Exploring the Potential of Green Synthesized Sr0.8Ce0.2Fe0.8Co0.2O3 Using Orange and Lemon Extracts for Hybrid Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
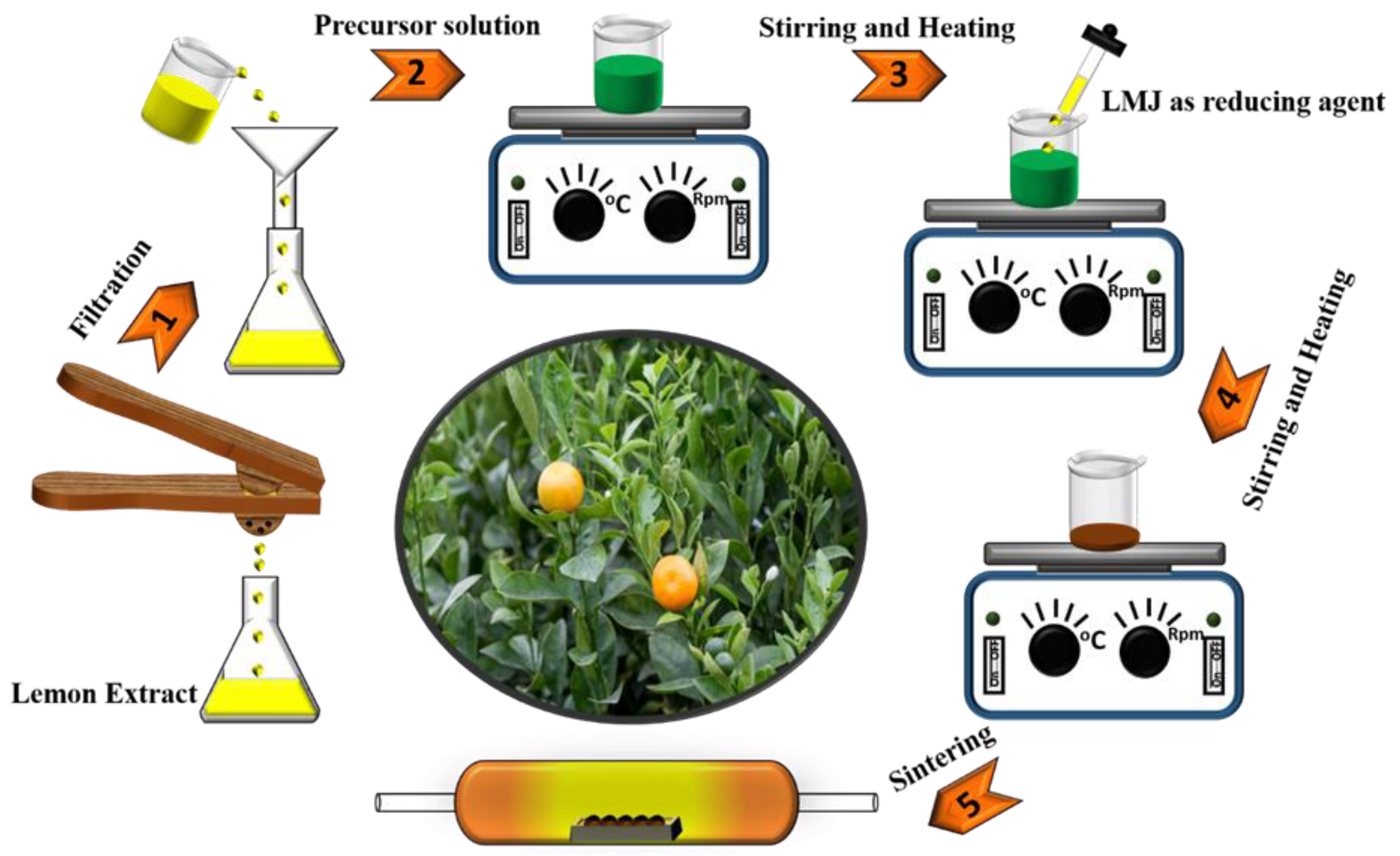
2.1. Powder Synthesis
2.2. Electrode Fabrication
2.3. Powder Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Crystal Structure
3.2. Chemical Bond Analysis
3.3. Photoluminescence
3.4. Morphological Study
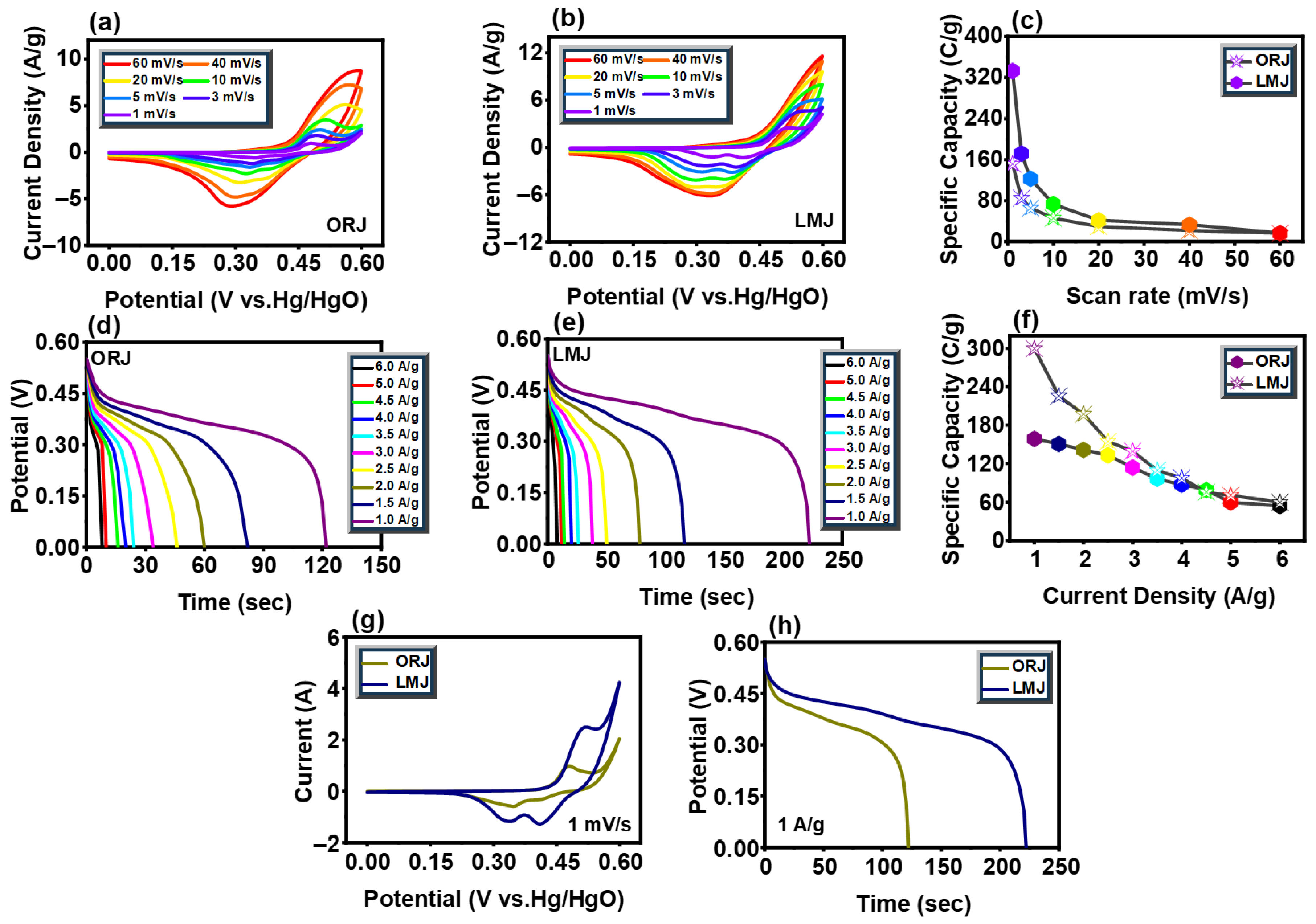
3.5. Electrochemical Performance in Three-Electrode Assembly
- Working Mechanism
- Oxygen Intercalation
- Surface Redox Pseudocapacitance
3.6. Electrochemical Performance in Two-Electrode Assembly
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhaouadi, R.; Al-Othman, A.; Aidan, A.A.; Tawalbeh, M.; Zannerni, R. A Characterization Study for the Properties of Dust Particles Collected on Photovoltaic (PV) Panels in Sharjah, United Arab Emirates. Renew. Energy 2021, 171, 133–140. [Google Scholar] [CrossRef]
- Mahmoud, M.; Ramadan, M.; Olabi, A.-G.; Pullen, K.; Naher, S. A Review of Mechanical Energy Storage Systems Combined with Wind and Solar Applications. Energy Convers. Manag. 2020, 210, 112670. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, H.; Jin, Y.; Wang, L.; Gao, F.; Lu, Q. Hybrid α-Fe2O3@ Ni(OH)2 Nanosheet Composite for High-Rate-Performance Supercapacitor Electrode. Sci. Rep. 2016, 6, 31751. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Mohanty, D.; Hung, I.-M.; Moniruzzaman, M.; Satpathy, S.K. Unleashing Recent Electrolyte Materials for Next-Generation Supercapacitor Applications: A Comprehensive Review. J. Energy Storage 2023, 72, 108352. [Google Scholar] [CrossRef]
- Du, N.; Zheng, W.; Li, X.; He, G.; Wang, L.; Shi, J. Nanosheet-Assembled NiS Hollow Structures with Double Shells and Controlled Shapes for High-Performance Supercapacitors. Chem. Eng. J. 2017, 323, 415–424. [Google Scholar] [CrossRef]
- Zhou, Y.; Maleski, K.; Anasori, B.; Thostenson, J.O.; Pang, Y.; Feng, Y.; Zeng, K.; Parker, C.B.; Zauscher, S.; Gogotsi, Y.; et al. Ti3C2Tx MXene-Reduced Graphene Oxide Composite Electrodes for Stretchable Supercapacitors. ACS Nano 2020, 14, 3576–3586. [Google Scholar] [CrossRef]
- Tatrari, G.; Karakoti, M.; Tewari, C.; Pandey, S.; Bohra, B.S.; Dandapat, A.; Sahoo, N.G. Solid Waste-Derived Carbon Nanomaterials for Supercapacitor Applications: A Recent Overview. Mater. Adv. 2021, 2, 1454–1484. [Google Scholar] [CrossRef]
- Maity, C.K.; Goswami, N.; Verma, K.; Sahoo, S.; Nayak, G.C. A Facile Synthesis of Boron Nitride Supported Zinc Cobalt Sulfide Nano Hybrid as High-Performance Pseudocapacitive Electrode Material for Asymmetric Supercapacitors. J. Energy Storage 2020, 32, 101993. [Google Scholar] [CrossRef]
- Oje, A.I.; Ogwu, A.A.; Mirzaeian, M.; Tsendzughul, N.; Oje, A.M. Pseudo-Capacitance of Silver Oxide Thin Film Electrodes in Ionic Liquid for Electrochemical Energy Applications. J. Sci. Adv. Mater. Devices 2019, 4, 213–222. [Google Scholar] [CrossRef]
- Li, X.; Wei, B. Supercapacitors Based on Nanostructured Carbon. Nano Energy 2013, 2, 159–173. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A.G. Heteroatom Doped High Porosity Carbon Nanomaterials as Electrodes for Energy Storage in Electrochemical Capacitors: A Review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Ponce, M.F.; Mamani, A.; Jerez, F.; Castilla, J.; Ramos, P.B.; Acosta, G.G.; Sardella, M.F.; Bavio, M.A. Activated Carbon from Olive Tree Pruning Residue for Symmetric Solid-State Supercapacitor. Energy 2022, 260, 125092. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Sahoo, R.; Pal, A.; Pal, T. Noble Metal–Transition Metal Oxides/Hydroxides: Desired Materials for Pseudocapacitor. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 395–430. [Google Scholar]
- Brezesinski, K.; Wang, J.; Haetge, J.; Reitz, C.; Steinmueller, S.O.; Tolbert, S.H.; Smarsly, B.M.; Dunn, B.; Brezesinski, T. Pseudocapacitive Contributions to Charge Storage in Highly Ordered Mesoporous Group V Transition Metal Oxides with Iso-Oriented Layered Nanocrystalline Domains. J. Am. Chem. Soc. 2010, 132, 6982–6990. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ahmed, F.; Ullah, N.; Ansari, S.A.; Hussain, S.; Ibrahim, A.A.; Qasem, H.; Kumar, S.A.; Alhamami, M.A.; Almehbad, N. Exploring the Potential of Reduced Graphene Oxide/Polyaniline (rGO@ PANI) Nanocomposites for High-Performance Supercapacitor Application. Electrochim. Acta 2024, 479, 143743. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Zhong, Y.; Xu, X.; Veder, J.-P.M.; Rowles, M.R.; Saunders, M.; Ran, R.; Shao, Z. Activation-Free Supercapacitor Electrode Based on Surface-Modified Sr2CoMo1-xNixO6-δ Perovskite. Chem. Eng. J. 2020, 390, 124645. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, J.; Li, X.; Yue, L.; Liu, Q.; Lu, S.; Asiri, A.M.; Hu, J.; Luo, Y.; Sun, X. Recent Advances in Perovskite Oxides as Electrode Materials for Supercapacitors. Chem. Commun. 2021, 57, 2343–2355. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M.D.F. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef]
- Mei, P.; Kaneti, Y.V.; Pramanik, M.; Takei, T.; Dag, Ö.; Sugahara, Y.; Yamauchi, Y. Two-Dimensional Mesoporous Vanadium Phosphate Nanosheets through Liquid Crystal Templating Method toward Supercapacitor Application. Nano Energy 2018, 52, 336–344. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The Role of Oxygen Vacancies of ABO 3 Perovskite Oxides in the Oxygen Reduction Reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Agarwal, A.; Sankapal, B.R. Metal Phosphides: Topical Advances in the Design of Supercapacitors. J. Mater. Chem. A 2021, 9, 20241–20276. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation Pseudocapacitance in Electrochemical Energy Storage: Recent Advances in Fundamental Understanding and Materials Development. Mater. Today Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chen, W.-C.; Chan, S.-H.; Su, W.-F. The Effect of Strontium and Barium Doping on Perovskite-Structured Energy Materials for Photovoltaic Applications. Appl. Surf. Sci. 2018, 429, 9–15. [Google Scholar] [CrossRef]
- Adimule, V.; Bhat, V.S.; Yallur, B.C.; Gowda, A.H.; Padova, P.D.; Hegde, G.; Toghan, A. Facile Synthesis of Novel SrO0.5: MnO0.5 Bimetallic Oxide Nanostructure as a High-Performance Electrode Material for Supercapacitors. Nanomater. Nanotechnol. 2022, 12, 184798042110640. [Google Scholar] [CrossRef]
- Liu, G.F.; Ma, P.P.; Qiao, Y.; Xu, R.H.; Hu, R.Y.; Liu, L.Y.; Jiang, G.H.; Demir, M. Perovskite SrCo1-xTixO3-δ as Anion-Intercalated Electrode Materials for Supercapacitors. J. Energy Storage 2022, 52, 104942. [Google Scholar] [CrossRef]
- Salas, M.A.S.; De Paoli, J.M.; Pérez, O.E.L.; Bajales, N.; Fuertes, V.C. Synthesis and Characterization of Alumina-Embedded SrCo0.95V0.05O3 Nanostructured Perovskite: An Attractive Material for Supercapacitor Devices. Microporous Mesoporous Mater. 2020, 293, 109797. [Google Scholar] [CrossRef]
- Ahangari, M.; Mostafaei, J.; Sayyah, A.; Mahmoudi, E.; Asghari, E.; Coruh, A.; Delibas, N.; Niaei, A. Investigation of Structural and Electrochemical Properties of SrFexCo1-xO3-δ Perovskite Oxides as a Supercapacitor Electrode Material. J. Energy Storage 2023, 63, 107034. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, G.; Xu, R.; Hu, R.; Liu, L.; Jiang, G.; Demir, M.; Ma, P. SrFe1-xZrxO3-δ Perovskite Oxides as Negative Electrodes for Supercapacitors. Electrochim. Acta 2023, 437, 141527. [Google Scholar] [CrossRef]
- Mendoza, R.; Oliva, J.; Padmasree, K.P.; Mtz-Enriquez, A.I.; Zakhidov, A.; Encinas, A. Using the Amorphous-Carbon Derived from Cigarette Filters for the Fabrication of Highly Efficient Flexible Supercapacitors and Role of the Sr3.2Y0.8Fe1.5Co1.5O10 Layered Perovskite to Enhance Their Electrochemical Performance. J. Energy Storage 2023, 60, 106539. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Supercapacitor Behavior of Cerium Oxide Nanoparticles in Neutral Aqueous Electrolytes. Energy Fuels 2015, 29, 8246–8253. [Google Scholar] [CrossRef]
- Ansari, A.A.; Adil, S.F.; Alam, M.; Ahmad, N.; Assal, M.E.; Labis, J.P.; Alwarthan, A. Catalytic Performance of the Ce-Doped LaCoO3 Perovskite Nanoparticles. Sci. Rep. 2020, 10, 15012. [Google Scholar] [CrossRef]
- Mohan, M.; Shetti, N.P.; Aminabhavi, T.M. Perovskites: A New Generation Electrode Materials for Storage Applications. J. Power Sources 2023, 574, 233166. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Chen, J.; Yang, H.; Xia, L.; Chen, J.; Duan, J.; Chen, Z. Effects of Chelating Agents on Electrochemical Properties of Na0.9Ni0.45Mn0.55O2 Cathode Materials. J. Alloys Compd. 2021, 855, 157485. [Google Scholar] [CrossRef]
- Priyadharsini, N.; Kasturi, P.R.; Shanmugavani, A.; Surendran, S.; Shanmugapriya, S.; Selvan, R.K. Effect of Chelating Agent on the Sol-Gel Thermolysis Synthesis of LiNiPO4 and Its Electrochemical Properties for Hybrid Capacitors. J. Phys. Chem. Solids 2018, 119, 183–192. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumar, K.D.; Kim, H.-J. Facile Preparation of a Highly Efficient NiZn2O4-NiO Nanoflower Composite Grown on Ni Foam as an Advanced Battery-Type Electrode Material for High-Performance Electrochemical Supercapacitors. Dalton Trans. 2020, 49, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.D.; Kumar, A.J.; Balachandran, S.; Kusmartsev, F.V.; Trabelsi, A.B.G.; Alkallas, F.H.; Nagarani, S.; Sethuraman, V.; Lee, B.-K. High-Performance Chrysanthemum Flower-like Structure of Ni Doped ZnO Nanoflowers for Pseudo-Supercapacitors. J. Energy Storage 2023, 72, 108441. [Google Scholar] [CrossRef]
- Fazal, A.; Iqbal, M.J.; Raza, M.A.; Almutairi, B.S.; Iqbal, M.Z.; Subhani, T.; Riaz, S.; Naseem, S. Binder-Free Hydrothermal Approach to Fabricate High-Performance Zinc Phosphate Electrode for Energy Storage Applications. Ceram. Int. 2024, 50, 2742–2753. [Google Scholar] [CrossRef]
- Hekmat, F.; Hosseini, H.; Shahrokhian, S.; Unalan, H.E. Hybrid Energy Storage Device from Binder-Free Zinc-Cobalt Sulfide Decorated Biomass-Derived Carbon Microspheres and Pyrolyzed Polyaniline Nanotube-Iron Oxide. Energy Storage Mater. 2020, 25, 621–635. [Google Scholar] [CrossRef]
- Zhao, F.; Xie, D.; Song, X.; Wu, H.; Zhang, Q.; Zou, J.; Zeng, X. Construction of Hydrangea-like Nickel Cobalt Sulfide through Efficient Microwave-Assisted Approach for Remarkable Supercapacitors. Appl. Surf. Sci. 2021, 539, 148260. [Google Scholar] [CrossRef]
- Tummino, M.L.; Liotta, L.F.; Magnacca, G.; Lo Faro, M.; Trocino, S.; Campagna Zignani, S.; Aricò, A.S.; Deganello, F. Sucrose-Assisted Solution Combustion Synthesis of Doped Strontium Ferrate Perovskite-Type Electrocatalysts: Primary Role of the Secondary Fuel. Catalysts 2020, 10, 134. [Google Scholar] [CrossRef]
- Tummino, M.L.; Vineis, C.; Varesano, A.; Liotta, L.F.; Rigoletto, M.; Laurenti, E.; Deganello, F. Sr0.85Ce0.15Fe0.67Co0.33-xCuxO3 Perovskite Oxides: Effect of B-Site Copper Codoping on the Physicochemical, Catalytic and Antibacterial Properties upon UV or Thermal Activation. Front. Environ. Eng. 2023, 2, 1249931. [Google Scholar] [CrossRef]
- Deganello, F.; Liotta, L.F.; Leonardi, S.G.; Neri, G. Electrochemical Properties of Ce-Doped SrFeO3 Perovskites-Modified Electrodes towards Hydrogen Peroxide Oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Prevot, A.B.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Markov, A.A.; Nikitin, S.S.; Leonidov, I.A.; Patrakeev, M.V. Oxygen and Electron Transport in Ce0.1Sr0.9FeO3-δ. Solid. State Ion. 2020, 344, 115131. [Google Scholar] [CrossRef]
- Wei, Y.; Gui, H.; Zhao, Z.; Li, J.; Liu, Y.; Xin, S.; Li, X.; Xie, W. Structure and Magnetic Properties of the Perovskite YCo0.5Fe0.5O3. AIP Adv. 2014, 4, 127134. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, X.; Meng, J.; Meng, J.; Meng, F. A Niobium and Tungsten Co-Doped SrFeO3-δ Perovskite as Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Ceram. Int. 2019, 45, 7351–7358. [Google Scholar] [CrossRef]
- Palenik, R.C.; Abboud, K.A.; Palenik, G.J. Bond Valence Sums and Structural Studies of Antimony Complexes Containing Sb Bonded Only to O Ligands. Inorganica Chim. Acta 2005, 358, 1034–1040. [Google Scholar] [CrossRef]
- Talaei, M.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Synthesis of Mesoporous CuFe2O4@ SiO2 Core-Shell Nanocomposite for Simultaneous Drug Release and Hyperthermia Applications. Ceram. Int. 2021, 47, 30287–30297. [Google Scholar] [CrossRef]
- Aydoghmish, S.M.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Facile Synthesis and Investigation of NiO-ZnO-Ag Nanocomposites as Efficient Photocatalysts for Degradation of Methylene Blue Dye. Ceram. Int. 2019, 45, 14934–14942. [Google Scholar] [CrossRef]
- Pecchi, G.; Campos, C.; Peña, O. Thermal Stability against Reduction of LaMn1−yCoyO3 Perovskites. Mater. Res. Bull. 2009, 44, 846–853. [Google Scholar] [CrossRef]
- Zheng, X.; Li, B.; Shen, L.; Cao, Y.; Zhan, Y.; Zheng, S.; Wang, S.; Jiang, L. Oxygen Vacancies Engineering of Fe Doped LaCoO3 Perovskite Catalysts for Efficient H2S Selective Oxidation. Appl. Catal. B Environ. 2023, 329, 122526. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Yuvakkumar, R.; Balamurugan, K.; Ahamad, T.; Khan, M.M.; Ramalingam, G.; Mohammed, M.K.; Ravi, G. Preparation of Fe-SnO2@ CeO2 Nanocomposite Electrode for Asymmetric Supercapacitor Device Performance Analysis. J. Energy Storage 2021, 36, 102402. [Google Scholar] [CrossRef]
- Manju; Jain, M.; Madas, S.; Vashishtha, P.; Rajput, P.; Gupta, G.; Kahaly, M.U.; Özdoğan, K.; Vij, A.; Thakur, A. Oxygen Vacancies Induced Photoluminescence in SrZnO2 Nanophosphors Probed by Theoretical and Experimental Analysis. Sci. Rep. 2020, 10, 17364. [Google Scholar]
- Suggana, P.; Kumar, E.P.; Reddy, K.C.; Lakshmaiah, M.V.; Joo, S.W.; Reddy, G.R. 2D Sheet to 1D Rod-like Morphology Regulated Self-Assembled ZnCo2O4 Microstructures under Mixed Solvent Conditions for Battery-Type Supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131423. [Google Scholar] [CrossRef]
- Seenivasan, S.; Shim, K.I.; Lim, C.; Kavinkumar, T.; Sivagurunathan, A.T.; Han, J.W.; Kim, D.-H. Boosting Pseudocapacitive Behavior of Supercapattery Electrodes by Incorporating a Schottky Junction for Ultrahigh Energy Density. Nano-Micro Lett. 2023, 15, 62. [Google Scholar] [CrossRef]
- Liang, M.; Zhao, M.; Wang, H.; Shen, J.; Song, X. Enhanced Cycling Stability of Hierarchical NiCo2S4@ Ni(OH)2@ PPy Core–Shell Nanotube Arrays for Aqueous Asymmetric Supercapacitors. J. Mater. Chem. A 2018, 6, 2482–2493. [Google Scholar] [CrossRef]
- William, J.J.; Balakrishnan, S.; Murugesan, M.; Gopalan, M.; Britten, A.J.; Mkandawire, M. Mesoporous β-Ag2MoO4 Nanopotatoes as Supercapacitor Electrodes. Mater. Adv. 2022, 3, 8288–8297. [Google Scholar] [CrossRef]
- Safari, M.; Mazloom, J.; Boustani, K.; Monemdjou, A. Hierarchical Fe2O3 Hexagonal Nanoplatelets Anchored on SnO2 Nanofibers for High-Performance Asymmetric Supercapacitor Device. Sci. Rep. 2022, 12, 14919. [Google Scholar] [CrossRef]
- Ahangari, M.; Mahmoodi, E.; Delibaş, N.; Mostafaei, J.; Asghari, E.; Niaei, A. Application of SrFeO3 Perovskite as Electrode Material for Supercapacitor and Investigation of Co-Doping Effect on the B-Site. Turk. J. Chem. 2022, 46, 1723–1732. [Google Scholar] [CrossRef]
- Liu, L.; Liu, G.; Wu, S.; He, J.; Zhou, Y.; Demir, M.; Huang, R.; Ruan, Z.; Jiang, G.; Ma, P. Fe-Substituted SrCoO3 Perovskites as Electrode Materials for Wide Temperature-Tolerant Supercapacitors. Ceram. Int. 2024, 50, 1970–1980. [Google Scholar] [CrossRef]
- Shafi, P.M.; Mohapatra, D.; Reddy, V.P.; Dhakal, G.; Kumar, D.R.; Tuma, D.; Brousse, T.; Shim, J.-J. Sr-and Fe-Substituted LaMnO3 Perovskite: Fundamental Insight and Possible Use in Asymmetric Hybrid Supercapacitor. Energy Storage Mater. 2022, 45, 119–129. [Google Scholar] [CrossRef]
- George, G.; Jackson, S.L.; Luo, C.Q.; Fang, D.; Luo, D.; Hu, D.; Wen, J.; Luo, Z. Effect of Doping on the Performance of High-Crystalline SrMnO3 Perovskite Nanofibers as a Supercapacitor Electrode. Ceram. Int. 2018, 44, 21982–21992. [Google Scholar] [CrossRef]
- Tomar, A.K.; Singh, G.; Sharma, R.K. Fabrication of a Mo-Doped Strontium Cobaltite Perovskite Hybrid Supercapacitor Cell with High Energy Density and Excellent Cycling Life. ChemSusChem 2018, 11, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Ajin, I.; Balamurugan, R.; Chandra Bose, A. Tailoring the Perovskite Structure to Acquire an Inorganic La2NiCrO6 Double Perovskite as an Efficient Energy Storage Application by Varying Molar Concentrations of Citric Acid. ACS Appl. Energy Mater. 2023, 6, 9764–9777. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Xu, W.; Wang, M.; Shao, R.; Sun, Y.; Lin, B. Mesoporous LaFeO3 Perovskite Derived from MOF Gel for All-Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2020, 386, 124030. [Google Scholar] [CrossRef]
- Raj, T.V.; Hoskeri, P.A.; Muralidhara, H.B.; Manjunatha, C.R.; Kumar, K.Y.; Raghu, M.S. Facile Synthesis of Perovskite Lanthanum Aluminate and Its Green Reduced Graphene Oxide Composite for High Performance Supercapacitors. J. Electroanal. Chem. 2020, 858, 113830. [Google Scholar] [CrossRef]







| Material | R-Factors | χ | R | CN | BVS | |||
|---|---|---|---|---|---|---|---|---|
| Rp | Rwp | Rexp | ||||||
| ORJ | Sr-O | 12.5 | 15.7 | 13.9 | 1.27 | 2.76 | 12 | 2.12 |
| Fe-O | 1.95 | 6 | 3.56 | |||||
| LMJ | Sr-O | 13.3 | 16.7 | 15.4 | 1.18 | 2.74 | 12 | 2.20 |
| Fe-O | 1.94 | 6 | 3.70 | |||||
| Sr. No | Material | Synthesis Method | Electrolyte | Capacity | Capacitance | Ref. |
|---|---|---|---|---|---|---|
| 1 | SrCo0.5Fe0.5O3 | Sol–gel | 1 M KOH | - | 219 F/g at 2 A/g | [60] |
| 2 | SrFe0.8Co0.2O3 | Combustion Sol–gel | 1 M KOH | - | 433 F/g at 2 A/g | [28] |
| 3 | SrCo0.9Fe0.1O3-δ | Solid-state Reaction | 1 M NaOH | - | 526 F/g at 1 A/g | [61] |
| 4 | La 0.7Sr0.3Mn0.5Fe0.5O3 | Sol–gel Auto-combustion | 3 M KOH | 330 C/g at 1 A/g | - | [62] |
| 5 | SrFe0.85Zr0.15O3-δ | Solid-state Reaction | 2 M KOH | - | 163 F/g at 0.5 A/g | [29] |
| 6 | Sr 0.8Ce 0.2Fe0.8Co0.2O3 (ORJ), (LMJ) | Auto-combustion | 1 M KOH | 158 C/g, 300 C/g at 1 A/g | 300 F/g, 544 F/g at 1 A/g | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazal, A.; Iqbal, M.J.; Raza, M.A.; Almutairi, B.S.; Zakaly, H.M.H.; Akhtar, N.; Irshad, M.; Riaz, S. Exploring the Potential of Green Synthesized Sr0.8Ce0.2Fe0.8Co0.2O3 Using Orange and Lemon Extracts for Hybrid Supercapacitor Applications. Batteries 2025, 11, 310. https://doi.org/10.3390/batteries11080310
Fazal A, Iqbal MJ, Raza MA, Almutairi BS, Zakaly HMH, Akhtar N, Irshad M, Riaz S. Exploring the Potential of Green Synthesized Sr0.8Ce0.2Fe0.8Co0.2O3 Using Orange and Lemon Extracts for Hybrid Supercapacitor Applications. Batteries. 2025; 11(8):310. https://doi.org/10.3390/batteries11080310
Chicago/Turabian StyleFazal, Asmara, M. Javaid Iqbal, Mohsin Ali Raza, Badriah S. Almutairi, Hesham M. H. Zakaly, Naureen Akhtar, Muneeb Irshad, and Saira Riaz. 2025. "Exploring the Potential of Green Synthesized Sr0.8Ce0.2Fe0.8Co0.2O3 Using Orange and Lemon Extracts for Hybrid Supercapacitor Applications" Batteries 11, no. 8: 310. https://doi.org/10.3390/batteries11080310
APA StyleFazal, A., Iqbal, M. J., Raza, M. A., Almutairi, B. S., Zakaly, H. M. H., Akhtar, N., Irshad, M., & Riaz, S. (2025). Exploring the Potential of Green Synthesized Sr0.8Ce0.2Fe0.8Co0.2O3 Using Orange and Lemon Extracts for Hybrid Supercapacitor Applications. Batteries, 11(8), 310. https://doi.org/10.3390/batteries11080310









