Luffa-like Interconnective Porous Nanofiber with Anchored Co/CoCr2O4 Hybrid Nanoparticles for Zinc–Air Batteries
Abstract
1. Introduction
2. Results and Discussion
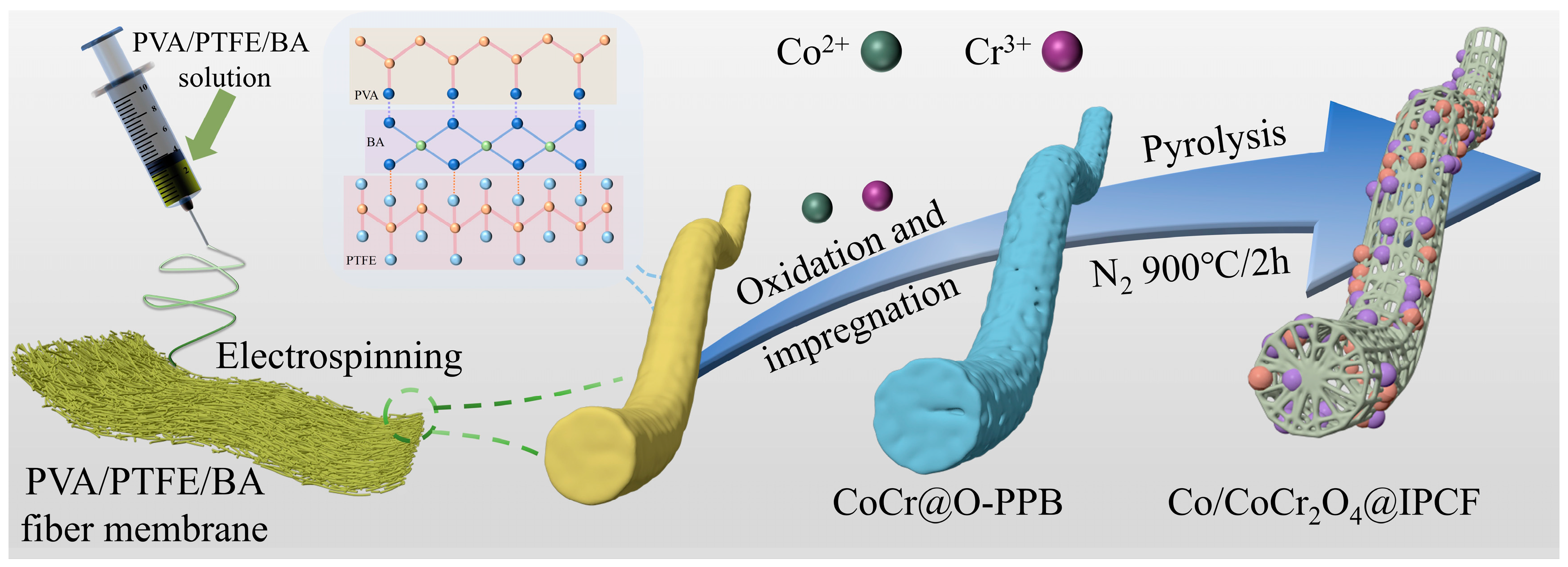
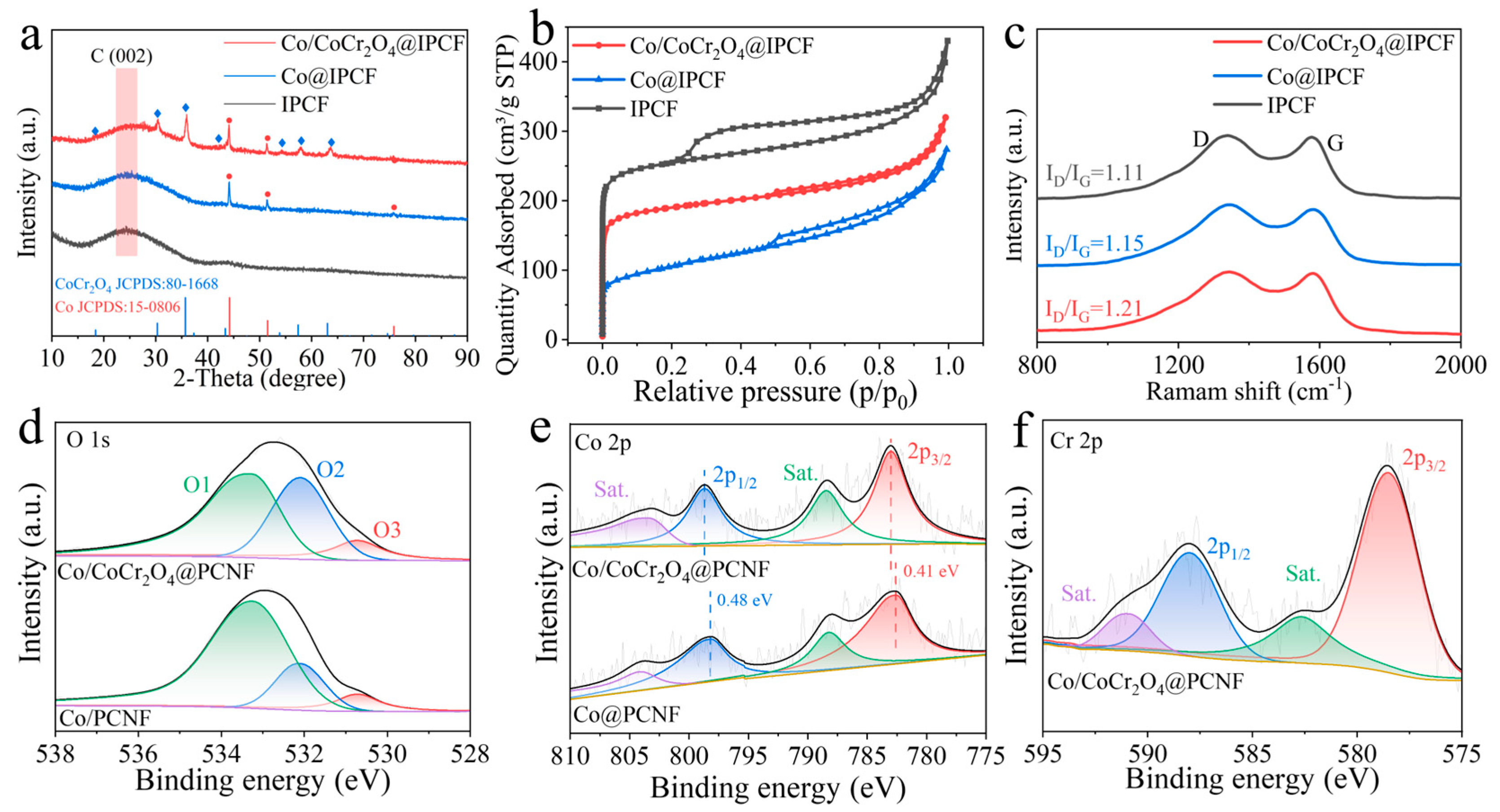
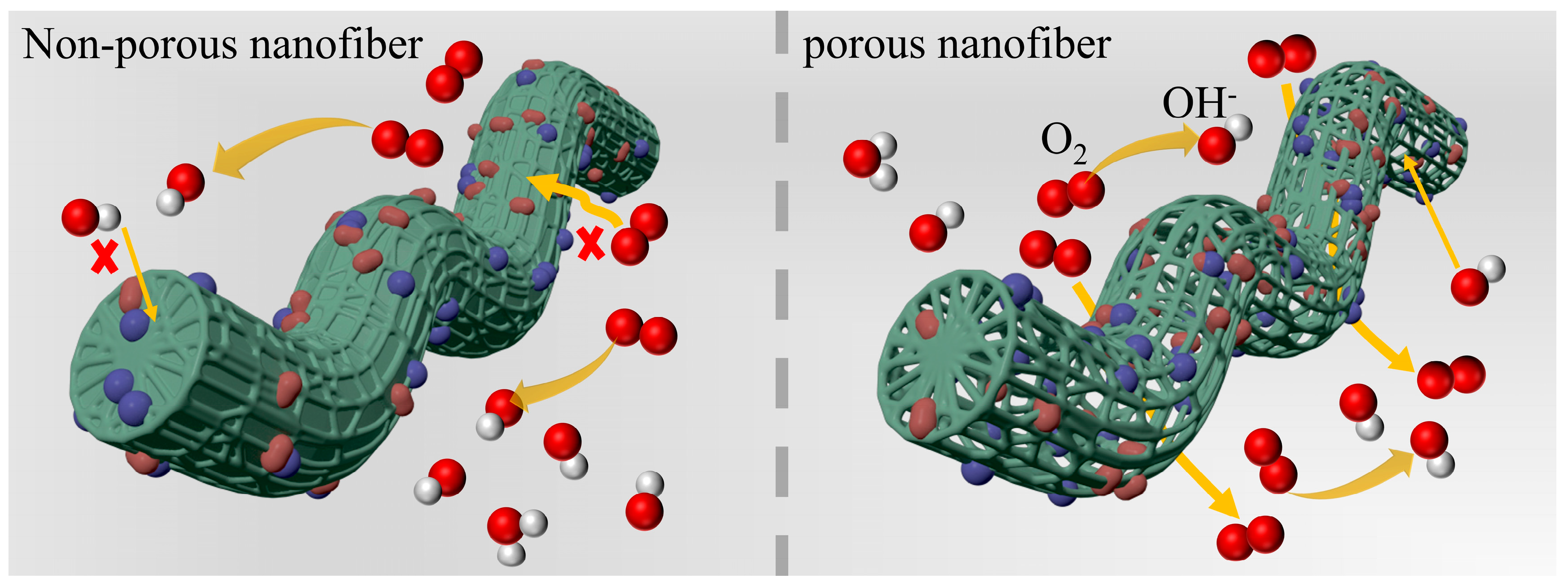
2.1. Preparation and Structural Characterization of Catalysts
2.2. Electrocatalytic Activities of ORR and OER
2.3. Performance of Zinc–Air Batteries
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Liu, B.; Liu, D.; Guo, P.F.; Liu, J.; Wang, R.R.; Guo, Y.H.; Tu, X.; Pan, H.G.; Sun, D.L.; et al. Alloying Co Species into Ordered and Interconnected Macroporous Carbon Polyhedra for Efficient Oxygen Reduction Reaction in Rechargeable Zinc-Air Batteries. Adv. Mater. 2022, 34, 2109605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, X.; Liu, X.; Liu, X.; Alwarappan, S. Transformation of Biomass Precursor into Both High-Efficiency Cathode Catalyst and Solid Electrolyte for Rechargeable Zn-Air Batteries. Small 2025, 2502395. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, J.; Mosa, J.; Aparicio, M. Binder-Free Fe-N-C-O Bifunctional Electrocatalyst in Nickel Foam for Aqueous Zinc–Air Batteries. Batteries 2025, 11, 159. [Google Scholar] [CrossRef]
- Liu, H.B.; Xie, R.X.; Niu, Z.Q.; Jia, Q.H.; Yang, L.; Wang, S.T.; Cao, D.P. Two-in-one strategy to construct bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. Chin. J. Catal. 2022, 43, 2906–2912. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Z.; Ye, Q.T.; Yang, Z.B.; Xu, R.J.; Kong, K.X.; Zhang, Y.F.; Yan, T.; Liu, Y.P.; Pan, Z.J.; et al. Construction of Fe Nanoclusters/Nanoparticles to Engineer FeN4 Sites on Multichannel Porous Carbon Fibers for Boosting Oxygen Reduction Reaction. Adv. Funct. Mater. 2024, 34, 2315150. [Google Scholar] [CrossRef]
- Arif, M.; Mahsud, A.; Muhmood, T.; Deepak, F.L. Design, synthesis, and electronic structure modulation of ORR electrocatalysts. J. Environ. Chem. Eng. 2024, 12, 113417. [Google Scholar] [CrossRef]
- Qin, J.Y.; Liu, H.; Zou, P.C.; Zhang, R.; Wang, C.Y.; Xin, H.L. Altering Ligand Fields in Single-Atom Sites through Second-Shell Anion Modulation Boosts the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2022, 144, 2197–2207. [Google Scholar] [CrossRef]
- Li, Z.J.; Ji, S.Q.; Wang, C.; Liu, H.X.; Leng, L.P.; Du, L.; Gao, J.C.; Qiao, M.; Horton, J.H.; Wang, Y. Geometric and Electronic Engineering of Atomically Dispersed Copper-Cobalt Diatomic Sites for Synergistic Promotion of Bifunctional Oxygen Electrocatalysis in Zinc-Air Batteries. Adv. Mater. 2023, 35, 2300905. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.G.; Kubal, J.; Sen, F.G.; Zou, J.X.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.J.; Liu, D.J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Yang, G.G.; Zhu, J.W.; Yuan, P.F.; Hu, Y.F.; Qu, G.; Lu, B.A.; Xue, X.Y.; Yin, H.B.; Cheng, W.Z.; Cheng, J.Q.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734. [Google Scholar] [CrossRef]
- Yan, J.H.; Dong, K.Q.; Zhang, Y.Y.; Wang, X.; Aboalhassan, A.A.; Yu, J.Y.; Ding, B. Multifunctional flexible membranes from sponge-like porous carbon nanofibers with high conductivity. Nat. Commun. 2019, 10, 5584. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiao, Y.H.; Ge, Y.F.; Gao, D.G.; Zhang, Y.G.; Li, Z.P.; Han, Y.D. Constructing Fe-N-doped porous carbon nanofibers for a pH-universal ORR and switchable, superior Zn-air batteries. J. Mater. Chem. A 2024, 12, 2004–2010. [Google Scholar] [CrossRef]
- Cheng, Q.Q.; Yang, L.J.; Zou, L.L.; Zou, Z.Q.; Chen, C.; Hu, Z.; Yang, H. Single Cobalt Atom and N Codoped Carbon Nanofibers as Highly Durable Electrocatalyst for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, F.C.; Li, S.L.; Liu, J.; Dong, L.Z.; Wang, Z.M.; Lan, Y.Q. Cobalt@Nitrogen-Doped Porous Carbon Fiber Derived from the Electrospun Fiber of Bimetal-Organic Framework for Highly Active Oxygen Reduction. Small Methods 2018, 2, 1800049. [Google Scholar] [CrossRef]
- Han, Z.L.; Chai, S.Y.; Zhu, Y.Q.; Yao, X.Y.; Yang, L.F.; Du, C.L.; Ma, X.L.; Cao, C.B.; Zou, M.S. Pre-coordination anchoring strategy for modulating the coordination structure of iron single atoms and ORR performance. Chem. Eng. J. 2024, 502, 157943. [Google Scholar] [CrossRef]
- Liu, M.L.; Zhao, Z.P.; Duan, X.F.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, 1802234. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Li, S.; Sun, C. Porous Perovskite La0.6Sr0.4Co0.8Mn0.2O3 Nanofibers Loaded with RuO2 Nanosheets as an Efficient and Durable Bifunctional Catalyst for Rechargeable Li–O2 Batteries. ACS Catal. 2017, 7, 7737–7747. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Kumar, P.; Zhu, X.F.; Daiyan, R.; Bedford, N.M.; Wu, K.H.; Han, Z.J.; Zhang, T.R.; Amal, R.; Lu, X.Y. Electronically Modified Atomic Sites Within a Multicomponent Co/Cu Composite for Efficient Oxygen Electroreduction. Adv. Energy Mater. 2021, 11, 2100303. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Lu, Z.K.; Qiao, Z.L.; Xing, M.H.; Han, L.K.; Wang, S.T.; Cao, D.P. Long-Range Regulation of Se Doping for Oxygen Reduction of Atomically Dispersed Sb Catalysts for Ultralow-Temperature Solid-State Zn-Air Batteries. ACS Catal. 2023, 13, 7122–7131. [Google Scholar] [CrossRef]
- Li, X.; Deng, C.; Kong, Y.; Huo, Q.; Mi, L.; Sun, J.; Cao, J.; Shao, J.; Chen, X.; Zhou, W.; et al. Unlocking the Transition of Electrochemical Water Oxidation Mechanism Induced by Heteroatom Doping. Angew. Chem. Int. Ed. 2023, 62, e202309732. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.-J.; Feng, X.; Ma, Z.; Ma, Z.-F.; Xue, Y. Progress of Pt and iron-group transition metal alloy catalysts with high ORR activity for PEMFCs. J. Electroanal. Chem. 2024, 959, 118165. [Google Scholar] [CrossRef]
- Garapati, M.S.; Sundara, R. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2019, 44, 10951–10963. [Google Scholar] [CrossRef]
- Liu, H.J.; Sun, F.M.; Yang, L.; Chen, M.; Wang, H.J. Gaining insight into the impact of electronic property and interface electrostatic field on ORR kinetics in alloy engineering via theoretical prognostication and experimental validation. Colloid Interface Sci. 2023, 652, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, G.L.; Zhao, H.Y.; Howlett, P.C.; Wang, X.A.; Fang, J. Earthworm-Inspired Co/Co3O4/CoF2@NSC Nanofibrous Electrocatalyst with Confined Channels for Enhanced ORR/OER Performance. Adv. Mater. 2024, 36, 2311272. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Q.; Wang, J.A.; Sun, Y.X.; Peng, L.C.; Li, C.J. Interface engineering of Co3O4/CeO2 heterostructure in-situ embedded in Co/N-doped carbon nanofibers integrating oxygen vacancies as effective oxygen cathode catalyst for Li-O2 battery. Chem. Eng. J. 2023, 452, 139317. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Wang, H.-Y.; Feng, L.; Zhu, J.-Z.; Liu, J.-X.; Li, W.-X. Crystal-Phase Engineering in Heterogeneous Catalysis. Chem. Rev. 2024, 124, 164–209. [Google Scholar] [CrossRef]
- Chen, X.; Pu, J.; Hu, X.H.; Yao, Y.C.; Dou, Y.B.; Jiang, J.J.; Zhang, W.J. Janus Hollow Nanofiber with Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. Small 2022, 18, 2200578. [Google Scholar] [CrossRef]
- Wang, G.; Gao, H.J.; Yan, Z.R.; Li, L.; Li, Q.X.; Fan, J.; Zhao, Y.X.; Deng, N.P.; Kang, W.M.; Cheng, B.W. Copper nanodot-embedded nitrogen and fluorine co-doped porous carbon nanofibers as advanced electrocatalysts for rechargeable zinc-air batteries. Colloid Interface Sci. 2023, 647, 163–173. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, Y.Y.; Liu, S.L.; Zhou, L.M.; Wu, X.L.; Guo, X.J.; Zhang, A.Q.; Zhang, P.X.; Li, B.J.; Jiang, J.C. Mn-doped Co nanoparticles on wood-derived monolithic carbon for rechargeable zinc-air batteries. J. Mater. Chem. A 2023, 11, 22951–22959. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, R.; Du, G.; Wang, H.; Hou, M.; Zhang, W.; Sun, P.; Chen, T. Hierarchically porous Fe/N/S/C nanospheres with high-content of Fe-Nx for enhanced ORR and Zn-air battery performance. Green Energy Environ. 2023, 8, 1693–1702. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, C.; Wang, F. Effect of Porous and Highly N,O-Doped Amorphous Carbon Materials with Defects Generated by KOH Etching on the Stability of ORR Catalysts. Energy Fuels 2024, 38, 18010–18017. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, W.; Lou, Y.; Ma, J.; Yao, W.; Zong, R.; Zhu, Y. Encapsulate α-MnO2 nanofiber within graphene layer to tune surface electronic structure for efficient ozone decomposition. Nat. Commun. 2021, 12, 4152. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sun, Y.; Guo, S.; Li, C. Highly efficient construction of hollow Co–Nx nanocube cage dispersion implanted with porous carbonized nanofibers for Li–O2 batteries. J. Mater. Chem. A 2022, 10, 740–751. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, X.; Sun, Y.; Li, C. Electrospun ZIF-derived cavity porous carbon nanofibers as a freestanding cathode for lithium–oxygen batteries with ultralow overpotential. Nanoscale 2021, 13, 16477–16486. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Dong, J.; Sun, H.; Wang, X.; Fang, J.; Zhuang, Z.; Tian, S.; Sun, X. Co(CN)3 catalysts with well-defined coordination structure for the oxygen reduction reaction. Nat. Catal. 2023, 6, 1164–1173. [Google Scholar] [CrossRef]
- Xie, Q.; Cai, Z.; Li, P.; Zhou, D.; Bi, Y.; Xiong, X.; Hu, E.; Li, Y.; Kuang, Y.; Sun, X. Layered double hydroxides with atomic-scale defects for superior electrocataiysis. Nano Res. 2018, 11, 4524–4534. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, L.; Shi, R.; Lu, M.; Wang, P. Unraveling the Synergistic Effect of Hollow Sea Urchin-like Ag-Co3O4 Heterostructures in Electrochemical Dehydrogenation Reactions: Interface-Enhanced Electron Transfer and Desorption Process. ACS Catal. 2025, 15, 7966–7978. [Google Scholar] [CrossRef]
- Chen, S.; Ma, Y.Y.; Zhang, L.; Zhang, Y.Y.; Chen, Y.H.; Zhang, X.H.; Yan, J.H. The Contact Interface Electronic Coupling of Cobalt and Zirconia Enables Stable and Highly Efficient 4e− Oxygen Reduction Reaction Catalysis. Small 2024, 20, 2307278. [Google Scholar] [CrossRef]
- Douka, A.I.; Xu, Y.Y.; Yang, H.; Zaman, S.; Yan, Y.; Liu, H.F.; Salam, M.A.; Xia, B.Y. A Zeolitic-Imidazole Frameworks-Derived Interconnected Macroporous Carbon Matrix for Efficient Oxygen Electrocatalysis in Rechargeable Zinc-Air Batteries. Adv. Mater. 2020, 32, 2002170. [Google Scholar] [CrossRef]
- Lu, B.L.; Lin, F.C.; Jiang, X.; Cheng, J.J.; Lu, Q.L.; Song, J.B.; Chen, C.; Huang, B. One-Pot Assembly of Microfibrillated Cellulose Reinforced PVA-Borax Hydrogels with Self-Healing and pH-Responsive Properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Wang, Z.K.; Pan, Q.M. An Omni-Healable Supercapacitor Integrated in Dynamically Cross-Linked Polymer Networks. Adv. Funct. Mater. 2017, 27, 1700690. [Google Scholar] [CrossRef]
- Lei, S.J.; Liu, L.; Wang, C.Y.; Shen, X.L.; Wang, C.N.; Guo, D.H.; Zeng, S.Y.; Cheng, B.C.; Xiao, Y.H.; Zhou, L. A facile in situ reduction route for preparation of spinel CoCr2O4 polycrystalline nanosheets and their magnetic properties. Crystengcomm 2014, 16, 277–286. [Google Scholar] [CrossRef]
- Guo, M.X.; Tang, B.B.; Zhang, H.M.; Yin, S.H.; Jiang, W.; Zhang, Y.M.; Li, M.Y.; Wang, H.; Jiao, L.Q. A high efficiency CoCr2O4/carbon nanotubes nanocomposite electrocatalyst for dye-sensitised solar cells. Chem. Commun. 2014, 50, 7356–7358. [Google Scholar] [CrossRef] [PubMed]
- Rahbar-Shamskar, K.; Azar, P.A.; Rashidi, A.; Baniyaghoob, S.; Yousefi, M. Synthesis of micro/mesoporous carbon adsorbents by in-situ fast pyrolysis of reed for recovering gasoline vapor. J. Clean. Prod. 2020, 259, 120832. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Guo, P.F.; Li, H.Z.; Fei, B.; Guo, Y.H.; Pan, H.G.; Sun, D.L.; Fang, F.; Wu, R.B. Co/CoP Heterojunction on Hierarchically Ordered Porous Carbon as a Highly Efficient Electrocatalyst for Hydrogen and Oxygen Evolution. Adv. Energy Mater. 2021, 11, 2102134. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Yin, H.J.; Wang, Y.; Chuang, C.H.; Xing, L.; Dong, M.Y.; Lu, Y.R.; Casillas-Garcia, G.; Zheng, Y.L.; Chen, S.; et al. Coexisting Single-Atomic Fe and Ni Sites on Hierarchically Ordered Porous Carbon as a Highly Efficient ORR Electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, Y.; Zhang, H.; Wu, Y. Preparation of manganese-doped cubic carbon electrode based on ZIF-8 and its capacitive deionization performance for fluoride removal. Sep. Purif. Technol. 2024, 328, 125046. [Google Scholar] [CrossRef]
- Meng, F.L.; Wang, Z.L.; Zhong, H.X.; Wang, J.; Yan, J.M.; Zhang, X.B. Reactive Multifunctional Template-Induced Preparation of Fe-N-Doped Mesoporous Carbon Microspheres Towards Highly Efficient Electrocatalysts for Oxygen Reduction. Adv. Mater. 2016, 28, 7948–7955. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Su, X.T.; Zhang, H.M.; Yin, H.J.; Liu, P.R.; Yang, H.G.; Wang, D.; Tang, Z.Y.; Wang, Y.; Zhao, H.J. Strongly Coupled CoCr2O4/Carbon Nanosheets as High Performance Electrocatalysts for Oxygen Evolution Reaction. Small 2016, 12, 2866–2871. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Xiong, R.; Zhong, L.; Song, Y.; Xu, J.; Xiao, Y.; Cheng, B.; Lei, S. CoCr2O4 Nanoparticles with Abundant Oxygen Vacancies: A New Photothermal Platform for Efficient Solar Evaporation. ACS Mater. Lett. 2023, 5, 1992–2001. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Z.; Sun, Y.; Wu, J.; Pan, X.; Sun, J. Steering Bidirectional Sulfur Redox via Geometric/Electronic Mediator Comodulation for Li–S Batteries. ACS Nano 2023, 17, 6002–6010. [Google Scholar] [CrossRef] [PubMed]
- Khalil, T.E.; Soliman, S.M.; Khalil, N.A.; El-Faham, A.; Foro, S.; El-Dissouky, A. Synthesis, structure, X-ray photoelectron spectroscopy (XPS), and antimicrobial, anticancer, and antioxidant activities of Co (III) complexes based on the antihypertensive hydralazine. Appl. Organomet. Chem. 2022, 36, e6565. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Liu, H.B.; Qiao, Z.L.; Qiao, K.W.; Sun, P.P.; Xu, H.X.; Wang, S.T.; Cao, D.P. Yolk-like Pt nanoparticles as cathode catalysts for low-Pt-loading proton-exchange membrane fuel cells. Mater. Today Energy 2022, 27, 101043. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xu, N.; Li, Y. High-entropy spinel oxides as efficient ORR catalysts towards enhanced kinetics for zinc-air batteries. J. Energy Storage 2025, 123, 116784. [Google Scholar] [CrossRef]
- Chang, Q.; He, F.; Zhang, Z.; Fu, X.; Wang, Y.; Huang, C.; Li, Y. Self-Organized Integrated Electrocatalyst on Oxygen Conversion for Highly Durable Zinc-Air Batteries. Angew. Chem. Int. Ed. 2025, 64, e202416664. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Jiang, G.; Mamaghani, A.H.; Sy, S.; Gao, R.; Jiang, Y.; Deng, Y.; Bai, Z.; Yang, L.; et al. Three-dimensionally ordered mesoporous Co3O4 decorated with Mg as bifunctional oxygen electrocatalysts for high-performance zinc-air batteries. Nano Energy 2022, 100, 107425. [Google Scholar] [CrossRef]
- Li, J.; Wan, T.; Li, J.; Zhang, Z.; Wang, Y.; Liu, G. Three-dimensionally ordered mesoporous trimetal sulfide as efficient electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2022, 575, 151728. [Google Scholar] [CrossRef]
- Xu, C.-X.; Zhang, J.-J.; Dou, H.-R.; Li, Y.-Z.; Li, D.-M.; Zhang, Y.-J.; Liu, B.; Inbaraj, P.; Huo, P.-P. Fe4N particles embedded in nitrogen-doped electrospun carbon nanofibers as efficient ORR catalysts for zinc-air battery. Rare Met. 2025, 44, 3156–3169. [Google Scholar] [CrossRef]
- Yasin, G.; Ibrahim, S.; Ibraheem, S.; Ali, S.; Iqbal, R.; Kumar, A.; Tabish, M.; Slimani, Y.; Nguyen, T.A.; Xu, H.; et al. Defective/graphitic synergy in a heteroatom-interlinked-triggered metal-free electrocatalyst for high-performance rechargeable zinc–air batteries. J. Mater. Chem. A 2021, 9, 18222–18230. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Mi, H.; Li, Y.; Deng, L.; Zhang, Q.; He, C.; Ren, X. Bifunctional oxygen electrocatalysis on ultra-thin Co9S8/MnS carbon nanosheets for all-solid-state zinc–air batteries. J. Mater. Chem. A 2021, 9, 22635–22642. [Google Scholar] [CrossRef]
- Akbarian, P.; Eshghi, A.; Asadi, A.; Kheirmand, M. Pd–Co3O4/acetylene black nanocomposite as an efficient and robust bifunctional ORR/OER electrocatalyst for rechargeable zinc-air batteries. Int. J. Hydrogen Energy 2024, 91, 1103–1112. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Lan, F.; Wang, Y.; Jiang, H.; Wu, X.; Huang, Y.; Li, R.; Jiang, Q.; Gao, D.; et al. Breaking the Stability-Activity Trade-off of Oxygen Electrocatalyst by Gallium Bilateral-Regulation for High-Performance Zinc-Air Batteries. Angew. Chem. Int. Ed. 2025, 64, e202420481. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, X.; Ge, L.; Zheng, Z.; Zhu, Y.; Zhou, C.; Yuan, J.; Zhu, S.; Gu, Y.; Zhou, W.; et al. N, S co-doped carbon with embedment of FeNi alloy as bifunctional oxygen electrocatalysts for rechargeable Zinc-air batteries. Carbon 2023, 202, 141–149. [Google Scholar] [CrossRef]
- Qi, Y.; Liang, Q.; Song, K.; Zhou, X.; Liu, M.; Li, W.; Liu, F.; Jiang, Z.; Zou, X.; Chen, Z.; et al. Optimizing high-coordination shell of Co-based single-atom catalysts for efficient ORR and zinc-air batteries. J. Energy Chem. 2024, 95, 306–314. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Tian, S.; Yang, J.; Li, J.; Wang, X.; Wang, L.; Chen, C.; Zhang, P.; Yang, Q. Nanoporous Fe and Co Dually Doped Carbon Nanotube-Based Oxygen Electrocatalysts for Efficient Zinc–Air Batteries. ACS Appl. Nano Mater. 2024, 7, 13536–13546. [Google Scholar] [CrossRef]
- Cao, X.; Gao, Y.; Wang, Z.; Zeng, H.; Song, Y.; Tang, S.; Luo, L.; Gong, S. FeNiCrCoMn High-Entropy Alloy Nanoparticles Loaded on Carbon Nanotubes as Bifunctional Oxygen Catalysts for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 32365–32375. [Google Scholar] [CrossRef]
- Allwyn, N.; Gokulnath, S.; Sathish, M. In-Situ Nanoarchitectonics of Fe/Co LDH over Cobalt-Enriched N-Doped Carbon Cookies as Facile Oxygen Redox Electrocatalysts for High-Rate Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 20360–20374. [Google Scholar] [CrossRef]
- Deng, Y.; Du, J.; Zhu, Y.; Zhao, L.; Wang, H.; Gong, Y.; Jin, J.; He, B.; Wang, R. Interface engineering of Ruddlesden–Popper perovskite/CeO2/carbon heterojunction for rechargeable zinc-air batteries. J. Colloid Interface Sci. 2024, 653, 1775–1784. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Zheng, Y.; Shen, M.; Wen, H.; Ma, R. Nickel-iron layered double hydroxides interlinked by N-doped carbon network as bifunctional electrocatalysts for rechargeable zinc-air batteries. Diam. Relat. Mater. 2024, 141, 110596. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, H.; Jiang, J.; Zhao, T.; Liu, S.; Wu, C.; Xu, G.; Zhang, L. In-situ nitrogen-doped carbon nanotube-encapsulated Co9S8 nanoparticles as self-supporting bifunctional air electrodes for zinc-air batteries. J. Mater. Sci. Technol. 2025, 222, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, R.; Zhu, X.; Li, H.; Wang, Y.; Wang, J.; Wang, F.; Lan, H. Strain-Regulated Pt–NiO@Ni Sub-Micron Particles Achieving Bifunctional Electrocatalysis for Zinc–Air Battery. Small 2023, 19, 2301640. [Google Scholar] [CrossRef]
- Feng, Y.; Song, K.; Zhang, W.; Zhou, X.; Yoo, S.J.; Kim, J.-G.; Qiao, S.; Qi, Y.; Zou, X.; Chen, Z.; et al. Efficient ORR catalysts for zinc-air battery: Biomass-derived ultra-stable Co nanoparticles wrapped with graphitic layers via optimizing electron transfer. J. Energy Chem. 2022, 70, 211–218. [Google Scholar] [CrossRef]
- Radwan, A.; Jin, H.; Liu, B.; Chen, Z.; Wu, Q.; Zhao, X.; He, D.; Mu, S. 3D-ZIF scaffold derived carbon encapsulated iron nitride as a synergistic catalyst for ORR and zinc-air battery cathodes. Carbon 2021, 171, 368–375. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Zhang, S.; Chen, Z.; Peng, W.; Li, Y.; Fan, X. MOF-on-MOF-derived FeCo@NC OER&ORR bifunctional electrocatalysts for zinc-air batteries. J. Colloid Interface Sci. 2025, 677, 800–811. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G.; Liu, B.; Liu, Y.; Zhang, X.; Cao, D.; Zhang, X. Luffa-like Interconnective Porous Nanofiber with Anchored Co/CoCr2O4 Hybrid Nanoparticles for Zinc–Air Batteries. Batteries 2025, 11, 306. https://doi.org/10.3390/batteries11080306
Jin G, Liu B, Liu Y, Zhang X, Cao D, Zhang X. Luffa-like Interconnective Porous Nanofiber with Anchored Co/CoCr2O4 Hybrid Nanoparticles for Zinc–Air Batteries. Batteries. 2025; 11(8):306. https://doi.org/10.3390/batteries11080306
Chicago/Turabian StyleJin, Guoqiang, Bin Liu, Yan Liu, Xueting Zhang, Dapeng Cao, and Xiuling Zhang. 2025. "Luffa-like Interconnective Porous Nanofiber with Anchored Co/CoCr2O4 Hybrid Nanoparticles for Zinc–Air Batteries" Batteries 11, no. 8: 306. https://doi.org/10.3390/batteries11080306
APA StyleJin, G., Liu, B., Liu, Y., Zhang, X., Cao, D., & Zhang, X. (2025). Luffa-like Interconnective Porous Nanofiber with Anchored Co/CoCr2O4 Hybrid Nanoparticles for Zinc–Air Batteries. Batteries, 11(8), 306. https://doi.org/10.3390/batteries11080306




