Various Technologies to Mitigate Volume Expansion of Silicon Anode Materials in Lithium-Ion Batteries
Abstract
1. Potential and Challenges of Silicon Anodes
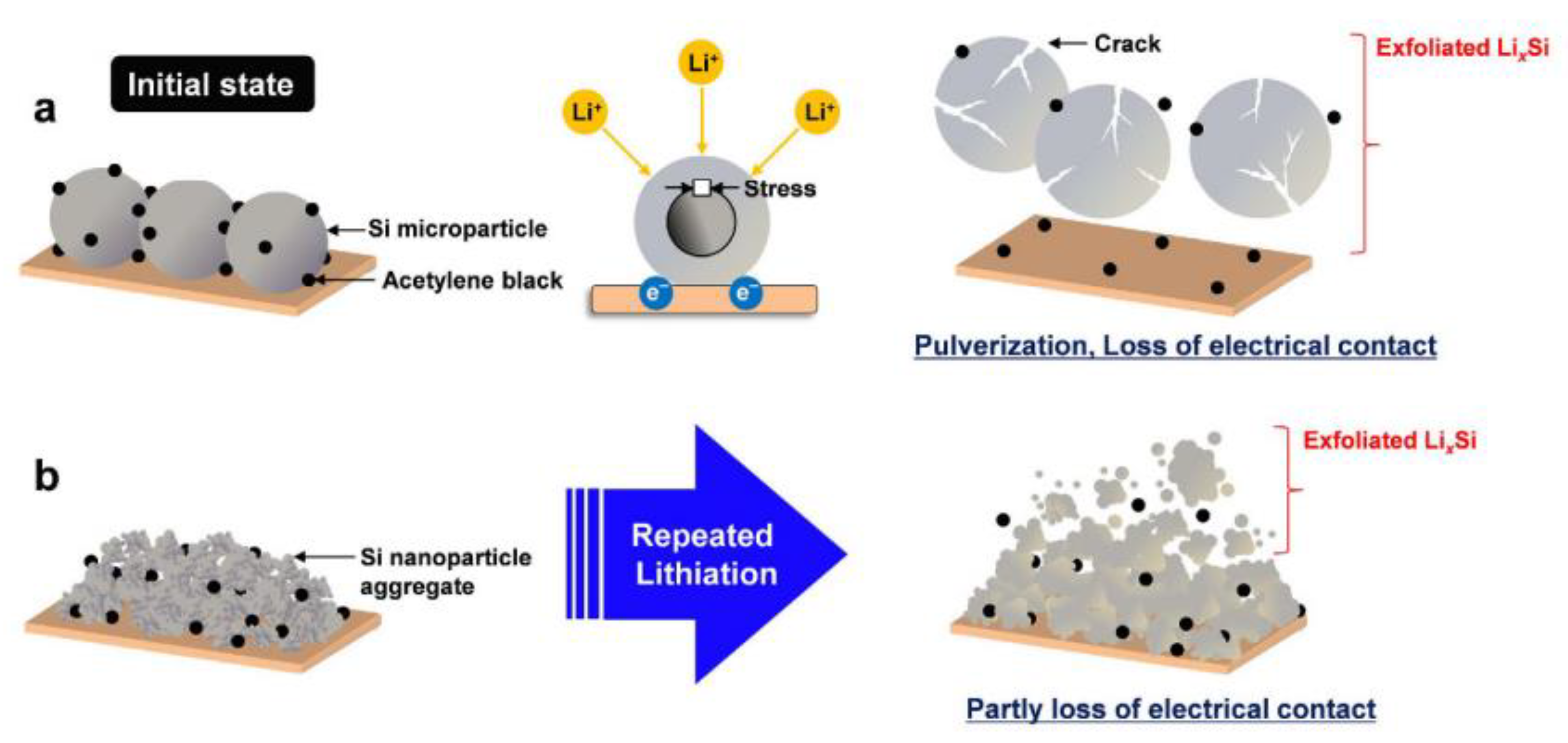
2. Concept and Principles of Hybrid Material Systems
2.1. Definition of Hybrid Material Systems
2.2. Working Mechanisms of Hybrid Material Systems in Silicon Anodes
3. Types and Characteristics of Hybrid Material Systems
3.1. Classification According to Constituent Materials
3.1.1. Silicon–Carbon Hybrid Systems
3.1.2. Silicon–Metal Oxide Hybrid Systems
3.1.3. Silicon–2D Material Hybrid Systems
3.1.4. Silicon–Conductive Polymer Hybrid Systems
3.2. Classification According to Structural Characteristics
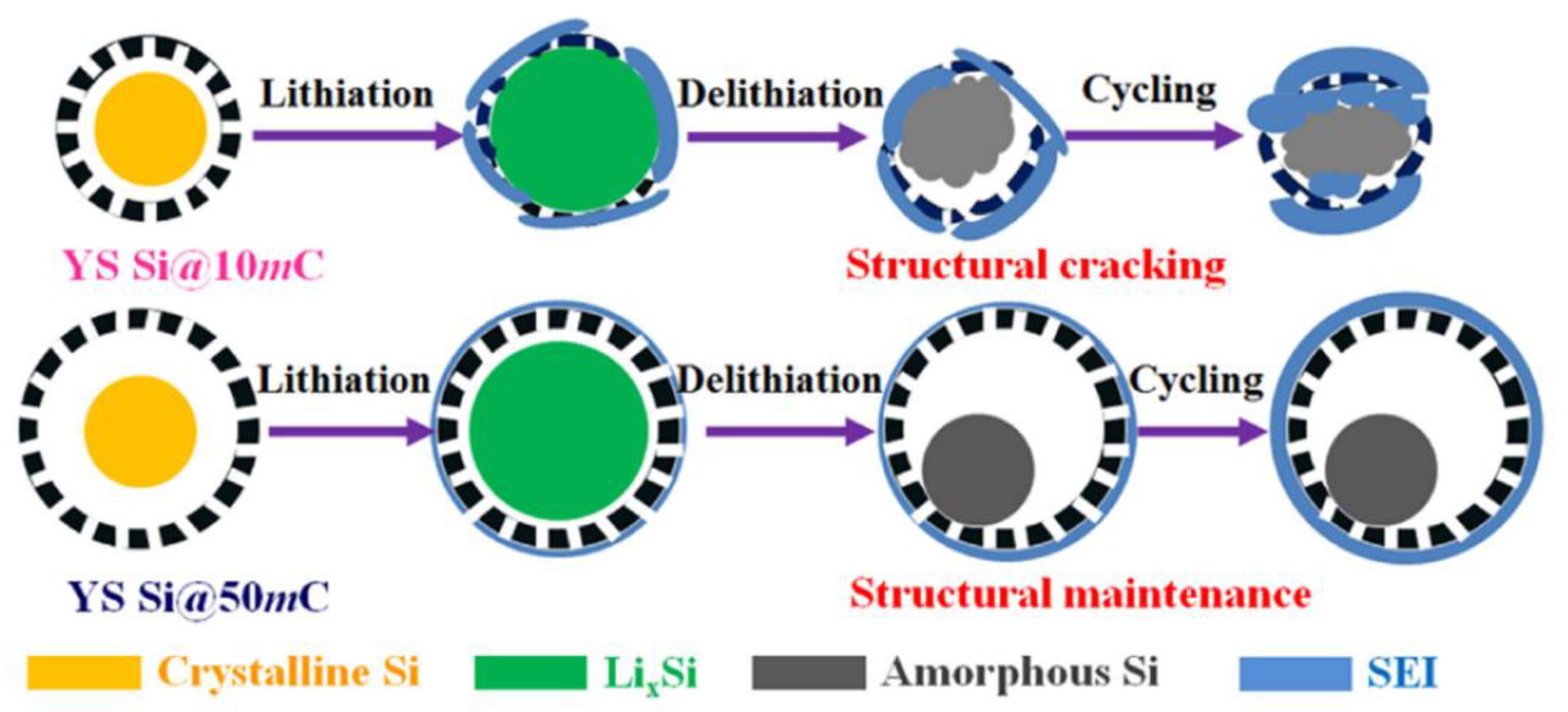
3.2.1. Core–Shell Hybrid Structures
3.2.2. Sandwich Hybrid Structures
3.2.3. Three-Dimensional Network Hybrid Structures
4. Synthesis Methods for Hybrid Material Systems
4.1. Solution-Based Synthesis Methods
4.2. Vapor Deposition Methods
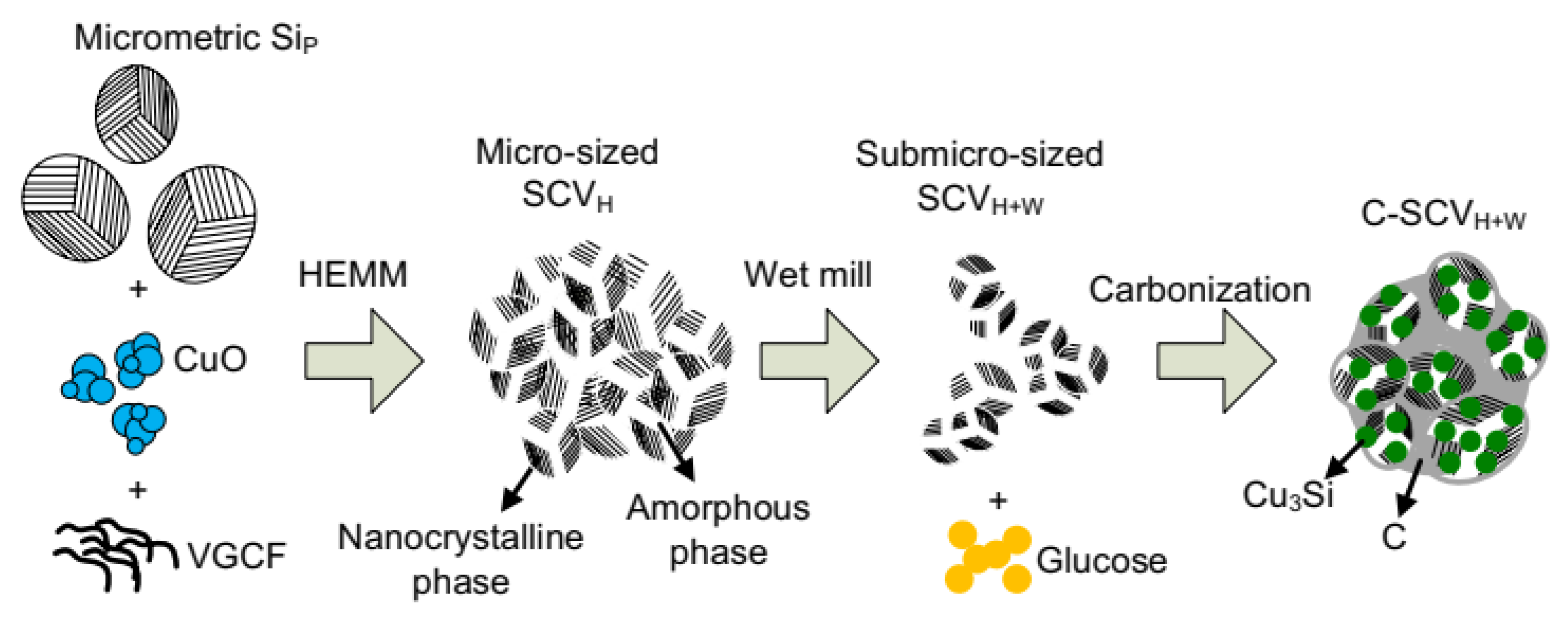
4.3. Mechanical Synthesis Methods
4.4. Hybrid Process Methods
5. Advantages, Disadvantages, and Challenges of Hybrid Material Systems
5.1. Advantages of Hybrid Material Systems
5.2. Disadvantages and Limitations of Hybrid Material Systems
5.3. Challenges to Overcome and Possible Solutions
5.4. Recent Industrial Progress and Commercial Cases
6. Conclusions and Future Perspectives
6.1. Conclusions
6.2. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIB | Lithium-ion battery |
| EV | Electric vehicle |
| ESS | Energy storage systems |
| SEI | Solid electrolyte interphase |
| CNTs | Carbon nanotubes |
| CNFs | Carbon nanofibers |
| MOx | Metal oxides |
| MXene | Mn+1XnTx (M: early transition metal, X: carbon or nitrogen, Tx: surface functional groups, n = 1–4) |
| h-BN | Hexagonal boron nitride |
| TMD | Transition metal dichalcogenide |
| CP | Conductive polymer |
| PANI | Polyaniline |
| PPy | Polypyrrole |
| PEDOT:PPS | Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate |
| CVD | Chemical vapor deposition |
| PVD | Physical vapor deposition |
| ALD | Atomic layer deposition |
| CE | Coulombic efficiency |
| ICE | Initial Coulombic efficiency |
References
- Yi, X.; Qi, G.; Liu, X.; Depcik, C.; Liu, L. Challenges and Strategies toward Anode Materials with Different Lithium Storage Mechanisms for Rechargeable Lithium Batteries. J. Energy Storage 2024, 95, 112480. [Google Scholar] [CrossRef]
- Wang, K.; Hua, W.; Huang, X.; Stenzel, D.; Wang, J.; Ding, Z.; Cui, Y.; Wang, Q.; Ehrenberg, H.; Breitung, B.; et al. Synergy of Cations in High Entropy Oxide Lithium Ion Battery Anode. Nat. Commun. 2023, 14, 1487. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Wang, J.; Kober, D.; Li, S.; Wang, X.; Shen, X.; Bekheet, M.F.; Gurlo, A. Polymer-Derived SiOC Integrated with a Graphene Aerogel as a Highly Stable Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2020, 12, 46045–46056. [Google Scholar] [CrossRef]
- Haro, M.; Kumar, P.; Zhao, J.; Koutsogiannis, P.; Porkovich, A.J.; Ziadi, Z.; Bouloumis, T.; Singh, V.; Juarez-Perez, E.J.; Toulkeridou, E.; et al. Nano-Vault Architecture Mitigates Stress in Silicon-Based Anodes for Lithium-Ion Batteries. Commun. Mater. 2021, 2, 16. [Google Scholar] [CrossRef]
- Riedel, O.; Düttmann, A.; Dühnen, S.; Kolny-Olesiak, J.; Gutsche, C.; Parisi, J.; Winter, M.; Knipper, M.; Placke, T. Surface-Modified Tin Nanoparticles and Their Electrochemical Performance in Lithium Ion Battery Cells. ACS Appl. Nano Mater. 2019, 2, 3577–3589. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Lv, W.; Wan, B.; Li, Y.; Wang, J.; Sun, S.; Yan, W.; Wang, J. Micro Lithium Batteries toward the Next-generation Smart Microsystems: A Review. Energy Storage Mater. 2025, 81, 104522. [Google Scholar] [CrossRef]
- Refino, A.D.; Eldona, C.; Hernandha, R.F.H.; Adhitama, E.; Sumboja, A.; Peiner, E.; Wasisto, H.S. Advances in 3D Silicon-based Lithium-ion Microbatteries. Commun. Mater. 2024, 5, 22. [Google Scholar] [CrossRef]
- Refino, A.D.; Yulianto, N.; Priyono, S.; Manawan, M.; Hernandha, R.F.H.; Adhitama, E.; Kottmeier, J.; Dietzel, A.; Peiner, E.; Wasisto, H.S. Carbon-coating Effect on the Performance of Photolithographically-structured Si Nanowires for Lithium-ion Microbattery Anodes. Commun. Mater. 2025, 6, 32. [Google Scholar] [CrossRef]
- Cui, Q.; Zhong, Y.; Pan, L.; Zhang, H.; Yang, Y.; Liu, D.; Teng, F.; Bando, Y.; Yao, J.; Wang, X. Recent Advances in Designing High-Capacity Anode Nanomaterials for Li-Ion Batteries and Their Atomic-Scale Storage Mechanism Studies. Adv. Sci. 2018, 5, 1700902. [Google Scholar] [CrossRef] [PubMed]
- Je, M.; Han, D.-Y.; Ryu, J.; Park, S. Constructing Pure Si Anodes for Advanced Lithium Batteries. Acc. Chem. Res. 2023, 56, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, P.; Liu, M.; Cheng, P.; Zhang, T.; Liu, J.; Liu, D.; He, D. To Achieve Controlled Specific Capacities of Silicon-Based Anodes for High-Performance Lithium-Ion Batteries. J. Alloys Compd. 2022, 905, 164189. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Wang, L.; Chen, Z.; Jin, Z. Stabilizing Porous Micro-sized Silicon Anodes via Construction of Tough Composite Interface Networks for High-energy-density Lithium-ion Batteries. Nano Res. 2024, 17, 9737–9745. [Google Scholar] [CrossRef]
- Li, H.; Yamaguchi, T.; Matsumoto, S.; Hoshikawa, H.; Kumagai, T.; Okamoto, N.L.; Ichitsubo, T. Circumventing Huge Volume Strain in Alloy Anodes of Lithium Batteries. Nat. Commun. 2020, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, Q.; Chen, Z.; Rong, C.; Chao, D. Application and Development of Silicon Anode Binders for Lithium-Ion Batteries. Materials 2023, 16, 4266. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Sano, T.; Tsuda, T.; Ui, K.; Oshima, Y.; Yamagata, M.; Ishikawa, M.; Haruta, M.; Doi, T.; Inaba, M.; et al. In Situ Scanning Electron Microscopy of Silicon Anode Reactions in Lithium-Ion Batteries during Charge/Discharge Processes. Sci. Rep. 2016, 6, 36153. [Google Scholar] [CrossRef]
- Gross, S.J.; Hsieh, M.-T.; Mumm, D.R.; Valdevit, L.; Mohraz, A. Alleviating Expansion-Induced Mechanical Degradation in Lithium-Ion Battery Silicon Anodes via Morphological Design. Extreme Mech. Lett. 2022, 54, 101746. [Google Scholar] [CrossRef]
- Müller, S.; Pietsch, P.; Brandt, B.-E.; Baade, P.; De Andrade, V.; De Carlo, F.; Wood, V. Quantification and Modeling of Mechanical Degradation in Lithium-Ion Batteries Based on Nanoscale Imaging. Nat. Commun. 2018, 9, 2340. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Ezzedine, M.; Jardali, F.; Florea, I.; Zamfir, M.-R.; Cojocaru, C.-S. Nanostructuring Strategies for Silicon-Based Anodes in Lithium-Ion Batteries: Tuning Areal Silicon Loading, SEI Formation/Irreversible Capacity Loss, Rate Capability Retention and Electrode Durability. Batteries Supercaps 2023, 6, e202200451. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, X.; Jin, Z. Interfacial Engineering of Porous SiOx@C Composite Anodes toward High-performance Lithium-ion Batteries. Chem. Eng. J. 2023, 474, 145960. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M.Ö.; Dogru, A.; Wang, J.; Rencheck, M.; Han, Y.; Wang, L.; Seydibeyoğlu, E.A.; Zhao, X.; Ong, K.; Shatkin, J.A.; et al. Review on Hybrid Reinforced Polymer Matrix Composites with Nanocellulose, Nanomaterials, and Other Fibers. Polymers 2023, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Occhicone, A.; De Gregorio, E.; Ricciotti, L.; Cioffi, R.; Ferone, C.; Tarallo, O. Geopolymer-Based Composite and Hybrid Materials: The Synergistic Interaction between Components. Sustain. Mater. Technol. 2025, 44, e01404. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Hou, S.-C.; Chen, T.-Y.; Wu, Y.-H.; Chen, H.-Y.; Lin, X.-D.; Chen, Y.-Q.; Huang, J.-L.; Chang, C.-C. Mechanochemical Synthesis of Si/Cu3Si-Based Composite as Negative Electrode Materials for Lithium Ion Battery. Sci. Rep. 2018, 8, 12695. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Z.; Zheng, C.; Zhang, L.; Wang, S.; Du, H. Revisiting the Core Problem Impeding the Commercialization of Silicon-Based Lithium-Ion Batteries. Energy Mater. Devices 2025, 3, 9370055. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, R.; Yang, J.G.; Wang, J.; Peng, Y. A Review on Mechanical, Electrical, Chemical, and Electrochemical Properties of Coating Materials for Silicon Anodes in Lithium-Ion Batteries. Small 2025, e06400. [Google Scholar] [CrossRef]
- Kong, X.; Xi, Z.; Wang, L.; Zhou, Y.; Liu, Y.; Wang, L.; Li, S.; Chen, X.; Wan, Z. Recent Progress in Silicon-Based Materials for Performance-Enhanced Lithium-Ion Batteries. Molecules 2023, 28, 2079. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, Q.; Jia, Y.; Liu, X.; Jiang, N.; Zhao, Y.; Wu, C.; Guan, L. Enhancing the Cycling Stability of Commercial Silicon Nanoparticles by Carbon Coating and Thin Layered Single-Walled Carbon Nanotube Webbing. J. Power Sources 2024, 602, 234338. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.-X.; Chou, S.-L.; Zhang, R.; Xu, Y.; Fan, J.; Zhang, W.-X.; Liu, H.K.; Zhao, D.; Dou, S.X. Yolk-Shell Silicon-Mesoporous Carbon Anode with Compact Solid Electrolyte Interphase Film for Superior Lithium-Ion Batteries. Nano Energy 2015, 18, 133–142. [Google Scholar] [CrossRef]
- Jin, B.; Liao, L.; Shen, X.; Mei, Z.; Du, Q.; Liang, L.; Lei, B.; Du, J. Advancement in Research on Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries. Metals 2025, 15, 386. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Q.; Li, X.; Tan, X.; Liu, Q.; Lu, X.; Cao, B.; Yuan, X.; Li, Y.; Shen, L.; et al. Spheres of Graphene and Carbon Nanotubes Embedding Silicon as Mechanically Resilient Anodes for Lithium-Ion Batteries. Nano Lett. 2022, 22, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Y.; Mo, Z.; Lei, X.; Xie, X.; Xue, X.; Qin, H.; Jiang, H. Research Progress of Silicon-Based Anode Materials for Lithium-Ion Batteries. RSC Adv. 2025, 15, 10731–10753. [Google Scholar] [CrossRef] [PubMed]
- Vorauer, T.; Schöggl, J.; Sanadhya, S.G.; Poluektov, M.; Widanage, W.D.; Figiel, L.; Schädler, S.; Tordoff, B.; Fuchsbichler, B.; Koller, S.; et al. Impact of Solid-Electrolyte Interphase Reformation on Capacity Loss in Silicon-Based Lithium-Ion Batteries. Commun. Mater. 2023, 4, 44. [Google Scholar] [CrossRef]
- Li, J.; Fan, S.; Xiu, H.; Wu, H.; Huang, S.; Wang, S.; Yin, D.; Deng, Z.; Xiong, C. TiO2-Coated Silicon Nanoparticle Core–Shell Structure for High-Capacity Lithium-Ion Battery Anode Materials. Nanomaterials 2023, 13, 1144. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liu, Z.; Leung, K.; Chen, L.-Q.; Lu, P.; Qi, Y. Connecting the Irreversible Capacity Loss in Li-Ion Batteries with the Electronic Insulating Properties of Solid Electrolyte Interphase (SEI) Components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Wang, T.; Liu, Y.; Qiao, Y.; Lu, X.; Qi, M.; Jin, Z. Fluorinated MXene-engineered LiF-rich Solid Electrolyte Interphase and Hierarchical Confinement Strategy Enabling High Performance Micro-sized Silicon Anodes. Nano Res. 2025. [Google Scholar] [CrossRef]
- Toki, G.F.I.; Hossain, M.K.; Rehman, W.U.; Manj, R.Z.A.; Wang, L.; Yang, J. Recent Progress and Challenges in Silicon-Based Anode Materials for Lithium-Ion Batteries. Ind. Chem. Mater. 2024, 2, 226–269. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Liu, Y.; Wang, H.; Jin, Z. Synergistic Engineering of Micron-sized Porous Silicon Anodes via Ge Doping and Liquid Metal Alloy Modification for High-energy-density Lithium-ion Batteries. J. Mater. Chem. A 2025, 13, 14346–14352. [Google Scholar] [CrossRef]
- Khan, M.; Yan, S.; Ali, M.; Mahmood, F.; Zheng, Y.; Li, G.; Liu, J.; Song, X.; Wang, Y. Innovative Solutions for High Performance Silicon Anodes in Lithium Ion Batteries: Overcoming Challenges and Real World Applications. Nano-Micro Lett. 2024, 16, 179. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Electrospun Silicon Nanoparticle/Porous Carbon Hybrid Nanofibers for Lithium-Ion Batteries. Small 2013, 9, 2684–2688. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Sun, C.; Wang, Z.; Li, Y.; Zhang, D. Durable Flexible Dual-layer and Free-standing Silicon/Carbon Composite Anode for Lithium-ion Batteries. J. Alloys Compd. 2023, 932, 167687. [Google Scholar] [CrossRef]
- Li, P.; Miao, C.; Yi, D.; Wei, Y.; Chen, T.; Wu, W. Pomegranate like Silicon–carbon Composites Prepared from Lignin-derived Phenolic Resins as Anode Materials for Lithium-ion Batteries. New J. Chem. 2023, 47, 16855–16863. [Google Scholar] [CrossRef]
- Ma, L.; Fu, X.; Zhao, F.; Yu, L.; Su, W.; Wei, L.; Tang, G.; Wang, Y.; Wu, F.; Guo, X. Microsized Silicon/Carbon Composite Anodes through In Situ Polymerization of Phenolic Resin onto Silicon Microparticles for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 4989–4999. [Google Scholar] [CrossRef]
- Xie, J.; Tong, L.; Su, L.; Xu, Y.; Wang, L.; Wang, Y. Core-shell Yolk-shell Si@C@Void@C Nanohybrids as Advanced Lithium Ion Battery Anodes with Good Electronic Conductivity and Corrosion Resistance. J. Power. Sources 2017, 342, 529–536. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Gang, Y.; Garakani, M.A.; Abouali, S.; Huang, J.-Q.; Kim, J.-K. Carbon-coated Mesoporous Silicon Microsphere Anodes with Greatly Reduced Volume Expansion. J. Mater. Chem. A 2016, 4, 6098–6106. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Zhang, S.; Cao, G.; Zhao, X. Scalable Preparation of Silicon@Graphite/Carbon Microspheres as High-performance Lithium-ion Battery Anode Materials. RSC Adv. 2016, 6, 69882–69888. [Google Scholar] [CrossRef]
- Su, H.; Li, X.; Liu, C.; Shang, Y.; Liu, H. Scalable Synthesis of Micrometer-sized Porous Silicon/carbon Composites for High-stability Lithium-ion Battery Anodes. Chem. Eng. J. 2023, 451, 138394. [Google Scholar] [CrossRef]
- Chen, Y.; Du, N.; Zhang, H.; Yang, D. Porous Si@C Coaxial Nanotubes: Layer-by-layer Assembly on ZnO Nanorod Templates and Application to Lithium-ion Batteries. CrystEngComm 2017, 19, 1220–1229. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Zhang, H.; He, Y.-B.; Li, B.; Ke, L.; Lv, W.; Du, H.; Yang, Q.-H.; Kang, F. Multilayered Silicon Embedded Porous Carbon/Graphene Hybrid Film as a High Performance Anode. Carbon 2015, 84, 434–443. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Si, W.; Xi, L.; Eichler, B.; Yan, C.; Schmidt, O.G. Sandwich Nanoarchitecture of Si/Reduced Graphene Oxide Bilayer Nanomembranes for Li-Ion Batteries with Long Cycle Life. ACS Nano 2015, 9, 1198–1205. [Google Scholar] [CrossRef]
- Sheem, J.M.; Koo, J.K.; Ha, C.; Kim, Y.M.; Kim, Y.U.; Nah, J.H.; Kim, Y.-J. Graphene-Coated Si/C Composites for High-Density Electrodes: Mitigating Silicon Degradation and Enhancing Cycle Life in Lithium-Ion Batteries. Appl. Surf. Sci. Adv. 2025, 26, 100715. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Wang, W.; Zhang, Y.; Yu, N.; Lu, C. Silicon/Carbon Nanotubes Anode for Lithium-Ion Batteries: Synthesis, Interface and Electrochemical Performance. Surf. Interfaces 2024, 48, 104223. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, Y.; Chang, A.-Y.; Liao, Y.; Zhang, S.; Wen, X.; Wang, S. Nanofiber-in-Microfiber Carbon/Silicon Composite Anode with High Silicon Content for Lithium-Ion Batteries. Carbon 2023, 203, 436–444. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xia, X.H.; Wang, X.L.; Mai, Y.J.; Shi, S.J.; Tang, Y.Y.; Li, L.; Tu, J.P. Silicon/Graphene-Sheet Hybrid Film as Anode for Lithium Ion Batteries. Electrochem. Commun. 2012, 23, 17–20. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, C.; Fu, A.; Chen, L.; Pei, F.; He, Y.; Fang, X.; Qu, B.; Chen, X.; Ng, A.M.C.; et al. A Three-Dimensional Network of Graphene/Silicon/Graphene Sandwich Sheets as Anode for Li-Ion Battery. Thin Solid Films 2020, 693, 137702. [Google Scholar] [CrossRef]
- Cong, R.; Choi, J.-Y.; Song, J.-B.; Jo, M.; Lee, H.; Lee, C.-S. Characteristics and Electrochemical Performances of Silicon/Carbon Nanofiber/Graphene Composite Films as Anode Materials for Binder-Free Lithium-Ion Batteries. Sci. Rep. 2021, 11, 139. [Google Scholar] [CrossRef]
- Güneş, F. A Direct Synthesis of Si-Nanowires on 3D Porous Graphene as a High Performance Anode Material for Li-Ion Batteries. RSC Adv. 2016, 6, 1678–1685. [Google Scholar] [CrossRef]
- Gohier, A.; Laik, B.; Kim, K.H.; Maurice, J.L.; Pereira-Ramos, J.P.; Cojocaru, C.S.; Van, P.T. High-Rate Capability Silicon Decorated Vertically Aligned Carbon Nanotubes for Li-Ion Batteries. Adv. Mater. 2012, 24, 2592–2597. [Google Scholar] [CrossRef]
- Liu, W.; Su, S.; Wang, Y.; Wang, H.; Wang, F.; Wang, G.; Qu, M.; Peng, G.; Xie, Z. Constructing a Stable Conductive Network for High-Performance Silicon-Based Anode in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 10703–10713. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, N.; Lin, W.; Wang, H.; Li, J.; Ma, L.; Wang, X.; Zhang, D.; Cao, Z. Carbon-Encapsulated Silicon Ordered Nanofiber Membranes as High-Performance Anode Material for Lithium-Ion Batteries. J. Alloys Compd. 2025, 1010, 177012. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Zhao, H.; Cai, C.; Zhang, Z.; Hu, W.; Dong, H.; Ding, S. Embedding Silicon Nanoparticle in Porous Carbon Fiber for Highly Stable Lithium-Ion Battery Anode. Mater. Lett. 2024, 361, 136015. [Google Scholar] [CrossRef]
- She, Z.; Gad, M.; Ma, Z.; Li, Y.; Pope, M.A. Enhanced Cycle Stability of Crumpled Graphene-Encapsulated Silicon Anodes via Polydopamine Sealing. ACS Omega 2021, 6, 12293–12305. [Google Scholar] [CrossRef]
- Cao, Y.; Su, M.; Zhang, X.; Lin, Q.; Bi, T. A 3D Network-Based Hierarchical Porous Carbon/Si@C Composite as Anode Material for Lithium-Ion Battery with Desirable Performance. J. Energy Storage 2024, 98, 112988. [Google Scholar] [CrossRef]
- Kim, S.K.; Chang, H.K.; Kim, C.M.; Yoo, H.D.; Kim, H.S.; Jang, H.D. Fabrication of Ternary Silicon–Carbon Nanotubes–Graphene Composites by Co-Assembly in Evaporating Droplets for Enhanced Electrochemical Energy Storage. J. Alloys Compd. 2018, 751, 43–48. [Google Scholar] [CrossRef]
- Min, K.S.; Kim, K.T.; An, H.L.; Go, Y.H.; Lee, Y.E.; Lim, D.W.; Baeck, S.H. Yolk-Shell-Structured SiO2@N,P Co-Doped Carbon Spheres as Highly Stable Anode Materials for Lithium Ion Batteries. J. Power Sources 2022, 543, 231849. [Google Scholar] [CrossRef]
- Pappas, G.S.; Ferrari, S.; Huang, X.; Bhagat, R.; Haddleton, D.M.; Wan, C. Heteroatom Doped-Carbon Nanospheres as Anodes in Lithium Ion Batteries. Materials 2016, 9, 35. [Google Scholar] [CrossRef]
- Schwan, J.; Nava, G.; Mangolini, L. Critical Barriers to the Large Scale Commercialization of Silicon-containing Batteries. Nanoscale Adv. 2020, 2, 4368–4389. [Google Scholar] [CrossRef]
- Kang, H.E.; Ko, J.; Song, S.G.; Yoon, Y.S. Recent Progress in Utilizing Carbon Nanotubes and Graphene to Relieve Volume Expansion and Increase Electrical Conductivity of Si-based Composite Anodes for Lithium-ion Batteries. Carbon 2024, 219, 118800. [Google Scholar] [CrossRef]
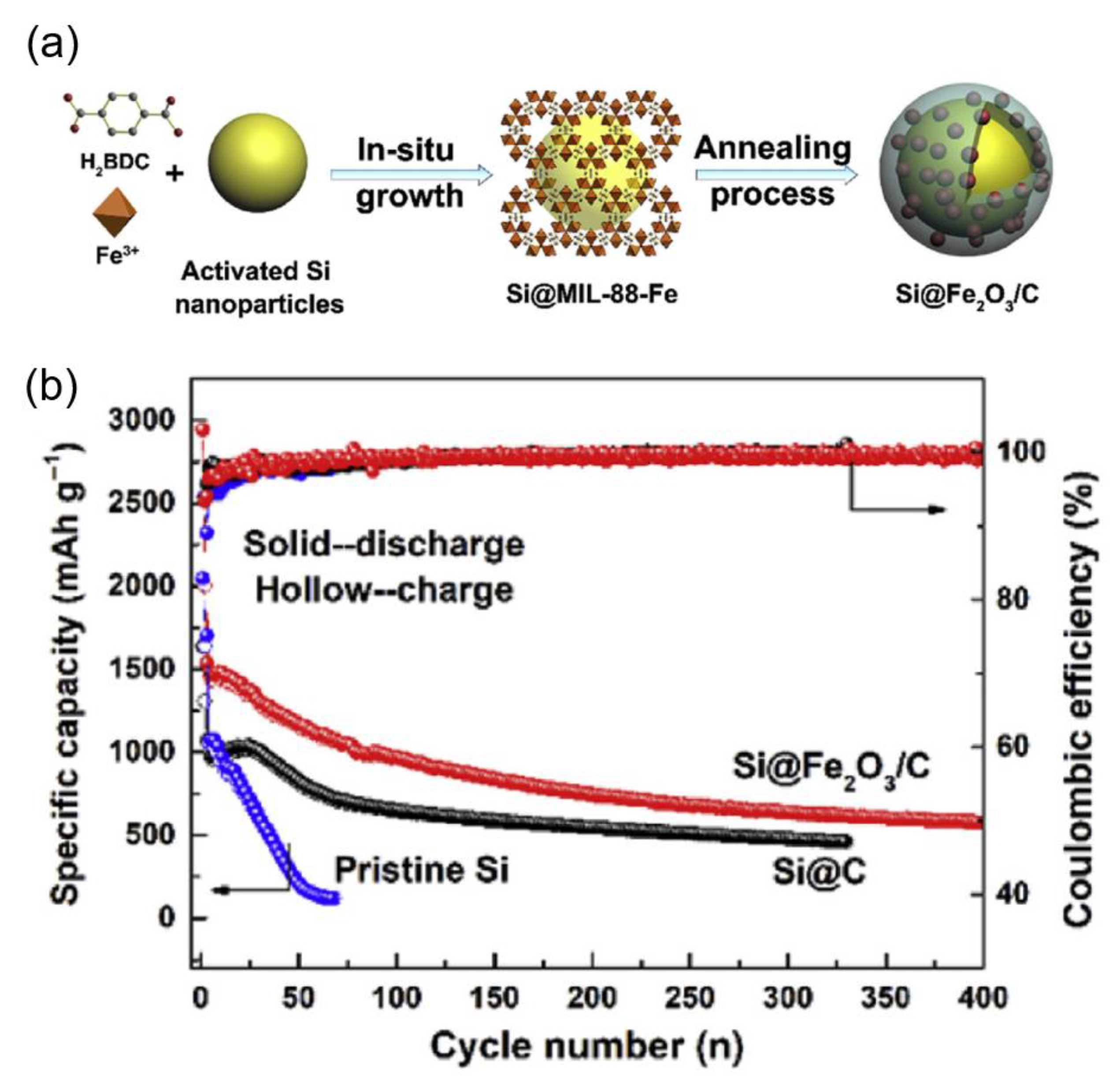
- Chen, Y.; Yan, Y.; Liu, X.; Zhao, Y.; Wu, X.; Zhou, J.; Wang, Z. Porous Si/Fe2O3 Dual Network Anode for Lithium–Ion Battery Application. Nanomaterials 2020, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, R.; Yang, R.; Ji, W.; Jiang, M.; Ding, W.; Peng, L. Mixed Molybdenum Oxides with Superior Performances as an Advanced Anode Material for Lithium-Ion Batteries. Sci. Rep. 2017, 7, 44697. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Lin, R. Improving the Electrochemical Performance of Silicon Materials by SnO2 through Structural Design and Conductivity. Appl. Surf. Sci. 2022, 581, 152230. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, C.L.; Venkateswarlu, M.; Hwang, B.J. Influence of TiO2 Nano-Particles on the Transport Properties of Composite Polymer Electrolyte for Lithium-Ion Batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Li, W.; Wang, L.; Fan, Y.; Jiang, W.; Luo, W.; Wang, Y.; Kong, B.; Selomulya, C.; et al. Amorphous TiO2 Shells: A Vital Elastic Buffering Layer on Silicon Nanoparticles for High-Performance and Safe Lithium Storage. Adv. Mater. 2017, 29, 1700523. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Huang, Z.; Huang, K.; Qi, X.; Zhong, J. Synthesis of Si/TiO2 Core–Shell Nanoparticles as Anode Material for High Performance Lithium Ion Batteries. J. Mater. Sci. Mater. Electron. 2016, 27, 12813–12819. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.; Yang, Z.; Zhang, C.; Dang, F.; Liu, Y.; Jin, F.; Chen, X. A Carbon-Doped Anatase TiO2-Based Flexible Silicon Anode with High-Performance and Stability for Flexible Lithium-Ion Battery. J. Power Sources 2020, 466, 228339. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Kalisvaart, P.; Cui, K.; Kohandehghan, A.; Kupsta, M.; Olsen, B.; Mitlin, D. ALD TiO2 Coated Silicon Nanowires for Lithium Ion Battery Anodes with Enhanced Cycling Stability and Coulombic Efficiency. Phys. Chem. Chem. Phys. 2013, 15, 13646–13657. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sireesha, P.; Shang, J.; McCloy, J.S.; Liu, J.; Zhong, W.-H. Converting Commercial Fe2O3 to Effective Anode Material Using Glucose as “Etching” Agent. Ceram. Int. 2023, 49, 32652–32662. [Google Scholar] [CrossRef]
- Zhou, M.; Gordin, M.L.; Chen, S.; Xu, T.; Song, J.; Lv, D.; Wang, D. Enhanced Performance of SiO/Fe2O3 Composite as an Anode for Rechargeable Li-Ion Batteries. Electrochem. Commun. 2013, 28, 79–82. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Li, Y.; Wu, X.; Jin, C.; Wang, Z. Synthesis of Si/Fe2O3-Anchored rGO Frameworks as High-Performance Anodes for Li-Ion Batteries. Int. J. Mol. Sci. 2021, 22, 11041. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, C.; He, J.; Yang, S.; Liu, Z.; Wang, Q. Fe2O3/C-Modified Si Nanoparticles as Anode Material for High-Performance Lithium-Ion Batteries. J. Alloys Compd. 2019, 795, 284–290. [Google Scholar] [CrossRef]
- Wang, B.; Su, D.; Park, J.; Ahn, H.; Wang, G. Graphene-Supported SnO2 Nanoparticles Prepared by a Solvothermal Approach for an Enhanced Electrochemical Performance in Lithium-Ion Batteries. Nanoscale Res. Lett. 2012, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, S.; Du, M.; Mi, J.; Gao, D.-C.; Hao, L.; Jiang, L.-J.; Luo, M.; Jiang, W.-Q.; Li, F.; et al. Preparation of Double-Shell Si@SnO2@C Nanocomposite as Anode for Lithium-Ion Batteries by Hydrothermal Method. Rare Met. 2023, 42, 2972–2981. [Google Scholar] [CrossRef]
- Ma, T.; Yu, X.; Li, H.; Zhang, W.; Cheng, X.; Zhu, W.; Qiu, X. High Volumetric Capacity of Hollow Structured SnO2@Si Nanospheres for Lithium-Ion Batteries. Nano Lett. 2017, 17, 3959–3964. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Wei, B.; Ding, K.; Zhang, Y.; Shi, X.; Zhou, J. A Novel Composite Anode Material of Si–SnO2–Graphene Prepared in Air for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 11701–11714. [Google Scholar] [CrossRef]
- Han, J.; Kong, D.; Lv, W.; Tang, D.-M.; Han, D.; Zhang, C.; Liu, D.; Xiao, Z.; Zhang, X.; Xiao, J.; et al. Caging Tin Oxide in Three-dimensional Graphene Networks for Superior Volumetric Lithium Storage. Nat. Commun. 2018, 9, 402. [Google Scholar] [CrossRef]
- Yuca, N.; Kalafat, I.; Guney, E.; Cetin, B.; Taskin, O.S. Self-Healing Systems in Silicon Anodes for Li-Ion Batteries. Materials 2022, 15, 2392. [Google Scholar] [CrossRef]
- Wenelska, K.; Ottmann, A.; Schneider, P.; Thauer, E.; Klingeler, R.; Mijowska, E. Hollow Carbon Sphere/Metal Oxide Nanocomposites Anodes for Lithium-Ion Batteries. Energy 2016, 103, 100–106. [Google Scholar] [CrossRef]
- Huertas, Z.C.; Settipani, D.; Flox, C.; Morante, J.R.; Kallio, T.; Biendicho, J.J. High Performance Silicon Electrode Enabled by Titanicone Coating. Sci. Rep. 2022, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yang, H.; Chen, G.Z. Enhanced Performance of Silicon Negative Electrodes Composited with Titanium Carbide Based MXenes for Lithium-Ion Batteries. Nanoenergy Adv. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Enhanced Reversible Li-ion Storage in Si@Ti3C2 MXene Nanocomposite. Electrochem. Commun. 2018, 97, 16–21. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Xia, Y.; Wu, G.; Chen, C.; Wang, J.; Rao, P.; Dong, A. Facile Electrostatic Assembly of Si@MXene Superstructures for Enhanced Lithium-ion Storage. J. Colloid Interface Sci. 2020, 580, 68–76. [Google Scholar] [CrossRef]
- Zhang, C.J.; Park, S.-H.; Seral-Ascaso, A.; Barwich, S.; McEvoy, N.; Boland, C.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. High Capacity Silicon Anodes Enabled by MXene Viscous Aqueous Ink. Nat. Commun. 2019, 10, 849. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Marriam, I.; Tebyetekerwa, M.; Chen, H.; Chathuranga, H.; Motta, N.; Alarco, J.A.; He, Z.-J.; Zheng, J.-C.; Du, A.; Yan, C. Few-Layer MoS2 Nanosheets with and without Silicon Nanoparticles as Anodes for Lithium-Ion Batteries. J. Mater. Chem. A 2023, 11, 2670–2678. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional Building Blocks for Future Materials and Devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, J.; Chen, X.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Enhanced Cycling Performance of Si–MXene Nanohybrids as Anode for High Performance Lithium Ion Batteries. Chem. Eng. J. 2019, 378, 122212. [Google Scholar] [CrossRef]
- Kong, S.; Liu, C.; Ren, J.; Wang, T.; Geng, X.; Yuan, Y.; Zhao, C.; Zhao, C.; Yang, L. The Synergistic Effect of Cross-Linked and Electrostatic Self-Assembly Si/MXene Composites Anode for Highly Efficient Lithium-Ion Battery. Coatings 2024, 14, 1210. [Google Scholar] [CrossRef]
- Zhou, Y.; Kucheyev, S.O.; Wan, L.F. First-Principles Elucidation of Defect-Mediated Li Transport in Hexagonal Boron Nitride. Phys. Chem. Chem. Phys. 2025, 27, 3997–4003. [Google Scholar] [CrossRef]
- Yadav, D.; Jung, J.-H.; Lee, Y.; Kim, T.Y.-S.; Park, E.; Choi, K.-I.; Cha, J.; Song, W.-J.; Choi, J.-H.; Doo, S.; et al. Enhancement of Coulombic Efficiency and Capacity of Li-Ion Batteries Using a Boron Nitride Nanotubes-Dispersed Electrolyte with High Ionic Conductivity. ACS Mater. Lett. 2023, 5, 2648–2655. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B. Surface-Coating Strategies of Si-Negative Electrode Materials in Lithium-Ion Batteries. Batteries 2024, 10, 327. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition Metal Dichalcogenides (MoS2, MoSe2, WS2 and WSe2) Exfoliation Technique Has Strong Influence upon Their Capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Kawade, U.V.; Ambalkar, A.A.; Panmand, R.P.; Kalubarme, R.S.; Kadam, S.R.; Naik, S.D.; Kulkarni, M.V.; Kale, B.B. Silicon Nanoparticle-Sandwiched Ultrathin MoS2–Graphene Layers as an Anode Material for Li-Ion Batteries. Mater. Chem. Front. 2019, 3, 587–596. [Google Scholar] [CrossRef]
- Wang, J.; Malgras, V.; Sugahara, Y.; Yamauchi, Y. Electrochemical Energy Storage Performance of 2D Nanoarchitectured Hybrid Materials. Nat. Commun. 2021, 12, 3563. [Google Scholar] [CrossRef]
- He, Y.; Shen, X.; Zhang, Y. Layered 2D Materials in Batteries. ACS Appl. Nano Mater. 2024, 7, 27907–27939. [Google Scholar] [CrossRef]
- Ka, N.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Balqis, F.; Eldona, C.; Laksono, B.T.; Aini, Q.; Hamid, F.H.; Wasisto, H.S.; Sumboja, A. Conductive Polymer Frameworks in Silicon Anodes for Advanced Lithium-Ion Batteries. ACS Appl. Polym. Mater. 2023, 5, 4933–4952. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-Ion Battery Anodes by In-Situ Polymerization of Conducting Hydrogel to Conformally Coat Silicon Nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.; Yang, L.; Chen, T.; Li, J.; Pan, H.; Yang, Y.; Wang, Z. Carbon Free Silicon/Polyaniline Hybrid Anodes with 3D Conductive Structures for Superior Lithium-Ion Batteries. Chem. Commun. 2020, 56, 2328–2331. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Wang, Y.; Zhang, T.; Yue, H.; Li, T.; Li, W.; Li, H.; Hao, Y.; Gao, Y. Fabrication of Polypyrrole-Coated Silicon Nanoparticle Composite Electrode for Lithium-Ion Battery. Ionics 2024, 30, 7869–7879. [Google Scholar] [CrossRef]
- Yue, L.; Wang, S.; Zhao, X.; Zhang, L. Nano-Silicon Composites Using Poly(3,4-Ethylenedioxythiophene):Poly(styrenesulfonate) as Elastic Polymer Matrix and Carbon Source for Lithium-Ion Battery Anode. J. Mater. Chem. 2012, 22, 1094–1099. [Google Scholar] [CrossRef]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of Polyaniline Content and Protonating Dopants on Electroconductive Composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef]
- Tu, J.; Hu, L.; Wang, W.; Hou, J.; Zhu, H.; Jiao, S. In-Situ Synthesis of Silicon/Polyaniline Core/Shell and Its Electrochemical Performance for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1916–A1921. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Z.; Mu, D.; Wu, B.; Bi, J.; Zhang, L.; Yang, H.; Wu, H.; Wu, F. Hierarchical Void Structured Si/PANi/C Hybrid Anode Material for High-Performance Lithium-Ion Batteries. Electrochim. Acta 2019, 300, 341–348. [Google Scholar] [CrossRef]
- Eldona, C.; Hawari, N.H.; Hamid, F.H.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. A Free-Standing Polyaniline/Silicon Nanowire Forest as the Anode for Lithium-Ion Batteries. Chem. Asian J. 2022, 17, e202200946. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-J.; Zuo, P.-J.; Cheng, X.-Q.; Xu, Y.-H.; Yin, G.-P. Nano-Silicon/Polyaniline Composite for Lithium Storage. Electrochem. Commun. 2010, 12, 1572–1575. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and Factor Affecting on the Conductivity of Polypyrrole: A Short Review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Guo, Z.P.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Study of Silicon/Polypyrrole Composite as Anode Materials for Li-Ion Batteries. J. Power Sources 2005, 146, 448–451. [Google Scholar] [CrossRef]
- Du, F.-H.; Li, B.; Fu, W.; Xiong, Y.-J.; Wang, K.-X.; Chen, J.-S. Surface Binding of Polypyrrole on Porous Silicon Hollow Nanospheres for Li-Ion Battery Anodes with High Structure Stability. Adv. Mater. 2014, 26, 6145–6150. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, S.; Kim, J.-H. Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode. Polymers 2022, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Wang, J.; Han, K. Micro-Sized Porous Silicon@PEDOT with High Rate Capacity and Stability for Li-Ion Battery Anode. Mater. Lett. 2021, 293, 129712. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef]
- Dhason, M.V.A.; Bhattacharya, I.; Mansour, M.; Lazer, S.J.; Banik, T.; Soyoye, B. Conductive Polymers in Si Anodes for Lithium-Ion Batteries: Advancements, Challenges and Future Aspects. Mater. Today Energy 2025, 51, 101897. [Google Scholar] [CrossRef]
- Maji, R.; Salvador, M.A.; Ruini, A.; Magri, R.; Degoli, E. A First-Principles Study of Self-Healing Binders for Next-Generation Si-Based Lithium-Ion Batteries. Mater. Today Chem. 2023, 29, 101474. [Google Scholar] [CrossRef]
- Hwa, Y.; Kim, W.-S.; Hong, S.-H.; Sohn, H.-J. High Capacity and Rate Capability of Core–Shell Structured Nano-Si/C Anode for Li-Ion Batteries. Electrochim. Acta 2012, 71, 201–205. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Q.; Luo, Z.; Liu, A.; Li, H.; Zhong, M.; Zhang, Z. Sandwich Network Structure Silicon/Carbon Anode Material for Lithium-Ion Batteries Based on Bacterial Cellulose. Ionics 2024, 30, 135–144. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, S.; Mu, D.; Wu, B.; Liu, Q.; Zhao, Z.; Wu, F. A Three-Dimensional Network Structure Si/C Anode for Li-Ion Batteries. J. Mater. Sci. 2017, 52, 10950–10958. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Tang, J.-J.; Yang, J.; Xie, J.; Ma, L.-L. Silicon@Carbon Hollow Core–Shell Heterostructures Novel Anode Materials for Lithium Ion Batteries. Electrochim. Acta 2013, 87, 663–668. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X. Nanosized Core/Shell Silicon@Carbon Anode Material for Lithium Ion Batteries with Polyvinylidene Fluoride as Carbon Source. J. Mater. Chem. 2010, 20, 3216–3220. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Hsieh, C.-C.; Liu, W.-R. Synthesis of Double Core–Shell Carbon/Silicon/Graphite Composite Anode Materials for Lithium-Ion Batteries. Surf. Coat. Technol. 2020, 387, 125528. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, Y.; Qian, Z.; Qu, M.; Liu, Z. An Innovative Strategy for Constructing Multicore Yolk-Shell Si/C Anodes for Lithium-Ion Batteries. J. Colloid Interface Sci. 2025, 684, 678–689. [Google Scholar] [CrossRef]
- Bi, X.; Tang, T.; Shi, X.; Ge, X.; Wu, W.; Zhang, Z.; Wang, J. One-Step Synthesis of Multi-Core–Void@Shell Structured Silicon Anode for High-Performance Lithium-Ion Batteries. Small 2022, 18, 2200796. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Ruan, T.; Gao, T.; Song, R.; Jin, F.; Zhou, Y.; Wang, D.; Liu, H.; Dou, S. Construction of Structure-Tunable Si@Void@C Anode Materials for Lithium-Ion Batteries through Controlling the Growth Kinetics of Resin. ACS Nano 2019, 13, 12219–12229. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Hu, G.; Zhang, Q. Interfacial Self-Assembled Si@SiOx@C Microclusters with High Tap Density for High-Performance Li-Ion Batteries. Mater. Today Energy 2022, 29, 101090. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, H.; Na, R.; Wang, D.; Shan, Z.; Tian, J. Facile Preparation of Void-Buffered Si@TiO2/C Microspheres for High-Capacity Lithium Ion Battery Anodes. Electrochim. Acta 2020, 337, 135841. [Google Scholar] [CrossRef]
- Lee, J.-I.; Ko, Y.; Shin, M.; Song, H.-K.; Choi, N.-S.; Kim, M.G.; Park, S. High-performance Silicon-based Multicomponent Battery Anodes Produced via Synergistic Coupling of Multifunctional Coating Layers. Energy Environ. Sci. 2015, 8, 2075–2084. [Google Scholar] [CrossRef]
- An, W.; He, P.; Xiao, C.; Guo, E.; Pang, C.; He, X.; Ren, J.; Yuan, G.; Du, N.; Yang, D. Hierarchical Carbon Shell Compositing Microscale Silicon Skeleton as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 4976–4985. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, T.; Liu, Y.; Sun, L. Mechanically Robust and Interface-Stable pSi@GO–CNTs@C Anode via Multi-Level Confinement for Long-Cycling Lithium-Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 12920–12928. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, B.; Chen, Y.; Liu, H. Enhanced Electrochemical Performance of Si/C Anode by a Facile Surface Modification Strategy. Appl. Surf. Sci. 2017, 425, 614–621. [Google Scholar]
- Chen, Y.; Huang, J.; Chen, Z.; Zeng, H.; Wu, Z.; Yang, H.; Chen, L.; Sun, Q.; Qian, W. Electrospun Sandwich-Structured C@Si/C@C as Anode for Advanced Lithium-Ion Batteries. Appl. Surf. Sci. 2025, 699, 163139. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Ji, J.; Qiu, B.; Zhang, K.; Liu, Z. Superior Cycling Performance of a Sandwich Structure Si/C Anode for Lithium Ion Batteries. RSC Adv. 2016, 6, 12107–12113. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Zhang, Q.; Feng, L.; Wen, G.; Qin, L.-C.; Wang, D. Using Sandwiched Silicon/Reduced Graphene Oxide Composites as Anodes for Lithium-Ion Batteries. Molecules 2024, 29, 2178. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-Y.; Sung, K.-W.; Ahn, H.-J. Layer-by-Layer-Structured Silicon-Based Electrode Design for Ultrafast Lithium-Ion Batteries. Korean J. Chem. Eng. 2025, 42, 1045–1053. [Google Scholar] [CrossRef]
- Chang, J.; Huang, X.; Zhou, G.; Cui, S.; Hallac, P.B.; Jiang, J.; Hurley, P.T.; Chen, J. Multilayered Si Nanoparticle/Reduced Graphene Oxide Hybrid as a High-Performance Lithium-Ion Battery Anode. Adv. Mater. 2014, 26, 758–764. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Gao, H.; Li, M.; Wan, H.; Kong, X.; Liu, X.; Chen, G.; Chen, Z. Monolithic Layered Silicon Composed of a Crystalline–Amorphous Network for Sustainable Lithium-Ion Battery Anodes. ACS Nano 2024, 18, 15671–15680. [Google Scholar] [CrossRef]
- Di, F.; Gu, X.; Chu, Y.; Li, L.; Geng, X.; Sun, C.; Zhou, W.; Zhang, H.; Zhao, H.; Tao, L.; et al. Enhanced Stability and Kinetic Performance of Sandwich Si Anode Constructed by Carbon Nanotube and Silicon Carbide for Lithium-Ion Battery. J. Colloid Interface Sci. 2024, 670, 204–214. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Q.; Feng, X.; Yan, J.; Wang, L.; He, L.; Zhang, L.; Wang, G. Carbon-Coated Si Nanoparticles Anchored on Three-Dimensional Carbon Nanotube Matrix for High-Energy Stable Lithium-Ion Batteries. Batteries 2023, 9, 118. [Google Scholar] [CrossRef]
- Yang, B.; Liu, F.; Liu, Y.; Dong, J.; Liu, M.; Wang, S.; Zhang, L. Self-Assembled Three-Dimensional Si/Carbon Frameworks as Promising Lithium-Ion Battery Anode. J. Power Sources 2023, 553, 232274. [Google Scholar] [CrossRef]
- Zhang, K.; Xia, Y.; Yang, Z.; Fu, R.; Shen, C.; Liu, Z. Structure-Preserved 3D Porous Silicon/Reduced Graphene Oxide Materials as Anodes for Li-Ion Batteries. RSC Adv. 2017, 7, 24305–24311. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Li, A.; Yu, Y.; Chen, X.; Song, H. Three-Dimensional Hierarchical Porous Structures Constructed by Two-Stage MXene-Wrapped Si Nanoparticles for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 48718–48728. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, X.; Li, X.; Jia, D.; Li, D.; Ma, Z.; Li, J. Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries. Materials 2022, 15, 3190. [Google Scholar] [CrossRef]
- Chen, W.-T.; Muruganantham, R.; Liu, W.-R. Construction of 3D Porous Graphene Aerogel Wrapped Silicon Composite as Anode Materials for High-Efficient Lithium-Ion Storage. Surf. Coat. Technol. 2022, 434, 128147. [Google Scholar] [CrossRef]
- Wang, W.; Favors, Z.; Ionescu, R.; Ye, R.; Hosseini Bay, H.; Ozkan, M.; Ozkan, C.S. Monodisperse Porous Silicon Spheres as Anode Materials for Lithium Ion Batteries. Sci. Rep. 2015, 5, 8781. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Electrospun Carbon Nanofibers Containing Silicon Particles as an Energy-Storage Medium. Carbon 2009, 47, 3219–3226. [Google Scholar] [CrossRef]
- Li, Q.; Yin, L.; Gao, X. Reduction Chemical Reaction Synthesized Scalable 3D Porous Silicon/Carbon Hybrid Architectures as Anode Materials for Lithium Ion Batteries with Enhanced Electrochemical Performance. RSC Adv. 2015, 5, 35598–35607. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Z.-S. Modeling Li-Ion Concentration Distribution and Stress of Porous Electrode Particles Considering Binder and Direct Particle Contact. J. Energy Storage 2021, 44, 103315. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, P.; Li, Z.; Shen, X.; Yu, Y.; Yu, J. Hierarchical Porous Structured Si/C Anode Material for Lithium-Ion Batteries by Dual Encapsulating Layers for Enhanced Lithium-Ion and Electron Transports Rates. Small 2025, 21, 2407276. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z.; et al. Inward Lithium-Ion Breathing of Hierarchically Porous Silicon Anodes. Nat. Commun. 2015, 6, 8844. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bao, P.; Huang, X.; Sun, B.; Wang, G. Hierarchical 3D Mesoporous Silicon@Graphene Nanoarchitectures for Lithium Ion Batteries with Superior Performance. Nano Res. 2014, 7, 85–94. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Gaspard, L.; Au, H.; Mattevi, C.; Titirici, M.-M.; Crespo-Ribadeneyra, M. Nature-Inspired Batteries: From Biomaterials to Biomimetic Design Strategies. Green Chem. 2024, 26, 6944–6958. [Google Scholar] [CrossRef]
- Beydaghi, H.; Abouali, S.; Thorat, S.B.; Del Rio Castillo, A.E.; Bellani, S.; Lauciello, S.; Gentiluomo, S.; Pellegrini, V.; Bonaccorso, F. 3D Printed Silicon–Few Layer Graphene Anode for Advanced Li-Ion Batteries. RSC Adv. 2021, 11, 35051–35060. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Liang, Y.-Z.; Bhat, A.L.; Su, Y.-S. Green Synthesis of Graphene Flake/Silicon Composite Anode for Lithium-Ion Batteries Using a Ball-Mill-Derived Mechanical Transfer Technique. ACS Appl. Energy Mater. 2024, 7, 10574–10583. [Google Scholar] [CrossRef]
- Tiano, A.L.; Koenigsmann, C.; Santulli, A.C.; Wong, S.S. Solution-Based Synthetic Strategies for One-Dimensional Metal-Containing Nanostructures. Chem. Commun. 2010, 46, 8093–8130. [Google Scholar] [CrossRef] [PubMed]
- Hwa, Y.; Kim, W.-S.; Yu, B.-C.; Hong, S.-H.; Sohn, H.-J. Enhancement of the Cyclability of a Si Anode through Co3O4 Coating by the Sol–Gel Method. J. Phys. Chem. C 2013, 117, 7013–7017. [Google Scholar] [CrossRef]
- Ruttert, M.; Holtstiege, F.; Hüsker, J.; Börner, M.; Winter, M.; Placke, T. Hydrothermal-Derived Carbon as a Stabilizing Matrix for Improved Cycling Performance of Silicon-Based Anodes for Lithium-Ion Full Cells. Beilstein J. Nanotechnol. 2018, 9, 2381–2395. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, M.; Lucht, B.L.; Saha, A.; Guduru, P.R.; Bose, A. High Capacity, Stable Silicon/Carbon Anodes for Lithium-Ion Batteries Prepared Using Emulsion-Templated Directed Assembly. ACS Appl. Mater. Interfaces 2014, 6, 4678–4683. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Jiang, X.; Zhao, L.; Wang, P.; Zhang, Y. Rational Design of SiOx Based Anode Materials for Next Generation Lithium-ion Batteries. Mater. Adv. 2024, 5, 896–919. [Google Scholar] [CrossRef]
- Wan, W.; Mai, Y.; Guo, D.; Hou, G.; Dai, X.; Gu, Y.; Li, S.; Wu, F. A Novel Sol-gel Process to Encapsulate Micron Silicon with a Uniformly Ni-doped Graphite Carbon Layer by Coupling for Use in Lithium Ion Batteries. Synth. Met. 2021, 274, 116717. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Guan, P.; Lv, C.; Yang, C.; Han, N.; Wang, X.; Song, G.; Peng, Z. One-Pot Sol-Gel Synthesis of Si/C Yolk-Shell Anodes for High Performance Lithium-Ion Batteries. J. Alloys Compd. 2020, 835, 155135. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal Technology for Smart Materials. Int. Mater. Rev. 2016, 61, 477–509. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Niu, Y.; Ren, J.; Hou, H. Facile Hydrothermal Synthesis of Double Shelled Si@SnO2@C as Advanced Cathode for High-Temperature Lithium Batteries. J. Alloys Compd. 2021, 858, 157661. [Google Scholar] [CrossRef]
- Mery, A.; Chenavier, Y.; Marcucci, C.; Benayad, A.; Alper, J.P.; Dubois, L.; Haon, C.; Herlin-Boime, N.; Sadki, S.; Duclairoir, F. Toward the Improvement of Silicon-Based Composite Electrodes via an In-Situ Si@C-Graphene Composite Synthesis for Li-Ion Battery Applications. Materials 2023, 16, 2451. [Google Scholar] [CrossRef]
- Qin, J.; Wu, M.; Feng, T.; Chen, C.; Tu, C.; Li, X.; Duan, C.; Xia, D.; Wang, D. High Rate Capability and Long Cycling Life of Graphene-Coated Silicon Composite Anodes for Lithium Ion Batteries. Electrochim. Acta 2017, 256, 259–266. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion Method: A Novel Route to Synthesize Organic and Inorganic Nanomaterials: 1st Nano Update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Barbé, C.J.A.; Kong, L. Formation of Silica Nanoparticles in Microemulsions. Langmuir 2007, 23, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fu, L.; Gao, J.; Yang, L.; Wu, Y.; Wu, H. Core-Shell Si/C Nanocomposite as Anode Material for Lithium Ion Batteries. Pure Appl. Chem. 2006, 78, 1889–1895. [Google Scholar] [CrossRef]
- Lajoie, L.; Fabiano-Tixier, A.-S.; Chemat, F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fujisawa, K.; Lin, Z.; Lei, Y.; Mondschein, J.S.; Terrones, M.; Schaak, R.E. Low-Temperature Solution Synthesis of Transition Metal Dichalcogenide Alloys with Tunable Optical Properties. J. Am. Chem. Soc. 2017, 139, 11096–11105. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Xu, J.; Liang, J.; Zhou, L. Microwave–Ultrasound Assisted Synthesis of β-FeOOH and Its Catalytic Property in a Photo-Fenton-Like Process. Ultrason. Sonochem. 2015, 27, 287–295. [Google Scholar] [CrossRef]
- Barrino, F. Hybrid Organic–Inorganic Materials Prepared by Sol–Gel and Sol–Gel-Coating Method for Biomedical Use: Study and Synthetic Review of Synthesis and Properties. Coatings 2024, 14, 425. [Google Scholar] [CrossRef]
- Yu, C.; Li, X.; Ma, T.; Rong, J.; Zhang, R.; Shaffer, J.; An, Y.; Liu, Q.; Wei, B.; Jiang, H. Silicon Thin Films as Anodes for High-Performance Lithium-Ion Batteries with Effective Stress Relaxation. Adv. Energy Mater. 2012, 2, 68–73. [Google Scholar] [CrossRef]
- González, N.; García, T.; Morant, C.; Barrio, R. Fine-Tuning Intrinsic and Doped Hydrogenated Amorphous Silicon Thin-Film Anodes Deposited by PECVD to Enhance Capacity and Stability in Lithium-Ion Batteries. Nanomaterials 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Puig-Pey González, J.; Lamure, A.; Senocq, F. Polyimide (PI) Films by Chemical Vapor Deposition (CVD): Novel Design, Experiments and Characterization. Surf. Coat. Technol. 2007, 201, 9437–9441. [Google Scholar] [CrossRef][Green Version]
- Kwak, W.; Kim, R.; Lee, J.; Park, H.; Ha, J.; Choi, J. Chemical Vapor Deposition Carbon Coating of SiOx Anode for Li-Ion Batteries: Significance of Carbon Precursor Selection and Deposition Temperature. ACS Omega 2025, 10, 2553–2560. [Google Scholar] [CrossRef]
- Keller, C.; Djezzar, Y.; Wang, J.; Karuppiah, S.; Lapertot, G.; Haon, C.; Chenevier, P. Easy Diameter Tuning of Silicon Nanowires with Low-Cost SnO2-Catalyzed Growth for Lithium-Ion Batteries. Nanomaterials 2022, 12, 2601. [Google Scholar] [CrossRef]
- Wang, L.; Li, N.; Chen, H.-S.; Song, W.-L. Influence of Carbon Sources on the Performance of Carbon-Coated Nano-Silicon. Chin. Phys. B 2023, 32, 108201. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Das, S.; Brennhagen, A.; Cavallo, C.; Killi, V.A.-L.K.; Jensen, I.J.T.; Thøgersen, A.; Mæhlen, J.P.; Lai, S.Y.; Nilsen, O.; Koposov, A.Y. Directing SEI Formation on Si-Based Electrodes Using Atomic Layer Deposition. Chem. Commun. 2024, 60, 15011–15014. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Wang, S.-S.; Liu, C.; Wu, D.; Li, A.-D. Atomic Layer Deposition of ZnO/TiO2 Nanolaminates as Ultra-Long Life Anode Material for Lithium-Ion Batteries. Sci. Rep. 2019, 9, 11526. [Google Scholar] [CrossRef]
- Hy, S.; Chen, Y.-H.; Cheng, H.-M.; Pan, C.-J.; Cheng, J.-H.; Rick, J.; Hwang, B.-J. Stabilizing Nanosized Si Anodes with the Synergetic Usage of Atomic Layer Deposition and Electrolyte Additives for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 13801–13807. [Google Scholar] [CrossRef]
- Garg, R.; Gonuguntla, S.; Sk, S.; Iqbal, M.S.; Dada, A.O.; Pal, U.; Ahmadipour, M. Sputtering Thin Films: Materials, Applications, Challenges and Future Directions. Curr. Opin. Solid State Mater. Sci. 2024, 330, 103203. [Google Scholar] [CrossRef]
- Evshchik, E.; Novikov, D.; Levchenko, A.; Nefedkin, S.; Shikhovtseva, A.V.; Bushkova, O.V.; Dobrovolsky, Y.A. Magnetron Sputtering Silicon Thin Film Electrodes for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2018, 13, 2860–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, Z.-L.; Zhang, X.; Li, C. Si@Cu Composite Anode Material Prepared by Magnetron Sputtering for High-Capacity Lithium-Ion Batteries. Int. J. Hydrogen Energy 2022, 47, 4766–4771. [Google Scholar] [CrossRef]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a High-Power Si@Graphite Anode for Lithium Ion Batteries through a Wet Ball Milling Process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef]
- Koraag, P.Y.E.; Firdaus, A.M.; Hawari, N.H.; Refino, A.D.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries 2022, 8, 165. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon–Carbon Composite Anodes from Industrial Battery Grade Silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lan, J.-L.; Xue, M.; Yu, Y.; Yang, X. Two-Step Ball-Milling Synthesis of a Si/SiOx/C Composite Electrode for Lithium Ion Batteries with Excellent Long-Term Cycling Stability. RSC Adv. 2017, 7, 36697–36704. [Google Scholar] [CrossRef]
- Sarwat, S.G. Contamination in Wet-Ball Milling. Powder Metall. 2017, 60, 267–272. [Google Scholar] [CrossRef]
- Gao, P.; Fan, X.; Sun, D.; Zeng, G.; Wang, Q.; Wang, Q. Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants. Water 2024, 16, 1639. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lu, L.; Yap, S.M. Prediction of the Amount of PCA for Mechanical Milling. J. Mater. Process. Technol. 1999, 89–90, 260–265. [Google Scholar] [CrossRef]
- Shang, X.; Wang, X.; Chen, S. Effects of Ball Milling Processing Conditions and Alloy Components on the Synthesis of Cu–Nb and Cu–Mo Alloys. Materials 2019, 12, 1224. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Ban, B.; Shi, J.; Wang, Q.; Chen, J. A Simple Method to Fabricate Size and Porosity Tunable Si by Al–Si Alloy as Lithium Ion Battery Anode Material. Electrochim. Acta 2020, 345, 136242. [Google Scholar] [CrossRef]
- Gomez-Romero, P.; Pokhriyal, A.; Rueda-García, D.; Bengoa, L.N.; González-Gil, R.M. Hybrid Materials: A Metareview. Chem. Mater. 2024, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wild, J.F.; Chen, J.J.; Yang, Y. Modeling Silane Deposition in Nanoporous Carbon for High-Capacity Si/C Composite Anodes. Energy Mater. Adv. 2024, 5, 0111. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Zamfir, M.R.; Duong, L.D.; Lee, Y.H.; Bondavalli, P.; Pribat, D. Alumina-Coated Silicon-Based Nanowire Arrays for High Quality Li-Ion Battery Anodes. J. Mater. Chem. 2012, 22, 24618–24626. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Xiao, F.M.; Tang, R.H.; Sun, T. Synthesis and Electrochemical Performance of Silicon/Carbon Composite Anodes through Ball Milling and Spray Drying Pyrolysis Process. Mater. Sci. Forum 2018, 914, 56–63. [Google Scholar] [CrossRef]
- Si, Q.; Hanai, K.; Ichikawa, T.; Phillipps, M.B.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Improvement of Cyclic Behavior of a Ball-Milled SiO and Carbon Nanofiber Composite Anode for Lithium-Ion Batteries. J. Power Sources 2011, 196, 9774–9779. [Google Scholar] [CrossRef]
- Li, B.; Yao, F.; Bae, J.J.; Chang, J.; Zamfir, M.R.; Le, D.T.; Pham, D.T.; Yue, H.; Lee, Y.H. Hollow Carbon Nanospheres/Silicon/Alumina Core–Shell Film as an Anode for Lithium-Ion Batteries. Sci. Rep. 2015, 5, 7659. [Google Scholar] [CrossRef]
- Morand, L.; Iraki, T.; Dornheim, J.; Sandfeld, S.; Link, N.; Helm, D. Machine Learning for Structure-Guided Materials and Process Design. Mater. Des. 2024, 248, 113453. [Google Scholar] [CrossRef]
- Qin, G.; Jia, Z.; Sun, S.; Wu, H.; Hu, K.; Liu, D.; Gao, Y.; Chen, J. Carbon-Coated Si Nanosheets as Anode Materials for High-Performance Lithium-Ion Batteries. ACS Appl. Nano Mater. 2024, 7, 7595–7604. [Google Scholar] [CrossRef]
- Saint, J.; Morcrette, M.; Larcher, D.; Laffont, L.; Beattie, S.; Pérès, J.-P.; Talaga, D.; Couzi, M.; Tarascon, J.-M. Towards a Fundamental Understanding of the Improved Electrochemical Performance of Silicon–Carbon Composites. Adv. Funct. Mater. 2007, 17, 1765–1774. [Google Scholar] [CrossRef]
- John, J.; Gangaja, B.; Nair, S.V.; Santhanagopalan, D. Conformal Coating of TiO2 Shell on Silicon Nanoparticles for Improved Electrochemical Performance in Li-Ion Battery Applications. Electrochim. Acta 2017, 235, 191–199. [Google Scholar] [CrossRef]
- Basak, S.; Tavabi, A.H.; Dzieciol, K.; Migunov, V.; Arszelewska, V.; Tempel, H.; Kungl, H.; Kelder, E.M.; Wagemaker, M.; George, C.; et al. Operando Transmission Electron Microscopy of Battery Cycling: Thickness Dependent Breaking of TiO2 Coating on Si/SiO2 Nanoparticles. Chem. Commun. 2022, 58, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Feng, K.; Park, H.W.; Hassan, F.M.; Elsayed, A.R.; Yu, A.; Xiao, X.; Chen, Z. Graphene Wrapped Silicon Nanocomposites for Enhanced Electrochemical Performance in Lithium Ion Batteries. Electrochim. Acta 2014, 130, 127–134. [Google Scholar] [CrossRef]
- Costa, A.L.A.; Liebertseder, M.; Gambaryan-Roisman, T.; Esken, D.; Menzel, F. A Comparative Study of Dry-Coated Fumed Metal Oxides for Enhanced Cycling Performance of SiOx/C Anodes in Lithium-Ion Batteries. Electrochem. Commun. 2025, 177, 107941. [Google Scholar] [CrossRef]
- Fan, X.-M.; Zhang, X.-H.; Hu, G.-R.; Zhang, B.; He, Z.-J.; Li, Y.-J.; Zheng, J.-C. Single-Walled Carbon Nanotube as Conductive Additive for SiO/C Composite Electrodes in Pouch-Type Lithium-Ion Batteries. Ionics 2019, 25, 5805–5813. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, J.; Ye, C.; Liu, M.; Wang, C.; Wu, S.; Tong, Q.; Zhu, M.; Gao, F. Strategies for Controlling or Releasing the Influence Due to the Volume Expansion of Silicon inside Si−C Composite Anode for High-Performance Lithium-Ion Batteries. Materials 2022, 15, 4264. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, Z.; Li, J.; Meng, Y.; Huang, X. Evaluation of the Electrochemical and Expansion Performances of the Sn-Si/Graphite Composite Electrode for the Industrial Use. Energy Mater. 2022, 2, 200004. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, L.; Zhong, B.; Qu, G. Research Progress on the Structural Design and Optimization of Silicon Anodes for Lithium-Ion Batteries: A Mini-Review. Coatings 2023, 13, 1502. [Google Scholar] [CrossRef]
- Duan, H.; Xu, H.; Wu, Q.; Zhu, L.; Zhang, Y.; Yin, B.; He, H. Silicon/Graphite/Amorphous Carbon as Anode Materials for Lithium Secondary Batteries. Molecules 2023, 28, 464. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takatsu, M.; Okuno, R.; Kato, A.; Takahashi, M. Nanoporous Silicon Fiber Networks in a Composite Anode for All-Solid-State Batteries with Superior Cycling Performance. Sci. Rep. 2023, 13, 17051. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical Porous Silicon Structures with Extraordinary Mechanical Strength as High-Performance Lithium-Ion Battery Anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Q.; Shi, L.; Wang, Y.; Wang, T.; Liu, D.; He, D. Low-Cost Micron-Scale Silicon-Based Anodes for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 7545–7555. [Google Scholar] [CrossRef]
- Maddipatla, R.; Loka, C.; Choi, W.J.; Lee, K.-S. Nanocomposite of Si/C Anode Material Prepared by Hybrid Process of High-Energy Mechanical Milling and Carbonization for Li-Ion Secondary Batteries. Appl. Sci. 2018, 8, 2140. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, P.; Tian, Y.; Fang, H.; Zhang, Q. One-Step Chemical Vapor Deposition Synthesis of Si NWs@C Core/Shell Anodes without Additional Catalysts by the Oxide-Assisted Growth Mechanism for Lithium-Ion Batteries. Dalton Trans. 2024, 53, 9052–9061. [Google Scholar] [CrossRef]
- Du, C.; Chen, M.; Wang, L.; Yin, G. Covalently-Functionalizing Synthesis of Si@C Core–Shell Nanocomposites as High-Capacity Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 15692–15697. [Google Scholar] [CrossRef]
- Shen, B.H.; Wang, S.; Tenhaeff, W.E. Ultrathin Conformal Polycyclosiloxane Films to Improve Silicon Cycling Stability. Sci. Adv. 2019, 5, eaaw4856. [Google Scholar] [CrossRef]
- Peng, B.; Bao, W.; Sun, K.; Xiao, J. Enhancing Silicon Anode Performance in Lithium-Ion Batteries Through Hybrid Artificial SEI Layer and Prelithiation. Nanomaterials 2025, 15, 690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable High-Capacity and High-Rate Silicon-Based Lithium Battery Anodes upon Two-Dimensional Covalent Encapsulation. Nat. Commun. 2020, 11, 3826. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lombardo, T.; Xu, J.; Ngandjong, A.C.; Franco, A.A. An Experimentally-Validated 3D Electrochemical Model Revealing Electrode Manufacturing Parameters’ Effects on Battery Performance. Energy Storage Mater. 2023, 54, 156–163. [Google Scholar] [CrossRef]



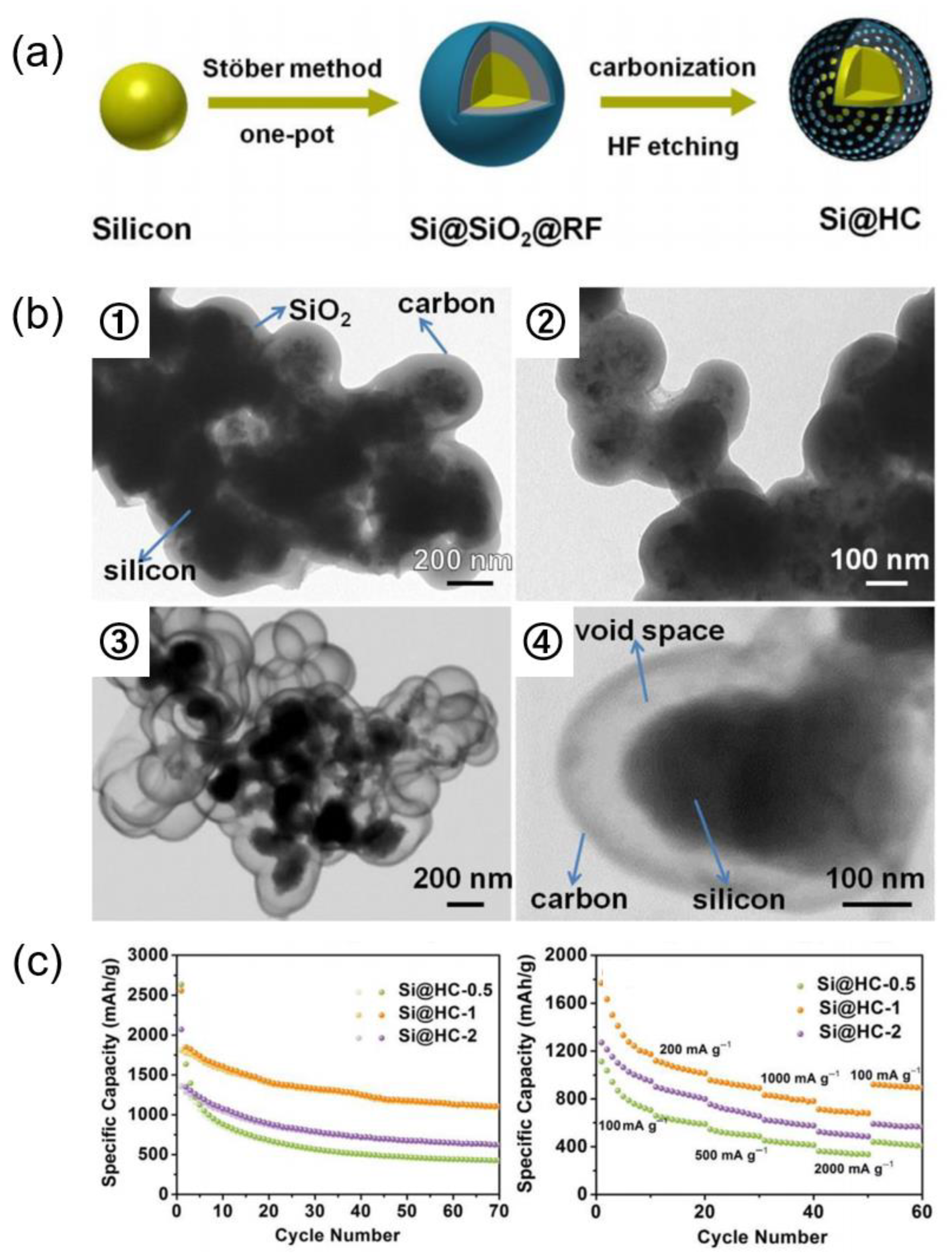
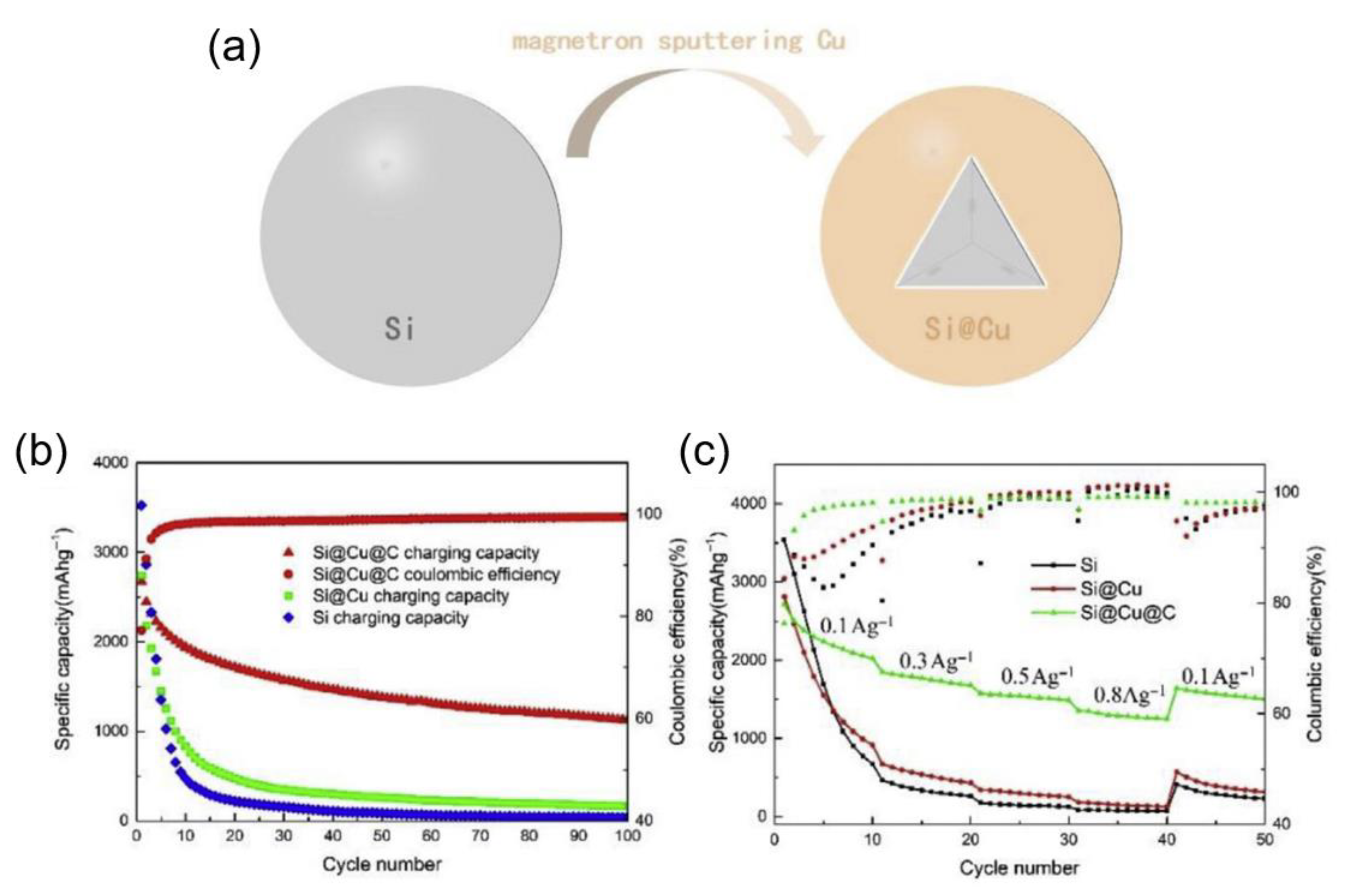
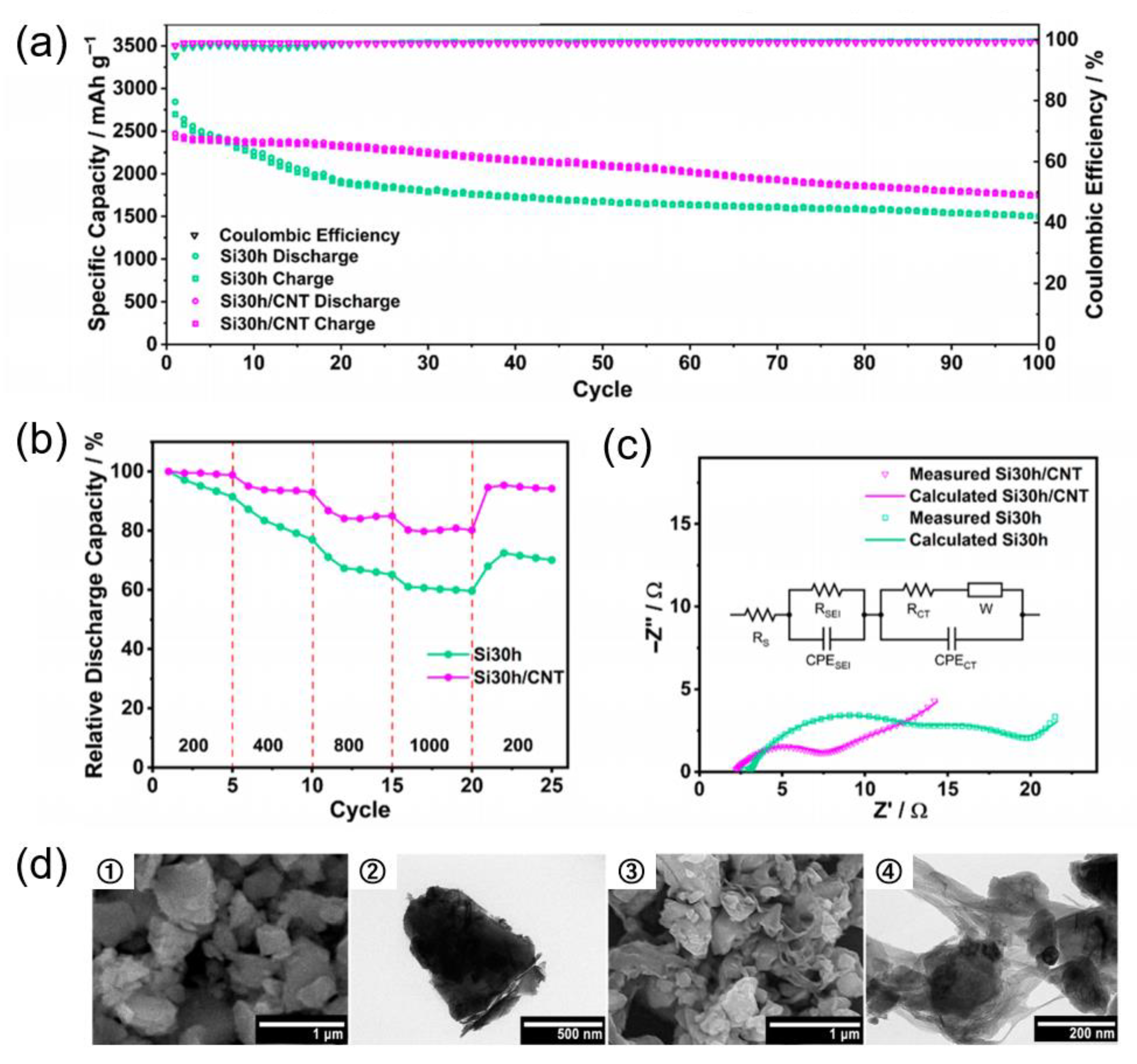
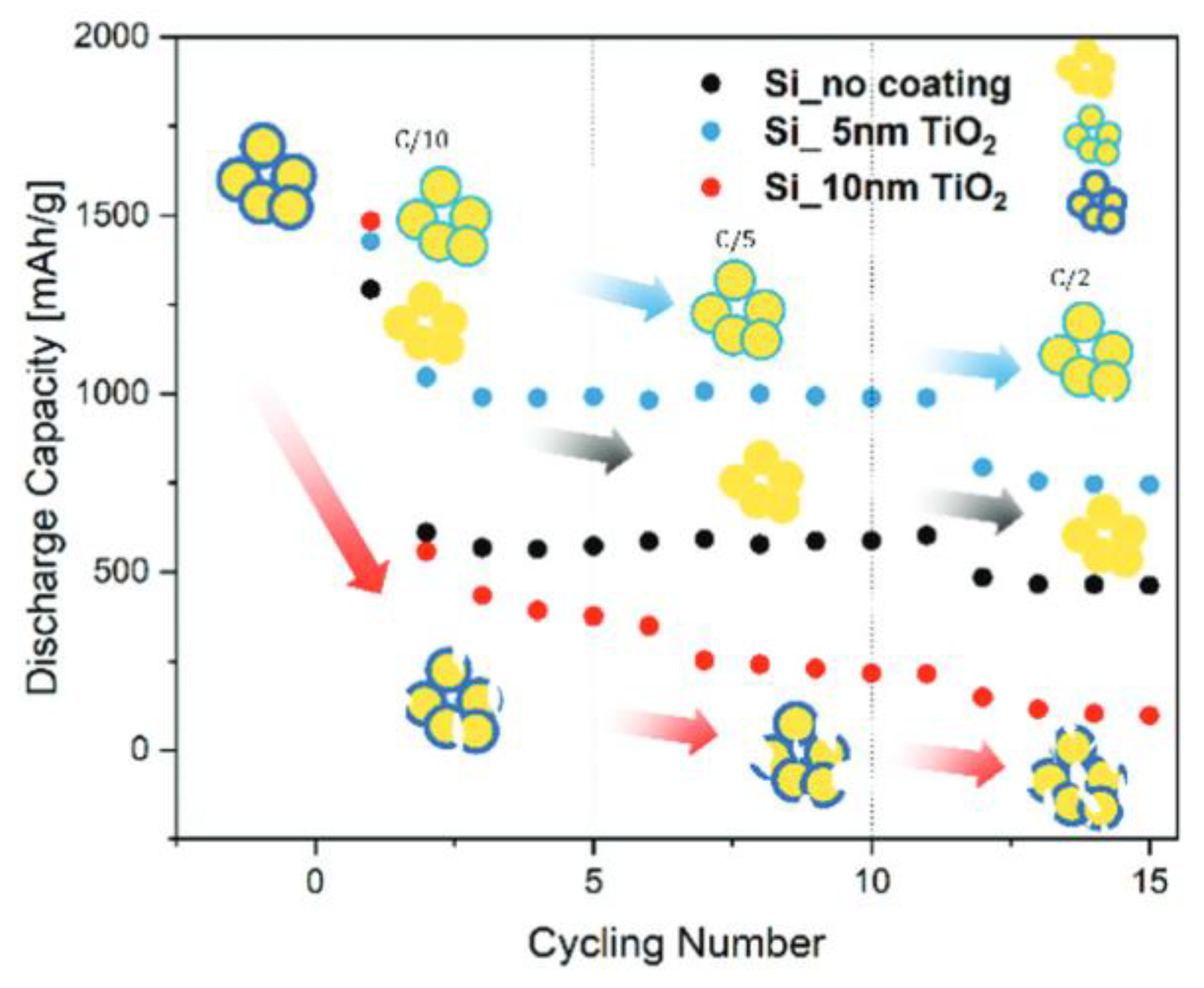
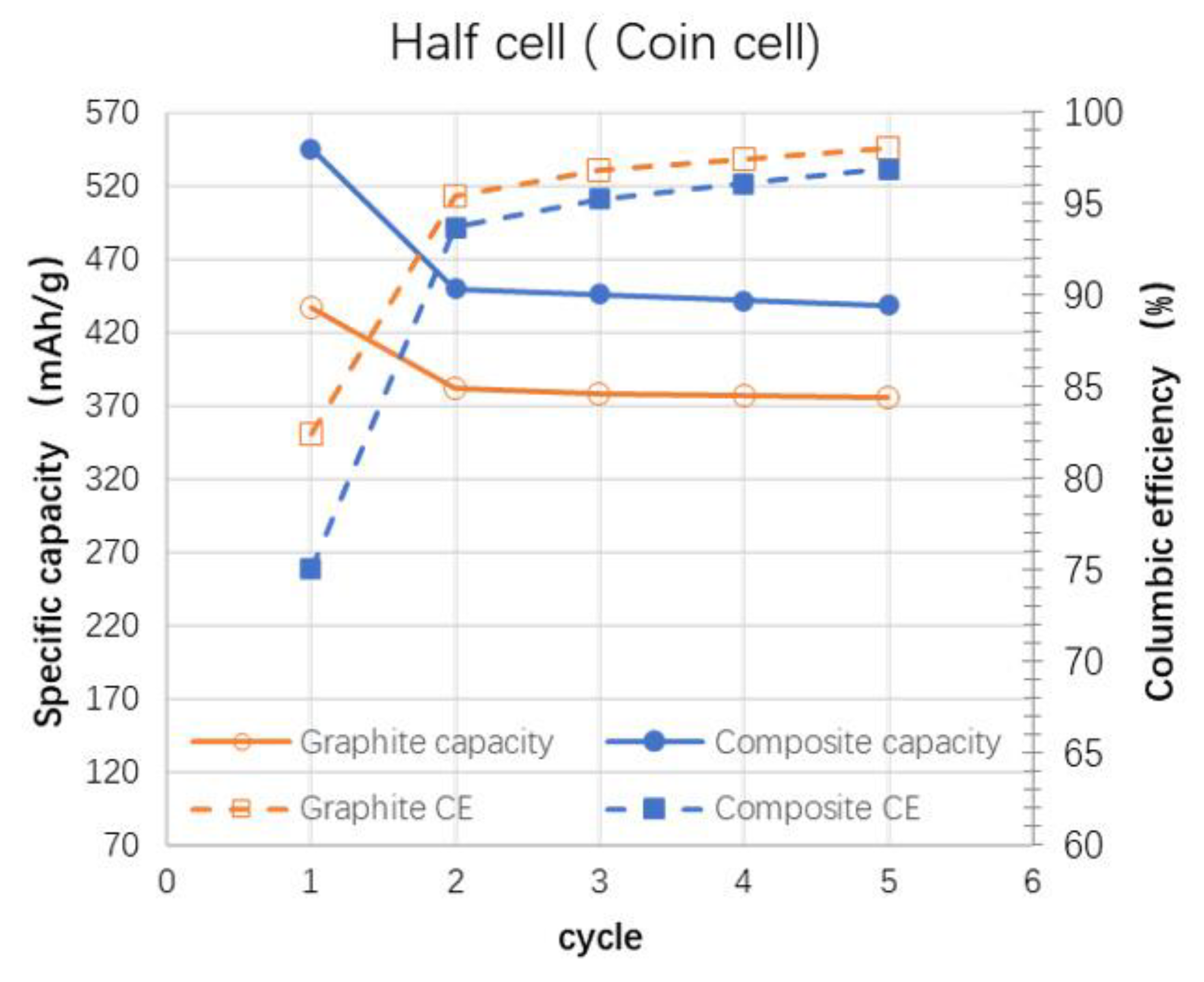
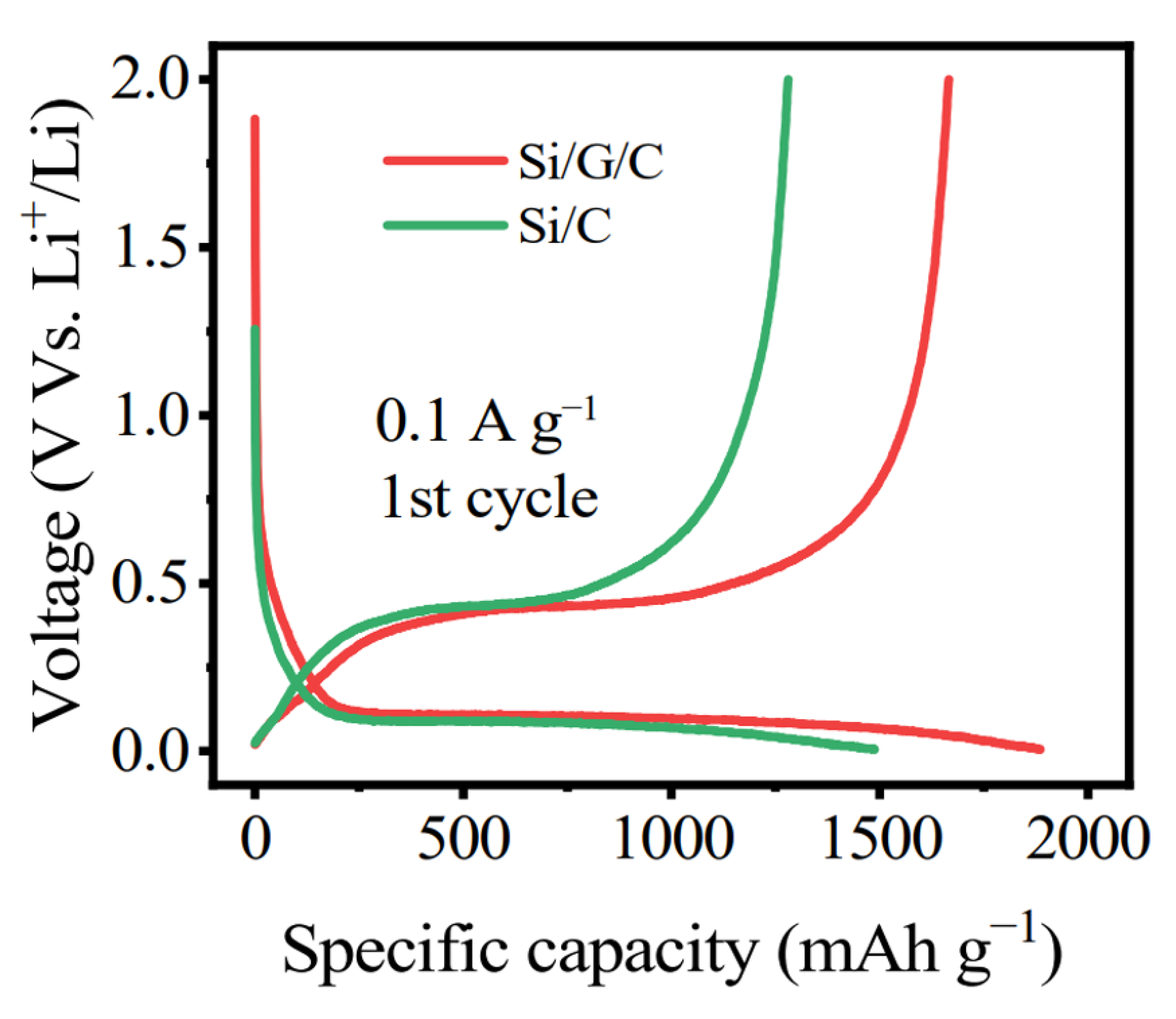
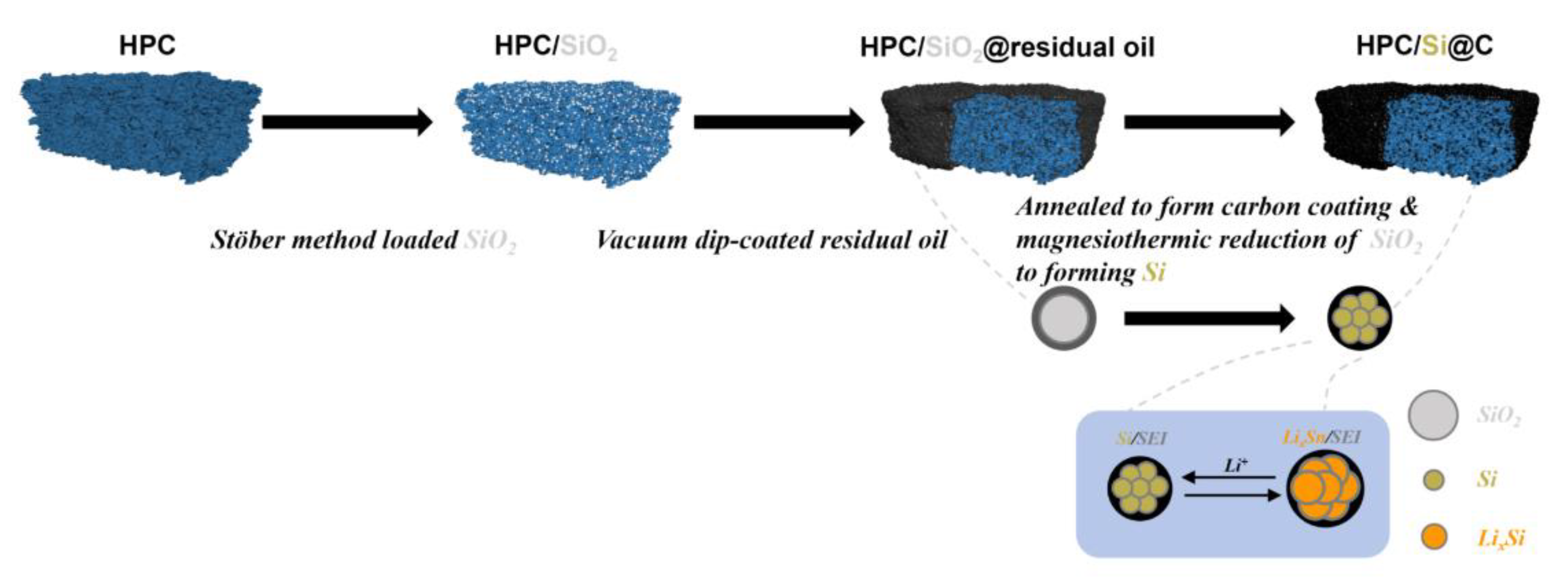
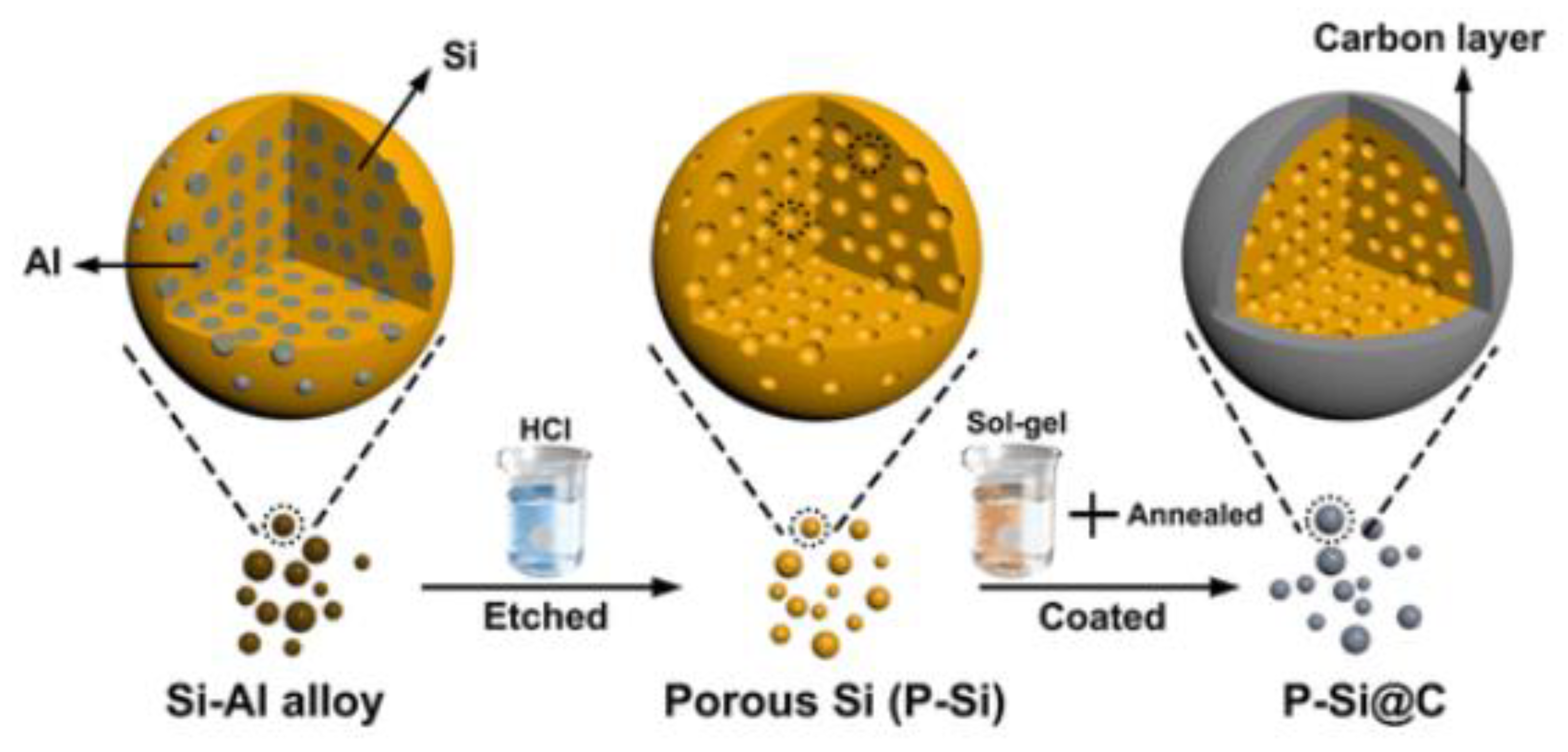
| Si Anodes | Current Density (A/g) | Cycle Number | Remaining Capacity (mAh/g) | Source (1st Author, Year, [Ref. No.]) |
|---|---|---|---|---|
| Silicon-Carbon | ||||
| Si/C | 0.1 | 100 | 941.1 | Zhang, 2023, [42] |
| Si/C | 0.5 | 100 | 605.43 | Li, 2023, [43] |
| Si/C | 2.0 | 400 | 1283 | Ma, 2023, [44] |
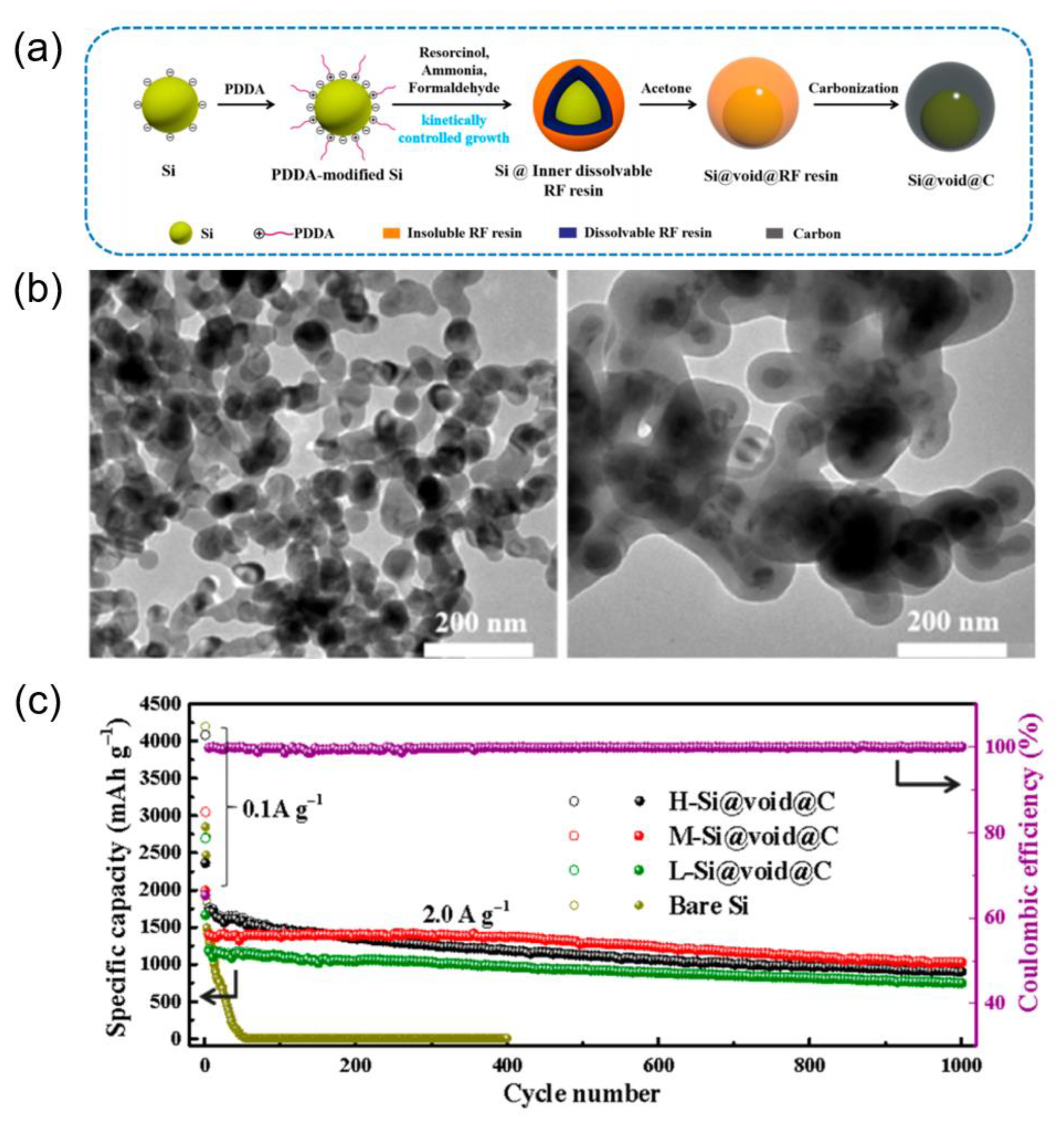
| Si@C@void@C | 0.5 | 50 | 1366 | Xie, 2017, [45] |
| Meso-Si/C | 1.0 | 1000 | 990 | Xu, 2016, [46] |
| Si/graphite/C | 0.5 | 300 | ~400 | Wang, 2016, [47] |
| M-pSi@C | 1.0 | 250 | 1702 | Su, 2023, [48] |
| Porous Si/C nanotubes | 0.2 | 200 | 1300 | Chen, 2017, [49] |
| Si/C/graphene | 0.2 | 100 | 760 | Wu, 2015, [50] |
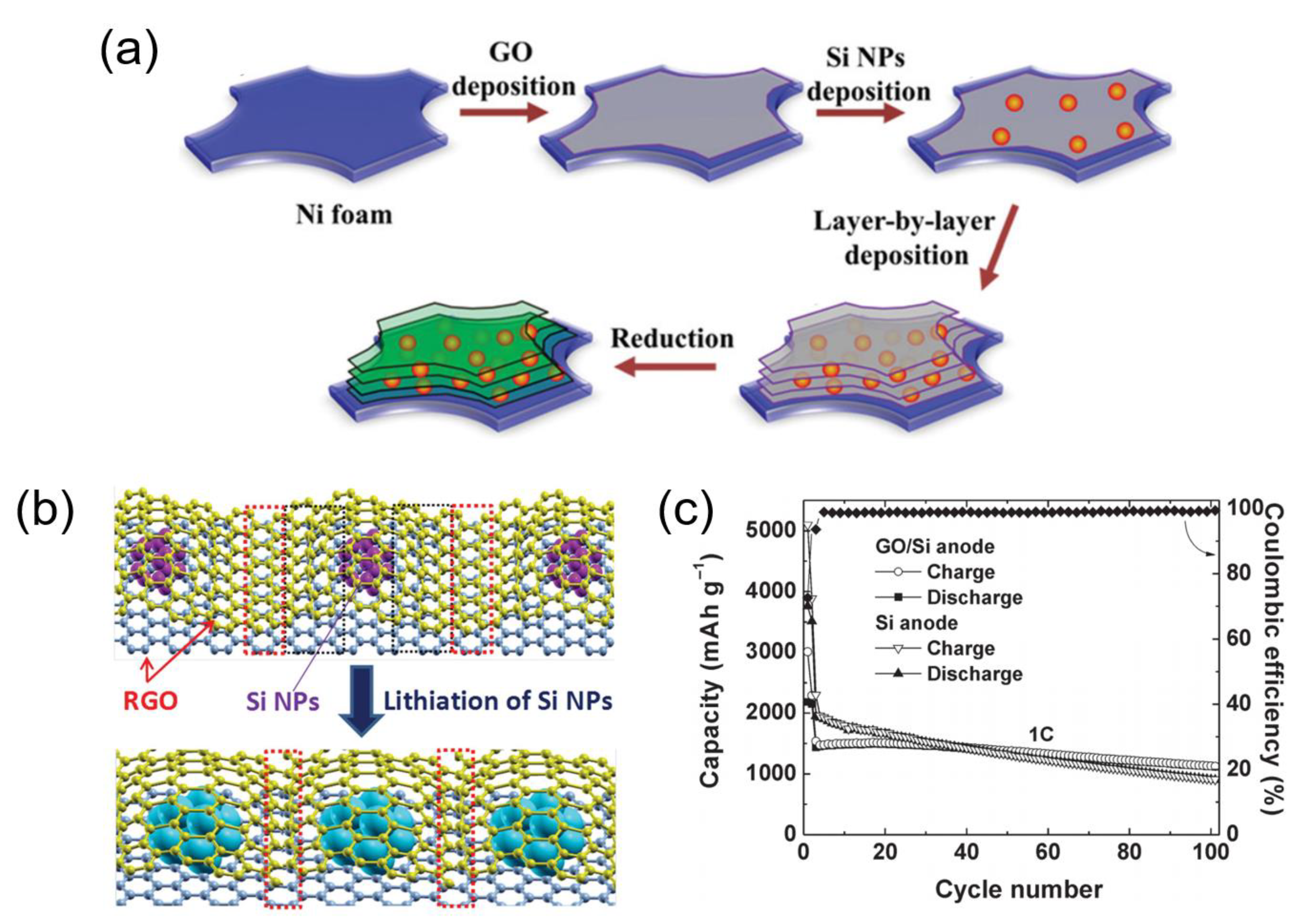
| Si/rGO | 0.1 | 100 | 1433 | Liu, 2015, [51] |
| Si/CNTs | 5.0 | 1000 | 612.3 | Guo, 2024, [53] |
| Si/CNTs | 10 | 100 | 800 | Gohier, 2012, [59] |
| Si@(POH-AOCNTs) | 1.0 | 500 | 1195.8 | Liu, 2024, [60] |
| Si@CNFs | 1.0 | 100 | 658.1 | Zhang, 2025, [61] |
| cpDOPA-crGO-Si | 1.0 | 200 | 1038 | She, 2021, [63] |
| HPC/Si@C | 2.0 | 500 | 358 | Cao, 2024, [64] |
| SiO2@NPC Y.S. | 0.1 | 300 | 705 | Min, 2022, [66] |
| Si Anodes. | Current Density (A/g) | Cycle Number | Remaining Capacity (mAh/g) | Source (1st Author, Year, [Ref. No.]) |
|---|---|---|---|---|
| Silicon-Metal Oxide | ||||
| Porous Si/Fe2O3 | 0.2 | 100 | 697.2 | Chen, 2020, [70] |
| Si@SnO2 | 0.2 | 200 | 1926 | Zhu, 2022, [72] |
| Si@a-TiO2 | 0.42 | 200 | 1720 | Yang, 2017, [74] |
| Si/TiO2 | 0.1 | 100 | 1010.7 | Li, 2016, [75] |
| TiO2/SiNWs | 0.1 | 100 | ~1700 | Lotfabad, 2013, [77] |
| SiO/Fe2O3 | 0.16 | 50 | 1335 | Zhou, 2013, [79] |
| Si/Fe2O3@rGO | 0.2 | 200 | 1744.5 | Yan, 2021, [80] |
| Si@Fe2O3/C | 1.0 | 300 | 680.7 | Wang, 2019, [81] |
| Si-SnO2-graphene | 0.1 | 20 | 350 | Li, 2017, [85] |
| TiGL@Si | 1.0 | 350 | 1200 | Huertas, 2022, [89] |
| Si Anodes | Current Density (A/g) | Cycle Number | Remaining Capacity (mAh/g) | Source (1st Author, Year, [Ref. No.]) |
|---|---|---|---|---|
| Silicon-2D Material | ||||
| Si@Ti3C2 Mxene | 0.2 | 150 | 188 | Kong, 2018, [91] |
| Si@Ti3C2 Mxene | 1.0 | 200 | 1342.8 | Yang, 2020, [92] |
| nSi/MX-N | 1.5 | 70 | 1106 | Zhang, 2019, [93] |
| Si/Mxene | 0.2 | 100 | 2118 | Tian, 2019, [94] |
| MoS2@Si | 0.1 | 300 | 1223 | Marriam, 2023, [95] |
| Si/d-Ti3C2 | 0.5 | 200 | 1130 | Zhu, 2019, [97] |
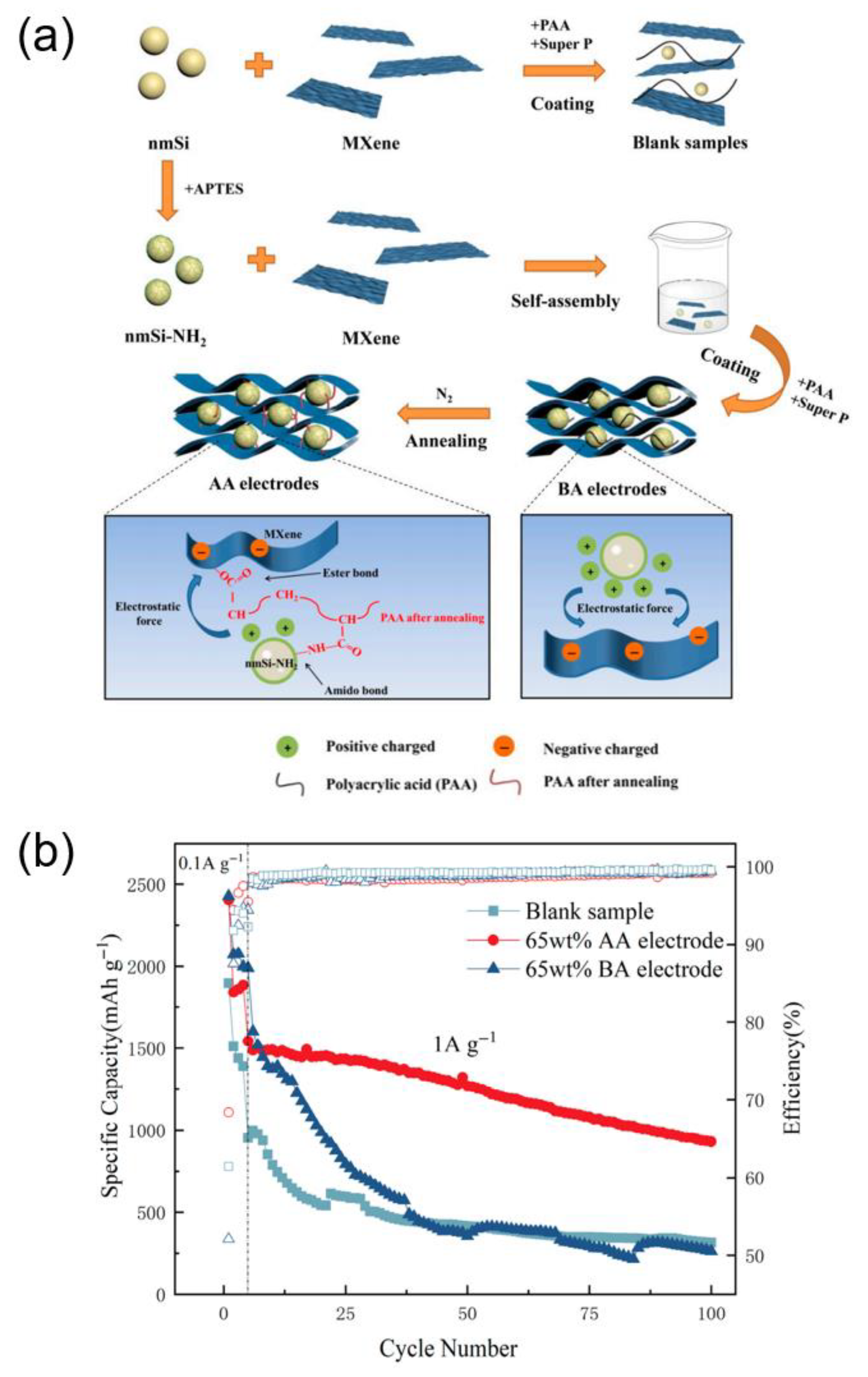
| nmSi-NH2/MXene | 1.0 | 100 | 929.5 | Kong, 2024, [98] |
| Si/MoS2-G | 0.2 | 90 | 923 | Kawade, 2019, [103] |
| Si Anodes | Current Density (A/g) | Cycle Number | Remaining Capacity (mAh/g) | Source (1st Author, Year, [Ref. No.]) |
|---|---|---|---|---|
| Silicon-Conductive Polymer | ||||
| SiNP/PANi | 1.0 | 1000 | 1200 | Wu, 2013, [108] |
| Si-oxalic acid-PANI | 1.0 | 1000 | 610 | Zhou, 2020, [109] |
| Si@Ppy | 1.0 | 500 | 1047 | Zhang, 2024, [110] |
| Si/PANI | 1.0 | 100 | 545.3 | Tu, 2013, [113] |
| Si/PANi/C | 0.1 | 200 | 1470 | Mu, 2019, [114] |
| Si/PANi | 0.1 | 25 | 1840 | Cai, 2010, [116] |
| Si@PEDOT | 0.5 | 100 | 1439.8 | Li, 2021, [121] |
| PEDOT:PSS/SiNP | 1.0 | 100 | 1950 | Higgins, 2016, [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.; Kwon, T. Various Technologies to Mitigate Volume Expansion of Silicon Anode Materials in Lithium-Ion Batteries. Batteries 2025, 11, 346. https://doi.org/10.3390/batteries11090346
Jang J, Kwon T. Various Technologies to Mitigate Volume Expansion of Silicon Anode Materials in Lithium-Ion Batteries. Batteries. 2025; 11(9):346. https://doi.org/10.3390/batteries11090346
Chicago/Turabian StyleJang, Jihun, and Taegyun Kwon. 2025. "Various Technologies to Mitigate Volume Expansion of Silicon Anode Materials in Lithium-Ion Batteries" Batteries 11, no. 9: 346. https://doi.org/10.3390/batteries11090346
APA StyleJang, J., & Kwon, T. (2025). Various Technologies to Mitigate Volume Expansion of Silicon Anode Materials in Lithium-Ion Batteries. Batteries, 11(9), 346. https://doi.org/10.3390/batteries11090346







