Thermal Runaway Hazards of Ternary Lithium-Ion Batteries Under Different Ambient Pressure Environments
Abstract
1. Introduction
2. Experimental Design
2.1. Cell Samples
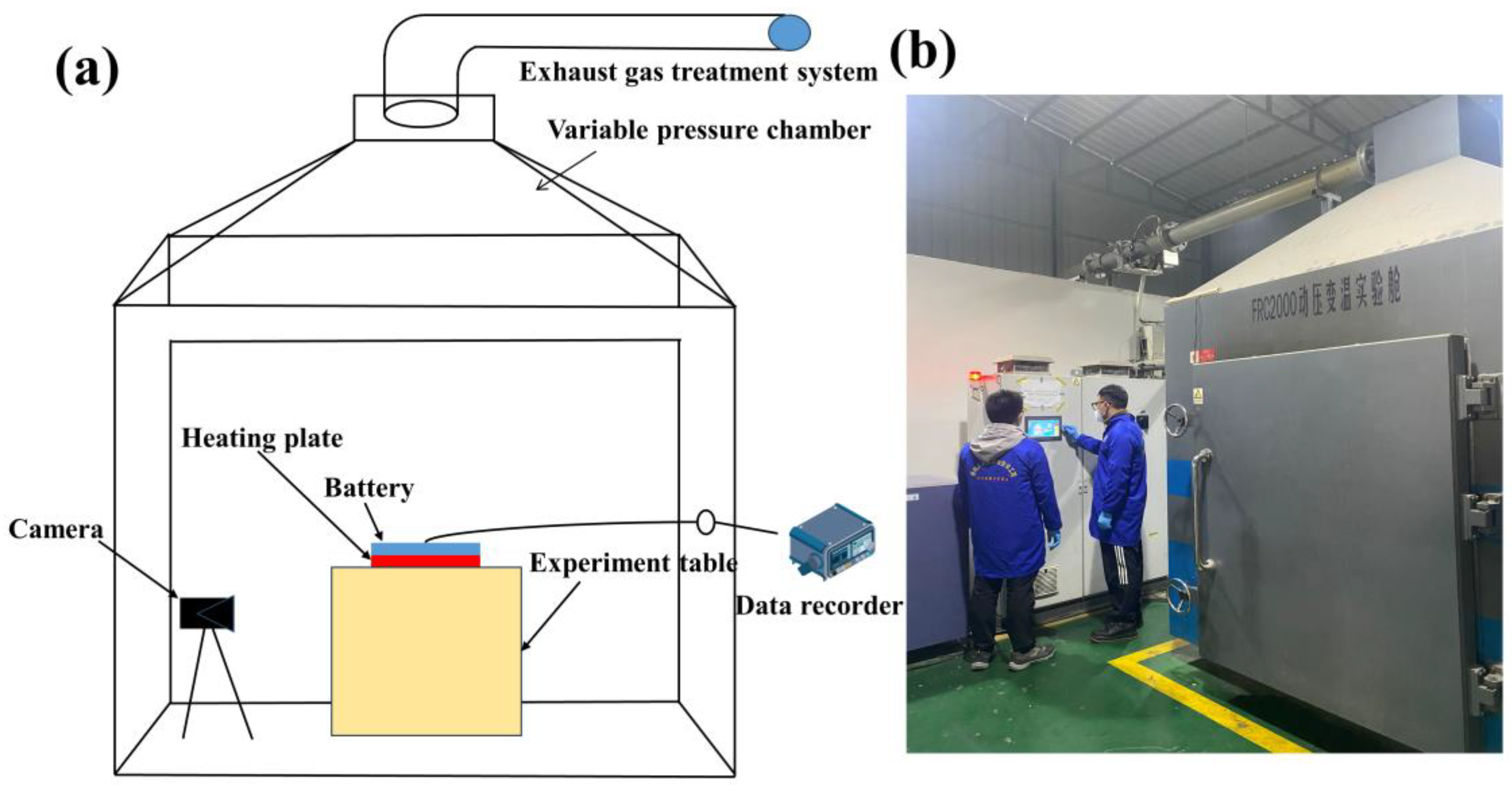
2.2. Experimental Setup
2.3. Materials Characterizations
3. Results and Discussion
3.1. Thermal Characteristic of Lithium-Ion Battery
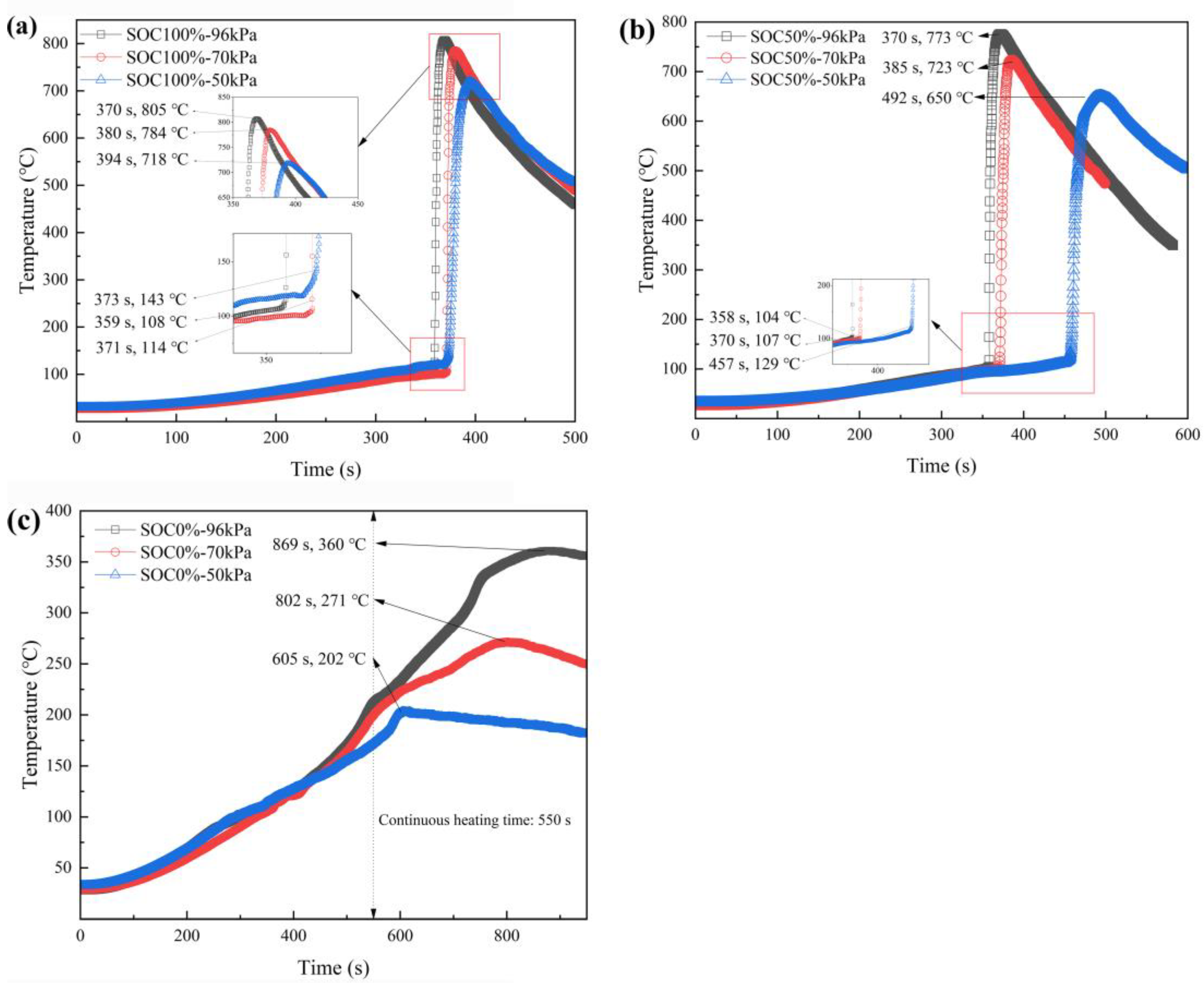
3.2. Characteristic Temperatures of Battery Thermal Runaway
3.2.1. Thermal Runaway Triggering Temperature
3.2.2. Effect of SOCs on Battery Thermal Runaway
3.2.3. Influence of Ambient Pressure on the Thermal Runaway Peak Temperatures
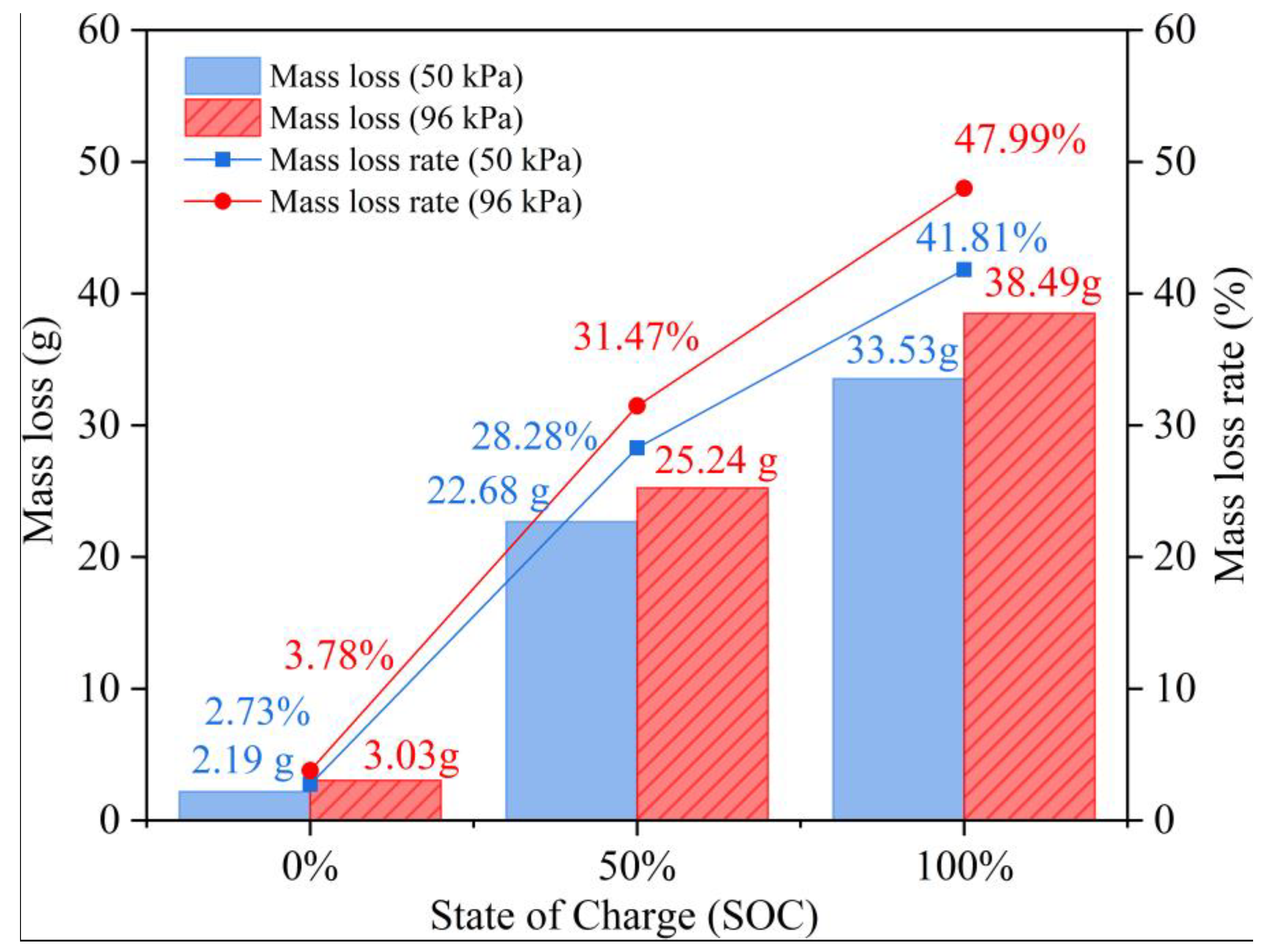
3.3. Effect of Pressure Environments on Battery Thermal Runaway Behavior
- I.
- Initial stage. Preliminary heating of the battery happened.
- II.
- Deformation stage. At this stage, partial irreversible pyrolysis reactions occurred inside the battery, generating large amounts of flammable gas [25]. Combined with the physical vaporization of the electrolyte solvent, internal pressure caused visible deformation of the battery. However, the aluminum laminate film had not yet ruptured, and no large-scale internal short circuit occurred.
- III.
- Jet stage. It was observed that, after the local rupture of aluminum-laminated film, a small amount of smoke was first released, and then sparks were ejected, which led to significant jet behavior. The trigger time of the eruption was negatively correlated with the ambient pressure. The trigger time was 370 s at 100%SOC-50kPa, which was 16 s and 85 s later than those at 50%SOC-96kPa and 50%SOC-50kPa, respectively. Furthermore, the position of the erupt port was random, which made it difficult to predict the flame injection direction. This phenomenon was influenced by many factors, including the geometry of the battery, the location of aluminum film packaging, and the fixing way of battery and heater. In this experiment, the erupt ports mainly appeared on the nearby side of battery tab.
- IV.
- Combustion and explosion stage. As a pressure relief point, the nozzle caused violent combustion and explosion. At this time, the heat production rate of the battery was significantly higher than the heat dissipation capacity, and it reached the critical state of thermal runaway under the action of thermal accumulation and pressure difference. The low ambient pressure delayed the trigger time of combustion and explosion. The explosion intensity of 50%SOC-50kPa was obviously weaker than the other five experiments. Notably, the batteries for SOC0%-96kPa, SOC0%-70kPa, and SOC0%-50kPa scenes did not undergo thermal runaway throughout the entire process, so the full video recording displayed as a black screen. This indicated that, in the fully discharged state, even the low-pressure environment would not trigger the risk of thermal runaway.
- V.
- Injection fire stage. Combustible mixed gas formed by vaporization of the electrolyte continued to burn.
- VI.
- Attenuation stage. Energy release was depleted, the thermal runaway process concluded, and the battery suffered irreversible damage overall.
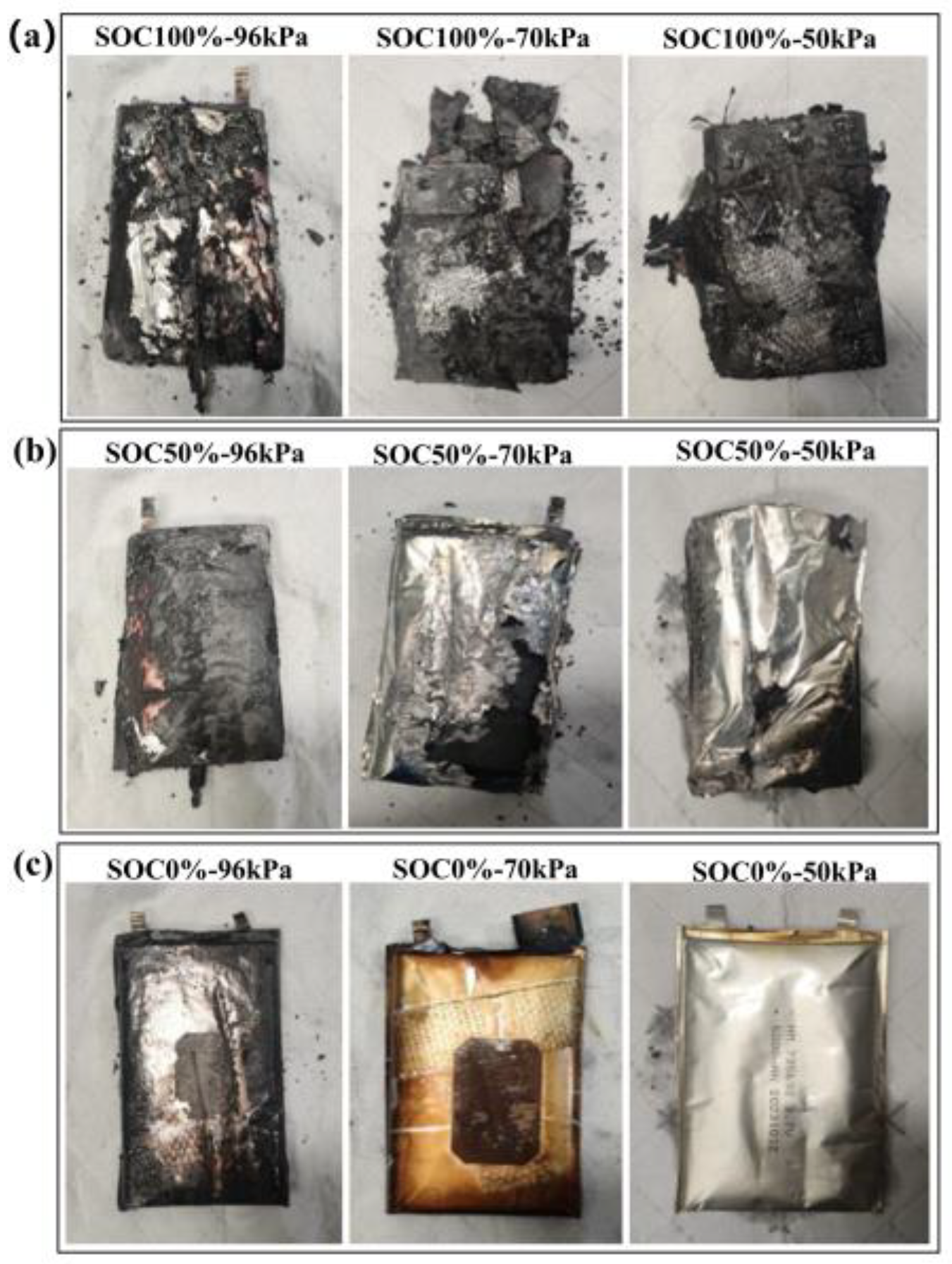
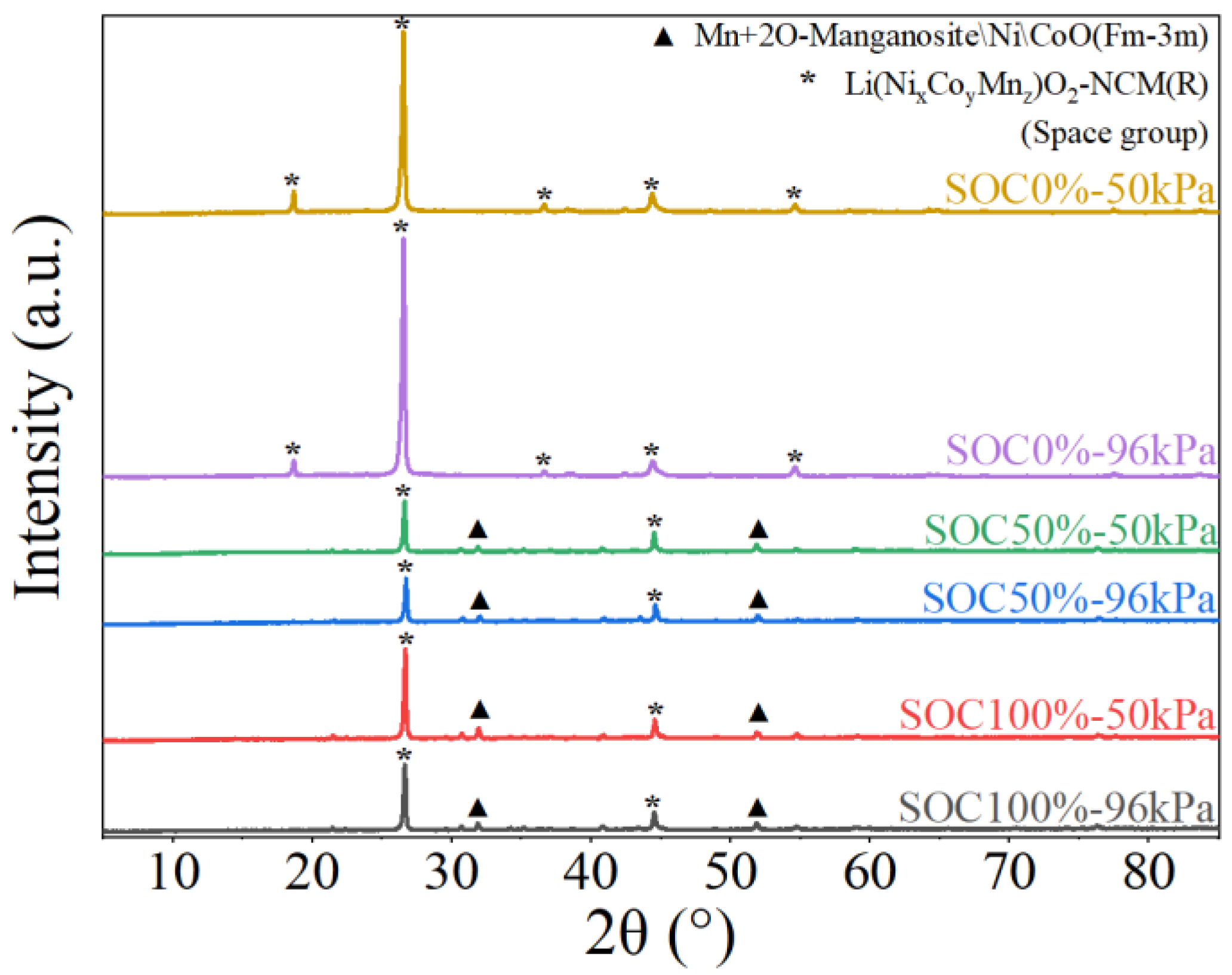
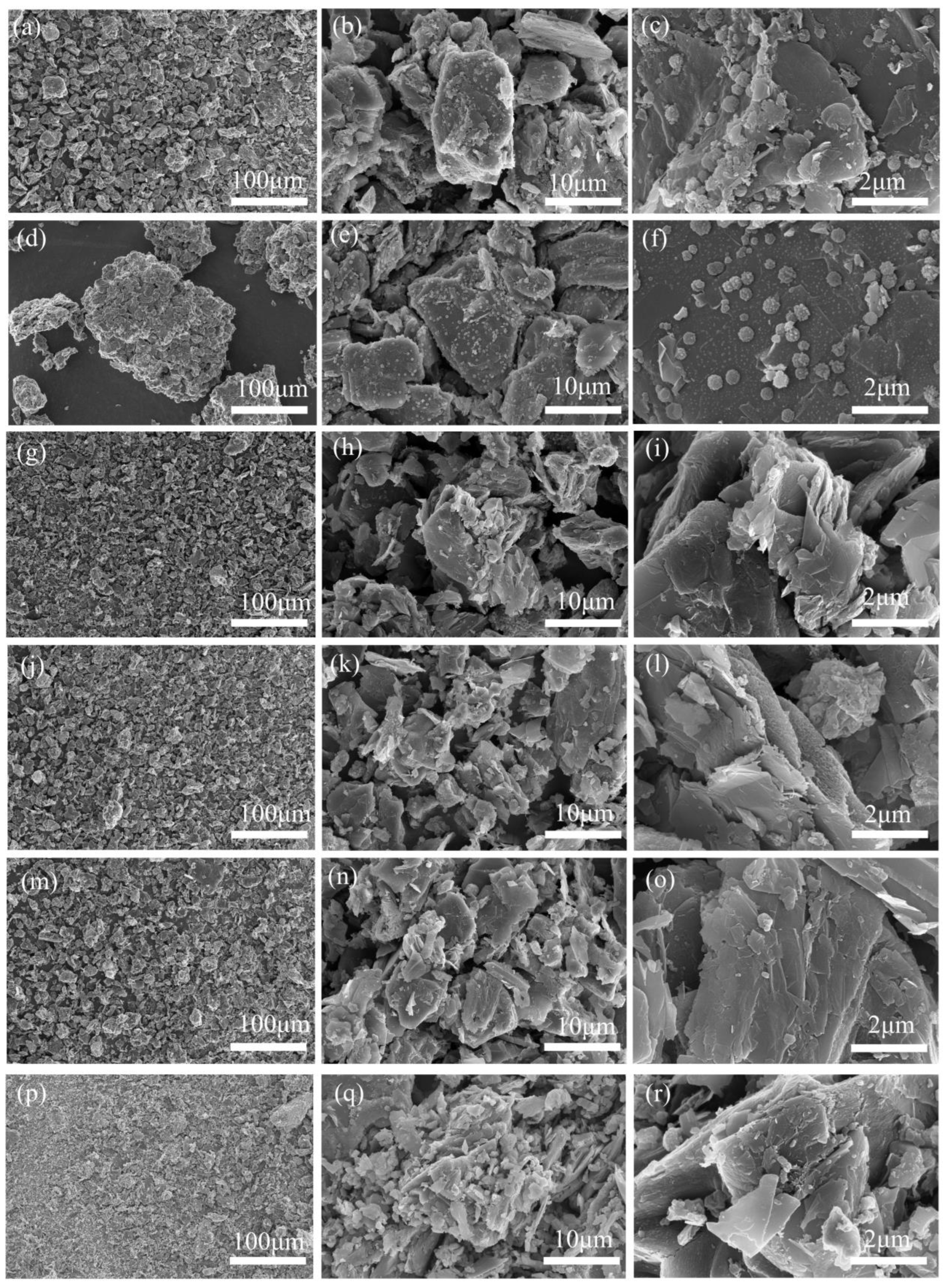
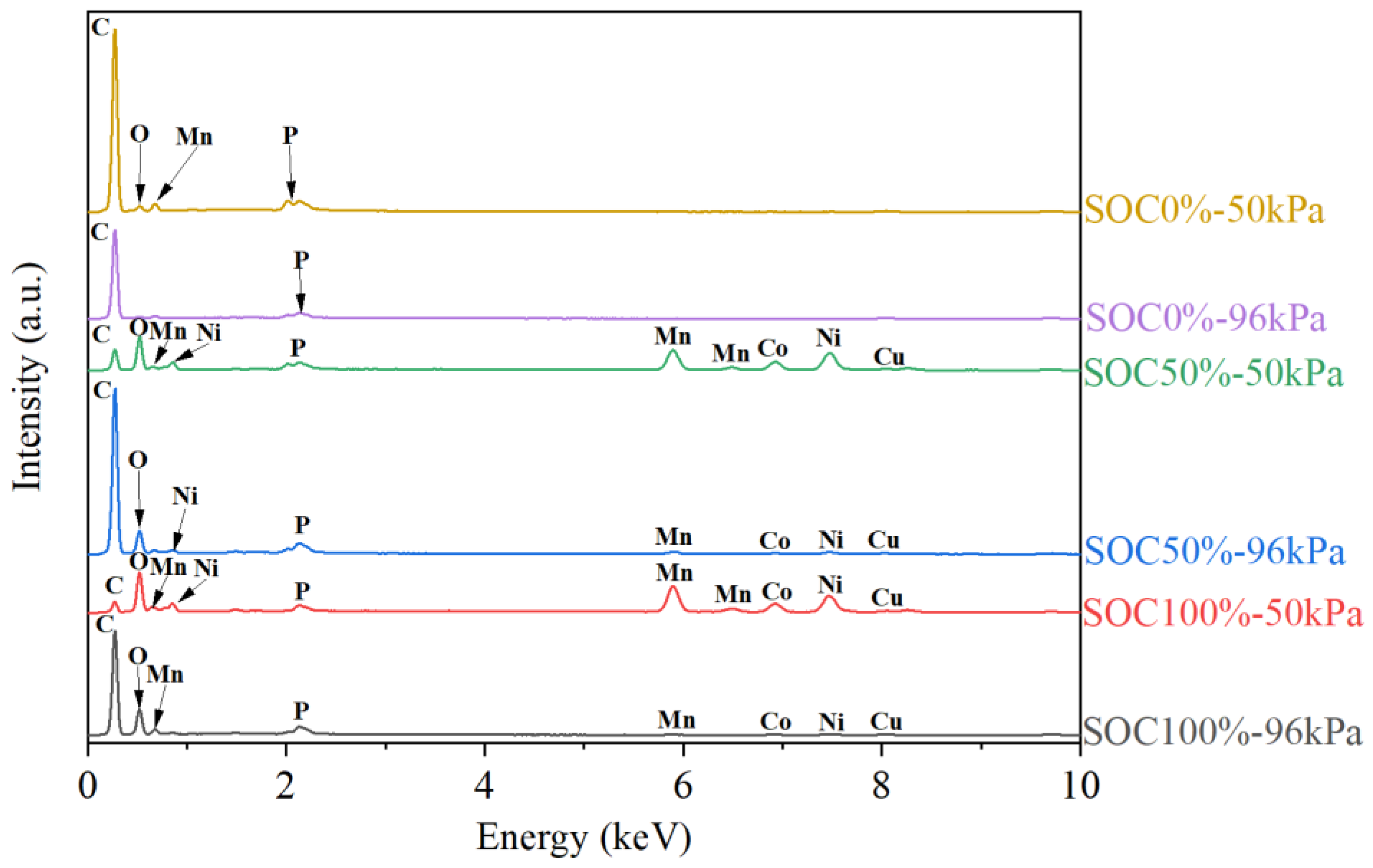
3.4. Analysis of Residues After Battery Thermal Runaway
3.5. Mechanism Analysis of Influence of Ambient Pressure and SOCs on Battery Thermal Runaway
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouyang, D.; Liu, B.; Huang, J.; Wang, Z. Degradation and safety performance of lithium-ion cells under high-rate charging/discharging scenarios. Process Saf. Environ. Prot. 2024, 185, 76–85. [Google Scholar] [CrossRef]
- Urquizo, J.; Singh, P. A review of health estimation methods for Lithium-ion batteries in Electric Vehicles and their relevance for Battery Energy Storage Systems. J. Energy Storage 2023, 73, 109194. [Google Scholar] [CrossRef]
- Mo, C.; Xie, J.; Zhang, G.; Zou, Z.; Yang, X. All-climate battery thermal management system integrating units-assembled phase change material module with forced air convection. Energy 2024, 294, 130642. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, Z.; Miao, S.; Wang, Q.; Sun, L.; Lu, H. Explosion hazards study of grid-scale lithium-ion battery energy storage station. J. Energy Storage 2021, 42, 102987. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating thermal runaway of lithium-ion batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Huang, W.; He, X.; Wang, L.; Ouyang, M. Challenges and opportunities to mitigate the catastrophic thermal runaway of high-energy batteries. Adv. Energy Mater. 2023, 13, 2203841. [Google Scholar] [CrossRef]
- Zhi, M.; Fan, R.; Zheng, L.; Yue, S.; Pan, Z.; Sun, Q.; Liu, Q. Experimental investigation on hydrated salt phase change material for lithium-ion battery thermal management and thermal runaway mitigation. Energy 2024, 307, 132685. [Google Scholar] [CrossRef]
- Qin, P.; Sun, J.; Wang, Q. A new method to explore thermal and venting behavior of lithium-ion battery thermal runaway. J. Power Sources 2021, 486, 229357. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Wang, Q. Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter. J. Energy Storage 2020, 28, 101232. [Google Scholar] [CrossRef]
- Jindal, P.; Bhattacharya, J. Understanding the thermal runaway behavior of Li-ion batteries through experimental techniques. J. Electrochem. Soc. 2019, 166, A2165. [Google Scholar] [CrossRef]
- Chen, M.; Ouyang, D.; Weng, J.; Liu, J.; Wang, J. Environmental pressure effects on thermal runaway and fire behaviors of lithium-ion battery with different cathodes and state of charge. Process Saf. Environ. Prot. 2019, 130, 250–256. [Google Scholar] [CrossRef]
- Meng, D.; Weng, J.; Wang, J. Experimental Investigation on Thermal Runaway of Lithium-Ion Batteries under Low Pressure and Low Temperature. Batteries 2024, 10, 243. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhi, M.; Zhao, C.; Jia, J.; Lv, P.; Xie, S.; Chen, X. Lithium-ion battery thermal runaway propagation characteristics under 20 kPa with different airflow rates. Fire Technol. 2023, 59, 1157–1179. [Google Scholar] [CrossRef]
- Liu, Q.; Yi, X.; Han, X. Effect of different arrangement on thermal runaway characteristics of 18650 lithium ion batteries under the typical pressure in civil aviation transportation. Fire Technol. 2020, 56, 2509–2523. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Shi, L.; Cheng, X.; Zhang, H. Ignition and combustion characteristics of lithium ion batteries under low atmospheric pressure. Energy 2018, 161, 38–45. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Liu, L.; Zhang, Z.; Hao, J.; Wang, Q.; Wang, S. Study on the thermal runaway and its propagation of lithium-ion batteries under low pressure. Fire Technol. 2020, 56, 2427–2440. [Google Scholar] [CrossRef]
- Jia, Z.; Huang, Z.; Zhai, H.; Qin, P.; Zhang, Y.; Li, Y.; Wang, Q. Experimental investigation on thermal runaway propagation of 18,650 lithium-ion battery modules with two cathode materials at low pressure. Energy 2022, 251, 123925. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Wang, H.; Jia, J.; Xie, S.; Zhi, M.; Fu, J.; Sun, Q. Influence of ambient pressure and heating power on the thermal runaway features of lithium-ion battery. J. Electrochem. Energy Convers. Storage 2021, 18, 021014. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Zhang, D.; Wang, Z.; Liu, Y. Experimental and Computational Analyses of Thermal Runaway Behavior of Lithium Ion Pouch Battery at Low Ambient Pressure. J. Electrochem. Energy Convers. Storage 2023, 20, 041007. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhi, M.; Chen, X.; Lv, P.; He, Y. Thermal characteristics of thermal runaway for pouch lithium-ion battery with different state of charges under various ambient pressures. J. Power Sources 2022, 527, 231175. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Xu, C.; Huang, X. Thermal runaway propagation in linear battery module under low atmospheric pressure. Appl. Therm. Eng. 2022, 216, 119086. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Li, Z.; Liu, J.; Xu, C.; Huang, X. Thermal runaway characteristics and failure criticality of massive ternary Li-ion battery piles in low-pressure storage and transport. Process Saf. Environ. Prot. 2021, 155, 486–497. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, J.; Li, H.; Xu, C.; Li, Y.; Wang, H.; Lu, L.; Dai, F.; Yu, R.; et al. Experimental Research on Thermal-Venting Characteristics of the Failure 280 Ah LiFePO4 Battery: Atmospheric Pressure Impacts and Safety Assessment. Batteries 2024, 10, 270. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Yin, B.; Shi, B.; Li, Z.; Yu, J. Effect of ambient pressure on the fire characteristics of lithium-ion battery energy storage container. J. Loss Prev. Process Ind. 2024, 92, 105459. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Wang, D.; Ren, X.; Lyu, S.; Fan, J.; Lv, S.; Zhu, S.; Li, N.; Wang, Y. Understanding Thermal Runaway in Lithium-Ion Batteries: Trigger, Mechanism, and Early Warning Strategies. J. Electrochem. Soc. 2024, 171, 120527. [Google Scholar] [CrossRef]
- Elsner, F.; Gerhards, P.; Berrier, G.; Vincent, R.; Dubourg, S.; Pischinger, S. Detailed Characterization of Thermal Runaway Particle Emissions from a Prismatic NMC622 Lithium-Ion Battery. Batteries 2025, 11, 225. [Google Scholar] [CrossRef]
- Ju, X.; Hou, X.; Liu, Z.; Du, L.; Zhang, L.; Xie, T.; Paillard, E.; Wang, T.; Winter, M.; Li, J. Revealing the Effect of High Ni Content in Li-Rich Cathode Materials: Mitigating Voltage Decay or Increasing Intrinsic Reactivity. Small 2023, 19, 2207328. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yi, Y.; Fang, B.; Yang, P.; Wang, T.; Liu, P.; Qu, L.; Li, M.; Zhang, S. Design strategies of safe electrolytes for preventing thermal runaway in lithium ion batteries. Chem. Mater. 2020, 32, 9821–9848. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Size (length × width × height) | 115 mm × 65 mm × 4 mm |
| Weight (g) | 80.20 |
| Cathode material | Li(Ni0.5Co0.3Mn0.2)O2 |
| Anode material | Graphite |
| Nominal capacity (mAh) | 5000 |
| Nominal voltage (V) | 3.7 |
| Charge cutoff voltage (V) | 4.25 |
| Discharge cutoff voltage (V) | 2.7 |
| Scenes | TTR/°C | Scenes | TTR/°C |
|---|---|---|---|
| 100%SOC-96kPa | 108 | 50%SOC-96kPa | 104 |
| 100%SOC-70kPa | 114 | 50%SOC-70kPa | 107 |
| 100%SOC-50kPa | 143 | 50%SOC-50kPa | 129 |
| Scenes | Elemental Amounts (at%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | Cu | P | F | Ni | Co | Mn | |
| 0%SOC-50kPa | 89.87 | 5.16 | 0.01 | 0.39 | 0.48 | 3.98 | 0.04 | 0.02 | 0.05 |
| 0%SOC-96kPa | 92.09 | 4.09 | 0.02 | 0.49 | 0.17 | 3.08 | 0.01 | 0.02 | 0.03 |
| 50%SOC-50kPa | 50.27 | 25.18 | 0.17 | 1.03 | 0.55 | 2.44 | 9.87 | 4.02 | 6.47 |
| 50%SOC-96kPa | 79.93 | 16.64 | 0.10 | 0.36 | 0.02 | 1.73 | 0.58 | 0.24 | 0.40 |
| 100%SOC-50kPa | 33.79 | 31.14 | 0.65 | 1.11 | 0.00 | 4.79 | 12.19 | 5.34 | 10.99 |
| 100%SOC-96kPa | 71.97 | 22.74 | 0.19 | 0.37 | 0.00 | 4.08 | 0.40 | 0.14 | 0.11 |
| Scenes | Tpeak/°C | Scenes | Tpeak/°C | Scenes | Tpeak/°C |
|---|---|---|---|---|---|
| 100%SOC-96kPa | 805 | 50%SOC-96kPa | 773 | 0%SOC-96kPa | 360 |
| 100%SOC-70kPa | 784 | 50%SOC-70kPa | 723 | 0%SOC-70kPa | 271 |
| 100%SOC-50kPa | 718 | 50%SOC-50kPa | 650 | 0%SOC-50kPa | 202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Zhi, M.; Sun, Q.; Zhang, M.; Zhang, K. Thermal Runaway Hazards of Ternary Lithium-Ion Batteries Under Different Ambient Pressure Environments. Batteries 2025, 11, 339. https://doi.org/10.3390/batteries11090339
Cui H, Zhi M, Sun Q, Zhang M, Zhang K. Thermal Runaway Hazards of Ternary Lithium-Ion Batteries Under Different Ambient Pressure Environments. Batteries. 2025; 11(9):339. https://doi.org/10.3390/batteries11090339
Chicago/Turabian StyleCui, Huajian, Maoyong Zhi, Qiang Sun, Mingge Zhang, and Kenan Zhang. 2025. "Thermal Runaway Hazards of Ternary Lithium-Ion Batteries Under Different Ambient Pressure Environments" Batteries 11, no. 9: 339. https://doi.org/10.3390/batteries11090339
APA StyleCui, H., Zhi, M., Sun, Q., Zhang, M., & Zhang, K. (2025). Thermal Runaway Hazards of Ternary Lithium-Ion Batteries Under Different Ambient Pressure Environments. Batteries, 11(9), 339. https://doi.org/10.3390/batteries11090339







