Cu-Substituted Na3V2(PO4)3/C Composites as High-Rate, Long-Cycle Cathodes for Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
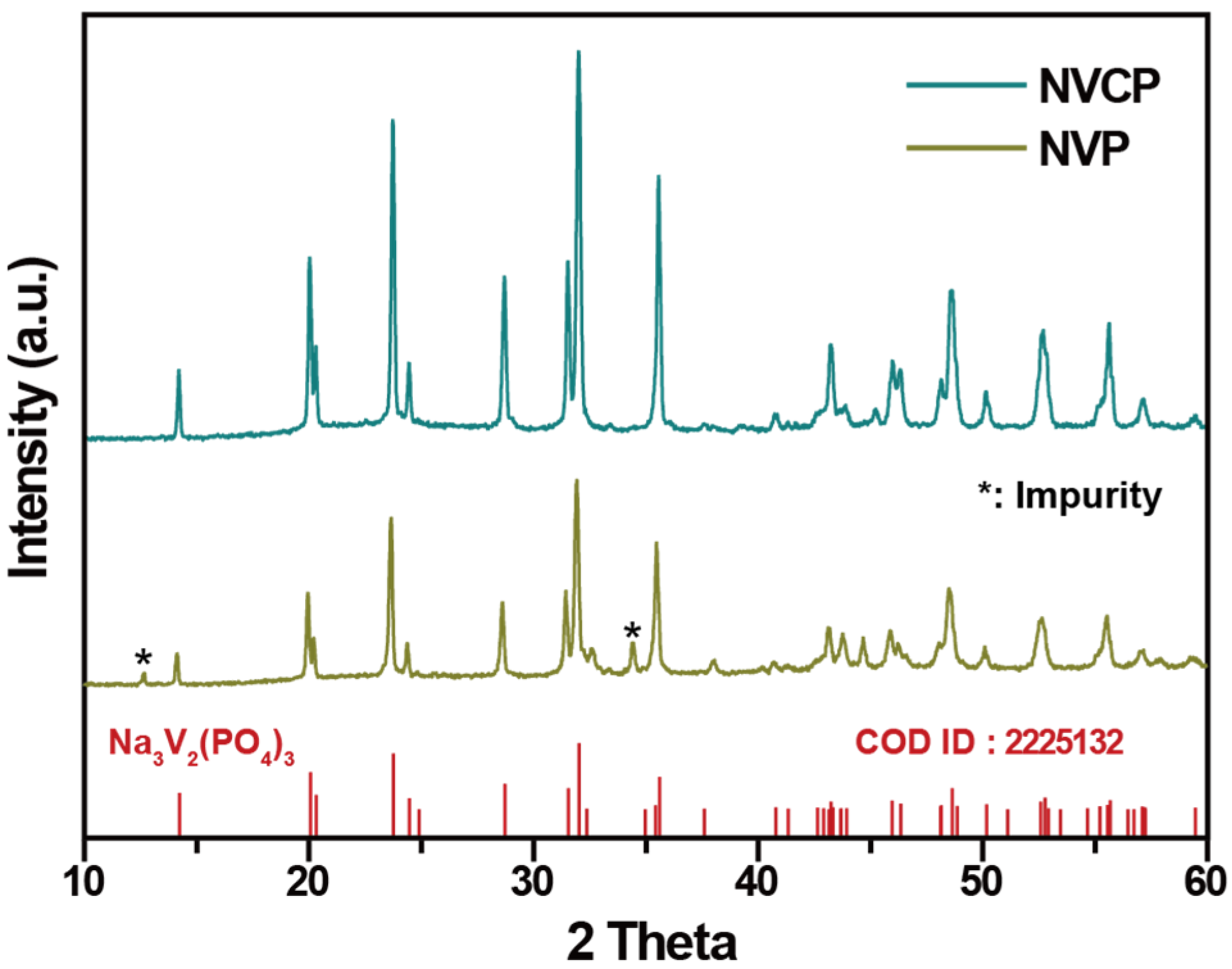
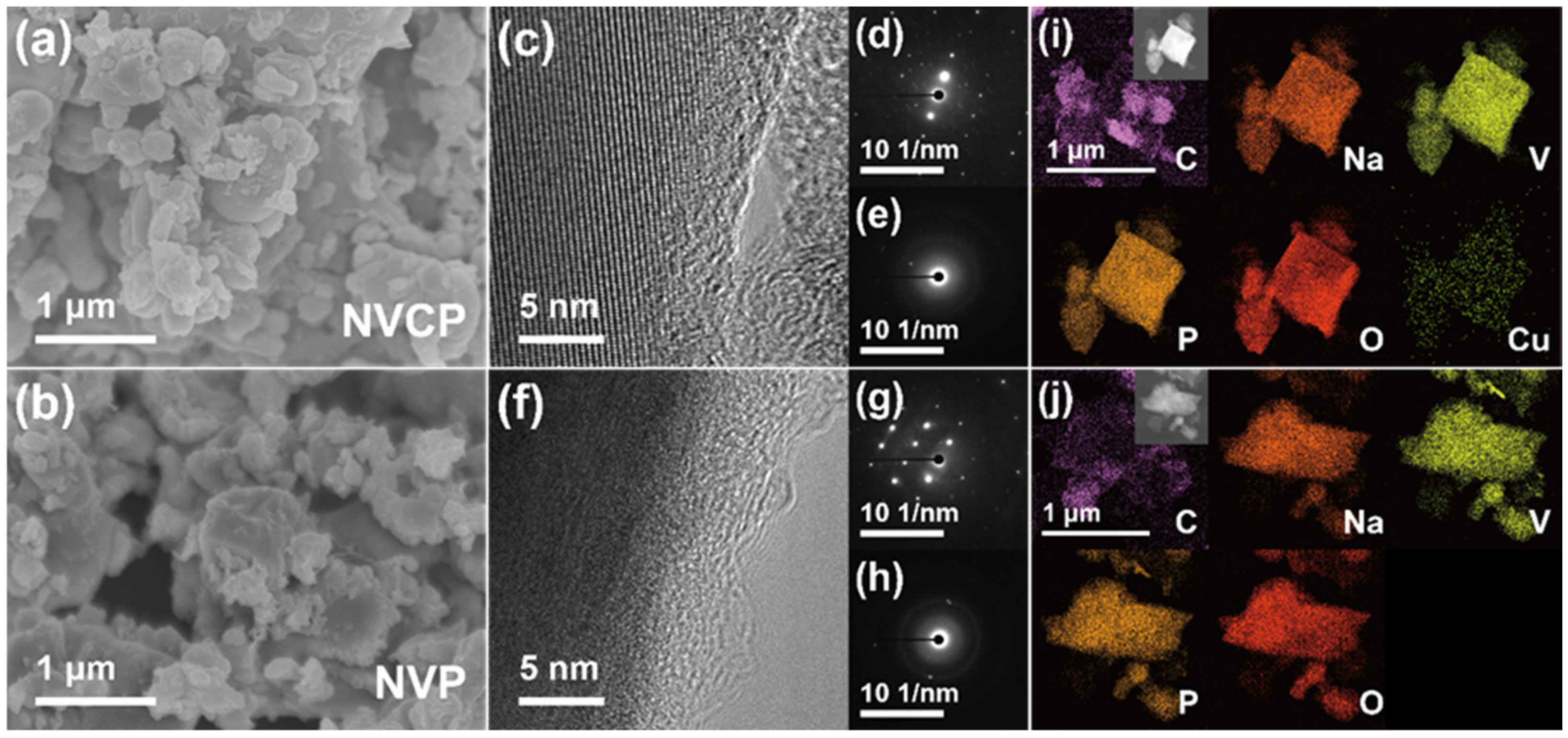
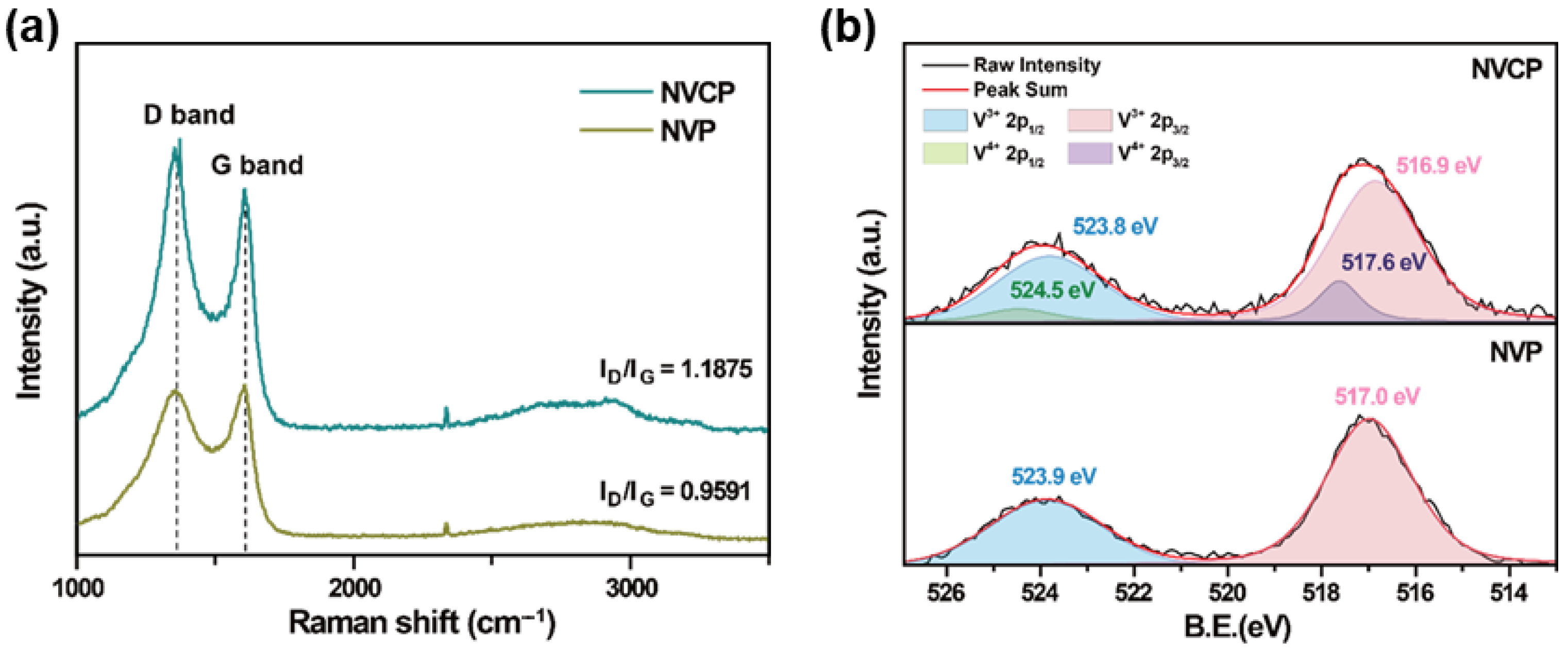
3.1. Structural and Morphological Characterization
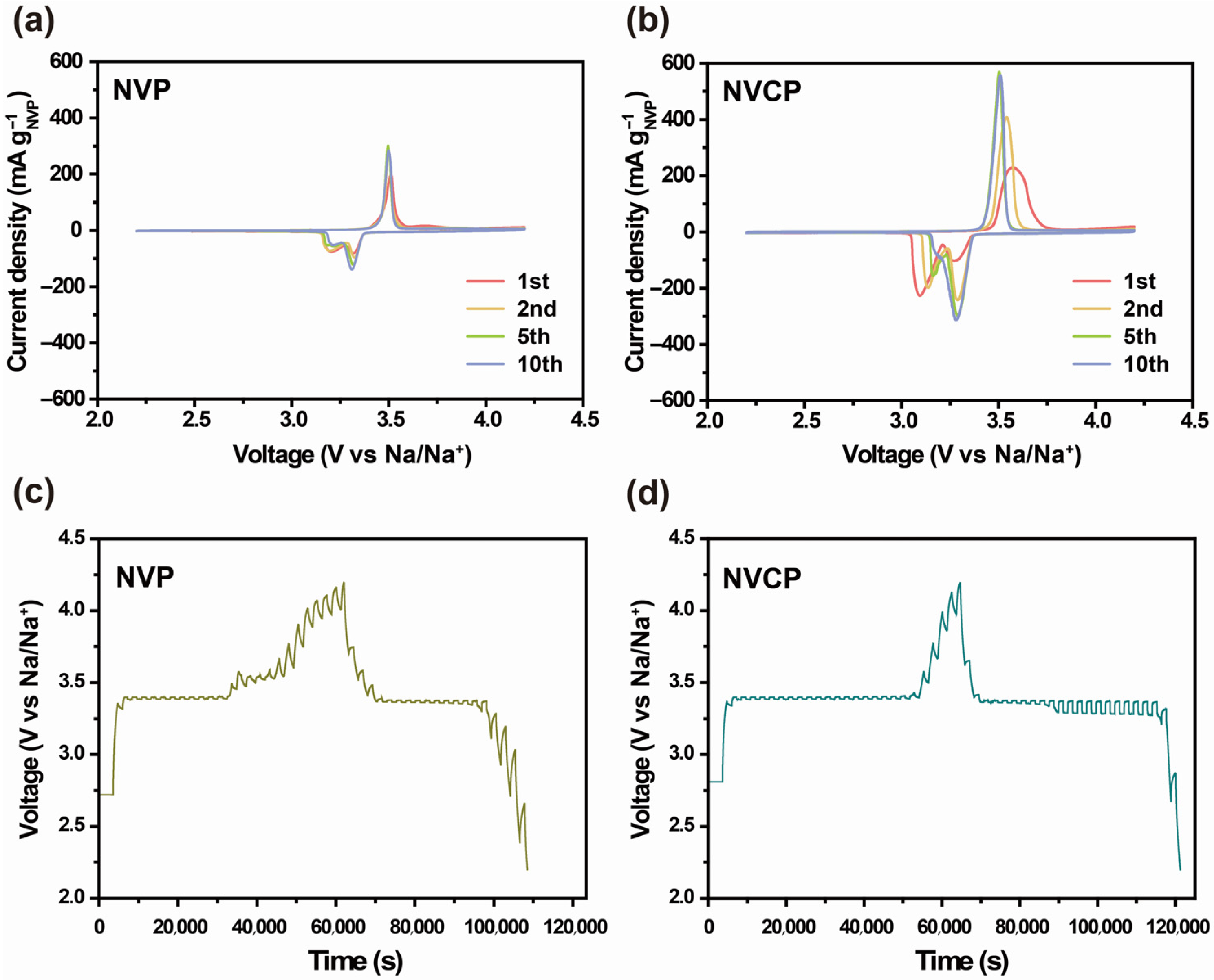
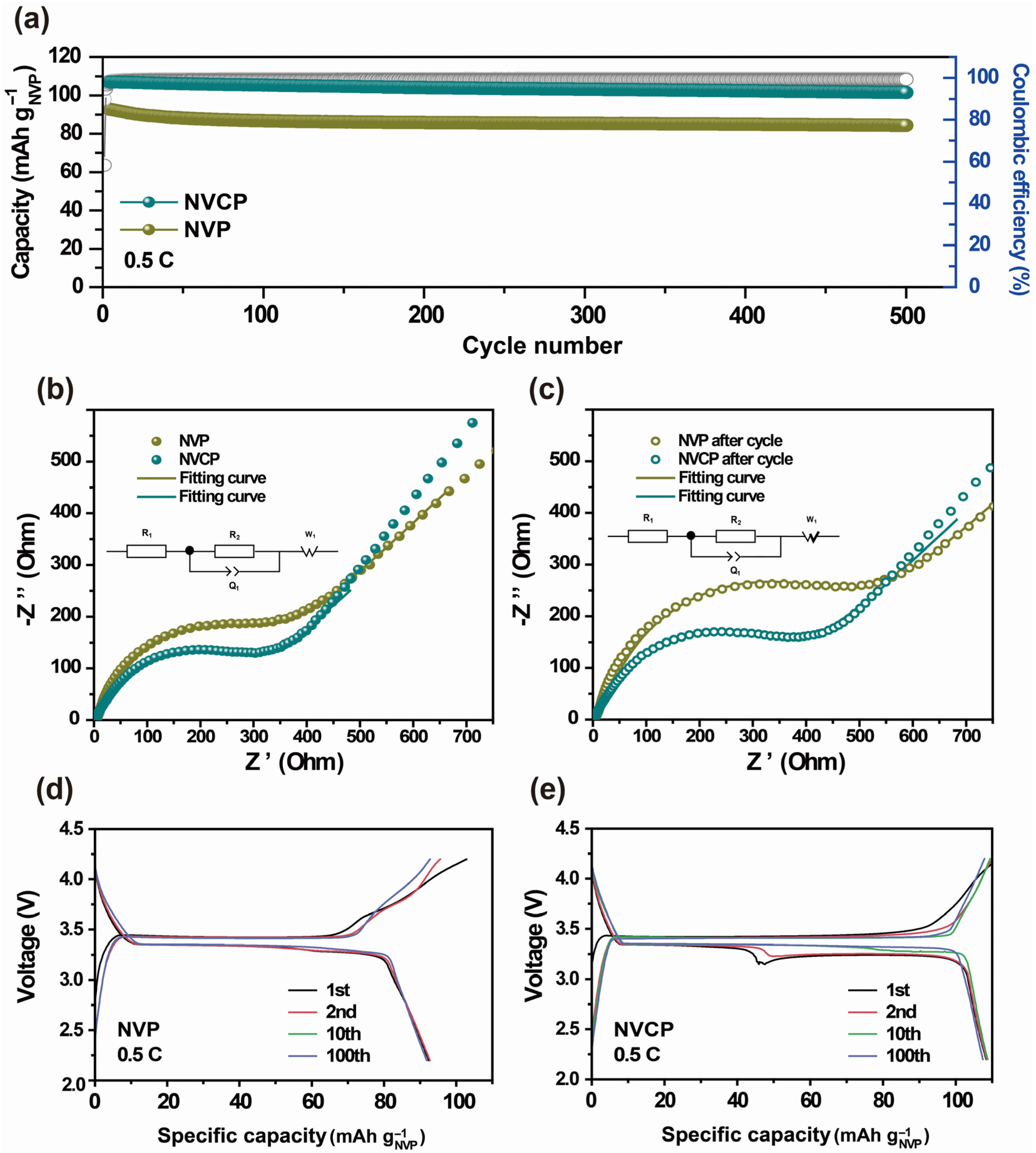
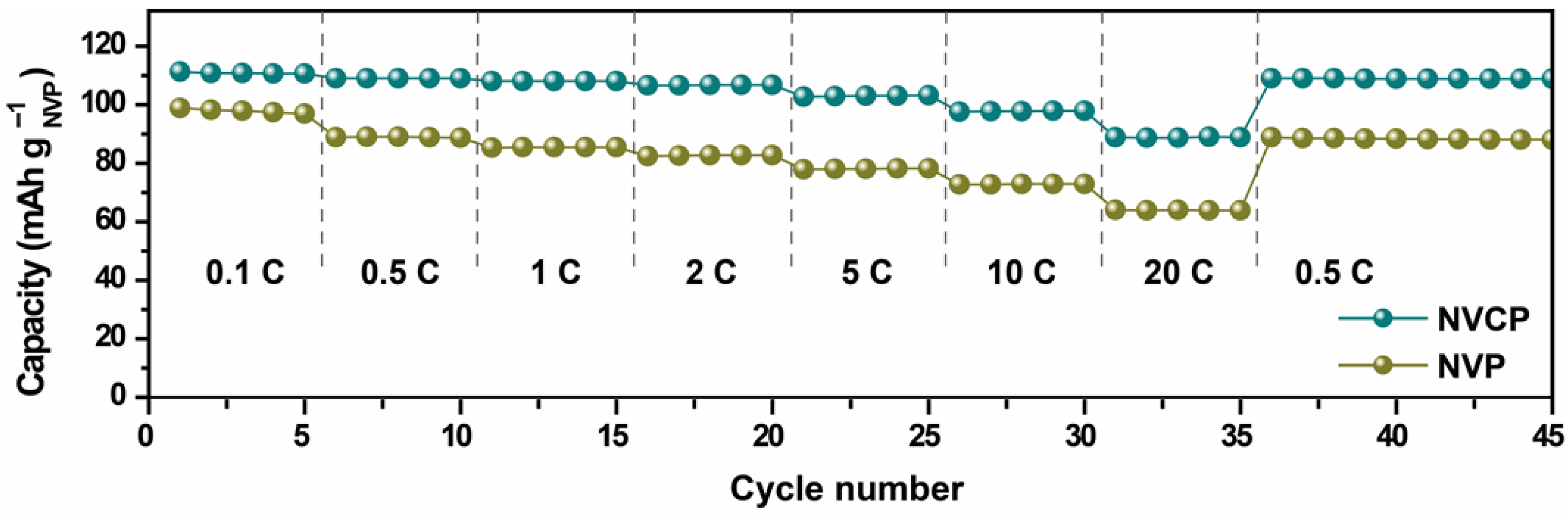
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mat. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, J.; Guo, Y.; Zhu, H.; Huang, X. Research Progress on Na3V2(PO4)3 Cathode Material of Sodium Ion Battery. Front. Chem. 2020, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, K.; Lv, X.; Shi, K.; Wang, Y.; Nian, Z.; Li, Y.; Wang, L.; Dai, L.; He, Z. Recent advances of NASICON-Na3V2(PO4)3 as cathode for sodium-ion batteries: Synthesis, modifications, and perspectives. J. Alloy. Compd. 2021, 867, 159060. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liang, Q.; Xu, L.; Ding, C.; Liu, Y.; Gao, Y. Optimization Strategies of Na3V2(PO4)3 Cathode Materials for Sodium-Ion Batteries. Nano-Micro Lett. 2024, 17, 33. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.; Wang, H.; Zhou, T.; Xie, H.; Ren, Y. Synthesis and electrochemical performances of Na3V2(PO4)2F3/C composites as cathode materials for sodium ion batteries. RSC Adv. 2019, 9, 30628–30636. [Google Scholar] [CrossRef]
- Bag, S.; Murarka, H.; Zhou, C.; Bhattacharya, A.; Jokhakar, D.; Pol, V.G.; Thangadurai, V. Understanding the Na-Ion Storage Mechanism in Na3+xV2−xMx(PO4)3 (M = Ni2+, Co2+, Mg2+; x = 0.1–0.5) Cathodes. ACS Appl. Energ. Mater. 2020, 3, 8475–8486. [Google Scholar] [CrossRef]
- Cushing, B.L.; Goodenough, J.B. Li2NaV2(PO4)3: A 3.7 V Lithium-Insertion Cathode with the Rhombohedral NASICON Structure. J. Solid State Chem. 2001, 162, 176–181. [Google Scholar] [CrossRef]
- Du, K.; Guo, H.; Hu, G.; Peng, Z.; Cao, Y. Na3V2(PO4)3 as cathode material for hybrid lithium ion batteries. J. Power Sources 2013, 223, 284–288. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Jian, Z.; Yuan, C.; Han, W.; Lu, X.; Gu, L.; Xi, X.; Hu, Y.S.; Li, H.; Chen, W.; Chen, D.; et al. Atomic Structure and Kinetics of NASICON NaxV2(PO4)3 Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 4265–4272. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, Y. Heterojunction of Y3+-substituted Na3V2(PO4)3-NaYO2 accelerating kinetics with superior performance for full sodium-ion batteries. J. Colloid Interface Sci. 2024, 654, 1163–1176. [Google Scholar] [CrossRef]
- Lalère, F.; Seznec, V.; Courty, M.; David, R.; Chotard, J.N.; Masquelier, C. Improving the energy density of Na3V2(PO4)3-based positive electrodes through V/Al substitution. J. Mater. Chem. A 2015, 3, 16198–16205. [Google Scholar] [CrossRef]
- Akçay, T.; Häringer, M.; Pfeifer, K.; Anhalt, J.; Binder, J.R.; Dsoke, S.; Kramer, D.; Mönig, R. Na3V2(PO4)3—A Highly Promising Anode and Cathode Material for Sodium-Ion Batteries. ACS Appl. Energ. Mater. 2021, 4, 12688–12695. [Google Scholar] [CrossRef]
- Jian, Z.; Han, W.; Lu, X.; Yang, H.; Hu, Y.S.; Zhou, J.; Zhou, Z.; Li, J.; Chen, W.; Chen, D.; et al. Superior Electrochemical Performance and Storage Mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2012, 3, 156–160. [Google Scholar] [CrossRef]
- Duan, W.; Zhu, Z.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F.; Chen, J. Na3V2(PO4)3@C core–shell nanocomposites for rechargeable sodium-ion batteries. J. Mater. Chem. A 2014, 2, 8668–8675. [Google Scholar] [CrossRef]
- Fang, J.; Wang, S.; Yao, X.; Hu, X.; Wang, Y.; Wang, H. Ration design of porous Mn-doped Na3V2(PO4)3 cathode for high rate and super stable sodium-ion batteries. Electrochim. Acta 2019, 295, 262–269. [Google Scholar] [CrossRef]
- Ghosh, S.; Barman, N.; Patra, B.; Senguttuvan, P. Structural and Electrochemical Sodium (De)intercalation Properties of Carbon-Coated NASICON-Na3+yV2−yMny(PO4)3 Cathodes for Na-Ion Batteries. Adv. Energy Sustain. Res. 2022, 3, 2200081. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, L.; Pan, H.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, L. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Feng, P.; Wang, W.; Wang, K.; Cheng, S.; Jiang, K. Na3V2(PO4)3/C synthesized by a facile solid-phase method assisted with agarose as a high-performance cathode for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 10261–10268. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Wang, J.; Miao, L.; Li, C.; Han, J.; Huang, Y. Superior Na-ion storage achieved by Ti substitution in Na3V2(PO4)3. Energy Storage Mater. 2018, 15, 108–115. [Google Scholar] [CrossRef]
- Mai, B.; Xing, B.; Yue, Y.; Cai, N.; Cai, C.; Lian, S.; Fan, H.; Yan, M.; Zhu, T.; Hu, P.; et al. Cr-doped Na3V2(PO4)3@C enables high-capacity with V2+/V5+ reaction and stable sodium storage. J. Mater. Sci. Technol. 2023, 165, 1–7. [Google Scholar] [CrossRef]
- Shi, H.; Guo, L.; Chen, Y. Unraveling the modified mechanism of ruthenium substitution on Na3V2(PO4)3 with superior rate capability and ultralong cyclic performance. J. Colloid Interface Sci. 2024, 664, 487–499. [Google Scholar] [CrossRef]
- Lv, Z.Q.; Zhang, Y.L.; Liu, Z.Q.; Qi, X.; Xu, Y.B.; Cui, Y.M.; Xu, W.L.; Yang, Z.L.; Zheng, Q. Carbon coated Na3+xV2−xCux(PO4)3@C cathode for high-performance sodium ion batteries. J. Colloid Interface Sci. 2024, 666, 540–546. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Yu, S.; Wu, K.; Zhang, F.; Gao, P. Cu2+ substitution regulating Na3V2(PO4)3 with solid SEI membrane for superior electrochemical performance. Dalton Trans. 2025, 54, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yang, Y.; Chen, J.; Jing, M.; Li, F.; Banks, C.E. First exploration of Na-ion migration pathways in the NASICON structure Na3V2(PO4)3. J. Mater. Chem. A 2014, 2, 5358–5362. [Google Scholar] [CrossRef]
- Ragul, S.; Prabakaran, A.; Sujithkrishnan, E.; Kannadasan, K.; Elumalai, P. Sodium-ion battery using a NASICON-type Na3V2(PO4)3 cathode: Quantification of diffusive and capacitive Na+ charge storage. New J. Chem. 2024, 48, 12323–12335. [Google Scholar] [CrossRef]
- Zhang, D.; Feng, P.; Xu, B.; Li, Z.; Qiao, J.; Zhou, J.; Chang, C. High Rate Performance of Na3V2−xCux(PO4)3/C Cathodes for Sodium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3563–A3569. [Google Scholar] [CrossRef]
- Cong, J.; Luo, S.-h.; Li, P.; Li, K.; Li, P.; Yan, S.; Qian, L.; Liu, X. Ultracapacity Properties of the Refined Structure in Na-Rich Na3.4V2(PO4)3/C as Sodium-Ion Battery Cathodes by Tapping the Na-Vacancy Potential. ACS Sustain. Chem. Eng. 2023, 11, 16341–16353. [Google Scholar] [CrossRef]
- Hwang, G.; Kim, J.M.; Hong, D.; Kim, C.K.; Choi, N.S.; Lee, S.Y.; Park, S. Multifunctional natural agarose as an alternative material for high-performance rechargeable lithium-ion batteries. Green Chem. 2016, 18, 2710–2716. [Google Scholar] [CrossRef]
- Xiong, W.; Tu, Z.; Yin, Z.; Zhang, X.; Hu, X.; Wu, Y. Supported Ionic Liquid Gel Membranes Enhanced by Ionization Modification for Sodium Metal Batteries. ACS Sustain. Chem. Eng. 2021, 9, 12100–12108. [Google Scholar] [CrossRef]
- Wei, C.; Luo, F.; Zhang, C.; Gao, H.; Niu, J.; Ma, W.; Bai, Y.; Zhang, Z. Voltage window-dependent electrochemical performance and reaction mechanisms of Na3V2(PO4)3 cathode for high-capacity sodium ion batteries. Ionics 2019, 26, 2343–2351. [Google Scholar] [CrossRef]
- Chen, R.; Butenko, D.S.; Li, S.; Li, D.; Zhang, X.; Cao, J.; Ogorodnyk, I.V.; Klyui, N.I.; Han, W.; Zatovsky, I.V. Effects of low doping on the improvement of cathode materials Na3+xV2−xMx(PO4)3 (M = Co2+, Cu2+; x = 0.01−0.05) for SIBs. J. Mater. Chem. A 2021, 9, 17380–17389. [Google Scholar] [CrossRef]
- Liu, Y.; Rong, X.H.; Bai, R.; Xiao, R.J.; Xu, C.L.; Zhang, C.; Xu, J.P.; Yin, W.; Zhang, Q.H.; Liang, X.M.; et al. Identifying the intrinsic anti-site defect in manganese-rich NASICON-type cathodes. Nat. Energy 2023, 8, 1088–1096. [Google Scholar] [CrossRef]
- Seshan, V.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Atomic-Scale Study of NASICON Type Electrode Material: Defects, Dopants and Sodium-Ion Migration in NaV(PO). Physchem 2024, 5, 1. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, X.; Ren, Y.; Wang, H.; Ding, J.; Zhou, S.; Ding, X.; Chen, Y. Enhanced sodium ion storage performance of Na3V2(PO4)3 with N-doped carbon by folic acid as carbon-nitrogen source. J. Alloy. Compd. 2018, 732, 454–459. [Google Scholar] [CrossRef]
- Liang, E.J.; Ding, P.; Zhang, H.R.; Guo, X.Y.; Du, Z.L. Synthesis and correlation study on the morphology and Raman spectra of CNx nanotubes by thermal decomposition of ferrocene/ethylenediamine. Diam. Relat. Mat. 2004, 13, 69–73. [Google Scholar] [CrossRef]
- Aragón, M.J.; Lavela, P.; Ortiz, G.F.; Tirado, J.L. Effect of Iron Substitution in the Electrochemical Performance of Na3V2(PO4)3 as Cathode for Na-Ion Batteries. J. Electrochem. Soc. 2015, 162, A3077–A3083. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Yao, Y.; Zhu, H.; Chen, Q.; Sun, Q.; Banks, C.E. A promising Na3V2(PO4)3 cathode for use in the construction of high energy batteries. Phys. Chem. Chem. Phys. 2014, 16, 3055–3061. [Google Scholar] [CrossRef]
- Ishado, Y.; Inoishi, A.; Okada, S. Exploring Factors Limiting Three-Na+ Extraction from Na3V2(PO4)3. Electrochemistry 2020, 88, 457–462. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.Y.; Zhou, C.G.; Chen, Y.L. Concerted Ion-Exchange Mechanism for Sodium Diffusion and Its Promotion in Na3V2(PO4)3 Framework. J. Phys. Chem C 2018, 122, 16649–16654. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The First Report on Excellent Cycling Stability and Superior Rate Capability of Na3V2(PO4)3 for Sodium Ion Batteries. Adv. Energy Mater. 2012, 3, 444–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-J.; Kim, Y.G.; Jeong, S.H.; Lee, S.J.; Jung, Y.H.; Kim, J.-H. Cu-Substituted Na3V2(PO4)3/C Composites as High-Rate, Long-Cycle Cathodes for Sodium-Ion Batteries. Batteries 2025, 11, 308. https://doi.org/10.3390/batteries11080308
Choi H-J, Kim YG, Jeong SH, Lee SJ, Jung YH, Kim J-H. Cu-Substituted Na3V2(PO4)3/C Composites as High-Rate, Long-Cycle Cathodes for Sodium-Ion Batteries. Batteries. 2025; 11(8):308. https://doi.org/10.3390/batteries11080308
Chicago/Turabian StyleChoi, Hyeon-Jun, Yu Gyeong Kim, Su Hwan Jeong, Sang Jun Lee, Young Hwa Jung, and Joo-Hyung Kim. 2025. "Cu-Substituted Na3V2(PO4)3/C Composites as High-Rate, Long-Cycle Cathodes for Sodium-Ion Batteries" Batteries 11, no. 8: 308. https://doi.org/10.3390/batteries11080308
APA StyleChoi, H.-J., Kim, Y. G., Jeong, S. H., Lee, S. J., Jung, Y. H., & Kim, J.-H. (2025). Cu-Substituted Na3V2(PO4)3/C Composites as High-Rate, Long-Cycle Cathodes for Sodium-Ion Batteries. Batteries, 11(8), 308. https://doi.org/10.3390/batteries11080308






