Abstract
In this study, the effect of lithium plating (LP) on the thermal properties of lithium-ion batteries (LIBs) was investigated. A large-format pouch 64.6 Ah cell with a graphite-SiOx/NMC chemistry was artificially aged (AA_LP) in the laboratory under specific conditions to induce LP on the anode. For thermal behavior analysis, temperature ramp experiments were conducted in a nitrogen-filled steel container on the cycled cell, as well as on fresh and real-life aged cells with the same specifications. Characteristic temperatures, such as first venting and safety critical temperatures, were monitored; additionally, the exhaust gas composition was analyzed using Fourier transform infrared spectroscopy (FTIR) and gas chromatography. It was revealed that the voltage decay of the cells started well before any safety-critical temperature, and the first venting of the AA_LP cell was significantly reduced to 112 °C in comparison to the fresh and real-life aged cells, in which it occurred at 130 °C and 134 °C, respectively. The earlier venting of the AA_LP cell was attributed to the reaction of the plated metallic lithium and the electrolyte. The safety-critical temperature rate (>10 °C/min) occurred at 160.9 °C for AA_LP and at around 159.1 °C for the fresh and real-life aged cells. The maximum temperatures reached were 616 °C, 553 °C, and 566 °C for the fresh, real-life aged, and AA_LP cells, respectively. No significant difference was observed in the exhaust gas after the thermal runaway for the tested cells.
1. Introduction
Lithium-ion batteries (LIBs) have become the predominant energy storage technology in various applications, ranging from consumer electronics to electric vehicles (EVs), due to their high energy density, long cycle life, and reliability [1,2]. However, the increasing application of LIBs has raised critical concerns about their safety, especially in high-energy applications. Among the different safety risks, thermal runaway (TR) remains one of the most severe and crucial regarding the safety of LIBs, characterized by a rapid and uncontrollable release of energy due to exothermic reactions, leading to fires or explosions [3,4,5]. Many critical factors influencing the safety of LIBs arise due to aging during the application period, such as electrolyte decomposition, solid electrolyte interphase growth, current collector corrosion, and lithium plating [6,7,8].
Lithium plating (LP) is a specific degradation phenomenon that occurs under certain charging conditions, such as high charging rates, low temperatures, and high states of charge (SOCs) [9,10,11,12]. During LP, metallic lithium (Li) is deposited on the anode surface rather than lithium ions intercalating into the anode’s active material [13,14]. This process is recognized as a critical safety concern due to its direct impact on TR behavior [15,16,17]. Metallic lithium is highly reactive, particularly when exposed to electrolytes, air, or moisture, leading to gas formation and thermal instability [17]. The formation of irreversible plated lithium, especially dendritic lithium, increases the likelihood of internal short circuits (ISCs) [18,19,20]. Furthermore, the dendritic morphology of plated lithium can puncture the separator, creating conditions prone to TR initiation [18,19,20].
In the literature, the TR behavior of LIBs is very well reported [3,4,21,22,23,24,25,26]. A limited number of studies have been published that investigated the effect of LP on the thermal behavior of LIBs, and an even lower number of those studies dealt with high-energy density LIBs. Friesen et al. [27] investigated the safety behavior of fresh and low-temperature-cycled till state of health (SOH) 70% 18,650 type 2.2 Ah cells with a graphite/NMC chemistry using extended volume accelerated rate calorimetry (EV-ARC) with the heat–wait–seek (HWS) method. A nail penetration test and post-mortem analysis were also conducted. It was reported that fresh cells showed a state-of-charge (SOC) dependency on onset temperature for exothermic reactions and thermal runaway, whereas cycled cells showed no SOC dependency, and the onset temperature for exothermic reactions and thermal runaway was significantly reduced in the presence of LP. Bröner et al. [28] investigated the effect of aging on the thermal stability of 18650-type graphite/NMC cells using EV-ARC with HWS; multiple cells were cycled to SOH 90%, 80%, and 70% at 20 °C and 45 °C. They further supported their findings by confirming the presence of metallic lithium (Li) after cycling at 20 °C through post-mortem analysis. In these cells, a decrease in the onset temperature of the exothermic reaction was observed, along with a lower SOH. In contrast, cells cycled at 45 °C showed no reduction in onset temperature; instead, an increase was detected, consistent with the absence of metallic Li. Waldmann et al. [15] also investigated 18650-type cells with graphite/NCA chemistry using EV-ARC with HWS. Cells were cycled at 0 °C and 25 °C to SOH 80%. The authors reported that thermal runaway was triggered by exothermic reactions between metallic Li and the electrolyte. Tests were conducted 1.5 h after cycling as well as 8 days after cycling. Cells tested shortly after cycling 1.5 h exhibited more severe TR in comparison to cells tested after 8 days. Across all tested cells, the maximum temperature reached during thermal runaway was lower than that of fresh cells, which was attributed to the reduced electrochemical content of aged cells (i.e., their lower SOH).
Li et al. [16] investigated graphite/NMC 28.8 Ah pouch cells to study TR. Cells were cycled at C-rates of 0.33 C, 1.5 C, and 3 C, followed by EV-ARC testing. The post-mortem analyses confirmed the presence of metallic Li. For cells cycled at 3 °C, the chemical reaction between metallic Li and the electrolyte was identified as the trigger for TR, accompanied by a significant reduction in onset temperature. In a follow-up study, Li et al. [17] examined graphite/NMC 28.8 Ah pouch cells and 51 Ah prismatic cells to investigate battery eruption behavior and gas generation after fast charging. EV-ARC and hot-box tests involving electrolyte vs. Li and electrolyte vs. lithiated anode were also conducted in order to understand the reactions in detail. The authors reported that fast charging reduced thermal stability due to LP, resulting in markedly lower eruption temperatures and earlier gas release. Table 1 summarizes available studies on the influence of LP on the thermal behavior of commercial LIBs.

Table 1.
A comparison table of available literature on the effect of lithium plating on the thermal properties of commercial LIBs.
The authors identified the gap in knowledge for the influence of LP on the thermal behavior of large-format cells with graphite-SiOx/NMC chemistry. Furthermore, detailed analyses of gases released during TR in LIBs affected by LP remain scarce in the literature, representing another critical knowledge gap. While several studies have investigated TR gas composition in detail [29,30], none reported the presence of LP in the examined cells. We consider this gap particularly important for automotive applications, where LP may occur under conditions such as fast charging in cold climates. Such scenarios increase the likelihood of catastrophic failure in high–energy density cells, posing significant safety risks to EV passengers. In the event of a crash, LP could trigger ISC, leading to TR. This not only endangers passengers through the risk of fire and explosion but also exposes them to potentially toxic gases if they are injured and trapped inside the EV.
In our prior work, LP was systematically identified in graphite-SiOx/NMC cells using electrochemical non-destructive techniques [31], including incremental capacity analysis (ICA), voltage relaxation profile, and differential voltage analysis (DVA). These methods were complemented by a range of post-mortem analyses [32], such as inductively coupled plasma optical emission spectroscopy (ICP-OES), nuclear magnetic resonance (NMR), and scanning electron microscopy (SEM). Building on this foundation, the present study investigates the effects of LP on the TR behavior of graphite-SiOx/NMC cells. Specifically, we examine the interplay between LP-induced active material degradation, gas evolution, temperature rise rates, and internal short-circuit dynamics. This work aims to provide a deeper understanding of the safety risks associated with LP, thereby informing the development of safer cell designs and optimized operational strategies. Ultimately, these findings contribute to the broader objective of enhancing LIB safety in high–energy applications.
2. Materials and Methods
The cells investigated in this study were graphite-SiOx/NMC pouch cells with a nominal capacity of 64.6 Ah and a nominal energy density of 263 Wh/kg. The same cell type was previously used to study the formation and identification of lithium plating (LP) under different operational boundary conditions using both non-destructive [31] and post-mortem analysis [32] methods. For artificial aging, the cells were cycled using a CC-CV charge protocol with 1.5 C at 10 °C until SOH dropped below 85%. The cycling procedure consisted of 20 cycles, with relaxation time after charging in cycles 1, 5, 10, 15, and 20. The remaining cycles were performed without relaxation. The relaxation time was adopted for the purpose of voltage relaxation profile analysis, as described in our previous studies [31,32]. A uniform pretension of 300 N was applied across the cell surface to replicate stack pressure, while a localized higher pretension was introduced at the center of the cell using a thin indenter placed between the cell and pretension plate. This localized mechanical stress increased the likelihood of LP formation in that region, consistent with observations reported by Fuchs et al. [11]. For the thermal analysis, thermocouples were placed particularly in this area, as shown in Figure 1b. In addition to fresh (F) and artificially cycled cells (AA_LP), real-aged (RA) cells were also tested. The RA cells were extracted from an electric vehicle that had accumulated 160,000 km of driving [32]. The RA cell was used as an additional reference thermal behavior in addition to the F cell, to compare with the AA_LP cell.
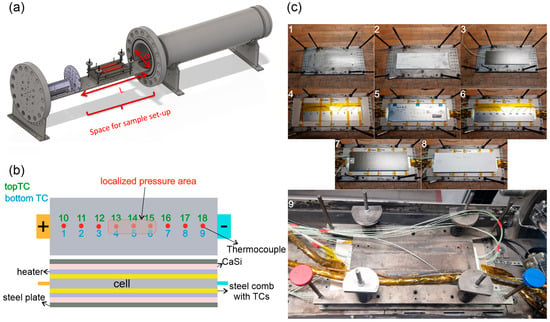
Figure 1.
Steel container is illustrated along with cell and test setup. (a) test chamber with cell holder; (b) cell and holder layout with heaters and thermocouples; (c) cell mounting and holder preparation, the stepwise mounting setup preparation is also shown by ascending order of the figure numbers.
Table 2 provides an overview of the cells in their respective states of aging, along with the nomenclature used throughout the Results and Discussion section. One representative experiment was conducted for each type of cell history.

Table 2.
Nomenclature and aging histories of the cells used in this study.
After artificial aging of the cells to produce AA_LP cells, temperature ramp experiments were conducted to investigate thermal stability and TR behavior, with particular focus on the influence of LP. The experiments were performed in a stainless steel calorimeter (Figure 1a) with a free volume of 119.96–121 L, designed at Virtual Vehicle GmbH, Graz, Austria, to detect exothermic reactions, cell temperatures, and container pressure. The calorimeter is rated for 85 bar at 200 °C and equipped with gas feed lines for TR gas analysis. The cells were mounted in a dedicated holder, and the container was purged and filled with nitrogen (N2) prior to testing. The setup and experimental procedure were designed to detect the exothermic reaction in cells, such as first venting, self-heating, and TR, by applying a slow temperature ramp of 2 °C/min. All tests were conducted at SOC 100%.
The cell holder consisted of a symmetric stack with 9 layers: steel-plate-layer, CaSi-layer, heater-layer, steel-sheet-spacer with integrated thermocouples (TCs), cell-layer, steel-sheet-spacer with integrated thermocouples, heater-layer, CaSi-layer, steel-plate-layer, as shown in Figure 1c. The steel layers were compressed with the help of four springs on each corner of the sample holder, applying a pre-tension of 500 N on the cell, see Figure 1c. The CaSi (Calcium-silicate plates) layers provided thermal insulation, while the heater layers were electrically connected to a regulated power source. The steel interlayers, equipped with machined grooves, conducted heat from the heaters to the cell and housed the TCs to record surface temperature. Type-K TCs (±2.5 °C accuracy) were used to measure temperatures at 18 positions across the cell surface (Figure 1b). Additional TCs monitored vent gas and reactor gas temperatures. For the AA_LP cell, TCs 13, 14, and 15 were positioned in the localized high-pressure region (top), while TCs 3, 4, and 5 were placed below this area (bottom) (Figure 1a). Due to experimental constraints and the setup design, alternative measurement techniques described in the literature [33] could not be implemented.
The main steps of the TR-test workflow are:
- Cell-mass measurement
- The cell is mounted in the stack as described above, and the stack is put into the reactor
- The reactor is closed and flushed with N2
- The cell is charged to 100% SOC (at room temperature)
- Time delay between end-of-charge and start of TR test: (F: 5 h, RA: 3 h, AA: 14 h)
- Main test-execution phase: The heaters were switched on and regulated to heat with 2 °C/min until TR
- After TR, the gas composition is analyzed
- Release vent-gas, heat-out and evacuate, flush with air
- Open reactor
- Remove cell-remains from stack
- Cell-mass measurement
Several events and processes emerge during the execution phase:
- Voltage decay
- Start of bloating (gas production inside the cell)
- First venting (break of pouch seals and bloating ends)
- Start of self-heating
- Reaching safety-critical temperature
- Internal short circuit/voltage breakdown
- TR/Main venting and gas production
Voltage decay was defined as the first measurable decrease in cell voltage exceeding 1 mV during the experiment. The first venting was identified when the pouch casing ruptured and gas was released to the steel container. The safety-critical temperature was defined as the point at which the temperature rate exceeds 10 °C/min.
During testing, cell bloating and subsequent thickness reduction after TR venting occasionally led to poor thermal contact between the cell surface and the TCs due to loss of pre-tension. In this phase, hot exhaust gases could directly contact the surface-mounted TCs, causing a spike in the temperature. Due to the mentioned reasons, only single TCs that showed a uniform rise in temperature are used for maximum temperature and temperature rate analysis.
In the literature, multiple definitions of critical temperatures are reported, including self-heating and TR triggering temperatures, typically based on temperature rates, presented in Table 3. The self-heating temperature ranges from 70 °C to 150 °C, while TR triggering/safety critical temperatures usually occur above 150 °C. An exception was reported by Waldmann et al. [15] who observed TR initiation below 100 °C under specific conditions.

Table 3.
Reported critical temperature rates in the literature compared to the presented study.
The voltage breakdown temperature was defined as the temperature at which the cell voltage dropped below 90% of its initial value. In addition to maximum temperature and temperature rate analyses, average cell temperatures were used to evaluate overall cell behavior. Furthermore, TCs exhibiting unique behavior, such as those located in the localized high-pressure area TCs on the AA_LP cell, are also used for analysis, as shown in Figure 1b.
The gas composition was quantified using two complementary methods: Fourier-transform infrared spectroscopy (FTIR) and gas chromatography (GC). FTIR measurements were performed using a Bruker MATRIX-MG01 spectrometer by Bruker, Billerica, MA, USA, with a wavenumber resolution of 0.5 cm−1 and a 10 cm gas measurement chamber maintained at 190 °C. The implemented method allowed for the qualitative detection of various infrared active components, including CO2, CO, DEC, DMC, EMC, EC, CH4, C2H6, C2H4, C2H2, H2O, and HF. For chromatography, an Inficon microGC Fusion 2-module system by Inficon Holding AG, Bad Ragaz, Switzerland, equipped with two columns and thermal conductivity detectors (TCDs), was used. It was equipped with a Rt-Molsieve 5A column for the detection of H2, O2, N2, CH4, and CO, and a Rt-Q-Bond column for the detection of CO2, C2H6, C2H4, H2O, C3H6, C4H10, C6H14, DMC, EMC, MeOH, EtOH, EtO, EtAc, C6H6, and C6H5F. The upstream piping connecting the analysis equipment to the reactor was heated to 170 °C to prevent condensation of analytes. The FTIR used internal calibration references from the manufacturer, while the GC was calibrated in-house with standard gas mixtures. The measurement error for reported concentration is estimated to be approximately 2%. A similar experimental method used in this study was already used by Essl et al. [29]. The procedures for determining maximum cell surface temperature, safety critical temperature, and the calculation of the amount of gas have been described in detail by Golubkov et al. [35].
3. Results
The results of the temperature ramp experiments are presented in this section. With comparisons between cells to identify significant changes in behavior and assess safety-critical differences. During the experiments, all tested cells underwent a complete TR, resulting in total cell structure failure. As an example, Figure 2 illustrates the AA cell before and after the experiment. The pouch casing burst at multiple locations, although the initial rupture site could not be identified. The weaker points on the pouch were expected to be the pouch seams, especially along the longer side and near the tabs of the cell, as observed in Figure 2. Post-experiment examination reveals a major rupture of the pouch at this spot, in addition to other rupture points as indicated.

Figure 2.
Status of the cell structure before and after, the localized pressure area is also marked on the cell surface. Major pouch rupture points are identified and marked after the experiment.
Figure 3a shows the temperature recorded by the hottest TC on each of the tested cells. The exothermic TR reaction caused a rapid increase in cell-surface temperature, reaching 616 °C for the F cell, 553 °C for the RA cell, and 566 °C for the AA_LP cell. Further analysis of the AA_LP cell reveals that the localized high-pressure region exhibited a higher temperature compared to the rest of the cell surface (Figure 3b). This effect was confined to the high-pressure area, as no temperature increase was detected at TCs located along the sides or near the tabs. Importantly, this localized temperature rise did not trigger immediate TR in the AA_LP cell. In contrast, the F and RA cells did not show a comparable localized temperature increase. Instead, both exhibited a slight temperature decrease at 130 °C for F and 134 °C for RA. When comparing the temperature of the localized high-pressure area on AA_LP to the side region of the cell, it is observed that there is no temperature change on the side region, whereas a temperature decrease similar to those observed in the F and RA cells occurred near the tabs, see Figure 3b.
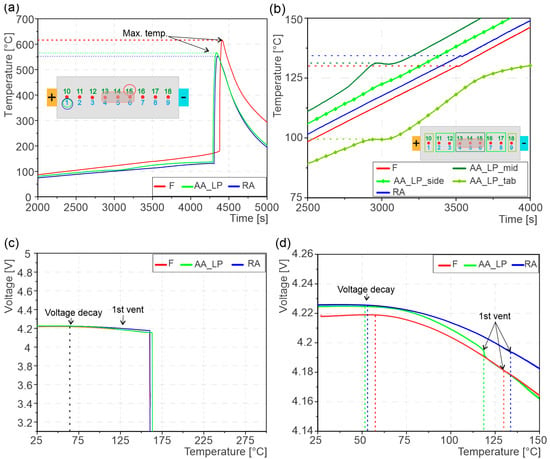
Figure 3.
(a) showing maximum cell-surface temperature during TR, the TCs are indicated on which the data was recorded; (b) cell surface temperature before 1st vent till TR, the temperature curves from localized pressure area of AA_LP cell and rest of the cell surface including F and RA cells; (c) cells voltage curve during tests showing the voltage decay, 1st venting and eventual ISC; (d) zoomed in of voltage curve showing detailed behavior of voltage curve.
The first venting of the cells coincides with the temperature changes described above. For the F cell, the first venting occurred at an average temperature of 130 °C, while the RA cell exhibited a slightly higher venting temperature of 134 °C. In contrast, the AA_LP cell experienced first venting at a significantly lower average temperature of 112 °C, see Figure 3d. Examining the first vent temperatures in Figure 3b, the first venting temperature on the localized area of the AA_LP cell is higher compared to F and RA cells. However, at the same time, the near tab venting temperature is lower; for this reason, the average first venting temperature of AA_LP cell was reduced to 112 °C.
The voltage behavior was monitored during temperature ramp experiments, as seen in Figure 3c,d. A voltage decay was observed for all cells already significantly before first venting and TR. The voltage decay occurs at 57 °C for F and 51 °C for AA_LP cell, whereas it occurs at 53 °C for RA cells. The cell voltage breakdown occurred at 160.1 °C for the F cell, at 163.2 °C for the AA_LP cell, and at 159.5 °C for the RA cell. The complete voltage breakdown happens around the same temperature as the safety critical temperature, pointing towards the fact that TR and voltage breakdown are simultaneous events. In contrast, the AA_LP cell exhibited a slight offset, suggesting that TR was initiated prior to voltage breakdown.
During the temperature ramp experiments, a constant temperature rate of 2 °C/min was realized with a closed-loop temperature control. The heaters were automatically turned off if the temperature rate exceeded this value and reactivated when the rate decreased. Figure 4a shows the heater power required to achieve the target heating for all three cells. For all cells, the energy required to achieve the same temperature increase rose monotonically with temperature. The heater power for the AA_LP cell remained lower than that of the F and RA cells at temperatures exceeding approximately 60 °C. The significant discontinuities in the heater power curves, observed at average temperatures between 112 °C and 135 °C, correspond to the first venting of the cells, see Figure 4a. This state is reached at the lowest temperature (112 °C) for the AA_LP cell and at or slightly above 130 °C for the F and RA cells, respectively. This change in heater power coincides with the average first venting temperature of the cells. From the first vent to thermal runaway, the curve of the AA_LP cell remains substantially below F and RA cells, resulting in a 16% reduction in the accumulated energy required to induce TR compared to the F and RA cells. Additionally, while the heater power decreased for the AA_LP cell after first venting, it continued to increase for the F and RA cells.
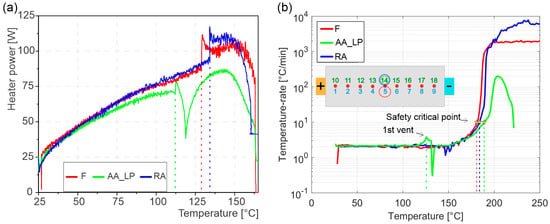
Figure 4.
(a) Heater power during the experiments, showing the change in heater power at 1st venting of the cells. Heater power is shown to decrease prior to the safety critical point; (b) temperature rates during experiments, 1st vent of AA_LP, and the safety-critical point of all cells are indicated.
The temperature rates shown in Figure 4b provide insight into the discontinuities observed in the heater power. At first vent of the AA_LP cell, the temperature rate exceeded the target ramp of 2 °C/min, reaching a peak of 3 °C/min at 124 °C (TC 14). This caused the heater current to decrease, as observed in Figure 4a. In contrast, the F and RA cells exhibited the opposite behavior: their temperature rates fell below the target ramp, prompting a slight increase in heater power before TR. The self-heating began at approximately 150 °C, when the temperature rate first exceeded 2 °C/min, as shown in Figure 4a,b. The start of self-heating can be identified in Figure 4a with a gradual decrease in heater power for all cells. Following the approach described earlier and consistent with the literature, the safety-critical temperature was defined as the point at which the temperature rate exceeded 10 °C/min. The average safety-critical temperatures were 159.1 °C for the F and RA cells and 160.9 °C for the AA_LP cell (Figure 4b). It can be observed that for the AA_LP cell, the safety critical temperature is lower than the temperature at which the voltage breakdown was observed. In the results, only average cell-surface temperature curves are presented, except in Figure 3a,b and Figure 4b, where temperature curves of specific TCs are shown.
The analysis of the exhaust gas produced during TR (see Figure 5a) revealed a similar distribution of major gas species across all three cells. CO2, CO, H2, C2H4, and CH4 collectively accounted for 93.0 ± 0.1 mol.% of the exhaust gas in all cases. Whereas AA_LP showed slightly higher CO2, F cell higher CO, and RA cell higher H2 concentrations. Additional gas components EMC, H2O, MeOH, C6H5F, C3H6, and C2H6 made up 5.8 ± 0.1 mol.%, with a notable increase in water content for the RA cell compared to the F and AA_LP cells. EMC and C6H5F showed a decreasing trend across the cells, F > AA_LP > RA. The remaining 1.2 ± 0.2 mol.% consisted of C6H6, EtO, EtOH, C2H2, DMC, C4H10, C3H8 and EtAc. Within this fraction, DMC in the AA_LP cell was more than double that in the F and RA cells. Detailed molar compositions are provided in Table A1 in Appendix A. The total amount of exhaust gas released during first venting and during TR is shown in Figure 5b. Interestingly, the aged cells displayed opposite trends compared to the F cell: the RA cell released 5% more gas, whereas the AA_LP cell released 7.5% less gas relative to the F cell. However, during first venting AA_LP cell released 23% more exhaust gas in comparison to the RA cell. The general decomposition reactions of hydrocarbons and graphite present in the cell, as previously described by Golubkov et al. [36], are given in the reactions 1-4 below:
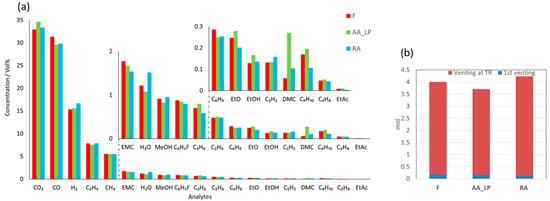
Figure 5.
The plots show the exhaust gases produced during TR and the relevant gas composition, supplemented by the total TR gas amount during these tests; (a) Composition of exhaust gas produced by the cells after TR. The insert figures show component gases with lower concentration; (b) The amount of exhaust gas produced during first vent and TR of the cell.
The cell masses before and after the experiments are summarized in Table 4. Both the AA_LP and RA cells exhibited higher effective mass loss compared to the F cell, showing a clear correlation with the state of health (SOH). Mass loss during the experiments occurred primarily through the release of vent gases and the ejection of fragmented or decomposed cell components during TR.

Table 4.
Cell mass comparison before and after the temperature ramp experiments.
4. Discussion
During the temperature ramp experiments, all the tested cells underwent TR, resulting in the complete destruction of the structure. The first venting event occurred at 130 °C for the F cell, 134 °C for the RA cell, and significantly earlier at 112 °C for the AA_LP cell. The reduction in venting onset temperature for the AA_LP cell can be attributed to reactions between plated metallic Li and the electrolyte, possibly initiated after solid electrolyte interphase (SEI) decomposition [16,17]. These reactions were also associated with a decrease in heater power. Based on the cycling conditions, it can be well assumed that most of the capacity loss is due to irreversible LP. However, other degradation mechanisms, such as SEI growth and gas formation, occur simultaneously and cannot be decoupled, making it impossible to directly quantify the contribution of plated Li to the observed temperature differences. Attempts to apply ICP-OES for mass balance analysis of metallic Li and other anode components proved unsuccessful, owing to the inhomogeneous Li distribution across the electrode stack [32]. The AA_LP cell also exhibited a higher temperature rise rate (3 °C/min) at the first venting compared to the F and RA cells (2 °C/min), which did not show a clear acceleration at this stage. This increase can also be attributed to the exothermic reactions between plated Li and the electrolyte. While these reactions provide additional energy during first venting, it is insufficient to trigger immediate TR. In contrast, F and RA cells exhibited a slight temperature drop at the first venting, attributed to electrolyte evaporation at elevated temperatures, which eventually caused pouch rupture [17]. Furthermore, pouch bloating prior to rupture may have disrupted thermocouple contact with the internal cell layers, resulting in a drop in the temperature and temperature rate.
In this study, the safety critical temperature is defined as the temperature at which its time derivative exceeds a threshold value of 10 °C/min. For the tested cells, the safety-critical temperatures were found to be in a similar range for F, RA, and AA_LP cells. Similar behavior was reported by Börner et al. [28], although their study employed cycling at 20 °C, whereas the present work used 10 °C. Our results, however, contrast with other reports on pouch cells, where the TR temperature was drastically reduced after LP [15,16,17,37]. We attribute this discrepancy to the reduced amount of metallic Li remaining in the AA_LP cell after exothermic reactions during the first venting, which prevented a significant shift in safety-critical temperature relative to the F and RA cells. Overall, the effect of LP on the safety-critical temperature in these experiments was not significant. Further, it has to be taken into account that studies in the literature employed HWS procedures to induce TR, whereas a constant temperature rate was applied in this work. The influence of these differing methodologies on the experimental outcomes remains unclear. Both aged cells show a clear lower maximum temperature compared with the F cell. This is consistent with literature findings [15] that lower SOH can result in lower peak temperatures in case of a TR, which is explained primarily by the reduced energy content that can be converted into heat. The AA_LP cell contradicts this statement; despite a clearly lower SOH (approximately 10% in comparison to RA), it showed a higher peak temperature. It is considered that, in the event of TR, the exothermic chemical reaction of plated Li in the AA_LP cell provides energy to break down the SEI around the metallic Li, exposing metallic Li to electrolyte and causing exothermic reactions. This leads to heat generation, resulting in higher peak cell surface temperature, despite the lower SOH of the AA_LP cell.
Analyzing the cell voltage breakdown, a clear indicator of ISC, revealed distinct behaviors between the tested cells. For the F and RA cells, the ISC coincides with the safety critical temperature, as can be seen in (Figure 3a and Figure 4b), consistent with literature reports that ISC contributes to the initiation of TR [16]. In contrast, the AA_LP cell exhibited ISC only after surpassing the safety-critical temperature, indicating that TR was triggered earlier, likely due to the presence of LP. Although the difference is marginal (approximately 2 °C corresponding to a delay of 12 s), this observation aligns with literature reporting that TR is triggered before ISC in cells affected by LP [16]. We attribute this to exothermic reactions between the plated metallic Li and the electrolyte, which generate sufficient heat to trigger TR in the AA_LP cell prior to ISC onset. By contrast, in the F and RA cells, TR appears to be driven primarily by heat generation associated with ISC. Additionally, it can be observed that the voltage decay of all tested cells began at significantly lower temperatures in comparison to first venting and the safety critical temperature. As this early decay occurs across all cells, it can be interpreted as cell degradation at elevated temperatures, most likely associated with electrode active material and electrolyte decomposition (Figure 3d).
The main exhaust gas products during TR originate from SEI and electrolyte decomposition [36,38,39]. A higher CO content in comparison to CO2 in Figure 5a suggests an incomplete combustion [29]. In the literature [29,34], higher CO2 and H2 content was reported for a fresh prismatic cell in comparison to pouch cells in this study, while CO levels were lower than those found in this study. These differences highlight the influence of cell geometry, energy content, and possibly electrolyte composition on TR gas generation. The reduced electrolyte solvent content (EMC) detected in the exhaust gas of AA_LP and RA cells can be attributed to lower SOH of the cells, since aged cells contain less electrolyte. he measured solvent fractions of AA_LP and RA cells are consistent with their respective SOC values. Water detected in the exhaust arises from the SEI and hydrogen fluoride (HF) reaction [36,38], as well as electrolyte oxidation. HF itself is generated when oxygen released from the cathode oxidizes both SEI and electrolyte (solute, solvent, salt). However, HF is expected to react with internal cell components before reaching gas analysis equipment; hence, no HF is detected. Fluorobenzene, in contrast, likely originates directly from the electrolyte [40,41] rather than from secondary reactions. In addition to the toxic gases CO and CO2, the exhaust also contained highly flammable species, including H2, CH4, and C2H4, in the exhaust gas present critical safety hazards, although no significant difference is found between the tested cells. The total amount of exhaust gas produced after TR was higher for F and RA cells as compared to AA_LP and is attributed to the lower SOH of the AA_LP cell, see Table 5. The loss of cell mass during thermal runaway is higher for AA_LP and RA in comparison to F cells, where lower SOH leads to higher mass loss. NMC degradation as a result of strong delithiation due to LP leads to cracks in the active material [42], causing excessive mass loss in the AA_LP cell. The most relevant safety-critical parameters of the tested cells are also compared in Table 5.

Table 5.
Most safety-relevant parameters of the cells’ thermal behavior during experiments.
5. Conclusions
Temperature ramp experiments were conducted on 64.6 Ah pouch LIBs with different aging histories, namely as fresh (F), real-life aged (RA), and artificially aged to induce lithium plating (AA_LP). The thermal behavior of RA and AA_LP cells was compared with that of F cells to evaluate the influence of aging on thermal safety, with particular emphasis on the role of lithium plating (LP) in high-energy LIBs. Additionally, exhaust gas analysis following thermal runaway (TR) was conducted to assess the impact of aging on gas composition. Based on the findings of this study, the following conclusions can be drawn:
- Early venting due to LP: Lithium plating (LP) was found to trigger the first venting event at significantly lower temperatures. In the AA_LP cell, localized regions with higher mechanical pressure exhibited rapid temperature increases, indicating exothermic reactions between plated Li and the electrolyte. Such effects were not observed in the F and RA cells.
- Safety critical temperature and TR behavior: Contrary to reports in the literature, the presence of plated Li did not reduce the safety critical temperature associated with the onset of TR. While the AA_LP cell exhibited moderately lower peak temperatures, its overall TR behavior was comparable to that of the F and RA cells. Nevertheless, the fact that exothermic reactions were initiated at much lower temperatures underscores LP as a critical degradation mode with significant implications for thermal safety.
- Triggering mechanism of TR: In the AA_LP cell, the internal short circuit occurred only after the onset of critical self-heating, indicating that exothermic reactions between plated metallic Li and the electrolyte were the primary trigger for TR. This finding reinforces the conclusion that LP represents a critical degradation mode with severe implications for LIB thermal safety.
- Gas analysis: Exhaust gas analysis revealed broadly similar compositions across all tested cells, with only minor variations in the concentrations of certain species such as H2, CO, and CO2, as a function of aging
- Cell mass loss: Both aged cells exhibited greater mass loss after TR compared to the F cell. Previous work [42] demonstrated clear evidence of cathode active material degradation in RA cells, and similar effects are likely present in the AA_LP cell [43]. Such degradation of cathode active material provides a plausible explanation for the increased mass loss, despite the lower overall TR severity observed in terms of peak temperature and total exhaust gas volume.
This work contributes to ongoing research on the influence of lithium plating (LP) on the operational safety of Li-ion batteries, with a particular emphasis on thermal safety [31,32,43]. Beyond the identification of LP formation and its impact on cell behavior under crash loads, this study examined its effect on thermal stability and TR characteristics. In the analyzed high-energy automotive LIB pouch cells, the presence of LP was confirmed, and self-heating reactions were observed at temperatures where no such effects were detected for F or RA cells. Although this earlier onset of self-heating did not result in an earlier TR initiation or higher peak temperatures, it nevertheless represents a critical alteration of cell behavior with important implications for automotive safety. The earlier onset of voltage decay is another significant finding that advances the understanding of LIB thermal behavior and should be considered in future LIB and battery pack designs. However, the generalizability of these results is limited, as other studies have reported partially contradictory findings. Future investigations should therefore employ improved diagnostic methods, such as embedded temperature and pressure sensors in combination with thermal imaging, to provide more detailed insights. The effect of other possible influencing factors on TR should also be investigated in the future, such as the composition of the electrolyte and the effect of the test environment, e.g., ambient air instead of N2. However, we believe that the apparent variation and partial contradiction of findings in available literature still exhibit a significant gap in knowledge and underscore the need for further investigations.
Author Contributions
Conceptualization, S.M.A. and G.G.; methodology, S.M.A., G.G. and A.W.G.; software, S.M.A.; validation, S.M.A., O.K., A.W.G. and G.G.; formal analysis, S.M.A., A.W.G., O.K., and G.G.; investigation, S.M.A., A.W.G. and O.K.; resources, S.M.A.; data curation, S.M.A. and A.W.G.; writing—original draft preparation, S.M.A.; writing—review and editing, S.M.A., G.G., A.W.G., O.K., S.E. and C.E.; visualization, S.M.A.; supervision, G.G.; project administration, G.G. and C.E.; funding acquisition, C.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work originates from the research project SafeLIB. The COMET Project SafeLIB is funded within the framework of COMET—Competence Centers for Excellent Technologies (Grant agreement No. 882506) by BMK, BMDW, the Province of Upper Austria, the Province of Styria, as well as SFG. The COMET Program is managed by FFG. The authors thank the consortium members of the SafeLIB project for supporting this work. Supported by the Open Access Funding of the Graz University of Technology.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
Open Access Funding by the Graz University of Technology. The authors thank the consortium members of the SafeLIB project for their valuable input to this work.
Conflicts of Interest
The authors Andrey W. Golubkov and Oliver Korak were employed at Virtual Vehicle GmbH. Author Simon Erker was employed at AVL GmbH. Author Christian Ellersdorfer was also employed at Battery4Life GmbH. The remaining authors declare that the research was conducted in the absence of any financial or commercial relationships that can be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CaSi | Calcium silicate |
| EV-ARC | extended volume accelerated rate calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| HF | Hydrogen fluoride |
| HWS | heat-wait-seek |
| ICP-OES | inductively coupled plasma optical emission spectroscopy |
| ISC | Internal short circuit |
| Li | Lithium |
| LIBs | Lithium-ion batteries |
| LP | Lithium plating |
| NMR | Nuclear magnetic resonance |
| SEM | scanning electron microscope |
| SOC | State of charge |
| SOH | State of health—based on nominal capacity |
| TC | Thermocouple |
| TR | Thermal runaway |
Appendix A

Table A1.
Gas analysis.
Table A1.
Gas analysis.
| Chemical Formula | Name | CAS No. | mol.% | ||
|---|---|---|---|---|---|
| F | AA_LP | RA | |||
| CO2 | Carbon dioxide | 124-38-9 | 32.9 | 34.7 | 33.3 |
| CO | Carbon monoxide | 630-08-0 | 31.3 | 29.6 | 29.8 |
| H2 | Hydrogen | 1333-74-0 | 15.3 | 15.6 | 16.7 |
| C2H4 | Ethylene | 74-85-1 | 7.9 | 7.6 | 7.8 |
| CH4 | Methane | 74-82-8 | 5.5 | 5.5 | 5.5 |
| EMC | Ethyl Methyl Carbonate | 623-53-0 | 1.8 | 1.7 | 1.5 |
| H2O | Water | 7732-18-5 | 1.2 | 1.1 | 1.5 |
| MeOH | Methanol | 67-56-1 | 0.91 | 0.82 | 0.95 |
| C6H5F | Fluorobenzene | 462-06-6 | 0.88 | 0.84 | 0.79 |
| C3H6 | Propylene | 115-07-1 | 0.70 | 0.80 | 0.59 |
| C2H6 | Ethane | 74-84-0 | 0.48 | 0.50 | 0.48 |
| C6H6 | Benzene | 89-05-4 | 0.29 | 0.25 | 0.25 |
| EtO | Ethanol | 75-07-0 | 0.25 | 0.28 | 0.20 |
| EtOH | Ethanol | 64-17-5 | 0.13 | 0.17 | 0.14 |
| C2H2 | Acetylene | 74-86-2 | 0.13 | 0.13 | 0.16 |
| DMC | Dimethyl carbonate | 616-38-6 | 0.058 | 0.27 | 0.10 |
| C4H10 | Butane | 106-97-8 | 0.17 | 0.20 | 0.11 |
| C3H8 | Propane | 74-98-6 | 0.047 | 0.052 | 0.043 |
| EtAc | Ethyl acetate | 141-78-6 | 0.0083 | 0.010 | 0.12 |
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental Analysis of Thermal Runaway and Propagation in Lithium-Ion Battery Modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Legrand, N.; Knosp, B.; Desprez, P.; Lapicque, F.; Raël, S. Physical characterization of the charging process of a Li-ion battery and prediction of Li plating by electrochemical modelling. J. Power Sources 2014, 245, 208–216. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Kasper, M.; Grolleau, S.; Couceiro, C.G.; Trad, K.; Matadi, B.P.; Wohlfahrt-Mehrens, M. Interplay of Operational Parameters on Lithium Deposition in Lithium-Ion Cells: Systematic Measurements with Reconstructed 3-Electrode Pouch Full Cells. J. Electrochem. Soc. 2016, 163, A1232–A1238. [Google Scholar] [CrossRef]
- Fuchs, G.; Willenberg, L.; Ringbeck, F.; Sauer, D.U. Post-Mortem Analysis of Inhomogeneous Induced Pressure on Commercial Lithium-Ion Pouch Cells and Their Effects. Sustainability 2019, 11, 6738. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, C.; Li, H.; Du, J.; Xiong, R. Detecting undesired lithium plating on anodes for lithium-ion batteries—A review on the in-situ methods. Appl. Energy 2021, 300, 117386. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding undesirable anode lithium plating issues in lithium-ion batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Cai, W.; Yan, C.; Yao, Y.-X.; Xu, L.; Chen, X.-R.; Huang, J.-Q.; Zhang, Q. The Boundary of Lithium Plating in Graphite Electrode for Safe Lithium-Ion Batteries. Angew. Chem. Int. Ed Engl. 2021, 60, 13007–13012. [Google Scholar] [CrossRef]
- Waldmann, T.; Wohlfahrt-Mehrens, M. Effects of rest time after Li plating on safety behavior—ARC tests with commercial high-energy 18650 Li-ion cells. Electrochim. Acta 2017, 230, 454–460. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, X.; Feng, X.; Ren, D.; Li, Y.; Hou, J.; Wu, Y.; Du, J.; Lu, L.; Ouyang, M. Battery eruption triggered by plated lithium on an anode during thermal runaway after fast charging. Energy 2022, 239, 122097. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef]
- Orsini, F.; Du Pasquier, A.; Beaudoin, B.; Tarascon, J.; Trentin, M.; Langenhuizen, N.; de Beer, E.; Notten, P. In situ Scanning Electron Microscopy (SEM) observation of interfaces within plastic lithium batteries. J. Power Sources 1998, 76, 19–29. [Google Scholar] [CrossRef]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A three-dimensional thermal abuse model for lithium-ion cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Mandal, B.K.; Padhi, A.K.; Shi, Z.; Chakraborty, S.; Filler, R. Thermal runaway inhibitors for lithium battery electrolytes. J. Power Sources 2006, 161, 1341–1345. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Spotnitz, R.M.; Weaver, J.; Yeduvaka, G.; Doughty, D.H.; Roth, E.P. Simulation of abuse tolerance of lithium-ion battery packs. J. Power Sources 2007, 163, 1080–1086. [Google Scholar] [CrossRef]
- Fleischmann, C.; Weinschenk, C.; Madrzykowski, D.; Schraiber, A.; Gaudet, B. Quantifying the Fire Hazard from Li-Ion Battery Fires Caused by Thermal Runaway in E-scooters. Fire Technol. 2025, 61, 2865–2887. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Friesen, A.; Horsthemke, F.; Mönnighoff, X.; Brunklaus, G.; Krafft, R.; Börner, M.; Risthaus, T.; Winter, M.; Schappacher, F.M. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J. Power Sources 2016, 334, 1–11. [Google Scholar] [CrossRef]
- Börner, M.; Friesen, A.; Grützke, M.; Stenzel, Y.P.; Brunklaus, G.; Haetge, J.; Nowak, S.; Schappacher, F.M.; Winter, M. Correlation of aging and thermal stability of commercial 18650-type lithium ion batteries. J. Power Sources 2017, 342, 382–392. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Abbas, S.M.; Drießen, C.; Sprenger, M.; Ellersdorfer, C.; Hanzu, I.; Gstrein, G. Nondestructive Electrochemical Identification of Lithium Plating in High-Energy Automotive Batteries. ACS Omega 2025, 10, 13209–13217. [Google Scholar] [CrossRef]
- Abbas, S.M.; Jodlbauer, A.; Wilkening, M.; Helmar, W.; Ecker, J.V.; Ellersdorfer, C.; Gstrein, G.; Hanzu, I. Post-mortem identification of lithium plating in high energy automotive batteries. Sustain. Energy Fuels 2025. [Google Scholar] [CrossRef]
- Bello, I.T.; Raza, H.; Michael, A.T.; Muneeswara, M.; Tewari, N.; Bingsen, W.; Cheung, Y.N.; Choi, Z.; Boles, S.T. Charging Ahead: The Evolution and Reliability of Nickel-Zinc Battery Solutions. EcoMat 2025, 7, e12505. [Google Scholar] [CrossRef]
- Ren, D.; Liu, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components. Appl. Energy 2018, 228, 633–644. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Planteu, R.; Krohn, P.; Rasch, B.; Brunnsteiner, B.; Thaler, A.; Hacker, V. Thermal runaway of large automotive Li-ion batteries. RSC Adv. 2018, 8, 40172–40186. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Carter, R.E.; Love, C.T.; Mukherjee, P.P. Correlating lithium plating quantification with thermal safety characteristics of lithium-ion batteries. Energy Storage Mater. 2024, 66, 103214. [Google Scholar] [CrossRef]
- Onuki, M.; Kinoshita, S.; Sakata, Y.; Yanagidate, M.; Otake, Y.; Ue, M.; Deguchi, M. Identification of the Source of Evolved Gas in Li-Ion Batteries Using #2#1-labeled Solvents. J. Electrochem. Soc. 2008, 155, A794. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.; Xu, J.; Ning, F.; Wang, D. Investigation on gas generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Z.; Zhai, B.; Li, X.; Hu, W.; Zhang, H.; Cheng, S.; Xie, J. Fluorobenzene-based diluted highly concentrated carbonate electrolyte for practical high-voltage lithium metal batteries. J. Power Sources 2021, 506, 230086. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Z.; Zhong, W.; Ge, Z.; Li, L.; Lei, S.; Wu, Q.; Zhang, H.; Cheng, S.; Xie, J. Non-flammable fluorobenzene-diluted highly concentrated electrolytes enable high-performance Li-metal and Li-ion batteries. J. Colloid Interface Sci. 2022, 619, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Gstrein, G.; Abbas, S.M.; Ewert, E.; Wenzl, M.; Ellersdorfer, C. Safety-Critical Influence of Ageing on Mechanical Properties of Lithium-Ion Pouch Cells. Batteries 2025, 11, 99. [Google Scholar] [CrossRef]
- Abbas, S.M.; Gstrein, G.; Jauernig, A.D.; Schmid, A.; Michelini, E.; Hinterberger, M.; Ellersdorfer, C. Influence of Lithium Plating on the Mechanical Properties of Automotive High-Energy Pouch Batteries. Batteries 2025, 11, 330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).