NVPF Sodium-Ion Versus NMC and LFP Lithium-Ion Batteries in Thermal Runaway: Vent Gas Composition and Thermal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Cells
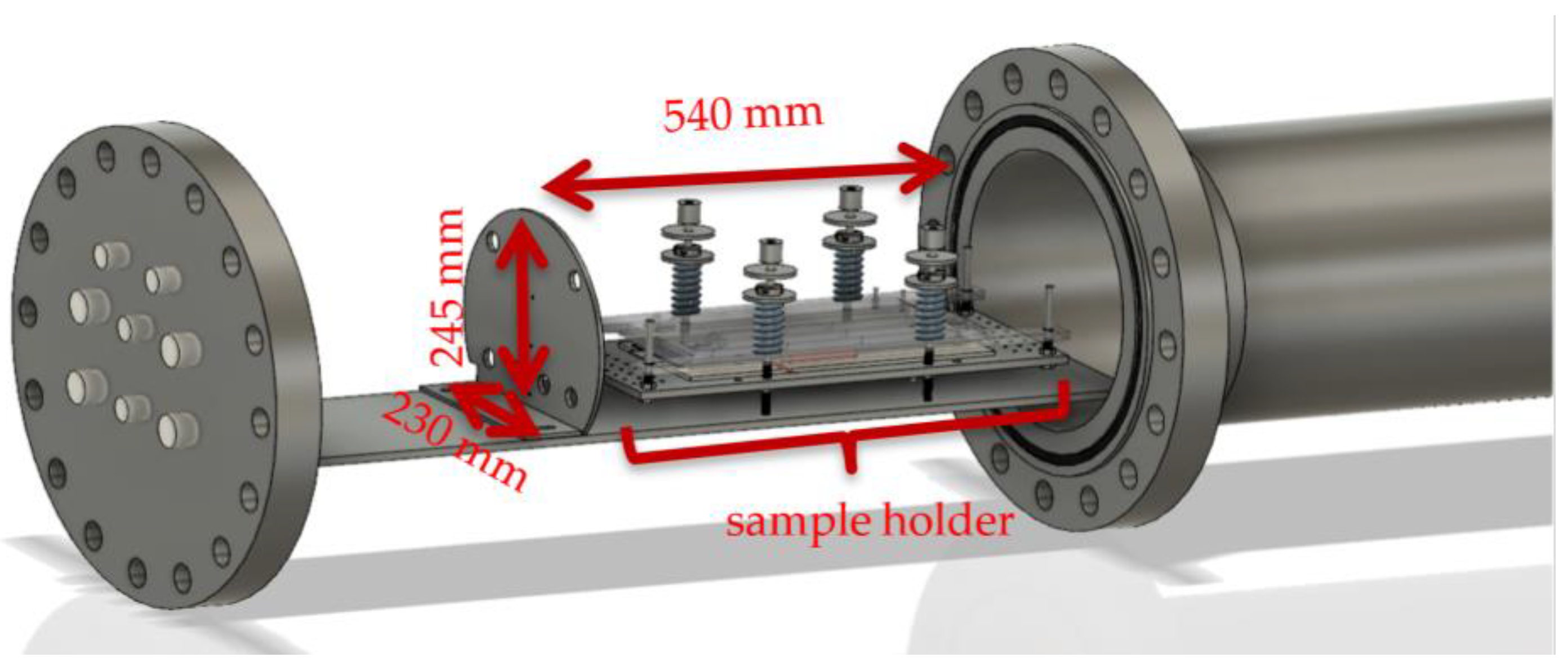
2.2. Conditions & Trigger
2.2.1. Overtemperature
2.2.2. Overcharge
2.2.3. Nail Penetration
2.2.4. Short Circuit
3. Results and Discussion
3.1. Influence of Different Cathode Materials
3.2. Influence of TR Triggers
4. Conclusions
- The NVPF cell with an energy density of 90 Wh/kg experiences the least destructive TR: it releases 0.05 mol/Ah of vent gas with a maximum vent gas temperature of 265 °C. Compositionally CO2, DMC, H2, CO, butane, ethene, methane and O2 are measured at >2%. The largest share is CO2, at 42%. Smaller amounts of H2 (15%) and CO (10%) were detected.
- The NMC cell has the highest energy density of 230 Wh/kg and showed the most destructive TR: the vent gas amounts to 0.07 mol/Ah and a maximum vent gas temperature of up to 1000 °C. Besides CO2 (24%), two prominent vent gas components are CO (36%) and H2 (19%).
- The LFP cell releases 0.02 mol/Ah, with an energy density of 173 Wh/kg. The vent gas contains H2 as the major gas component at 41%, followed by CO2 (27%) and a minor share of CO (8%). The vent gas temperature reaches a maximum of 446 °C.
- Overcharging the cell results in the highest amount of vent gas produced, at 1.23 mol (0.07 mol/Ah). It contains the highest share of H2 (23%) compared to the other triggers.
- The OT-1 and OT-2 side experiments resulted in 0.91 mol and 0.92 mol vent gas.
- The lowest amount of vent gas was produced by the externally short-circuited cell, at 0.14 mol.
- HF was solely recorded for the OT-2 side trigger (135.36 ppm); it is assumed that small amounts of HF were also produced for all other investigated cells and triggers, but it reacted with the ejected particles in the reactor and the analysis pathway.
- The highest vent gas temperature was recorded with the OT-2 side trigger (265 °C) and the lowest with the short trigger (94 °C). A maximum vent gas temperature of 259 °C was recorded for the OC trigger. The OT-1 side and nail-triggered cell reached maximum values of 199 °C and 162 °C.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMC | Dimethyl carbonate |
| EC | Ethyl carbonate |
| EMC | Ethyl methyl carbonate |
| FTIR | Fourier-Transformation-Infrared spectroscopy |
| GC | Gas chrmoatography |
| LFL | Lower flammability limit |
| Li-ion | Lithium ion |
| Nail | Nail penetration trigger method |
| Na-ion | Sodium ion |
| OC | Overcharge |
| OT-1 side | One sided overtemperature trigger method |
| OT-2 side | Two sided overtemperature trigger method |
| SEI | Solid electrolyte interface |
| Short | External short circuit trigger method |
| TR | Thermal runaway |
| UFL | Upper flammability limit |
References
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 38. [Google Scholar] [CrossRef]
- Bai, H.; Song, Z. Lithium-ion battery, sodium-ion battery, or redox-flow battery: A comprehensive comparison in renewable energy systems. J. Power Sources 2023, 580, 233426. [Google Scholar] [CrossRef]
- Bhutia, P.T.; Grugeon, S.; El Mejdoubi, A.; Laruelle, S.; Marlair, G. Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems. Batteries 2024, 10, 370. [Google Scholar] [CrossRef]
- Bhutia, P.T.; Grugeon, S.; Bertrand, J.-P.; Binotto, G.; Bordes, A.; El Mejdoubi, A.; Laruelle, S.; Marlair, G. Fire hazards of carbonate-based electrolytes for sodium-ion batteries: What changes from lithium-ion batteries? J. Power Sources 2024, 622, 235234. [Google Scholar] [CrossRef]
- Mohan, I.; Raj, A.; Shubham, K.; Lata, D.B.; Mandal, S.; Kumar, S. Potential of potassium and sodium-ion batteries as the future of energy storage: Recent progress in anodic materials. J. Energy Storage 2022, 55, 105625. [Google Scholar] [CrossRef]
- Boddu, V.R.R.; Puthusseri, D.; Shirage, P.M.; Mathur, P.; Pol, V.G. Layered NaxCoO2-based cathodes for advanced Na-ion batteries: Review on challenges and advancements. Ionics 2021, 27, 4549–4572. [Google Scholar] [CrossRef]
- Schöberl, J.; Ohneseit, S.; Schaeffler, S.; Förstermann, D.; Grahl, L.; Jossen, A.; Ziebert, C.; Lienkamp, M. Thermal runaway characterization of cylindrical lithium-ion and sodium-ion batteries with various sizes and energy contents. J. Power Sources 2025, 648, 237240. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- Mei, W.; Cheng, Z.; Wang, L.; Teng, A.; Li, Z.; Jin, K.; Sun, J.; Wang, Q. Thermal hazard comparison and assessment of Li-ion battery and Na-ion battery. J. Energy Chem. 2025, 102, 18–26. [Google Scholar] [CrossRef]
- Ping, P.; Ren, X.; Kong, D.; Gao, W.; Zhang, Y.; Yang, C.; Wang, G.; Feng, Z.; Guo, J.; Ren, J. Multi-scale thermal runaway analysis of sodium-ion batteries and comparative safety assessment with lithium-ion batteries. Compos. Part B Eng. 2025, 302, 112532. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Wang, J.; Wang, C.; He, X.; Cheng, Z.; Li, H.; Mei, W.; Wang, Q. Thermal runaway and gas venting behaviors of large-format prismatic sodium-ion battery. Energy Storage Mater. 2025, 77, 104197. [Google Scholar] [CrossRef]
- Wang, Z.; Yua, X.; Yin, B.; Xie, R.; Shia, B.; He, J.; Chen, J. Thermal runaway mechanisms and explosion risk evolution of sodium-ion batteries with varying states of charge in a confined chamber. Therm. Sci. Eng. Prog. 2025, 65, 103945. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Fuchs, A. Comparing Different Thermal Runaway Triggers for Two Automotive Lithium-Ion Battery Cell Types. J. Electrochem. Soc. 2020, 167, 130542. [Google Scholar] [CrossRef]
- Amano, K.O.A.; Hahn, S.-K.; Tschirschwitz, R.; Rappsilber, T.; Krause, U. An Experimental Investigation of Thermal Runaway and Gas Release of NMC Lithium-Ion Pouch Batteries Depending on the State of Charge Level. Batteries 2022, 8, 41. [Google Scholar] [CrossRef]
- Velumani, D.; Bansal, A. Thermal Behavior of Lithium- and Sodium-Ion Batteries: A Review on Heat Generation, Battery Degradation, Thermal Runway—Perspective and Future Directions. Energy Fuels 2022, 36, 14000–14029. [Google Scholar] [CrossRef]
- Bordes, A.; Marlair, G.; Zantman, A.; Chesnaye, A.; Le Lore, P.-A.; Lecocq, A. Safety Evaluation of a Sodium-Ion Cell: Assessment of Vent Gas Emissions under Thermal Runaway. ACS Energy Lett. 2022, 7, 3386–3391. [Google Scholar] [CrossRef]
- Dreyer, S.L.; Kondrakov, A.; Janek, J.; Brezesinski, T. In situ analysis of gas evolution in liquid- and solid-electrolyte-based batteries with current and next-generation cathode materials. J. Mater. Res. 2022, 37, 3146–3168. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.-M.; Trabesinger, S. Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry. Energy Storage Mater. 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, K.; Jiang, X.; Shu, C.-M.; Sun, X. Thermal Runaway Critical Threshold and Gas Release Safety Boundary of 18,650 Lithium-Ion Battery in State of Charge. Processes 2025, 13, 2175. [Google Scholar] [CrossRef]
- Sturk, D.; Rosell, L.; Blomqvist, P.; Tidblad, A.A. Analysis of Li-Ion Battery Gases Vented in an Inert Atmosphere Thermal Test Chamber. Batteries 2019, 5, 61. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Groth, K.M.; Al-Douri, A. Hydrogen safety, risk, and reliability analysis. In Hydrogen Economy, 2nd ed.; Scipioni, A., Manzardo, A., Ren, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 487–510. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 2010. [Google Scholar]
- Schöberl, J.; Ank, M.; Schreiber, M.; Wassiliadis, N.; Lienkamp, M. Thermal runaway propagation in automotive lithium-ion batteries with NMC-811 and LFP cathodes: Safety requirements and impact on system integration. Etransportation 2024, 19, 100305. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Wang, J.; Tong, X.; Yan, W. Lower explosion limit of the vented gases from Li-ion batteries thermal runaway in high temperature condition. J. Loss Prev. Process Ind. 2020, 63, 103992. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. Etransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Qi, C.; Wang, H.; Li, M.; Li, C.; Li, Y.; Shi, C.; Wei, N.; Wang, Y.; Zhang, H. Research on the Thermal Runaway Behavior and Flammability Limits of Sodium-Ion and Lithium-Ion Batteries. Batteries 2025, 11, 24. [Google Scholar] [CrossRef]
- Wang, K.; Wu, D.; Chang, C.; Zhang, J.; Ouyang, D.; Qian, X. Charging rate effect on overcharge-induced thermal runaway characteristics and gas venting behaviors for commercial lithium iron phosphate batteries. J. Clean. Prod. 2024, 434, 139992. [Google Scholar] [CrossRef]
- Samigullin, R.R.; Drozhzhin, O.A.; Antipov, E.V. Comparative Study of the Thermal Stability of Electrode Materials for Li-Ion and Na-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 14–19. [Google Scholar] [CrossRef]




| Parameter | NVPF | NMC | LFP |
|---|---|---|---|
| Design | Prismatic hard case | Prismatic hard case | Prismatic hard case |
| Cathode | Na3V2(PO4)2F3 | LiNi0.6Mn0.2Co0.2O2 | LiFePO4 |
| Anode | Hard carbon | Graphite | Graphite |
| Weight | 650 g | 960 g | 1350 g |
| Gravimetric energy density | 90 Wh/kg | 235 Wh/kg | 173 Wh/kg |
| Nominal voltage | 3.7 V | 3.7 V | 3.2 V |
| Aging state | Fresh, unused | Fresh, unused | Fresh, unused |
| Start SOC | 100% | 100% | 100% |
| Cell thickness | 26.7 mm | 28.0 mm | 26.8 mm |
| Triggers | OT-2 Side | OT-1 Side | OC | Nail | Short |
|---|---|---|---|---|---|
| Atmosphere | N2 | N2 | N2 | N2 | N2 |
| Heat ramp/°C·min−1 | 2 | 2 | - | - | - |
| SOC/% | 100 | 100 | >100 | 100 | 100 |
| Current/A | - | - | +300 | - | 1000 |
| Cell chemistry | NVPF, NMC, LFP | NVPF | NVPF | NVPF | NVPF |
| Gases | NVPF /mmol/Ah | NMC /mmol/Ah | LFP /mmol/Ah |
|---|---|---|---|
| H2 | 7.7 | 13.2 | 9.1 |
| CH4 | 1.0 | 5.2 | 1.1 |
| CO | 5.1 | 25.3 | 1.8 |
| CO2 | 21.5 | 16.8 | 6.0 |
| C2H4 | 3.1 | 3.5 | 0.7 |
| C2H6 | 0.1 | 0.3 | 0.2 |
| C2H2 | 0.0 | 0.3 | 0.0 |
| C3H6 | 0.0 | 0.0 | 0.0 |
| C3H8 | 0.0 | 0.0 | 0.0 |
| H2O | 0.4 | 2.1 | 1.1 |
| DEC | 0.0 | 0.0 | 0.0 |
| DMC | 7.7 | 2.1 | 0.9 |
| EMC | 0.0 | 0.0 | 0.0 |
| C6H14 | 0.0 | 0.2 | 0.1 |
| C4H10 | 2.6 | 1.3 | 0.9 |
| HF | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdigg, G.; Mair, C. NVPF Sodium-Ion Versus NMC and LFP Lithium-Ion Batteries in Thermal Runaway: Vent Gas Composition and Thermal Analysis. Batteries 2025, 11, 323. https://doi.org/10.3390/batteries11090323
Ferdigg G, Mair C. NVPF Sodium-Ion Versus NMC and LFP Lithium-Ion Batteries in Thermal Runaway: Vent Gas Composition and Thermal Analysis. Batteries. 2025; 11(9):323. https://doi.org/10.3390/batteries11090323
Chicago/Turabian StyleFerdigg, Gabriel, and Christiane Mair (Essl). 2025. "NVPF Sodium-Ion Versus NMC and LFP Lithium-Ion Batteries in Thermal Runaway: Vent Gas Composition and Thermal Analysis" Batteries 11, no. 9: 323. https://doi.org/10.3390/batteries11090323
APA StyleFerdigg, G., & Mair, C. (2025). NVPF Sodium-Ion Versus NMC and LFP Lithium-Ion Batteries in Thermal Runaway: Vent Gas Composition and Thermal Analysis. Batteries, 11(9), 323. https://doi.org/10.3390/batteries11090323







