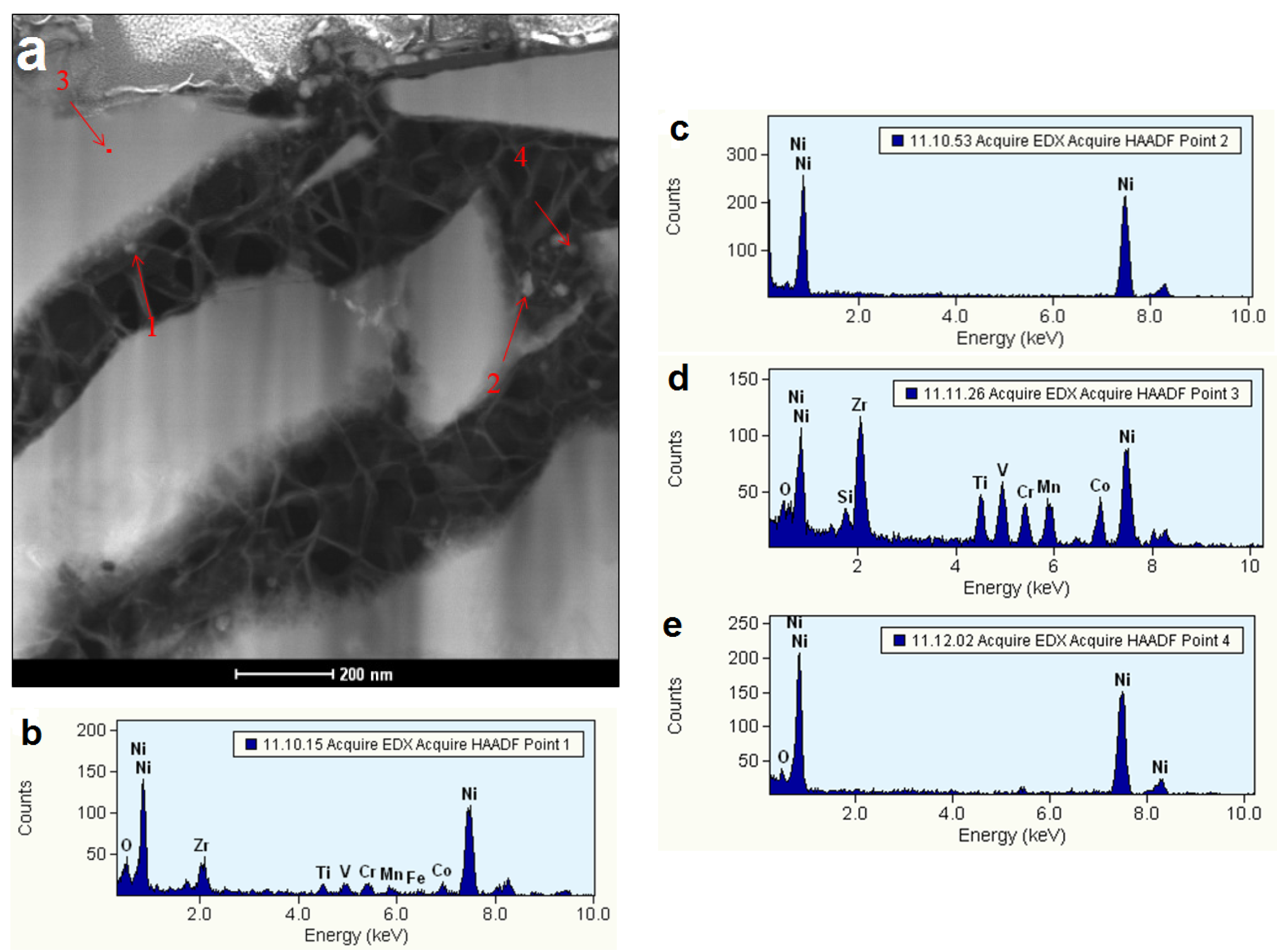
3.5. Electrochemically Active Surfaces on the B2 Phase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hybrid Electric Vehicle. Available online: https://en.wikipedia.org/wiki/Hybrid_electric_vehicle (accessed on 30 August 2015).
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel–metal hydride battery for industrial applications. J. Alloys Compd. 2013, 580, S378–S381. [Google Scholar] [CrossRef]
- Nishimura, K.; Takasaki, T.; Sakai, T. Introduction of large-sized nickel–metal hydride battery GIGACELL® for industrial applications. J. Alloys Compd. 2013, 580, S353–S358. [Google Scholar] [CrossRef]
- Kai, T.; Ishida, J.; Yasuoka, S.; Takeno, K. The effect of nickel-metal hydride battery’s characteristics with structure of the alloy. In Proceedings of the 54th Battery Symposium in Japan, Osaka, Japan, 6–9 October 2013; p. 210.
- Kang, J.; Yan, F.; Zhang, P.; Du, C. Comparison of comprehensive properties of Ni-MH (nickel-metal hydride) and Li-ion (lithium-ion) batteries in terms of energy efficiency. Energy 2014, 70, 618–625. [Google Scholar] [CrossRef]
- Ovshisnky, S.R.; Fetcenko, M.A.; Ross, J. A nickel metal hydride battery for electric vehicles. Science 1993, 260, 176–181. [Google Scholar]
- Dhar, S.K.; Ovshinsky, S.R.; Gifford, P.R.; Corrigan, D.A.; Fetcenko, M.A.; Venkatesan, S. Nickel/metal hydride technology for consumer and electric vehicle batteries—A review and up-date. J. Power Sources 1997, 65, 1–7. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, K.; Fetcenko, M.A. The influence of nickel-hydroxide composition and microstructure on the high-temperature performance of nickel metal hydride batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Fetcenko, M.A.; Ovshinsky, S.R.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in NiMH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Kong, F.; Kostecki, R.; Nadeau, G.; Song, X.; Zaghib, K.; Kinoshita, K.; McLarnon, F. In situ studies of SEI formation. J. Power Sources 2001, 97–98, 58–66. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Schranzhofer, H.; Bugajski, J.; Santner, H.J.; Korepp, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Sitte, W. Electrochemical impedance spectroscopy study of the SEI formation on graphite and metal electrodes. J. Power Sources 2006, 153, 391–395. [Google Scholar] [CrossRef]
- Bryngelsson, H.; Stjerndahl, M.; Gustafsson, T.; Edström, K. How dynamic is the SEI? J. Power Sources 2007, 174, 970–975. [Google Scholar] [CrossRef]
- Kim, S.; van Duin, A.C.T.; Shenoy, V.B. Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: A molecular dynamics study. J. Power Sources 2011, 196, 8590–8597. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Smith, K.A.; Kee, R.J. Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in Li–ion batteries. Electrochim. Acta 2011, 58, 33–43. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Schlapbach, L.; Stucki, F.; Seiler, A.; Siegmann, H.C. The formation of superparamagnetic metallic Ni and Fe particles at the surface of intermetallics by surface segregation. Surf. Sci. 1981, 106, 157–159. [Google Scholar] [CrossRef]
- Stucki, F.; Schlapbach, L. Magnetic properties of LaNi5, FeTi, Mg2Ni and their hydrides. J. Less Comm. Metal. 1980, 74, 143–151. [Google Scholar] [CrossRef]
- Stucki, F. Surface analysis by magnetization measurements on FeTi and Fe0.85Mn0.15Ti. J. Appl. Phys. 1982, 53, 2643–2644. [Google Scholar] [CrossRef]
- Kim, G.; Chun, C.; Lee, S.; Lee, J. A study on the microstructural change of surface of the intermetallic compound LaNi5 by hydrogen absorption. Scr. Metall. Mater. 1993, 29, 485–490. [Google Scholar] [CrossRef]
- Broom, D.P.; Kemali, M.; Ross, D.K. Magnetic properties of commercial metal hydride battery materials. J. Alloys Compd. 1999, 293–295, 255–259. [Google Scholar] [CrossRef]
- Tai, L.T.; Hang, B.T.; Thuy, N.P.; Hieh, T.D. Magnetic properties of LaNi5-based compounds. J. Magn. Magn. Mater. 2003, 262, 485–489. [Google Scholar] [CrossRef]
- Termsuksawad, P.; Niyomsoan, S.; Goldfarb, R.B.; Kaydanov, V.I.; Olson, D.L.; Mishra, B.; Gavra, Z. Measurement of hydrogen in alloys by magnetic and electronic techniques. J. Alloys Compd. 2004, 373, 86–95. [Google Scholar] [CrossRef]
- Li, W.K.; Ikeda, K.; Nakamori, Y.; Orimo, S.; Yakushiji, K.; Takanashi, K.; Ohyama, H.; Nakatsuji, K.; Dansui, Y. Size distribution of precipitated Ni clusters on the surface of an alkaline-treated LaNi5-based alloy. Acta Mater. 2007, 55, 481–485. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Pawlik, D.; Shen, H. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016. [Google Scholar] [CrossRef]
- Fetcenko, M.A.; Ovshinsky, S.R.; Young, K.; Reichman, B.; Fierro, C.; Koch, J.; Mays, W.; Ouchi, T.; Sommers, B.; Zallen, A. High catalytic activity disordered VTiZrNiCrCoMnAlSn hydrogen storage alloys for nickel–metal hydride batteries. J. Alloys Compd. 2002, 330–332, 752–759. [Google Scholar] [CrossRef]
- Maurel, F.; Knosp, B.; Backhaus-Ricoult, M. Characterization of corrosion products of AB5-type hydrogen storage alloys for nickel-metal hydride batteries. J. Electrochem. Soc. 2000, 147, 78–86. [Google Scholar] [CrossRef]
- Song, D.; Gao, X.; Zhang, Y.; Lin, D.; Zhou, Z.; Wang, G.; Shen, P. Surface analysis of a TiNiB hydrogen storage electrode. J. Alloys Compd. 1993, 199, 161–163. [Google Scholar]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface oxide of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Wong, D.F.; Wang, L. Structural, hydrogen storage, and electrochemical properties of Laves phase-related body-centered-cubic solid solution metal hydride alloys. Int. J. Hydrog. Energy 2014, 39, 21489–21499. [Google Scholar] [CrossRef]
- Wong, D.F.; Young, K.; Nei, J.; Wang, L.; Ng, K.Y.S. Effects of Nd-addition on the structural, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. J. Alloys Compd. 2015, 647, 507–518. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Blankenship, R. Improvement in −40 °C electrochemical properties of AB2 metal hydride alloy by silicon incorporation. J. Alloys Compd. 2013, 575, 65–72. [Google Scholar] [CrossRef]
- Chen, J.; Dou, S.X.; Bradhurst, D.; Liu, H.K. Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries. In Proceedings of the Twelfth Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 14–17 January 1997; IEEE: Piscataway, NJ, USA, 1997; pp. 313–316. [Google Scholar]
- Song, X.; Zhang, Z.; Zhang, X.B.; Lei, Y.Q.; Wang, Q.D. Effect of Ti Substitution on the microstructure and properties of Zr–Mn–V–Ni AB2 type hydride electrode alloys. J. Mater. Res. 1999, 14, 1279–1285. [Google Scholar] [CrossRef]
- Shi, Z.; Chumbley, S.; Laabs, F.C. Electron diffraction analysis of an AB2-type Laves phase for hydrogen battery applications. J. Alloys Compd. 2000, 312, 41–52. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Sequeira, C.; Zhang, Z. Microstructural evolution of body-centered cubic structure related Ti–Zr–Ni phases in non-stoichiometric Zr-based Zr–Ti–Mn–V–Ni hydride electrode alloys. J. Mater. Res. 2003, 18, 37–44. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Newbury, D.E.; Wang, K.; Bendersky, L.A.; Chiu, C.; Kattner, U.R.; Young, K.; Chao, B. Examination of Multiphase (Zr,Ti)(V,Cr,Mn,Ni)2 Ni-MH Electrode Alloys: Part I. Dendritic Solidification Structure. Metall. Mater. Trans. A 2010, 41, 2033–2047. [Google Scholar] [CrossRef]
- Bendersky, L.A.; Wang, K.; Boettinger, W.J.; Newbury, D.E.; Young, K.; Chao, B. Examination of Multiphase (Zr,Ti)(V,Cr,Mn,Ni)2 Ni-MH Electrode Alloys: Part II. Solid-State Transformation of the Interdendritic B2 Phase. Metall. Mater. Trans. A 2010, 41, 1891–1906. [Google Scholar] [CrossRef]
- Powder Diffraction File (PDF) Database; MSDS No. 00-014-0481; International Centre for Diffraction Data: Newtown Square, PA, USA, 2011.
- Young, K.; Reichman, B.; Fetcenko, M.A. Electrochemical performance of AB2 metal hydride alloys measured at −40 °C. J. Alloys Compd. 2013, 580, S349–S352. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.F.; Nei, J.; Reichman, B. Electrochemical properties of hypo-stoichiometric Y-doped AB2 metal hydride alloys at ultra-low temperature. J. Alloys Compd. 2015, 643, 17–27. [Google Scholar] [CrossRef]
| Origin | Al | Si | Ti | V | Cr | Mn | Co | Ni | Zr | Sn |
|---|---|---|---|---|---|---|---|---|---|---|
| Design | 0.4 | 4.0 | 12.0 | 10.0 | 7.5 | 8.1 | 8.0 | 28.2 | 21.5 | 0.3 |
| Surface | 0.7 | 4.2 | 11.2 | 10.1 | 7.5 | 8.4 | 8.2 | 29.3 | 20.3 | 0.1 |
| Location | Al | Si | Ti | V | Cr | Mn | Co | Ni | Zr |
|---|---|---|---|---|---|---|---|---|---|
| Area A (AB2 phase) | 1.25 | 5.23 | 9.77 | 13.06 | 12.47 | 9.86 | 8.60 | 23.59 | 16.12 |
| Area B (B2 phase) | ND | 0.19 | 24.08 | 1.08 | ND | 0.78 | 6.93 | 47.40 | 19.52 |
| Properties | Si5-AB2 | Si0-AB2 | AB5 | La-A2B7 | Nd-A2B7 | Mm-A2B7 |
|---|---|---|---|---|---|---|
| MS in emu·g−1 | 0.0324 | 0.0372 | 0.434 | 0.369 | 0.679 | 0.314 |
| H1/2 in kOe | 0.451 | 0.493 | 0.173 | 0.125 | 0.102 | 0.128 |
| References | This work | [33] | [30] | [30] | Nd-AB5 | [31] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-h.; Chao, B.; Nei, J. Microstructures of the Activated Si-Containing AB2 Metal Hydride Alloy Surface by Transmission Electron Microscope. Batteries 2016, 2, 4. https://doi.org/10.3390/batteries2010004
Young K-h, Chao B, Nei J. Microstructures of the Activated Si-Containing AB2 Metal Hydride Alloy Surface by Transmission Electron Microscope. Batteries. 2016; 2(1):4. https://doi.org/10.3390/batteries2010004
Chicago/Turabian StyleYoung, Kwo-hsiung, Benjamin Chao, and Jean Nei. 2016. "Microstructures of the Activated Si-Containing AB2 Metal Hydride Alloy Surface by Transmission Electron Microscope" Batteries 2, no. 1: 4. https://doi.org/10.3390/batteries2010004
APA StyleYoung, K.-h., Chao, B., & Nei, J. (2016). Microstructures of the Activated Si-Containing AB2 Metal Hydride Alloy Surface by Transmission Electron Microscope. Batteries, 2(1), 4. https://doi.org/10.3390/batteries2010004