Capacity Degradation Mechanisms in Nickel/Metal Hydride Batteries
Abstract
:1. Introduction
1.1. Significance of Nickel/Metal Hydride Batteries
1.2. Basic Structure of Nickel/Metal Hydride Battery
1.3. Experimental Methods Used in Failure Analysis
2. Capacity Degradation
2.1. Capacity Loss During Normal Cycling at Room Temperature
2.2. Capacity Loss During Long-Term Room Temperature Storage
2.3. Capacity Loss During High-Temperature Storage
2.4. Capacity Loss Due to Low-Temperature Cycling
2.5. Capacity Loss Due to High-Rate Cycling
2.6. Capacity Loss in a Multi-Cell Module
3. Methods to Improve Cycle Stability
3.1. Cell Design
3.2. Negative Electrode
3.3. Positive Electrode
3.4. Separator
3.5. Electrolyte
3.6. Other Components
4. Revival of Degraded/Failed Battery
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ouchi, T.; Young, K. Reviews on the Japanese Patents about nickel/metal hydride batteries. Batteries 2016. submitted for publication. [Google Scholar]
- Chang, S.; Nei, J.; Young, K. Reviews on the U.S. Patents about nickel/metal hydride batteries. Batteries 2016. submitted for publication. [Google Scholar]
- Young, K. Metal Hydrides. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Waltham, MA, USA, 2012. [Google Scholar] [CrossRef]
- Yasuoka, S.; Magari, Y.; Murata, T.; Tanaka, T.; Ishida, J.; Nakamura, H.; Nohma, T.; Kihara, M.; Baba, Y.; Teraoka, H. Development of high-capacity nickel-metal hydride batteries using superlattice hydrogen-absorbing alloys. J. Power Sources 2006, 156, 662–666. [Google Scholar] [CrossRef]
- Watada, M.; Kuzuhara, M.; Oshitani, M. Development trend of rechargeable nickel-metal hydride battery for replacement of dry cell. GS Yuasa Tech. Rep. 2006, 3, 46–53. [Google Scholar]
- Teraoka, H. Development of Low Self-Discharge Nickel-Metal Hydride Battery. Available online: http://www.scribd.com/doc/9704685/Teraoka-Article-En (accessed on 30 September 2015).
- Noréus, D. Substitution of Rechargeable NiCd Batteries. Available online: http://ec.europa.eu/environment/waste/studies/batteries/nicd.pdf (accessed on 30 September 2015).
- Yonezu, I.; Yasuoka, S.; Kitaoka, K.; Nagae, T.; Takee, M. Advanced Ni-MH Batteries for Hybrid Electric Vehicles Using Super-Lattice Alloys as A Negative Electrode Materials. In Proceedings of the 8th International Advanced Automotive Battery and Ultracapacitor Conference (AABC-08), Tampa, FL, USA, 14–16 May 2008.
- Young, K.; Wang, C.; Wang, L.Y.; Strunz, K. Electric Vehicle Battery Technologies. In Electric Vehicle Integration into Modern Power Networks; Garcia-Valle, R., Peҫas Lopes, J.A., Eds.; Springer: New York, NY, USA, 2013; pp. 15–56. [Google Scholar]
- Yano Economic Research Institute Ltd. The Investigation Results from World Market of Batteries for Vehicle Use. Available online: https://www.yano.co.jp/press/pdf/1346.pdf (accessed on 30 September 2015).
- Young, K.; Ng, K.Y.S.; Bendersky, L. A Technical Report of the Robust Affordable Next Generation Energy Storage System-BASF Program. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Meng, T.; Young, K.; Beglau, D.; Yan, S.; Zeng, P.; Cheng, M.M. Hydrogenated Amorphous Silicon Thin Film Anode for Proton Conducting Batteries. J. Power Sources 2016, 302, 31–38. [Google Scholar] [CrossRef]
- Takasaki, T.; Nishimura, K.; Saito, M.; Fukunaga, H.; Iwaki, T.; Sakai, T. Cobalt-free nickel-metal hydride battery for industrial applications. J. Alloys Compd. 2013, 580, S378–S381. [Google Scholar] [CrossRef]
- Nishimura, K.; Takasaki, T.; Sakai, T. Introduction of large-sized nickel-metal hydride battery GIGACELL® for industrial applications. J. Alloys Compd. 2013, 580, S353–S358. [Google Scholar] [CrossRef]
- Zelinsky, M.; Fetcenko, M.A.; Kusay, J.; Koch, J. Storage-Integrated PV Systems Using Advanced NiMH Battery Technology. In Proceedings of the 5th International Renewable Energy Storage Conference, Berlin, Germany, 22–24 November 2010.
- Wendler, M.; Hübner, G.; Krebs, M. Development of Printed Thin and Flexible Batteries. Available online: https://www.hdmstuttgart.de/international_circle/circular/issues/11_01/ICJ_04_32_wendler_huebner_krebs.pdf (accessed on 4 October 2015).
- Fetcenko, M.A.; Koch, J. Nickel-Metal Hydride Batteries. In Linden’s Handbook of Batteries, 4th ed.; Reddy, T.B., Ed.; McGraw-Hill: New York, NY, USA, 2011; pp. 22.1–22.50. [Google Scholar]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel Hydroxides and Related Materials: A Review of Their Structures, Synthesis and Properties. Proc. Math. Phys. Eng. Sci. 2015, 471. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Ruiz, F.C.; Martinez, P.S.; Castro, E.B.; Humana, R.; Peretti, H.A.; Visintin, A. Effect of electrolyte concentration on the electrochemical properties of an AB5-type alloy for Ni/MH batteries. Int. J. Hydrog. Energy 2013, 38, 240–245. [Google Scholar] [CrossRef]
- Karwowska, M.; Jarson, T.; Fijalkowski, K.J.; Leszczynski, P.J.; Rogilski, Z.; Czerwinski, A. Influence of electrolyte composition and temperature on behaviour of AB5 hydrogen storage alloy used as negative electrode in Ni/MH Batteries. J. Power Sources 2014, 263, 304–309. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Li, X.; Liu, J. Study on the storage performance improvement of Ni-MH cell. Chin. Battery Ind. 2001, 6, 18–20. [Google Scholar]
- Shinyama, K.; Harada, Y.; Maeda, R.; Nakamura, H.; Matsuta, S.; Nohma, T.; Yonezu, I. Effect of separators on the self-discharge reaction in nickel-metal hydride batteries. Res. Chem. Intermed. 2006, 32, 447–452. [Google Scholar] [CrossRef]
- Kong, L.; Chen, B.; Young, K.; Koch, J.; Chan, A.; Li, W. Effects of Al- and Mn-contents in the negative MH alloy on the self-discharge and long-term storage properties of Ni/MH battery. J. Power Sources 2012, 213, 128–139. [Google Scholar] [CrossRef]
- Zhou, X.; Young, K.; West, J.; Regalado, J.; Cherisol, K. Degradation mechanisms of high-energy bipolar nickel metal hydride battery with AB5 and A2B7 alloys. J. Alloys Compd. 2013, 580, S373–S377. [Google Scholar] [CrossRef]
- Young, K.; Koch, J.; Yasuoka, S.; Shen, H.; Bendersky, L.A. Mn in misch-metal based superlattice metal hydride alloy—Part 2 Ni/MH battery performance and failure mechanism. J. Power Sources 2015, 277, 433–442. [Google Scholar] [CrossRef]
- Bernard, P. Effects on the positive electrode of the corrosion of AB5 alloys in nickel-metal-hydride batteries. J. Electrochem. Soc. 1998, 145, 456–458. [Google Scholar] [CrossRef]
- Maurel, F.; Knosp, B.; Backhaus-Ricoult, M. Characterization of corrosion products of AB5-type hydrogen storage alloys for nickel-metal hydride batteries. J. Electrochem. Soc. 2000, 147, 78–86. [Google Scholar] [CrossRef]
- Wang, L.; Young, K.; Meng, T.; English, N.; Yasuoka, S. Partial substitution of cobalt for nickel in mixed rare earth metal based superlattice hydrogen absorbing alloy—Part 2 battery performance and failure mechanism. J. Alloys Compd. 2016, 664, 417–427. [Google Scholar] [CrossRef]
- Rajamathi, A.; Kamath, V.P.; Seshadri, R. Polymorphism in nickel hydroxide: Role of interstratification. J. Mater. Chem. 2000, 10, 503–506. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Liu, Y.; Nei, J. Microstructures of the oxides on the activated AB2 and AB5 metal hydride alloys surface. J. Alloys Compd. 2014, 606, 97–104. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Pawlik, D.; Shen, H. Transmission electron microscope studies in the surface oxide on the La-containing AB2 metal hydride alloy. J. Alloys Compd. 2016. submitted for publication. [Google Scholar]
- Young, K.; Chang, S.; Chao, B.; Nei, J. Microstructures of the activated Si-containing AB2 metal hydride alloy surface by transmission electron microscope. Batteries 2016, 2. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface of AB2, AB5, and A2B7 alloys. J. Alloys Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
- Ayari, M.; Paul-Boncour, V.; Lamloumi, J.; Percheron-Guégan, A.; Guillot, M. Study of the aging of LaNi3.55Mn0.4Al0.3(Co1−xFex)0.75 (0 ≤ x ≤1) compounds in Ni-MH batteries by SEM and magnetic measurements. J. Magn. Magn. Mater. 2005, 288, 374–383. [Google Scholar] [CrossRef]
- Qi, D.; Yang, Y.; Lin, Z. Investigation on degradation of storage characteristics of Ni-MH batteries. Chin. J. Power Sources 1998, 22, 236–239. [Google Scholar]
- Watanabe, N.; Arakawa, T.; Sasaki, Y.; Yamashita, T.; Koiwa, I. Influence of the memory effect on X-ray photoelectron spectroscopy and Raman scattering in positive electrode of Ni-MH batteries. J. Electrochem. Soc. 2012, 159, A1949–A1953. [Google Scholar] [CrossRef]
- Higuchi, E.; Otsuka, H.; Chiku, M.; Inoue, H. Effect of pretreatment on the surface structure of a Co(OH)2 electrode. J. Power Sources 2014, 248, 762–768. [Google Scholar] [CrossRef]
- Yan, D.; Suda, S. Electrochemical characteristics of LaNi4.7Al0.3 alloy activated by alkaline solution containing hydrazine. J. Alloys Compd. 1995, 223, 28–31. [Google Scholar] [CrossRef]
- Imoto, T.; Kato, K.; Higashiyama, N.; Kimoto, M.; Itoh, Y.; Nishio, K. Influence of surface treatment by HCL aqueous solution on electrochemical characteristics of a Mm(Ni-Co-Al-Mn)4.76 alloy for nickel-metal hydride battery. J. Alloys Compd. 1999, 282, 274–278. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Banik, A.; Koch, J.; Fetcenko, M.A. Improvement in the electrochemical properties of gas atomized AB2 metal hydride alloys by hydrogen annealing. Int. J. Hydrog. Energy 2011, 36, 3547–3555. [Google Scholar] [CrossRef]
- Etiemble, A.; Idrissi, H.; Meille, S.; Roué, L. In situ investigation of the volume change and pulverization of hydride materials for Ni-MH batteries by concomitant generated force and acoustic emission measurements. J. Power Sources 2012, 205, 500–505. [Google Scholar] [CrossRef]
- Etiemble, A.; Roue, L.; Idrissi, H. Study of Metal Electrodes for Ni-MH Batteries by Acoustic Emission. In Proceedings of the European Working Group on Acoustic Emission (EWAGE 2010), Vienna, Austria, 8–10 September 2010.
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Yang, S.; Yin, Y.; Chen, H.; Jia, J.; Zhang, M.; Ding, L. Charge/discharge characteristics and structural changing of spherical β-Ni(OH)2 prepared from different nickel salt. J. Electrochem. 2001, 7, 310–315. [Google Scholar]
- Bantignies, J.L.; Deabate, S.; Righi, A.; Rols, S.; Hermet, P.; Sauvajol, J.L.; Henn, F. New insight into the vibration behavior of nickel hydroxide and oxyhydroxide using inelastic neutron scattering, far/mid-infrared and Raman spectroscopies. J. Phys. Chem. C 2008, 112, 2193–2201. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Bock, C.; McDougall, B.R. Raman and infrared spectroscopy of α and β phases of thin nickel hydroxide films electrochemically formed on nickel. J. Phys. Chem. A 2012, 116, 6771–6784. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.S.; Lockwood, D.J.; Poirier, S.; Book, C.; MacDougall, B.R. Applications of in situ Raman spectroscopy for identifying nickel hydroxide materials and surface layers during chemical aging. ACS Appl. Mater. Interface 2014, 6, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, I.; Ishikawa, H. Electrochemical impedance spectra and deterioration mechanism of metal hydride electrodes. J. Electrochem. Soc. 1992, 139, L72–L73. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, J.; Liu, H.; Leng, Y.; Yuan, A.; Cao, C. Study on cycling deterioration of Ni-MH battery by electrochemical impedance spectroscopy (EIS). Chin. J. Power Sources 1999, 23, 62–63. [Google Scholar]
- Mo, Z.; Xu, P.; Han, X.; Zhao, L. Electrochemical impedance behavior of Ni-MH battery under different depth of discharge. Chin. J. Power Sources 2006, 30, 388–390. [Google Scholar]
- Guenne, L.L.; Benard, P. Life duration of Ni-MH cells for high power applications. J. Power Sources 2002, 105, 134–138. [Google Scholar] [CrossRef]
- Geng, M.; Han, J.; Feng, F.; Northwood, D.O. Charging/discharging stability of a metal hydride battery electrode. J. Electrochem. Soc. 1999, 146, 2371–2375. [Google Scholar] [CrossRef]
- Yuan, J.; Qi, J.; Tu, J.; Feng, H.; Chen, C.; Chen, W. Electrochemical analysis of failed Ni-MH batteries. Chin. J. Power Sources 2001, 25, 279–282. [Google Scholar]
- Yuan, X.; Xu, N. Electrochemical and hydrogen transport kinetic performance of MlNi3.75Co0.63Mn0.4Al0.2 metal hydride electrodes at various temperatures. J. Electrochem. Soc. 2002, 149, A407–A413. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Chen, Y.; Santos, D.M.F. Effects of temperature on the performance of the MmNi3.6Co0.7Mn0.4Al0.3 metal hydride electrode in alkaline solution. J. Electrochem. Soc. 2006, 153, A1863–A1867. [Google Scholar] [CrossRef]
- Gamboa, S.A.; Sebastian, P.J.; Feng, F.; Geng, M.; Northwood, D.O. Cyclic voltammetry investigation of a metal hydride electrode for nickel metal hydride batteries. J. Electrochem. Soc. 2002, 149, A137–A139. [Google Scholar] [CrossRef]
- Béléké, A.B.; Higuchi, E.; Inoue, H.; Mizuhata, M. Durability of nickel-metal hydride (Ni-MH) battery cathode using nickel-aluminum layered double hydroxide/carbon (Ni-AL LDH/C) composite. J. Power Sources 2014, 247, 572–578. [Google Scholar] [CrossRef]
- Lyons, M.E.G.; Doyle, R.L.; Godwin, I.; O’Brien, M.; Russell, L. Hydrous nickel oxide: Redox switching and the oxygen evolution reaction in aqueous alkaline solution. J. Electrochem. Soc. 2012, 159, H932–H944. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, G.; Li, A.; Liu, K. Electrochemical performance of AB5-type hydrogen storage alloy modified with Co3O4. Trans. Nonferrous Met. Soc. Chin. 2011, 21, 1428–1434. [Google Scholar] [CrossRef]
- Serrao, L.; Chehab, Z.; Guezennec, Y.; Rizzoni, G. An Aging Model of Ni-MH Batteries for Hybrid Electric Vehicles. In Proceedings of the IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 7–9 September 2005; pp. 78–85.
- Somogye, R.H. An Aging Model of Ni-MH Batteries for Use in Hybrid-Electric Vehicles. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2004. [Google Scholar]
- Picciano, N. Battery Aging and Characterization of Nickel Metal Hydride and Lead Acid Batteries. Bachelor’s Thesis, Ohio State University, Columbus, OH, USA, 2007. [Google Scholar]
- Ikoma, M.; Hoshina, Y.; Matsumoto, I. Self-discharge mechanism of sealed-type nickel/metal-hydride battery. J. Electrochem. Soc. 1996, 143, 1904–1907. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Koch, J.; Fetcenko, M.A. Compositional optimization of vanadium-free hypo-stoichiometric AB2 metal hydride alloy for Ni/MH battery application. J. Alloys Compd. 2012, 510, 97–106. [Google Scholar] [CrossRef]
- Guo, H.; Qiao, Y.; Zhang, H. Fast determination of micro short circuit in sintered MH-Ni battery. Chin. J. Power Sources 2010, 34, 608–609. [Google Scholar]
- Shinyama, K.; Magari, Y.; Kumagae, K.; Nakamura, H.; Nohma, T.; Takee, M.; Ishiwa, K. Deterioration mechanism of nickel metal-hydride batteries for hybrid electric vehicles. J. Power Sources 2005, 141, 193–197. [Google Scholar] [CrossRef]
- Zhu, W.H.; Zhu, Y.; Tatarchuk, B.J. Self-discharge characteristics and performance degradation of Ni-MH batteries for storage applications. Int. J. Hydrog. Energy 2014, 39, 19789–19798. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.-Y.; Lee, J.-Y. Self-discharge behaviour of sealed Ni-MH batteries using MmNi3.3+xCo0.7Al1.0−x anodes. J. Alloys Compd. 1996, 232, 197–203. [Google Scholar] [CrossRef]
- Wang, C.; Marrero-Rivera, M.; Serafini, D.A.; Baricuatro, J.H.; Soriaga, M.P.; Srinivasan, S. The self-discharge mechanism of AB5-type hydride electrodes in Ni/MH batteries. Int. J. Hydrog. Energy 2006, 31, 603–611. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Lee, S.; Lee, J. Self-discharge characteristics of sealed Ni-MH batteries using Zr1−xTixV0.8Ni1.6 anodes. J. Alloys Compd. 1995, 221, 174–179. [Google Scholar] [CrossRef]
- Jang, K.; Jung, J.; Kim, D.; Yu, J.; Lee, J. Self-discharge mechanism of vanadium-titanium metal hydride electrodes for Ni-MH rechargeable battery. J. Alloys Compd. 1998, 268, 290–294. [Google Scholar] [CrossRef]
- Yang, X.; Liaw, B.Y. Self-discharge and charge retention in AB2-based nickel metal hydride batteries. J. Electrochem. Soc. 2004, 151, A137–A143. [Google Scholar] [CrossRef]
- Fan, M.; Liao, W.; Wu, B.; Chen, H.; Jian, X. Temperature characteristic of Ni-MH battery used in EVs. Chin. Battery Ind. 2004, 9, 287–289. [Google Scholar]
- Huang, J.; Li, X. Factors affecting the self-discharge of Ni-MH batteries. Chin. Battery Ind. 2005, 10, 290–294. [Google Scholar]
- Zhu, W.H.; Zhu, Y.; Davis, Z.; Tatarchuk, B.J. Energy efficiency and capacity retention of Ni-MH batteries for storage applications. Appl. Energy 2013, 106, 307–313. [Google Scholar] [CrossRef]
- NiMH Battery Charging Algorithm. Available online: http://www.schaffler.com/whitepapers/NiMH Battery Algorithm.pdf (accessed on 4 October 2015).
- Kritzer, P. Separators for nickel metal hydride and nickel cadmium batteries designed to reduce self-discharge rates. J. Power Sources 2004, 137, 317–321. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Xu, Q.; Zhang, X.; Liu, Y. Assessment of separators in high rate discharge performance of Ni−MH battery. Chin. J. Process Eng. 2006, 6, 114–119. [Google Scholar]
- Li, X.; Song, Y.; Wang, L.; Xia, T.; Li, S. Self-discharge mechanism of Ni-MH battery by using acrylic acid grafted polypropylene separator. Int. J. Hydrog. Energy 2010, 35, 3798–3801. [Google Scholar] [CrossRef]
- Takasaki, T.; Nishimura, K.; Saito, M.; Iwaki, T.; Sakai, T. Cobalt-free materials for nickel-metal hydride battery: Self-discharge suppression and overdischarge resistance improvement. Electrochemistry 2013, 81, 553–558. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Tan, P.; Li, Y. Effects of electrolyte weight on self-discharge of Ni-MH battery. Chin. Battery Ind. 2008, 13, 299–302. [Google Scholar]
- Zai, Y.; Han, J.; Li, Y.; Lai, X.; Duan, Q. Influence of copper on the micro-short in Ni-MH battery. Chin. Battery Ind. 2012, 17, 78–80. [Google Scholar]
- Zha, Y.; Chen, X.; Li, L.; Yang, Z.; Wang, L. Effects of the negative current collector on the performance of the Ni-MH battery. Chin. Battery Ind. 2006, 11, 91–93. [Google Scholar]
- Cheng, S.; Zhang, J.; Yuan, A.; Ding, W.; Cao, C. Effects of separator on discharge capacity and cycle-life of Ni-MH battery. Chin. J. Power Sources 1999, 23, 10–12. [Google Scholar]
- Li, X.; Wang, X.; Dong, H.; Xia, T.; Wang, L.; Song, Y. Self-discharge performance of Ni-MH battery by using electrodes with hydrophilic/hydrophobic surface. J. Phys. Chem. Solids 2013, 74, 1756–1760. [Google Scholar] [CrossRef]
- Li, X.; Li, J. Effects of the surface treatment of formed positive electrode on self-discharge performance of Ni-MH batteries. Chin. Battery Ind. 2009, 14, 298–301. [Google Scholar]
- Feng, F.; Northwood, D.O. Self-discharge characteristics of a metal hydride electrode for Ni-MH rechargeable batteries. Int. J. Hydrog. Energy 2005, 30, 1367–1370. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J. Study on the performance of metal-hydride electrodes coated with Ni-B alloy. Contemp. Chem. Ind. 2014, 43, 1453–1455, 1463. [Google Scholar] [CrossRef]
- Deng, Z. Approach on influence factors of discharge oneself MH-Ni battery. Jiangxi Metal. 2003, 23, 137–139. [Google Scholar]
- Wang, Z.M.; Tsai, P.; Chan, S.L.I.; Zhou, H.Y.; Lin, K.S. Effects of electrolytes and temperature on high-rate discharge behavior of MmNi5-based hydrogen storage alloys. Int. J. Hydrog. Energy 2010, 35, 2033–2039. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Einerhand, R.E.F.; Daams, J.L.C. How to achieve long-term electrochemical cycling stability with hydride-forming electrode materials. J. Alloys Compd. 1995, 231, 604–610. [Google Scholar] [CrossRef]
- Dong, Q.; Yan, G.; Yu, C. Research on the cycle life of Ni-MH battery. Chin. J. Power Sources 2000, 24, 306–310. [Google Scholar]
- Yan, J.; Zhou, Z.; Li, Y.; Song, D.; Zhang, Y. Structure and property changes of positive and negative electrodes in Ni/MH batteries during charge/discharge cycles. J. Inorg. Chem. 1998, 14, 74–78. [Google Scholar]
- Tliha, M.; Mathlouthi, H.; Lamloumi, J.; Percheron-Guegan, A. AB5-type hydrogen storage alloy used as anodic materials in Ni-MH batteries. J. Alloys Compd. 2007, 436, 221–225. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, Y.; Liang, F.; Sun, L.; Yin, D.; Wu, Y.; Wang, L. High temperature performance of La0.6Ce0.4Ni3.45Co0.75Mn0.7Al0.1 hydrogen storage alloy for nickel/metal hydride batteries. Int. J. Hydrog. Energy 2014, 39, 13231–13239. [Google Scholar] [CrossRef]
- Meli, F.; Schlapbach, L. Surface analysis of AB5-type electrodes. J. Less Common Met. 1991, 172, 1252–1259. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wu, F.; Chen, S. Development of recycling and rusing process for nickel metal hydride batteries for HEV. J. Funct. Mater. 2007, 38, 1928–1932. [Google Scholar]
- Geng, M.; Hah, J.; Feng, F.; Northwood, D.O. Characteristics of the high-rate discharge capability of a nickel/metal hydride battery electrode. J. Electrochem. Soc. 1999, 146, 3591–3595. [Google Scholar] [CrossRef]
- Wang, C.; Jin, H.; Li, G.; Wang, R. Study on microstructures of La0.8Nd0.2Ni3.55Co0.75Mn0.4Al0.3 alloy during the charge-discharge cycle process. Chin. J. Power Sources 1998, 22, 65–67. [Google Scholar]
- Chen, Y.; Wei, J.; Gao, F.; Zhou, W.; Zhang, Y.; Zhou, Z. Discussing of invalidation factors of Ni-MH battery at high charge and discharge rate. Chin. J. Power Sources 2001, 25, 142–145. [Google Scholar]
- Maurel, F.; Hytch, M.J.; Knosp, B.; Backhaus-Ricoult, M. Formation of rare earth hydroxide nanotubes and whiskers as corrosion product of LaNi5-type alloys in aqueous KOH. Eur. Phys. J. Appl. Phys. 2000, 9, 205–213. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, H.; Gao, M.; Lei, Y.; Wang, Q. Degradation mechanism of the La-Mg-Ni-based metal hydride electrode La0.7Mg0.3Ni3.4Mn0.1. J. Electrochem. Soc. 2005, 152, A1089–A1095. [Google Scholar] [CrossRef]
- Xie, D.; Cheng, H. Study on the fading of MH-Ni batteries. Chin. Battery Ind. 2000, 5, 7–10. [Google Scholar]
- Xie, J.; Wang, S.; Xia, B.; Zhang, Q.; Shi, P. Research on the degradation of Ni-MH batteries (I)—Swelling of nickel electrode. Chin. J. Power Sources 1997, 21, 22–27. [Google Scholar]
- Singh, D. Characteristics and Effects of γ-NiOOH on cell performance and a method to quantify it in nickel electrodes. J. Electrochem. Soc. 1998, 145, 116–120. [Google Scholar] [CrossRef]
- Deng, X.; Huang, S.; Wang, Y.; Wang, J. Study on the shelf performance of Ni-MH batteries. Chin. Battery Ind. 2006, 11, 172–174. [Google Scholar]
- Durairajan, A.; Haran, B.S.; White, R.E.; Popov, B.N. Pulverization and corrosion studies of bare and cobalt-encapsulated metal hydride electrodes. J. Power Sources 2000, 87, 84–91. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; He, X.; Liu, J.; Chen, B.; Li, C. Investigation on internal resistance of Ni-MH battery during cycle life. Chin. Battery Ind. 2001, 6, 69–71. [Google Scholar]
- Wu, J.; Li, W.; Zai, Y. Relations of specific capacity of Ni-MH battery with self discharge and cycle life. Chin. Battery Ind. 2004, 9, 197–199. [Google Scholar]
- Sastry, A.M.; Choi, S.B.; Cheng, X. Damage in composite NiMH positive electrodes. J. Eng. Mater. Technol. 1998, 120, 280–283. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Lin, G.W.; Zhang, J.L.; Ge, J.S.; Shen, J.R. Degradation behavior of foamed nickel positive electrodes of Ni-MH batteries. J. Alloys Compd. 1999, 293–295, 795–798. [Google Scholar] [CrossRef]
- Young, K.; Wu, A.; Qiu, Z.; Tan, J.; Mays, W. Effects of H2O2 addition to the cell balance and self-discharge of Ni/MH batteries with AB5 and A2B7 alloys. Int. J. Hydrog. Energy 2012, 37, 9882–9891. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Z.; Tu, J. Electrochemical investigations of activation and degradation of hydrogen storage alloy electrodes in sealed Ni/MH battery. Int. J. Hydrog. Energy 2007, 27, 439–444. [Google Scholar] [CrossRef]
- Singh, P.; Wu, T.; Wendung, M.; Bendale, P.; Ware, J.; Ritter, D.; Zhang, L. Mechanisms causing capacity loss on long term storage in NiMH system. Mater. Res. Soc. Symp. Proc. 1998, 496, 25–36. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Z.; Zhao, S.; Geng, M.; Wang, Y.; Northwood, D.O. High-temperature characteristics of advanced Ni-MH batteries using nickel electrodes containing CaF2. J. Power Sources 2008, 175, 630–634. [Google Scholar] [CrossRef]
- Iwakura, C.; Kajiya, Y.; Yoneyama, H.; Sakai, T.; Oguro, K.; Ishikawa, H. Self-discharge mechanism of nickel-hydrogen batteries using metal hydride anodes. J. Electrochem. Soc. 1989, 136, 1351–1355. [Google Scholar] [CrossRef]
- Leblanc, P.; Blanchard, P.; Senyarich, S. Self-discharge of sealed nickel-metal hydride batteries. J. Electrochem. Soc. 1998, 145, 844–847. [Google Scholar] [CrossRef]
- Wang, C.S.; Marrero-Rivera, M.; Baricuatro, J.H.; Soriaga, M.P.; Serafini, D.; Srinivasan, S. Corrosion behavior of AB5-type hydride electrodes in alkaline electrolyte solution. J. Appl. Electrochem. 2003, 33, 325–331. [Google Scholar] [CrossRef]
- Rukpakavong, W.; Phillips, I.; Guan, L. Lifetime Estimation of Sensor Device with AA NiMH Batteries. Available online: http://www.ipcsit.com/vol55/018-ICICM2012-Contents.pdf (accessed on 04 October 2015).
- Bäuerlein, P.; Antonius, A.; Löffler, J.; Kümpers, J. Progress in high-power nickel–metal hydride batteries. J. Power Sources 2008, 176, 547–554. [Google Scholar] [CrossRef]
- Shi, F. The research of voltage’s accuracy and temperature effect on the MH-Ni battery group. Microcomput. Appl. 2013, 32, 60–62. [Google Scholar]
- Li, X.; Ma, L.; Lou, Y.; Li, F.; Xia, B. Storage performance of Ni-MH battery at state of discharge. Chin. J. Power Sources 2004, 28, 364–368. [Google Scholar]
- Notten, P.H.L.; Van Beek, J.R.G. Nickel-metal hydride batteries: From concept to characteristics. Chem. Ind. 2000, 54, 102–115. [Google Scholar]
- Li, L.; Wu, F.; Yang, K.; Wang, J.; Chen, S. The effects of overcharge on electrochemical performance of MH-Ni batteries. Mater. Rev. 2004, 18, 101–102. [Google Scholar]
- Hu, W.K.; Geng, M.M.; Gao, X.P.; Burchardt, T.; Gong, Z.X.; Noréus, D.; Nakstad, N.K. Effect of long-term overcharge and operated temperature on performance of rechargeable NiMH cells. J. Power Sources 2006, 159, 1478–1483. [Google Scholar] [CrossRef]
- Ye, Z.; Noréus, D. Oxygen and hydrogen gas recombination in NiMH cells. J. Power Sources 2012, 208, 232–236. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Wu, F.; Qiu, X.; Chen, S. Effects of low temperature on performance of hydrogen-storage alloys and electrolyte. Min. Metal. Eng. 2007, 27, 44–46. [Google Scholar]
- Guo, H.; Chao, M.; Chen, X.; Zhang, B.; Zhang, J. Study on the low temperature performance of MH-Ni battery. J. Henan Normal Univ. Nat. Sci. 2003, 31, 76–78. [Google Scholar]
- Liaw, B.Y.; Yang, X. Limiting mechanism on rapid charging Ni-MH batteries. Electrochim. Acta 2001, 47, 875–884. [Google Scholar] [CrossRef]
- Raju, M.; Ananth, M.V.; Vijayaraghavan, L. Rapid charging characterization of MmNi3.03Si0.85Co0.60Mn0.31Al0.08 alloy used as anodes in Ni-MH batteries. Int. J. Hydrog. Energy 2009, 34, 3500–3505. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Q.; Wang, F.; Hu, D. Effects of fast charging on efficiency of Ni-MH batteries. J. Beijing Technol. Bus. Univ. Nat. Sci. Ed. 2006, 24, 5–8. [Google Scholar]
- Taheri, P.; Yazdanpour, M.; Bahrami, M. Analytical assessment of the thermal behavior of nickel-metal hydride batteries during fast charging. J. Power Sources 2014, 245, 712–720. [Google Scholar] [CrossRef]
- Hou, X.; Xue, J.; Nan, J.; Xia, X.; Yang, M.; Cui, Y. The cycle life performance of MH-Ni batteries for high power applications. Acta Sci. Nat. Univ. Sunyats. 2005, 44, 77–80. [Google Scholar]
- Ayeb, A.; Otten, W.M.; Mank, J.G.; Notten, P.H.L. The hydrogen evolution and oxidation kinetics during overdischarging of sealed nickel-metal hydride batteries. J. Electrochem. Soc. 2006, 153, A2055–A2065. [Google Scholar] [CrossRef]
- Cha, C.; Yu, J.; Zhang, J. Comparative experimental study of gas evolution and gas consumption reactions in sealed Ni-Cd and Ni-MH cells. J. Power Sources 2004, 129, 347–357. [Google Scholar] [CrossRef]
- Cuscueta, D.J.; Salva, H.R.; Ghilarducci, A.A. Inner pressure characterization of a sealed nickel-metal hydride cell. J. Power Sources 2011, 196, 4067–4071. [Google Scholar] [CrossRef]
- Li, N. Methods to prevent explosion in MH-Ni battery. Chin. Battery Ind. 2003, 8, 277. [Google Scholar]
- Li, N.; Xu, Y. Discussion on explosion of MH-Ni batteries. Chin. Battery Ind. 2008, 13, 372–373. [Google Scholar]
- Zhu, X.; Ni, J.; Xu, W.; Huang, L.; Feng, S. Analysis of common malfunctions and maintenance of nickel metal hydride battery used in AGV. Log. Mater. Handl. 2013, 10, 156–158. [Google Scholar]
- Liu, C.; Li, J.; Chen, H.; Liu, J. The development of high performance SubC MH-Ni battery. J. Hunan Univ. Technol. 2012, 26, 64–67. [Google Scholar]
- Fetcenko, M.A.; Young, K.; Fierro, C. Hydrogen Storage Battery; Positive Nickel Electrode; Positive Electrode Active Material and Methods of Making. U.S. Patent 7,396,379, 8 July 2008. [Google Scholar]
- Li, X.; Tian, J.; Xia, T. A new way to reduce the consumption of hydrogen-absorbing alloy in Ni-MH battery. Chin. Battery Ind. 2011, 16, 74–78. [Google Scholar]
- Hu, W.; Shan, Z.; Tian, J. Impact of ratio of anode/cathode active materials on internal pressure of MH-Ni batteries. Chem. Ind. Eng. 2006, 23, 95–97. [Google Scholar]
- Sastry, A.M.; Cheng, X.; Wang, C. Mechanics of stochastic fibrous networks. J. Thermoplast. Compos. Mater. 1998, 3, 288–296. [Google Scholar] [CrossRef]
- Yu, T.; Zhai, Y.; Zhou, S.; Duan, Q. Effect of precharge on performance of Ni-MH battery. Chin. Battery Ind. 2005, 10, 74–76. [Google Scholar]
- Guiose, B.; Cuevas, F.; Décamps, B.; Leroy, E.; Percheron-Guégan, A. Microstructural analysis of the ageing of pseudo-binary (Ti,Zr)Ni intermetallic compounds as negative electrodes of Ni-MH batteries. Electrochim. Acta 2009, 54, 2781–2789. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Matsuyama, Y.; Kitani, R.; Suda, S. Corrosion and degradation behavior of Zr-based AB2 alloy electrodes during electrochemical cycling. J. Alloys Compd. 2000, 296, 201–208. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Kitani, R.; Suda, S. Improvement of electrochemical cyclic durability of Zr-based AB2 alloy electrodes. J. Alloys Compd. 2002, 330–332, 825–830. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Lee, J. Degradation mechanism of Ti-Zr-V-Mn-Ni metal hydride electrodes. J. Alloys Compd. 1997, 260, 201–207. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.; Cho, K.; Lee, J. The cycle life of Ti0.8Zr0.2V0.5Mn0.5−xCrxNi0.8 (x = 0 to 0.5) alloys for metal hydride electrodes of Ni-metal hydride rechargeable battery. J. Electrochem. Soc. 2000, 147, 2013–2017. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Electrochemical cycle life of Zr-based Laves phase alloys influenced by alloy stoichiometry and composition. J. Electrochem. Soc. 2002, 149, A537–A542. [Google Scholar] [CrossRef]
- Martíez, P.S.; Ruiz, F.C.; Visintin, A. Influence of different electrolyte concentrations on the performance of an AB2-type alloy. J. Electrochem. Soc. 2014, 161, A326–A329. [Google Scholar] [CrossRef]
- Rongeat, C.; Roué, L. On the cycle life improvement of amorphous MgNi-based alloy for Ni-MH batteries. J. Alloys Compd. 2005, 404–406, 679–681. [Google Scholar] [CrossRef]
- Ruggeri, S.; Roué, L. Correlation between charge input and cycle life of MgNi electrode for Ni-MH batteries. J. Power Sources 2003, 117, 260–266. [Google Scholar] [CrossRef]
- Rongeat, C.; Grosjean, M.-H.; Ruggeri, S.; Dehmas, M.; Bourlot, S.; Marcotte, S.; Roué, L. Evaluation of different approaches for improving the cycle life of MgNi-based electrodes for Ni-MH batteries. J. Power Sources 2006, 158, 747–753. [Google Scholar] [CrossRef]
- Goo, N.H.; Woo, J.H.; Lee, K.S. Mechanism of rapid degradation of nanostructured Mg2Ni hydrogen storage alloy electrode synthesized by mechanical alloying and the effect of mechanically coating with nickel. J. Alloys Compd. 1999, 288, 286–293. [Google Scholar] [CrossRef]
- Tsukahara, M.; Takahashi, K.; Isomura, A.; Sakai, T. Improvement of the cycle stability of vanadium-based alloy for nickel-metal hydride (Ni-MH) battery. J. Alloys Compd. 1999, 287, 215–220. [Google Scholar] [CrossRef]
- Bliznakov, S.; Lefterova, E.; Dimitrov, N.; Petrov, K.; Popov, A. A study of the Al content impact on the properties of MmNi4.4−xCo0.6Alx alloys as precursors for negative electrodes in NiMH batteries. J. Power Sources 2008, 176, 381–386. [Google Scholar]
- Zhou, W.; Ma, Z.; Wu, C.; Zhu, D.; Huang, L.; Chen, Y. The mechanism of suppressing capacity degradation of high-Al-AB5-type hydrogen storage alloys at 60 °C. Int. J. Hydrog. Energy 2016, 41, 1801–1810. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oguro, K.; Uehara, I.; Akiba, E. X-ray diffraction peak broadening and degradation in LaNi5-based alloys. Int. J. Hydrog. Energy 2000, 25, 531–537. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Yuan, H.; Zhang, Y. Effect of Al in Mm(NiCoMnAl)5 on the performance of the sealed MH/Ni cell. Chin. J. Appl. Chem. 1999, 16, 54–57. [Google Scholar]
- Zhang, Y.; Wang, X.; Chen, M.; Li, P.; Lin, Y.; Li, R. Design of chemical compositions on AB5-type hydrogen storage alloy with high quality. Met. Funct. Mater. 2002, 9, 1–6. [Google Scholar]
- Chandra, D.; Chien, W.; Talekar, A. Metal Hydrides for NiMH Battery Applications. Available online: http://www.sigmaaldrich.com/technical-documents/articles/material-matters/metal-hydrides-for.html (accessed on 1 October 2015).
- Qingxue, Z.; Joubert, J.-M.; Latroche, M.; Jun, D.; Percheron-Guégan, A. Influence of the rare earth composition on the properties of Ni-MH electrodes. J. Alloys Compd. 2003, 360, 290–293. [Google Scholar] [CrossRef]
- Guo, J.H.; Jiang, Z.T.; Duan, Q.S.; Liu, G.Z.; Zhang, J.H.; Xia, C.M.; Guo, S.Y. Influence of Ce and Nd contents of Ml(NiCoMnAl)5 alloy on the performances of Ni-MH batteries. J. Alloys Compd. 1999, 293–295, 821–824. [Google Scholar] [CrossRef]
- Sakai, T.; Miyamura, H.; Kuriyama, N.; Kato, A.; Oguro, K.; Ishikawa, H. Metal hydride anodes for nickel-hydrogen secondary battery. J. Electrochem. Soc. 1990, 137, 795–799. [Google Scholar] [CrossRef]
- Seo, C.; Choi, S.; Choi, J.; Park, C.; Lee, P.S.; Lee, J. Effect of Ti and Zr additions on the characteristics of AB5-type hydride electrode for Ni-MH secondary battery. Int. J. Hydrog. Energy 2003, 28, 317–327. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyay, R.K. Investigations of AB5-type negative electrode for nickel-metal hydride cell with regard to electrochemical and microstructural characteristics. J. Power Sources 2010, 195, 2996–3001. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Banik, A.; Koch, J.; Fetcenko, M.A.; Bendersky, L.A.; Wang, K.; Vaudin, M. Gas atomization of Cu-modified AB5 metal hydride alloys. J. Alloys Compd. 2011, 509, 4896–4904. [Google Scholar] [CrossRef]
- Spodaryk, M.; Shcherbakova, L.; Sameljuk, A.; Wichser, A.; Zakaznova-Herzog, V.; Holzer, M.; Braem, B.; Khyzhun, O.; Mauron, P.; Remhof, A.; et al. Description of the capacity degradation mechanism in LaNi5-based alloy electrodes. J. Alloys Compd. 2015, 621, 225–231. [Google Scholar] [CrossRef]
- Anderson, M.L.; Anderson, I.E. Gas atomization of metal hydrides for Ni-MH battery applications. J. Alloys Compd. 2000, 313, 47–52. [Google Scholar] [CrossRef]
- Bowman, R.C., Jr.; Witham, C.; Fultz, B.; Ratnakumar, B.V.; Ellis, T.W.; Anderson, I.E. Hydriding behavior of gas-atomized AB5 alloys. J. Alloys Compd. 1997, 253–254, 613–616. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Cheng, W.; Li, W.; Zhao, W. The influence of high-rite quenching on the cycle stability and the structure of the AB5-type hydrogen storage alloys with different Co content. J. Alloys Compd. 2001, 315, 218–223. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Wang, C. Investigations on ML(NiCoMnTi)5 alloys prepared with different solidification rates. J. Alloys Compd. 1998, 266, 300–306. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J.; Zhong, F.; Wang, X. Effect of nickel electroplating on negative electrode surface on cycle life and inner pressure of nickel hydride battery. Electroplat. Finish. 2009, 28, 9–13. [Google Scholar]
- Yang, K.; Wu, F.; Chen, S.; Zhang, C. Influences of Ni plated on electrodes on the cycle life and inner pressure of batteries. Min. Metal. Eng. 2006, 26, 74–76. [Google Scholar]
- Zhang, D.; Yuan, H.; Wang, G.; Zhou, Z.; Zhang, Y. Study on electrochemical characteristic of Cu-coated hydrogen storage alloy. J. Funct. Mater. Dev. 1998, 4, 167–174. [Google Scholar]
- Feng, F.; Northwood, D.O. Effect of surface modification on the performance of negative electrodes in Ni/MH batteries. Int. J. Hydrog. Energy 2004, 29, 955–960. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H. Effect of Cu addition on discharge performance of metal hydride electrode for Ni-MH battery. Rare Met. Lett. 2000, 3, 18–19. [Google Scholar]
- Raju, M.; Ananth, M.V.; Vijayaraghavan, L. Influence of electroless coatings of Cu, Ni-P and Co-P on MmNi3.25Al0.35Mn0.25Co0.66 alloy used as anodes in Ni-MH batteries. J. Alloys Compd. 2009, 475, 664–671. [Google Scholar] [CrossRef]
- Manimaran, K.; Ananth, M.V.; Raju, M.; Renganathan, N.G.; Ganesan, M.; Nithya, G. Electrochemical investigations on cobalt-microencapsulated MmNi2.38Al0.82Co0.66Si0.77Fe0.13Mn0.24 hydrogen storage alloys for Ni-MH batteries. Int. J. Hydrog. Energy 2010, 35, 4630–4637. [Google Scholar] [CrossRef]
- Visintin, A.; Tori, C.A.; Garaventta, G.; Triaca, W.E. The effect of palladium coating on hydrogen storage alloy electrodes for nickel/metal hydride batteries. J. Braz. Chem. Soc. 1997, 8, 125–129. [Google Scholar] [CrossRef]
- Lv, Y. A study on decorative electrochemical coating of hydrogen storage alloy powder with nickel-phosphorus alloy. J. Tangshan Coll. 2014, 27, 42–44. [Google Scholar]
- Bi, X.; Shan, Z.; Tian, J. Study on the performance of metal-hydride electrodes coated with Ni-S alloy in Ni-MH battery. Chin. J. Power Sources 2005, 29, 746–749. [Google Scholar]
- Casella, I.G.; Gatta, M. Electrodeposition and characterization of nickel-copper alloy films as electrode material in alkaline media. J. Electrochem. Soc. 2002, 149, B465–B471. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Pan, H.; Chen, C. Surface treatments of hydrogen storage alloy improve charge-discharge performances of Ni/MH battery. Acta Phys. Chim. Sin. 1998, 14, 742–746. [Google Scholar]
- Lee, H.; Yang, D.; Park, C.; Park, C.; Jang, H. Effects of surface modifications of the LMNi3.9Co0.6Mn0.3Al0.2 alloy in a KOH/NaBH4 solution upon its electrode characteristics within a Ni-MH secondary battery. Int. J. Hydrog. Energy 2009, 34, 481–486. [Google Scholar] [CrossRef]
- Li, C. Hydrogen storage alloy and fluorinated hydrating alloy batteries. Chin. J. Power Sources 1995, 19, 34–37. [Google Scholar]
- Sakashita, M.; Li, Z.P.; Suda, S. Fluorination mechanism and its effects on the electrochemical properties of metal hydrides. J. Alloys Compd. 1997, 253–254, 500–505. [Google Scholar] [CrossRef]
- Sun, Y.; Iwata, K.; Chiba, S.; Matsuyama, Y.; Suda, S. Studies on the properties and characteristics of the fluorinated AB5 hydrogen-absorbing electrode alloys. J. Alloys Compd. 1997, 253–254, 520–524. [Google Scholar] [CrossRef]
- Sun, Y.; Suda, S. Studies on the fluorination method for improving surface properties and characteristics of AB5-types of hydrides. J. Alloys Compd. 2002, 330–332, 627–631. [Google Scholar] [CrossRef]
- Chao, D.; Shi, P.; Zhang, S. Effect of surface modification on the electrochemical performances of LaNi5 hydrogen storage alloy in Ni/MH batteries. Mater. Chem. Phys. 2006, 98, 514–518. [Google Scholar]
- Lou, Y.; Ma, L.; Xia, B.; Yang, C. XRD study on microstructure of rare earth containing hydrogen storage alloy in Ni-MH battery. Rare Met. Mater. Eng. 2006, 35, 412–417. [Google Scholar]
- Pang, L.; Ye, P.; Guo, J. Methods of reducing MH-Ni battery internal pressure and increasing cycle life. Liaoning Chem. Ind. 1998, 27, 228–230. [Google Scholar]
- Sakai, T.; Miyamura, H.; Kuriyama, N.; Ishikawa, H.; Uehara, I. Rare-earth-based hydrogen storage alloys for rechargeable nickel-metal hydride batteries. J. Alloys Compd. 1993, 192, 155–157. [Google Scholar] [CrossRef]
- Gao, J.; Yan, X.L.; Zhao, Z.Y.; Chai, Y.J.; Hou, D.L. Effect of annealed treatment on microstructure and cyclic stability for La-Mg-Ni hydrogen storage alloys. J. Power Sources 2012, 209, 257–261. [Google Scholar] [CrossRef]
- Ma, J.; Pan, H.; Zhu, Y.; Li, S.; Chen, C. A novel method to improve the electrochemical properties of hydrogen storage electrode alloy. Acta Metal. Sin. 2001, 37, 57–60. [Google Scholar]
- Zhou, C.; Tao, M.; Yan, K.; Chen, Y.; Wu, C. Effects of nickel additive on the performance of the negative electrode for Ni-MH battery. Chin. J. Power Sources 2003, 27, 301–304. [Google Scholar]
- Jung, J.; Lee, S.; Kim, D.; Jang, K.; Lee, J. Effect of Cu powder as compacting material on the discharge characteristics of negative electrodes in Ni–MH batteries. J. Alloys Compd. 1998, 266, 271–275. [Google Scholar] [CrossRef]
- Mao, L.; Tong, J.; Shan, Z.; Yin, S.; Wu, F. Effect of Co additives on the cycle life of Ni-MH batteries. J. Alloys Compd. 1999, 293–295, 829–832. [Google Scholar] [CrossRef]
- Di, L.; Chen, L. Influence of the CoO in MH electrode on the performance of battery. Bull. Chin. Ceram. Soc. 2003, 3, 88–90. [Google Scholar]
- Chen, L.; Tian, Y.; Zhang, F. Influence of CoO addition on the MH electrode’s performance. J. Tianjin Inst. Technol. 2003, 19, 54–56. [Google Scholar]
- Meng, M.; Liu, M.; Deng, X.; Huang, S.; Zhan, J. Research on the cycle life of Ni-MH batteries. Chin. J. Power Sources 1998, 22, 155–157. [Google Scholar]
- Guo, J.; Li, W.; Meng, M. Study on effect of technology factors on the properties of Ni-MH battery. Chin. J. Power Sources 2000, 24, 319–321. [Google Scholar]
- Pang, L.; Zhang, J.; Guo, J.; Wang, B.; Li, K.; Duan, Q. Method of reducing internal pressure of Ni-MH battery and increasing cycle life. Chin. J. Power Sources 1999, 23, 158–160. [Google Scholar]
- Petrov, K.; Rostami, A.A.; Visintin, A.; Srinivasan, S. Optimization of composition and structure of metal-hydride electrodes. J. Electrochem. Soc. 1994, 141, 1747–1750. [Google Scholar] [CrossRef]
- Guo, W.; Xue, J.; Xu, Q.; Tang, Z. Effect of additive BC-1 on the performance of Ni-MH battery. Chin. J. Power Sources 2007, 31, 151–153. [Google Scholar]
- Cheng, Y.; Zhang, H.; Zhang, G.; Chen, Y.; Zhu, Q. Influence of addition of carbon nanotubes in the hydrogen storage alloy electrode on the performance of SC high power batteries. Acta Phys. Chim. Sin. 2008, 24, 527–532. [Google Scholar]
- Xie, F.; Dong, C.; Qian, W.; Zhai, Y.; Li, L.; Li, D. Electrochemical properties of nickel-metal hydride battery based on directly grown multiwalled carbon nanotubes. Int. J. Hydrog. Energy 2015, 40, 8935–8940. [Google Scholar] [CrossRef]
- Arnaud, O.; Le Guenne, L.; Audry, C.; Bernard, P. Effect of yttria content on corrosion of AB5-type alloys for nickel-metal hydride batteries. J. Electrochem. Soc. 2005, 152, A611–A616. [Google Scholar] [CrossRef]
- Tao, M.; Chen, Y.; Yan, K.; Zhou, C.; Wu, C.; Tu, M. Effect of rare earth oxides on the electrochemical performance of hydrogen storage electrode. Rare Met. Mater. Eng. 2005, 34, 552–556. [Google Scholar]
- Tanaka, T.; Kuzuhara, M.; Watada, M.; Oshitani, M. Effect of rare earth oxide additives on the performance of NiMH batteries. J. Alloys Compd. 2006, 408–412, 323–326. [Google Scholar] [CrossRef]
- Shinyama, K.; Nakamura, H.; Nohma, T.; Yonezu, I. Improvement of high- and low-temperature characteristics of nickel-metal hydride secondary batteries using rare-earth compounds. J. Alloys Compd. 2006, 408–412, 288–293. [Google Scholar] [CrossRef]
- Gamboa, S.A.; Sebastian, P.J.; Geng, M.; Northwood, D.O. Temperature, cycling, discharge current and self-discharge electrochemical studies to evaluate the performance of a pellet metal-hydride electrode. Int. J. Hydrog. Energy 2001, 26, 1315–1318. [Google Scholar] [CrossRef]
- Ma, G.; Guo, J.; Yan, Y.; Zhang, J.; Duan, Q.; Liu, G. Application of sintered metal hydride in Ni-MH battery. Chin. Battery Ind. 2003, 8, 113–115. [Google Scholar]
- Oshitani, M.; Yufu, H.; Takashima, K.; Tsuji, S.; Matsumaru, Y. Development of a pasted nickel electrode with high active material utilization. J. Electrochem. Soc. 1989, 136, 1590–1593. [Google Scholar] [CrossRef]
- Oshitani, M.; Sasaki, Y.; Takashima, K. Development of a nickel electrode having stable performance at various charge and discharge rates over a wide temperature range. J. Power Sources 1984, 12, 219–231. [Google Scholar] [CrossRef]
- Friebel, D.; Bajdich, M.; Yao, B.S.; Louie, M.W.; Miller, D.J.; Casalongue, H.S.; Mbuga, F.; Weng, T.; Nordlund, D.; Sokaras, D.; et al. On the chemical state of Co oxide electrocatalysts during alkaline water splitting. Phys. Chem. Chem. Phys. 2013, 15, 17460–17467. [Google Scholar] [CrossRef] [PubMed]
- Pralong, V.; Delahaye-Vidal, A.; Beaudoin, B.; Leriche, J.-B.; Tarascon, J.-M. Electrochemical behavior of cobalt hydroxide used as additive in the nickel hydroxide electrode. J. Eletrochem. Soc. 2000, 147, 1306–1313. [Google Scholar] [CrossRef]
- Otsuka, H.; Chiku, M.; Higuchi, E.; Inoue, H. Characterization of pretreated Co(OH)2-coated Ni(OH)2 positive electrode for Ni-MH batteries. ECS Trans. 2012, 41, 7–12. [Google Scholar]
- Pralong, P.; Chabre, Y.; Delahaye-Vidal, A.; Tarascon, J.-M. Study of the contribution of cobalt additive to the behavior of the nickel oxy-hydroxide electrode by potentiodynamic techniques. Solid State Ion. 2002, 147, 73–84. [Google Scholar] [CrossRef]
- Li, X.; Xia, T.; Dong, H.; Wie, Y. Study on the reduction behavior of CoOOH during the storage of nickel/metal-hydride battery. Mater. Chem. Phys. 2006, 100, 486–489. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, W.; Yu, L. Preparation of nickel hydroxide coated by cobalt hydroxide. Chin. J. Nonferr. Met. 2005, 15, 1775–1779. [Google Scholar]
- Chang, Z.; Tang, H.; Peng, P. Studies of electrochemical characteristics of nickel hydroxide with surface deposited cobalt. J. Funct. Mater. 2001, 32, 487–489. [Google Scholar]
- Yin, T. Surface modification of nickel hydroxide particles by micro-sized cobalt oxide hydroxide and properties as electrode materials. Surf. Coat. Technol. 2005, 200, 2376–2379. [Google Scholar]
- Ovshinsky, S.R.; Fetcenko, M.A.; Fierro, C.; Gifford, P.R.; Corrigan, D.A.; Benson, P.; Martin, F.J. Enhanced Nickel Hydroxide Positive Electrode Materials for Alkaline Rechargeable Electrochemical Cells. U.S. Patent 5,523,182, 4 June 1996. [Google Scholar]
- Yin, X.; Li, J.; Fu, J.; Gong, L.; Xia, X. Preparation, characterization and electrochemical performance of cobalt-substituted nickel hydroxide. Chem. Res. Appl. 2002, 14, 405–408. [Google Scholar]
- Tessier, C.; Faure, C.; Guerlou-Demourgues, L.; Denage, C.; Nabias, G.; Delmas, C. Electrochemical study of zinc-substituted nickel hydroxide. J. Electrochem. Soc. 2002, 149, A1136–A1145. [Google Scholar] [CrossRef]
- Fierro, C.; Zallen, A.; Koch, J.; Fetcenko, M.A. The influence of nickel-hydroxide composition and microstructure on the high-temperature performance of nickel metal hydride batteries. J. Electrochem. Soc. 2006, 153, A492–A496. [Google Scholar] [CrossRef]
- Zhang, S.; Han, Z. Influence of average grain size on the electrochemical properties of nickel hydroxide. Battery Ind. 1999, 4, 133–135. [Google Scholar]
- Li, W.; Jiang, C.; Wan, C. High-temperature performances of spherical nickel hydroxide coated with Yb(OH)3. J. Inorg. Mater. 2006, 21, 121–127. [Google Scholar]
- Ortiz, M.G.; Real, S.G.; Castro, E.B. Preparation and characterization of positive electrode of Ni-MH batteries with cobalt additives. Int. J. Hydrog. Energy 2014, 39, 8661–8666. [Google Scholar] [CrossRef]
- He, X.M.; Jiang, C.Y.; Li, W.; Wan, C.R. Co/Yb hydroxide coating of spherical Ni(OH)2 cathode materials for Ni-MH batteries at elevated temperatures. J. Electrochem. Soc. 2006, 153, A566–A569. [Google Scholar] [CrossRef]
- Han, E.; Xu, H.; Kang, H.; Feng, Z. Performance comparison between nano-sized and micro-sized spherical nickel hydroxide. J. Dalian Marit. Univ. 2008, 34, 87–90. [Google Scholar]
- Chen, Q.; Yi, S.; Zhao, M. Influence of nanometric zinc oxide on the electrochemical performance of nickel metal hydride batteries. Rare Met. Mater. Eng. 2010, 39, 39–42. [Google Scholar]
- Yuan, A.; Cheng, S.; Zhang, J.; Cao, C. Effects of metallic cobalt addition on the performance of pasted nickel electrodes. J. Power Sources 1999, 77, 178–182. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Yuan, H.; Zhou, Z.; Song, D.; Zhang, Y.; Zhu, L. Surface modification and electrochemical studies of spherical nickel hydroxide. J. Power Sources 1998, 72, 221–225. [Google Scholar] [CrossRef]
- Wang, X.; Leo, H.; Yang, H.; Sebastian, P.J.; Gamboa, S.A. Oxygen catalytic evolution reaction on nickel hydroxide electrode modified by electroless cobalt coating. Int. J. Hydrog. Energy 2004, 29, 967–972. [Google Scholar] [CrossRef]
- Li, Q. Study on capacity loss of Ni-MH batteries after storage at low potential. Chin. Battery Ind. 2006, 11, 303–306. [Google Scholar]
- Chang, Z.R.; Tang, H.; Chen, J.G. Surface modification of spherical nickel hydroxide for nickel electrodes. Electrochem. Commun. 1999, 1, 513–516. [Google Scholar] [CrossRef]
- Cheng, S.; Wenhau, L.; Zhang, J.; Cao, C. Electrochemical properties of the pasted nickel electrode using surface modified Ni(OH)2 powder as active material. J. Power Sources 2001, 101, 248–252. [Google Scholar]
- Hu, W.K.; Gao, X.P.; Gong, Z.X. Synthesis of CoOOH nanorods and application as coating materials of nickel hydroxide for high temperature Ni-MH cells. J. Phys. Chem. B 2005, 109, 5392–5394. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.D.; Gao, X.P.; Li, G.R.; Pan, G.L.; Yan, T.Y. Microstructure and electrochemical properties of Al-substituted nickel hydroxides modified with CoOOH nanoparticles. J. Phys. Chem. C 2007, 111, 17082–17087. [Google Scholar]
- Xia, X.; Zhang, W.; Huang, H.; Gan, Y.; Zhang, L. Effects of CoSO4 as additive on the electrochemical properties of nickel hydroxide electrode. Zhejiang Chem. Ind. 2007, 38, 1–4. [Google Scholar]
- Su, G.; He, Y.; Liu, K. Effects of Co3O4 as additive on the performance of metal hydride electrode and Ni–MH battery. Int. J. Hydrog. Energy 2012, 37, 11994–12002. [Google Scholar] [CrossRef]
- Nathira Begum, S.; Muralidharan, V.S.; Ahmed Basha, C. The influences of some additives on electrochemical behaviour of nickel electrodes. Int. J. Hydrog. Energy 2009, 34, 1548–1555. [Google Scholar] [CrossRef]
- Yuan, A.; Cheng, S.; Zhang, J.; Cao, C. The influence of calcium compounds on the behaviour of the nickel electrode. J. Power Sources 1998, 76, 36–40. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Wu, J.; Su, H. Effect of cupric oxide addition on the performance of nickel electrode. J. Power Sources 1999, 79, 86–90. [Google Scholar]
- Jung, K.; Yang, D.; Park, C.; Park, C.; Choi, J. Effects of the addition of ZnO and Y2O3 on the electrochemical characteristics of a Ni(OH)2 electrode in nickel-metal hydride secondary batteries. Int. J. Hydrog. Energy 2010, 35, 13073–13077. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Zhan, F. Study on the additives for inhibiting swelling of nickel electrode. Chin. J. Power Sources 2000, 24, 192–196. [Google Scholar]
- Chen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Nickel hydroxide as an active material for the positive electrode in rechargeable alkaline batteries. J. Electrochem. Soc. 1999, 146, 3606–3612. [Google Scholar] [CrossRef]
- Douin, M.; Guerlou-Demourgues, L.; Goubault, L.; Bernard, P.; Delmas, C. Effect of deep discharge on the electrochemical behavior of cobalt oxides and oxyhydroxides used as conductive additives in Ni-MH cells. J. Power Sources 2009, 193, 864–870. [Google Scholar] [CrossRef]
- Douin, M.; Guerlou-Demourgues, L.; Goubault, L.; Bernard, P.; Delmas, C. Influence of the electrolyte alkaline ions on the efficiency of the Na0.6CoO2 conductive additive in Ni-MH cells. J. Electrochem. Soc. 2008, 155, A945–A951. [Google Scholar] [CrossRef]
- Tronel, F.; Guerlou-Demourgues, L.; Basterreix, M.; Delmas, C. The Na0.60CoO2 phase, a potential conductive additive for the positive electrode of Ni–MH cells. J. Power Sources 2006, 158, 722–729. [Google Scholar] [CrossRef]
- Morioka, Y.; Narukawa, S.; Itou, T. State-of-the-art of alkaline rechargeable batteries. J. Power Sources 2001, 100, 107–116. [Google Scholar] [CrossRef]
- Ye, X.; Zhu, Y.; Wu, S.; Zhang, Z.; Zhou, Z.; Zheng, H.; Lin, X. Study on the preparation and electrochemical performance of rare earth doped nano-Ni(OH)2. J. Rare Earths 2011, 29, 787–792. [Google Scholar] [CrossRef]
- Ren, J.X.; Zhou, Z.; Gao, X.P.; Yan, J. Preparation of porous spherical α-Ni(OH)2 and enhancement of high-temperature electrochemical performances through yttrium addition. Eletrochim. Acta 2006, 52, 1120–1126. [Google Scholar] [CrossRef]
- Mi, X.; Gao, X.P.; Jiang, C.Y.; Geng, M.M.; Yan, Y.; Wan, C.R. High temperature performances of yttrium-doped spherical nickel hydroxide. Eletrochim. Acta 2004, 49, 3361–3366. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y. Effects of nano-sized Y2O3 additive on performance of Ni-MH battery. Chin. Battery Ind. 2008, 13, 226–228. [Google Scholar]
- Kaiya, H.; Ookawa, T. Improvement in cycle life performance of high capacity nickel-metal hydride battery. J. Alloys Compd. 1995, 231, 598–603. [Google Scholar] [CrossRef]
- Mi, X.; Ye, M.; Yan, J.; Wei, J.; Gao, X. High temperature performance of spherical nickel hydroxide with additive Y2O3. J. Rare Earths 2004, 22, 422–425. [Google Scholar]
- Wu, Q.D.; Liu, S.; Li, L.; Yan, T.Y.; Gao, X.P. High-temperature electrochemical performance of Al-α-nickel hydroxides modified by metallic cobalt or Y(OH)3. J. Power Sources 2009, 186, 521–527. [Google Scholar] [CrossRef]
- Fan, J.; Yang, Y.; Yu, P.; Chen, W.; Shao, H. Effects of surface coating of Y(OH)3 on the electrochemical performance of spherical Ni(OH)2. J. Power Sources 2007, 171, 981–989. [Google Scholar] [CrossRef]
- Oshitani, M.; Watada, M.; Shodai, K.; Kodama, M. Effect of lanthanide oxide additives on the high-temperature charge acceptance characteristics of pasted nickel electrodes. J. Electrochem. Soc. 2001, 148, A67–A73. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.; Li, Y.; Gao, X.; Yan, J. Effect of Lu2O3 on charge/discharge performances of spherical nickel hydroxide at high temperature. J. Rare Earths 2005, 23, 732–736. [Google Scholar]
- Li, J.; Shangguan, E.; Guo, D.; Li, Q.; Chang, Z.; Yuan, X.; Wang, H. Calcium metaborate as a cathode additive to improve the high-temperature properties of nickel hydroxide electrodes for nickel-metal hydride batteries. J. Power Sources 2014, 263, 110–117. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Chen, J. Synthesis, characterization, end electrochemical application of Ca(OH)2-, Co(OH)2- and Y(OH)3-coated Ni(OH)2 tubes. J. Phys. Chem. B 2005, 109, 14025–14032. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lei, L.; Chen, J.; Sun, Y. Improving the high-temperature performances of a layered double hydroxide, [Ni4Al(OH)10]NO3, through calcium hydroxide coatings. Eletrochim. Acta 2011, 56, 2862–2869. [Google Scholar] [CrossRef]
- Matsuda, H.; Ikoma, M. Nickel/metal-hydride battery for electric vehicles. Electrochemistry 1997, 65, 96–100. [Google Scholar]
- He, X.; Ren, J.; Li, W.; Jiang, C.; Wan, C. Ca3(PO4)2 coating of spherical Ni(OH)2 cathode materials for Ni–MH batteries at elevated temperature. Eletrochim. Acta 2011, 56, 4533–4536. [Google Scholar] [CrossRef]
- Guo, H.; Wang, W.; Zhang, W.; Wang, J. Effects of long-time storage in discharging state on the performance of Ni-MH batteries. Chin. Battery Ind. 2009, 14, 166–168. [Google Scholar]
- Fukunaga, H.; Kishimi, M.; Igarashi, N.; Ozaki, T.; Sakai, T. Non-foam-type nickel electrodes using various binders for Ni-MH batteries. J. Electrochem. Soc. 2005, 152, A42–A46. [Google Scholar] [CrossRef]
- Nishimura, K.; Takasaki, T.; Sakai, T. Development of high-capacity Ni(OH)2 electrode using granulation process. Stud. Sci. Technol. 2013, 2, 107–112. [Google Scholar]
- Fukunaga, H.; Kishimi, M.; Matsumoto, N.; Ozaki, T.; Sakai, T.; Tanaka, T.; Kishimoto, T. A nickel electrode with Ni-coated 3D steel sheet for hybrid electric vehicle applications. J. Electrochem. Soc. 2005, 152, A905–A912. [Google Scholar] [CrossRef]
- Xi, Z.; Zhang, J.; Wu, L.; Li, J.; Zhu, R.; Yang, Y.; Feng, P.; Zuo, C.; Shi, D. New type of anode material for the MH-Ni battery. Rare Met. Mater. Eng. 1999, 28, 371–374. [Google Scholar]
- Wang, D.; Wang, C.; Dai, C.; Sun, D. Study of Co-Ce coating and surface on pasted nickel electrodes substrate. Rare Met. 2006, 25, 47–50. [Google Scholar] [CrossRef]
- Kritzer, P.; Cook, J.A. Nonwoven as separators for alkaline batteries—An overview. J. Electrochem. Soc. 2007, 154, A481–A494. [Google Scholar] [CrossRef]
- Yu, T.; Zhai, Y.; Li, Q.; Duan, Q. Study on storage of Ni-MH battery. Chin. Battery Ind. 2005, 10, 149–152. [Google Scholar]
- Ni, J.; Wan, D.; Zhang, L. Influences of using separators made in China on Ni-MH battery performance in electric bicycle. Electr. Bicycle 2013, 6, 14–20. [Google Scholar]
- Cao, S.; Wang, H.; Jin, X. Study on the Sulfonation Treatment of ES Nonwoven Fabrics as Battery Separator. In Proceedings of the 7th National Conference of Functional Materials and Applications, Changsha, China, 15–18 October 2010; Zhao, G.-M., Ed.; Scientific Research Publishing: Merced, CA, USA; pp. 2075–2078.
- Furukawa, N. Development and commercialization of nickel-metal hydride secondary batteries. J. Power Sources 1994, 51, 45–59. [Google Scholar] [CrossRef]
- Du, H.; Liu, D.; Kong, Y.; Yang, J. Hydrophilic modification of polypropylene MH-Ni battery separator and its characterizations. Polym. Mater. Sci. Eng. 2013, 29, 101–104. [Google Scholar]
- Wang, F.; Wu, F.; Mao, L.; Zou, L.; Liu, Y.; Chen, S. Research on polymer-gel electrolyte bipolar nicker/metal hydride batteries. Mater. Rev. 2005, 19, 124–126. [Google Scholar]
- Cai, Z.; Gong, Z.; Geng, M.; Tang, Z. Study on a novel polymer gel improved separator in nickel/metal hydride battery. Chin. J. Power Sources 2005, 29, 142–145. [Google Scholar]
- Tian, Y.; Gao, H.; Wang, J.; Jin, X.; Wang, H. Preparation of hydroentangled CMC composite nonwoven fabrics as high performance separator for nickel metal hydride battery. Electrochim. Acta 2015, 177, 321–326. [Google Scholar] [CrossRef]
- Shigematsu, T.; Oku, Y.; Ishikawa, T. Nonwoven Fabric for Alkaline Battery Separator and Manufacture Thereof. Jpn. Patent Application H06–251760, 9 September 1994. [Google Scholar]
- Yao, Y.; Hu, J.; Yang, J.; Wang, Y.; Liang, Y. Preliminary study of making MH-Ni battery separator. Pap. Sci. Technol. 2009, 28, 76–80. [Google Scholar]
- Chen, Z.; Ju, Y.; Zhang, W.; Zhu, Y.; Li, L. Research in preparation and performance of a novel alkaline microporous polymer electrolyte. Mater. Rev. 2009, 23, 316–318, 334. [Google Scholar]
- Leblanc, P.; Jordy, C.; Knosp, B.; Blanchard, P.H. Mechanism of alloy corrosion and consequences on sealed nickel-metal hydride battery performance. J. Electrochem. Soc. 1998, 145, 860–863. [Google Scholar] [CrossRef]
- Khaldi, C.; Mathlouthi, H.; Lamloumi, J. A comparative study, of 1 M and 8 M KOH electrolyte concentrations, used in Ni-MH batteries. J. Alloys Compd. 2009, 469, 464–471. [Google Scholar] [CrossRef]
- Pei, L.; Yi, S.; He, Y.; Chen, Q. Effect of electrolyte formula on the self-discharge properties of nickel-metal hydride batteries. J. Guangdong Univ. Technol. 2008, 25, 10–12. [Google Scholar]
- Wang, C.; Marrero-Rivera, M.; Soriaga, M.P.; Serafini, D.; Srinivasan, S. Improvement in the cycle life of LaB5 metal hydride electrodes by addition of ZnO to alkaline electrolyte. Eletrochim. Acta 2002, 47, 1069–1078. [Google Scholar] [CrossRef]
- Matsuoka, M.; Asai, K.; Fukumoto, Y.; Iwakura, C. Electrochemical characterization of surface-modified negative electrodes consisting of hydrogen storage alloys. J. Alloys Compd. 1993, 192, 149–151. [Google Scholar] [CrossRef]
- Shangguan, E.; Wang, J.; Li, J.; Dan, G.; Chang, Z.; Yuan, X.; Wang, H. Enhancement of the high-temperature performance of advanced nickel-metal hydride batteries with NaOH electrolyte containing NaBO2. Int. J. Hydrog. Energy 2013, 38, 10616–10624. [Google Scholar] [CrossRef]
- Shangguan, E.; Li, J.; Guo, D.; Chang, Z.; Yuan, X.; Wang, H. Effects of different electrolytes containing Na2WO4 on the electrochemical performance of nickel hydroxide electrodes for nickel–metal hydride batteries. Int. J. Hydrog. Energy 2014, 39, 3412–3422. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Ai, X. Possible use of ferrocyanide as a redox additive for prevention of electrolyte decomposition in overcharged nickel batteries. Electrochim. Acta 2003, 48, 4033–4037. [Google Scholar] [CrossRef]
- Mao, L.; Wu, F.; Chen, S.; Wang, F.; Liu, Y. Application of gel polymer electrolytes in Ni/MH battery. J. Funct. Mater. 2005, 36, 1389–1390. [Google Scholar]
- Sun, S.; Song, J.; Shan, Z.; Hu, M.; Lei, L.; Chen, J.; Sun, Y. Electrochemical properties of a low molecular weight gel electrolyte for nickel/metal hydride cell. Eletrochim. Acta 2014, 130, 689–692. [Google Scholar] [CrossRef]
- Ju, Y.; Chen, Z.; Zhu, Y.; Li, L. Modification mechanism and research progress of polymer electrolytes in Ni/MH batteries. Mater. Rev. 2009, 23, 54–57. [Google Scholar]
- Yang, C. Polymer Ni-MH battery based on PEO-PVA-KOH polymer electrolyte. J. Power Sources 2002, 109, 22–31. [Google Scholar] [CrossRef]
- Vassal, N.; Salmon, E.; Fauvarque, J.-F. Nickel/metal hydride secondary batteries using an alkaline solid polymer electrolyte. J. Electrochem. Soc. 1999, 146, 20–26. [Google Scholar] [CrossRef]
- Iwakura, C.; Ikoma, K.; Nohara, S.; Furukawa, N. Charge-discharge and capacity retention characteristics of new type Ni/MH batteries using polymer hydrogel electrolyte. J. Electrochem. Soc. 2003, 150, A1623–A1627. [Google Scholar] [CrossRef]
- Wang, C.; Wallace, G.G. Flexible electrodes and electrolytes for energy storage. Electrochim. Acta 2015, 175, 87–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, H.; Yang, H.; Zhou, Z. Electrochemical stability of PAA alkaline polymer electrolyte and its application in Ni/MH secondary battery. Acta Sci. Nat. Univ. Nankaiensis 2008, 41, 57–61. [Google Scholar]
- Tang, Z.; Li, C.; Liu, Y. Research and development of polymer Ni/MH battery. Chem. Ind. Eng. Prog. 2006, 25, 1251–1255. [Google Scholar]
- Wu, R.; Lu, X.; Zhu, Y.; Li, L. Preparation and properties of PVA/PAAK alkaline polymer electrolytes for nickel metal hydride batteries. Polym. Mater. Sci. Eng. 2013, 29, 171–174. [Google Scholar]
- Lu, X.; Wu, R.; Zhu, Y.; Li, L. Preparation and properties of PVA-PAA-KOH alkaline polymer electrolyte membrane. J. Funct. Mater. 2013, 44, 590–594. [Google Scholar]
- Lu, X.; Wu, R.; Li, B.; Zhu, Y.; Li, L. Novel PVA/SiO2 alkaline micro-porous polymer electrolytes for polymer Ni-MH batteries. Acta Chim. Sin. 2013, 71, 427–432. [Google Scholar] [CrossRef]
- Vignarooban, K.; Dissanayake, M.A.K.L.; Albinsson, I.; Mellander, B.-E. Ionic conductivity enhancement in PEO:CuSCN solid polymer electrolyte by the incorporation of nickel-chloride. Solid State Ion. 2015, 278, 177–180. [Google Scholar] [CrossRef]
- Li, X.; Xia, B. Corrosion of AB5 alloy during the storage on the performance of nickel-metal-hydride battery. J. Alloys Compd. 2005, 391, 190–193. [Google Scholar] [CrossRef]
- Li, X.; Xia, B. Effect of charged-state storage on Ni-MH battery performance. Chin. J. Power Sources 2004, 28, 737–739. [Google Scholar]
- Zhang, Z.; Jia, C.; Xing, Z.; Li, L.; Ma, Y. Factors on storage performance of MH-Ni battery. J. Rare Earths 2004, 22, 347–348. [Google Scholar]
- Cao, M.; Zhang, J.; Du, P.; Wang, J. Investigation on performance of Ni-MH battery. Chin. J. Power Sources 2006, 30, 852–855. [Google Scholar]
- Jung, D.Y.; Lee, B.H.; Kim, S.W. Development of battery management system for nickel-metal hydride batteries in electric vehicle applications. J. Power Sources 2002, 109, 1–10. [Google Scholar] [CrossRef]
- Darcy, E.C. Investigation of the Response of NiMH Cells to Burp Charging. Ph.D. Thesis, University of Houston, Houston, TX, USA, 1998. [Google Scholar]
- Ma, S.; Yang, K.; Lui, Y.; Lan, Z. The effect of charge/discharge manner on properties of hydrogen storage alloy electrode. J. Guangxi Univ. Nat. Sci. Ed. 2006, 31, 205–207. [Google Scholar]
- Cao, S.; Ma, Y. Influences of the formation on the storage performance of high power MH/Ni battery. Sci. Technol. Baotou Steel Group Corp. 2008, 34, 50–52. [Google Scholar]
- Chen, B.; Li, X.; Li, X.; Zhang, Q. Method of reducing internal pressure of alkaline rechargeable battery. Chin. Battery Ind. 2000, 5, 211–213. [Google Scholar]
- Bai, Z.; Yu, X.; Lu, F. Sealing technology about cylindrical alkaline battery. Chin. J. Power Sources 2003, 27, 381–384. [Google Scholar]
- Yuan, J.; Chen, W. Research analysis on design optimization of cell sealing structure. Electr. Bicycl. 2015, 3, 10–11. [Google Scholar]
- Song, Q. Performance recovery of Ni-MH battery after long storing. Chin. Battery Ind. 2005, 10, 77–79. [Google Scholar]
- Xie, D.; Li, Q.; Wu, T. Discussion on some aspects of MH-Ni battery. Chin. Battery Ind. 2001, 6, 114–117. [Google Scholar]
- Li, N.; Meng, J. Capacity recovery of Ni-MH batteries after long-term storage. Chin. Battery Ind. 2008, 13, 95–96. [Google Scholar]
- Li, L.; Wu, F.; Chen, R.; Chen, S.; Wang, G. Regeneration of electrochemical performance for MH-Ni batteries. J. Funct. Mater. 2006, 37, 587–590. [Google Scholar]
- Minohara, T. Recycling Method of Nickel-Hydrogen Secondary Battery. U.S. Patent 6,077,622A, 20 June 2000. [Google Scholar]
- Wang, R.; Yan, J.; Zhou, Z.; Deng, B.; Gao, X.; Song, D. Regeneration of anode power from waste metal hydride/Ni rechargeable batteries. Chin. J. Appl. Chem. 2001, 18, 979–982. [Google Scholar]
- Nan, J.; Han, D.; Yang, M.; Cui, M. Dismantling, recovery, and reuse of spent nickel-metal hydride batteries. J. Electrochem. Soc. 2006, 153, A101–A105. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Yang, M.; Cui, M.; Hou, X.L. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 2006, 84, 75–80. [Google Scholar] [CrossRef]
- Tenorio, J.A.S.; Espinosa, D.C.R. Recovery of Ni-based alloys from spent NiMH battery. J. Power Sources 2002, 108, 70–73. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Bernardes, A.M.; Tenorio, J.A.S. Spent NiMH batteries: Characterization and metal recovery through mechanical processing. J. Power Sources 2006, 160, 1465–1470. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Wakui, Y.; Suzuki, T.M. Recovery of metal values from spent nickel-metal hydride rechargeable batteries. J. Power Sources 1999, 77, 116–122. [Google Scholar] [CrossRef]
- Rodrigues, L.; Mansur, M.B. Hydrometallurgical separation of rare earth elements, cobalt and nickel from spent nickel-metal-hydride batteries. J. Power Sources 2010, 195, 3735–3741. [Google Scholar] [CrossRef]
- Muller, T.; Friedrich, B. Development of a recycling process for nickel-metal hydride batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
- Rabah, M.A.; Farghaly, F.E.; Abd-El Motaleb, M.A. Recovery of nickel, cobalt and some salts from spent Ni-MH batteries. Waste Manag. 2008, 28, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.E.O.; Celante, V.G.; Lelis, M.F.F.; Freitas, M.B.J.G. Chemical and electrochemical recycling of the nickel, cobalt, zinc and manganese from the positives electrodes of spent Ni–MH batteries from mobile phones. J. Power Sources 2012, 218, 435–444. [Google Scholar] [CrossRef]
- Larsson, K.; Ekberg, C.; Ødegaard-Jensen, A. Dissolution and characterization of HEV NiMH batteries. Waste Manag. 2013, 33, 689–698. [Google Scholar] [CrossRef] [PubMed]
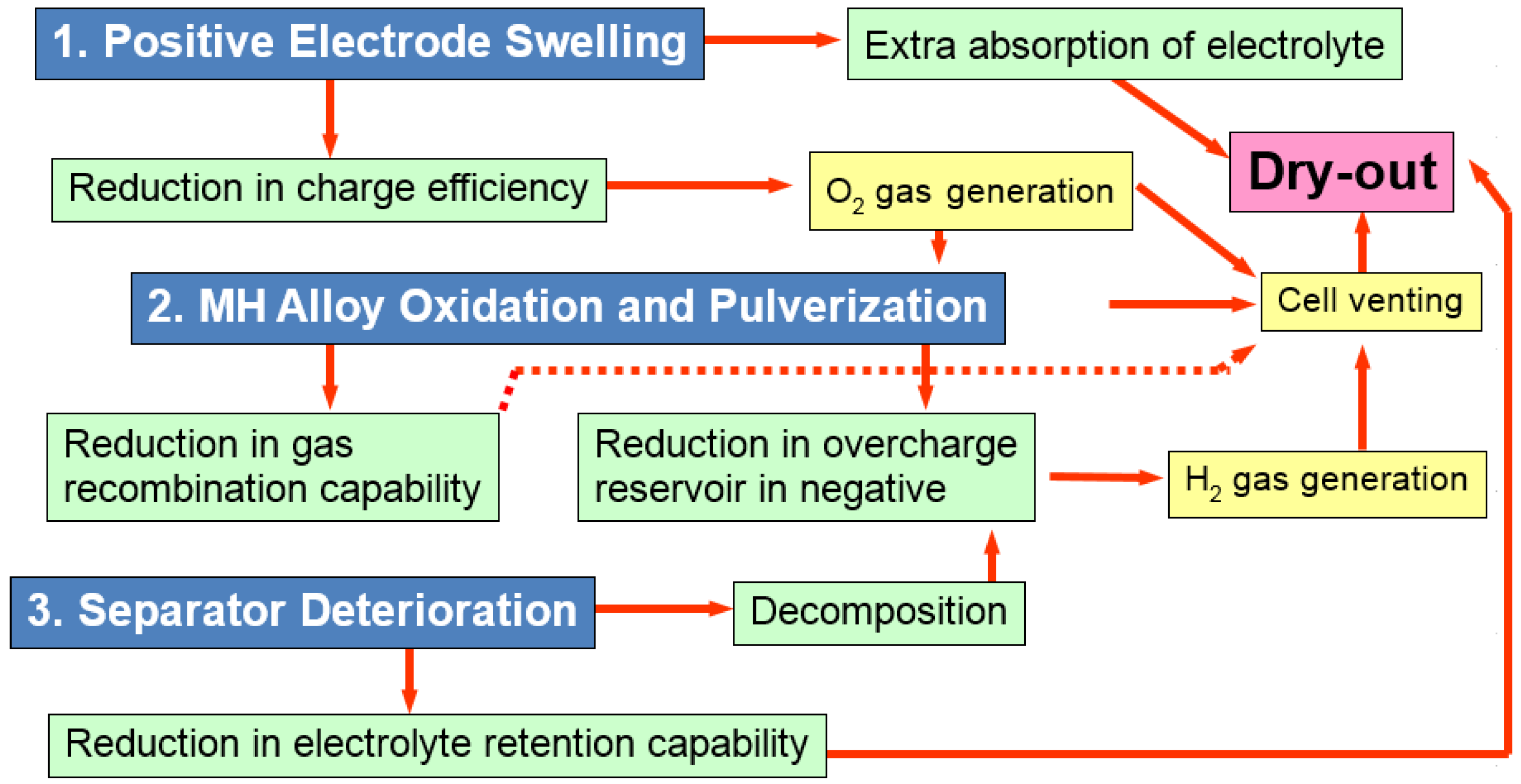
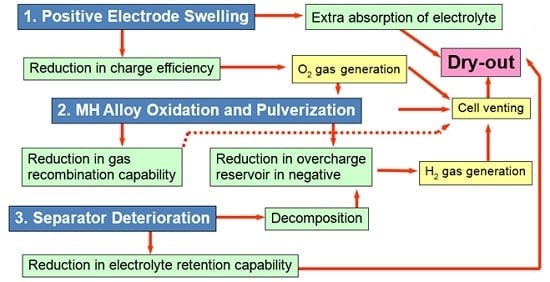
| Shape | Case material | Sealed | Manufacturability | Cost | Energy density | Heat dissipation | Abuse tolerance |
|---|---|---|---|---|---|---|---|
| Cylindrical | Metal | Yes | Easy | Low | High | Easy | High |
| Stick | Metal | Yes | Medium | Low | High | Easy | High |
| Prismatic | Metal | Yes | Medium | High | Low | Easy | Med |
| Prismatic | Plastic | Yes | Medium | High | Low | Hard | Med |
| Button | Metal | Yes | Easy | Low | Low | Easy | Low |
| Pouch | Al foil | Yes | Easy | Low | Very high | Easy | Low |
| Cylindrical/prismatic | Plastic/flooded | No | Easy | Low | Low | Hard | High |
| Symptom | Reasons | Possible causes |
|---|---|---|
| Battery short-circuit | Direct conducting path between two electrodes developed |
|
| Battery open-circuit | Breakage of inside connection |
|
| Battery abuse | Over-discharge and overcharge |
|
| Capacity decrease | Electrode degradation |
|
| Power decrease and impedance increase | Electrolyte dry-out |
|
| Electrode degradation |
| |
| Separator degradation |
| |
| Overheat during charge | Micro-shorting |
|
| White deposits | Electrolyte leak from venting |
|
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References |
|---|---|---|---|---|---|
| Use of a sulfonated separator | Removal of N-containing compounds | None | Modest | ⋆⋆⋆⋆⋆ | [22,78,79] |
| Use of an acrylic acid grafted PP separator | Reduction in Al- and Mn- debris formation in separator | None | None | ⋆⋆⋆⋆ | [80] |
| Removal of Co and Mn in A2B7 MH alloy | Reduction in debris formation in separator | None | None | ⋆⋆⋆⋆⋆ | [6,81] |
| Increase of the amount of electrolyte | Reduction in the hydrogen diffusion in electrolyte | None | None | ⋆⋆⋆⋆ | [82] |
| Removal of Cu-containing components | Reduction in micro-short | None | None | ⋆⋆⋆⋆⋆ | [83,84,85] |
| PTFE coating on positive electrode | Suppression of reaction between NiOOH and H2 | None | Negligible | ⋆⋆⋆⋆ | [86] |
| CMC solution dipping | Suppression of oxygen evolution | None | Negligible | ⋆⋆⋆⋆ | [87] |
| Micro-encapsulation of Cu on MH alloy | Decrease in H2 released from MH alloy | None | Modest | ⋆⋆⋆ | [88] |
| Ni-B alloy coating on MH alloy | Formation of a protection layer | None | Modest | ⋆⋆⋆ | [89] |
| Alkaline treatment of negative electrode | Reduction of leach-out of Mn and Al | None | Modest | ⋆⋆⋆⋆ | [90] |
| Addition of LiOH and NaOH into electrolyte | Reduction in electrolyte corrosion capabilities | None | None | ⋆⋆⋆⋆ | [75] |
| Addition of Al2(SO4)3 into electrolyte | Reduction in MH alloy corrosion | None | Negligible | ⋆⋆ | [91] |
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References |
|---|---|---|---|---|---|
| Pre-charge of the positive electrode | Reduction of ODR | None | Negligible | ⋆⋆⋆⋆⋆ | [142,143] |
| Increase in the n/p ratio | Trade-off of capacity for longer life | None | None | ⋆⋆⋆⋆⋆ | [134,144] |
| Optimization of electrolyte loading | Balance between cycle life and production yield | None | None | ⋆⋆⋆⋆ | [141] |
| Optimization of positive electrode thickness | Reduction in electrode breakage | None | None | ⋆⋆⋆⋆ | [145] |
| Pre-charge during the formation process | Protection of MH alloy | None | Negligible | ⋆⋆⋆ | [146] |
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References | |
|---|---|---|---|---|---|---|
| A. Alloy formula | Increase in Al-content | Increase in unit cell volume and reduction in lattice expansion during hydrogenation. Formation of Al2O3 protection layer on MH alloy. | None | None | ⋆⋆⋆⋆⋆ | [159,160,161,162] |
| Increase in Co-content | Reduction in hardness and prevention of La-migration onto surface | None | Modest | ⋆⋆⋆⋆⋆ | [163] | |
| Use of misch-metal instead of pure La | Increase in degree of disorder | None | Reduction | ⋆⋆⋆⋆⋆ | [164,165] | |
| Increase in Ce and Nd content | Increase in oxidation resistance | None | Modest | ⋆⋆⋆⋆⋆ | [166] | |
| Zr addition | Decrease in pulverization rate | None | Negligible | ⋆⋆⋆⋆⋆ | [167,168] | |
| Ti addition | Decrease in pulverization rate | None | Negligible | ⋆⋆⋆⋆ | [168,169] | |
| Use of hyper-stoichiometry | Reduction in pressure-concentration-temperature hysteresis and pulverization | None | None | ⋆⋆⋆⋆ | [92,163] | |
| B. Alloy preparation | Fast quenching-gas atomization | Distribution of stress from lattice expansion | None | Modest | ⋆⋆⋆⋆⋆ | [40,170,171,172,173] |
| Fast quenching-melt spin | Improvement in alloy homogeneity | None | Modest | ⋆⋆⋆⋆⋆ | [174,175] | |
| C. Surface treatment | Ni surface plating | Protection of alloy surface from oxidation and reduction in inner pressure | None | Modest | ⋆⋆⋆⋆⋆ | [176,177] |
| Cu coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [178,179,180,181] | |
| Co coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [182] | |
| Pd coating | Protection of alloy surface from oxidation | None | High | ⋆⋆⋆⋆ | [183] | |
| Ni-B alloy coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [89] | |
| Ni-P alloy coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [184] | |
| Ni-S alloy coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [185] | |
| Ni-Cu alloy coating | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆ | [186] | |
| Alkaline pre-activation | Formation of a Ni-rich surface | None | Modest | ⋆⋆⋆⋆⋆ | [187] | |
| KBH4 treatment | Formation of a Ni-rich surface | Toxic in contact with skin | Modest | ⋆⋆⋆⋆⋆ | [187,188] | |
| Surface fluorination | Protection of alloy surface from oxidation | None | Modest | ⋆⋆⋆⋆⋆ | [189,190,191,192] | |
| Cu and HF surface treatment | Formation of CuF2 protective layer on the surface | None | Modest | ⋆⋆⋆ | [193] | |
| D. Other treatments | AB5 annealing | Improvement in Mn homogeneity and reduction in inner pressure | None | Modest | ⋆⋆⋆⋆⋆ | [166,194,195,196] |
| La-A2B7 annealing | Improvement in phase homogeneity | None | Modest | ⋆⋆⋆⋆⋆ | [197] | |
| Magnetization | Improvement in mechanical integrity | None | Modest | ⋆⋆⋆ | [198] | |
| Ultrasound treatment | Reduction in pulverization | None | Modest | ⋆⋆⋆ | [128] | |
| E. Additives | Ni fine powder | Increase in mechanical integrity | None | Negligible | ⋆⋆⋆⋆ | [199] |
| Cu fine powder | Increase in mechanical integrity | None | Negligible | ⋆⋆⋆ | [200] | |
| Co-compounds | Increase in oxidation resistance | None | Modest | ⋆⋆⋆⋆ | [60,201,202,203] | |
| CMC:PVA (3:2) | Increase in mechanical integrity | None | Negligible | ⋆⋆⋆⋆ | [204] | |
| Ratio of binder to conductive additives | Increase in mechanical integrity | None | None | ⋆⋆⋆⋆ | [205] | |
| PTFE | Improvement in hydrogen gas absorption capability to reduce pressure | None | Negligible | ⋆⋆⋆⋆ | [206] | |
| Teflonized carbon | Creation of 3D conductive network | None | Negligible | ⋆⋆⋆⋆ | [207] | |
| HEC | Improvement in hydrogen gas absorption capability to reduce pressure | Very low toxicity if swallowed | Negligible | ⋆⋆⋆⋆ | [127,195] | |
| BC-1 (irigenin) | Improvement in gas recombination rate | None | Negligible | ⋆⋆⋆⋆ | [208] | |
| Carbon nanotube | Increase in mechanical integrity | None | Modest | ⋆⋆⋆⋆ | [209,210] | |
| Y2O3 | Improvement in corrosion resistance | None | Modest | ⋆⋆⋆⋆ | [211] | |
| Oxides of light RE | Improvement in corrosion resistance | None | Modest | ⋆⋆⋆⋆ | [212] | |
| Oxides of heavy RE | Improvement in corrosion resistance | None | Modest | ⋆⋆⋆⋆ | [213,214] | |
| F. Electrode type | Use of a pellet electrode | Increase in mechanical integrity | None | Reduction | ⋆⋆⋆ | [215] |
| Use of a sintered type electrode | Increase in mechanical integrity | None | Reduction | ⋆⋆⋆⋆ | [216] | |
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References | |
|---|---|---|---|---|---|---|
| A. Composition and particle size | Co-precipitation of Co | Increase in intrinsic conductivity | None | Modest | ⋆⋆⋆⋆⋆ | [228] |
| Co-precipitation of Zn | Prevention of γ-NiOOH formation | None | Negligible | ⋆⋆⋆⋆⋆ | [93,229] | |
| Co-precipitation of Mg and/or Ca | Improvement in high-temperature performance | None | Negligible | ⋆⋆⋆ | [230] | |
| New type of Ni-Al double layered hydroxide | High capacity α-Ni(OH)2/γ-NiOOH | None | Negligible | ⋆⋆⋆⋆ | [58] | |
| Increase in Ni(OH)2 crystallite size | Trade-off in activation | None | None | ⋆⋆⋆⋆ | [231] | |
| B. Surface coating | CoOOH coating | Enhancement in survival rate after long-term storage | None | Modest | ⋆⋆⋆⋆⋆ | [121,139,223] |
| Yb(OH)3 coating | Improvement in high-temperature performance | None | Modest | ⋆⋆⋆⋆ | [232] | |
| Electrode-less plating of Co | Improvement in Co-conductive network | None | Modest | ⋆⋆⋆⋆ | [233] | |
| Co/Yb hydroxide coating | Improvement in high-temperature performance | None | Modest | ⋆⋆⋆⋆ | [234] | |
| C. Additives | Nano-sized Ni(OH)2 | Increase in electrochemical reaction reversibility | None | None | ⋆⋆⋆⋆ | [235] |
| Nano-sized ZnO | Increase in the flexibility of the electrode | None | None | ⋆⋆⋆⋆ | [236] | |
| Co in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆ | [237,238,239] | |
| CoO in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆ | [110,240] | |
| Co(OH)2 in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆⋆ | [195,241,242] | |
| CoOOH in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆⋆ | [243,244] | |
| CoSO4 in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆ | [245] | |
| Co3O4 in paste | Formation of conductive Co-network | None | Modest | ⋆⋆⋆⋆ | [246] | |
| Co and CaCo3 | Prevention of oxygen evolution | None | Modest | ⋆⋆⋆⋆ | [247,248] | |
| CuO in paste | Uniform dispersion of Co-conductive network | None | Negligible | ⋆⋆⋆ | [249] | |
| ZnO in paste | Prevention of oxygen evolution | None | Negligible | ⋆⋆⋆ | [250,251] | |
| Zn(OH)2 in paste | Prevention of electrode swelling | None | Negligible | ⋆⋆⋆ | [252] | |
| Na0.6CoO2 | Formation of better conductive Co-network | None | Modest | ⋆⋆⋆⋆ | [253,254,255] | |
| RE | Decrease in oxidation rate of MH alloy | None | Modest | ⋆⋆⋆⋆⋆ | [256,257,258,259] | |
| Y2O3 | Decrease in oxidation rate of MH alloy | None | Modest | ⋆⋆⋆⋆⋆ | [23,250,260,261,262] | |
| Y(OH)3 | Decrease in oxidation rate of MH alloy | None | Modest | ⋆⋆⋆⋆⋆ | [263,264] | |
| Oxides of heavy RE | Improvement in corrosion resistance | None | Modest | ⋆⋆⋆⋆ | [213,265,266] | |
| Calcium metal borate | Prevention of oxygen evolution | None | Negligible | ⋆⋆⋆⋆ | [267] | |
| CaF2 | Improvement in high-temperature performance | None | Negligible | ⋆⋆⋆ | [116] | |
| Ca(OH)2 | Improvement in high-temperature performance | None | Negligible | ⋆⋆⋆ | [268,269] | |
| CaS | Improvement in high-temperature performance | Reacts with acid and releases toxic H2S gas | Negligible | ⋆⋆⋆ | [270] | |
| Ca3(PO4)2 | Improvement in high-temperature performance | None | Negligible | ⋆⋆⋆ | [271] | |
| D. Electrode process | Use of sintered electrode | Enhancement in survival rate after long-term storage | None | Reduction | ⋆⋆⋆⋆⋆ | [272] |
| Use of pasted electrode on NPPS | Increase in mechanical integrity | None | Reduction | ⋆⋆⋆ | [273] | |
| Use of granulated particles | Suppression of electrode swelling | None | None | ⋆⋆⋆⋆⋆ | [274] | |
| E. Substrate | Use of 3D Ni-plated steel sheet | Increase in power and cycle stability | None | Modest | ⋆⋆⋆⋆ | [275] |
| Use of Ni fiber felt | Increase in surface area and flexibility | None | Modest | ⋆⋆⋆⋆ | [276] | |
| Pre-coating of Co-Ce alloy | Increase in contact area between substrate and Ni(OH)2 | None | Modest | ⋆⋆⋆ | [277] | |
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References |
|---|---|---|---|---|---|
| Sulfonated separator | Reduction in N-compound shuttling effects | None | Modest | ⋆⋆⋆⋆⋆ | [23,279,280,281,282] |
| Grafted acrylic acid/PP | Improvement in electrolyte holding capability | None | Negligible | ⋆⋆⋆ | [283] |
| Polymer gel-type | Improvement in durability | None | Negligible | ⋆⋆⋆ | [284,285] |
| Hydroentangled CMC composite | Improvement in integrity | None | Negligible | ⋆⋆⋆ | [286] |
| EVOH | Improvement in integrity | Cytotoxic | Modest | ⋆⋆⋆ | [287,288] |
| AMPE | Improvement in voltage window | None | Modest | ⋆⋆ | [289] |
| Addition of a K-conducting solid oxide film | Elimination of cross-contamination from the negative electrode | None | High | ⋆⋆ | New idea |
| Method | Direct impact | Environmental impact | Cost impact | Effectiveness | References |
|---|---|---|---|---|---|
| Reduction in KOH concentration | Slow-down in alloy oxidation | None | None | ⋆⋆⋆⋆ | [291] |
| Replacement with NaOH | Slow-down in alloy oxidation | None | Negligible | ⋆⋆⋆⋆⋆ | [292] |
| ZnO additives | Slow-down in alloy oxidation | None | Negligible | ⋆⋆⋆ | [293] |
| LiOH additives | Prevention of K+ migrating into Ni(OH)2 and suppression of Fe-poisoning | None | Negligible | ⋆⋆⋆ | [93] |
| Al2(SO4)3 additives | Slow-down in alloy oxidation | None | Negligible | ⋆⋆⋆ | [91] |
| NaH2PO4 additives | Formation of a Ni-rich surface on MH alloy | None | Negligible | ⋆⋆⋆ | [294] |
| NaBO2 additives | Improvement of high-temperature cycle stability | None | Negligible | ⋆⋆⋆ | [295] |
| Na2WO4 additives | Increase in oxygen evolutionary potential | None | Negligible | ⋆⋆⋆ | [296] |
| K4Fe(CN)6 additives | Prevention of electrolyte decomposition | Highly toxic | Modest | ⋆⋆⋆ | [297] |
| Use of gel-type electrolyte | Reduction in corrosion and pulverization in the positive electrode | None | Modest | ⋆⋆⋆ | [298,299] |
| Use of polymer electrolyte | Wide voltage window and better mechanical integrity | None | Modest | ⋆⋆⋆⋆ | [300,301,302,303,304,305,306,307,308,309,310] |
| Method | Direct impact | Environmental Impact | Cost impact | Effectiveness | References |
|---|---|---|---|---|---|
| Install super water absorbing material at cell bottom | Reservoir for additional electrolyte | None | Negligible | ⋆⋆⋆⋆⋆ | [104] |
| Maintain cell OCV above 1.0 V | Prevention of Co dissolution and migration from the conductive network in the positive electrode | None | None | ⋆⋆⋆⋆ | [107,311,312] |
| Maintain cell OCV above 1.1 V | Prevention of Co dissolution and migration from the conductive network in the positive electrode | None | None | ⋆⋆⋆⋆⋆ | [313] |
| Reduction of depth of discharge | Prevention of swelling in the positive electrode | None | None | ⋆⋆⋆⋆⋆ | [110] |
| Reduction of number of shallow depth discharge | Prevention of memory effect | None | None | ⋆⋆⋆⋆⋆ | [314] |
| Implementation of an improved battery management system | Prevention of abuse | None | Modest | ⋆⋆⋆⋆⋆ | [315] |
| Pulse charging | Reduction in heat generated | None | Negligible | ⋆⋆⋆ | [316] |
| Optimization of formation parameters | Reduction in cell performance variation | None | None | ⋆⋆⋆⋆ | [317,318] |
| Battery sealing under vacuum | Reduction in inner pressure | None | Modest | ⋆⋆ | [319] |
| Improvement in sealing technology | Prevention of electrolyte leak | None | Negligible | ⋆⋆ | [320,321] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-h.; Yasuoka, S. Capacity Degradation Mechanisms in Nickel/Metal Hydride Batteries. Batteries 2016, 2, 3. https://doi.org/10.3390/batteries2010003
Young K-h, Yasuoka S. Capacity Degradation Mechanisms in Nickel/Metal Hydride Batteries. Batteries. 2016; 2(1):3. https://doi.org/10.3390/batteries2010003
Chicago/Turabian StyleYoung, Kwo-hsiung, and Shigekazu Yasuoka. 2016. "Capacity Degradation Mechanisms in Nickel/Metal Hydride Batteries" Batteries 2, no. 1: 3. https://doi.org/10.3390/batteries2010003
APA StyleYoung, K.-h., & Yasuoka, S. (2016). Capacity Degradation Mechanisms in Nickel/Metal Hydride Batteries. Batteries, 2(1), 3. https://doi.org/10.3390/batteries2010003