Experimental and Numerical Study of the Impact of Pressure During the Pyrolysis of Diethyl Carbonate and Ethyl Methyl Carbonate
Abstract
1. Introduction
2. Method
2.1. Shock-Tube Facility
2.2. CO Laser Absorption Diagnostic
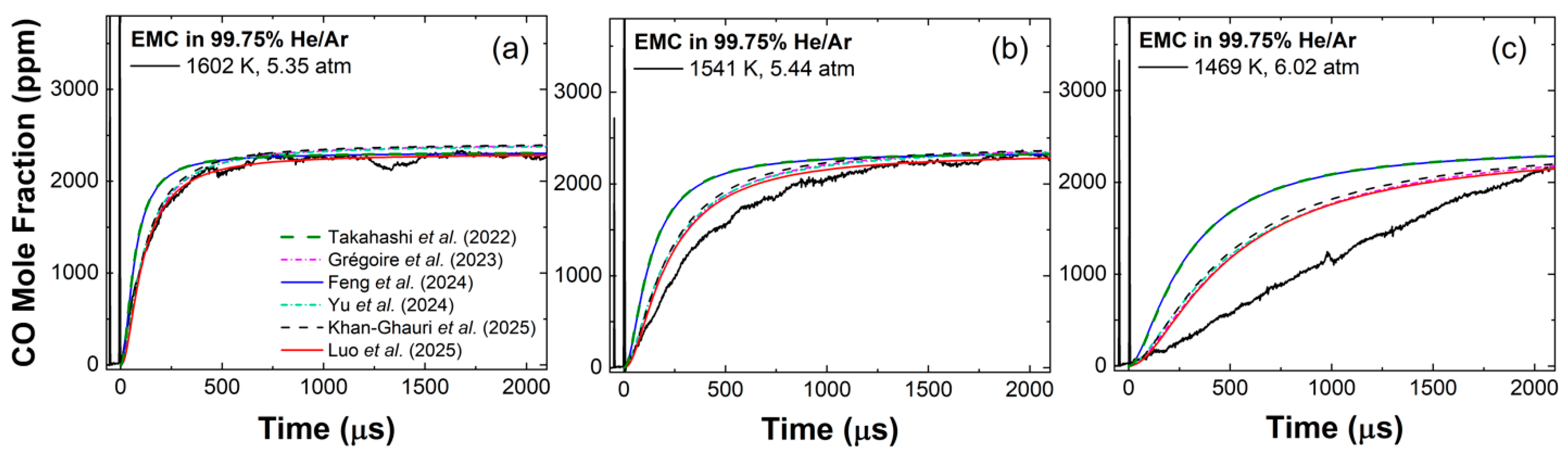
3. Results and Model Comparison
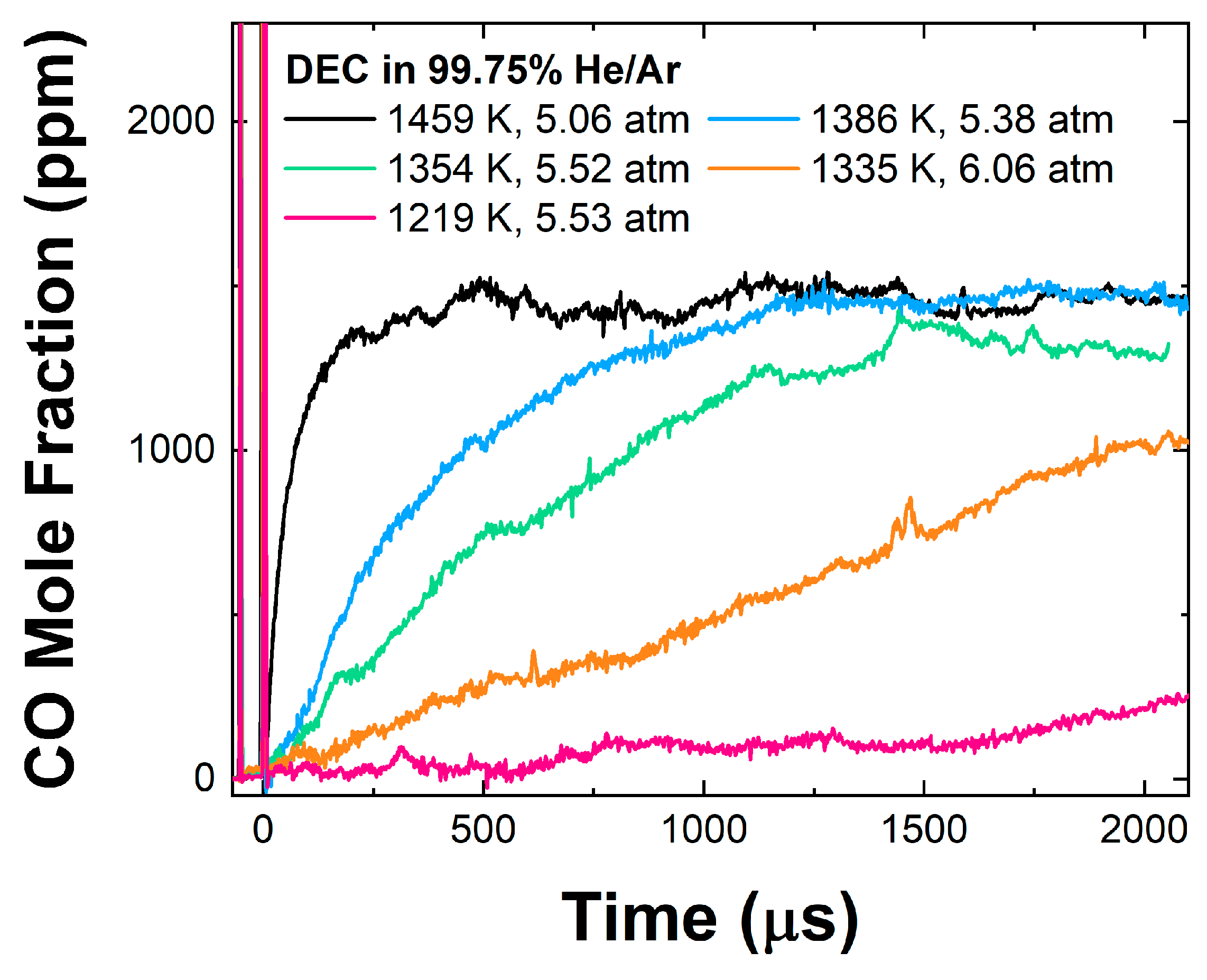
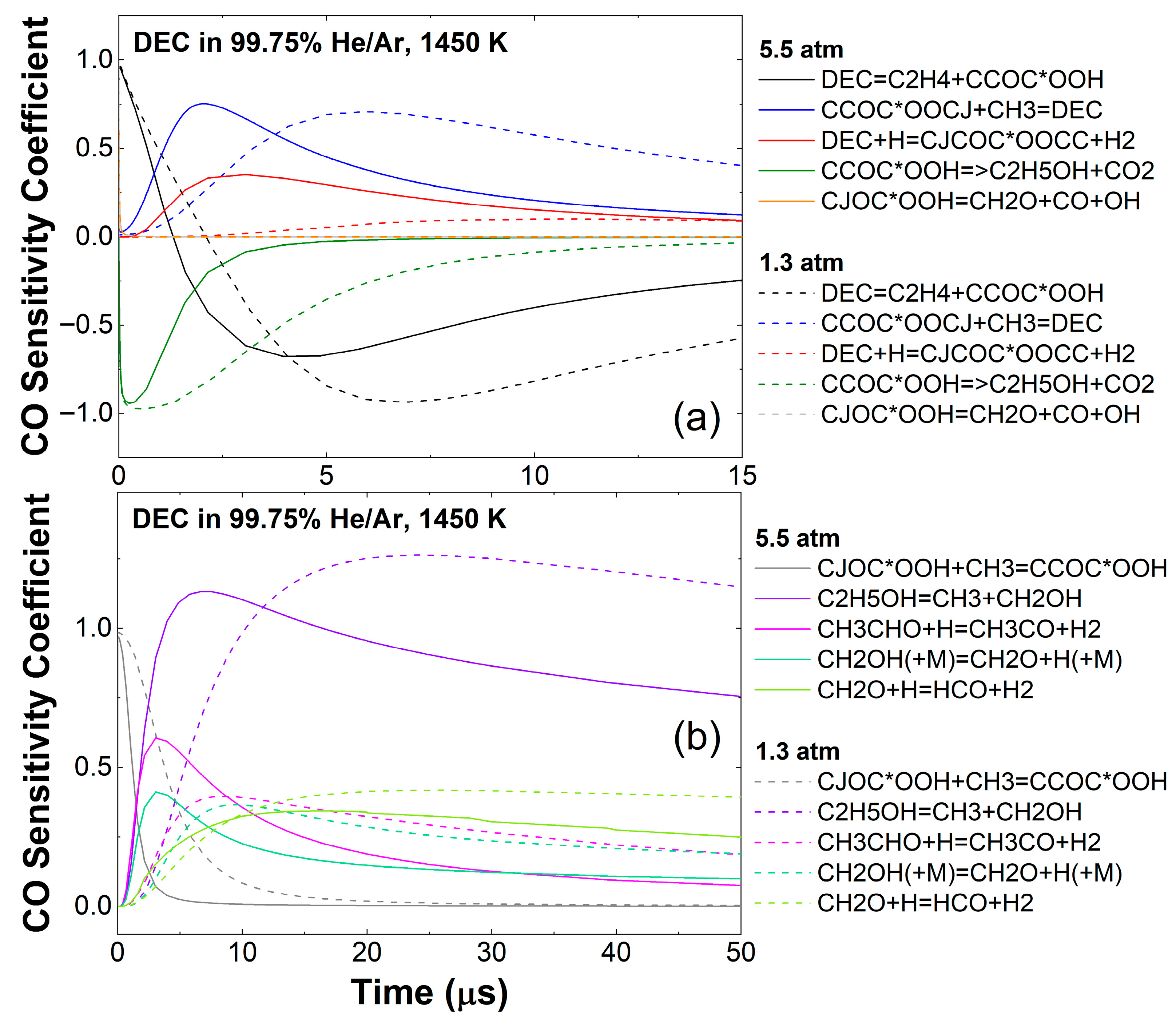
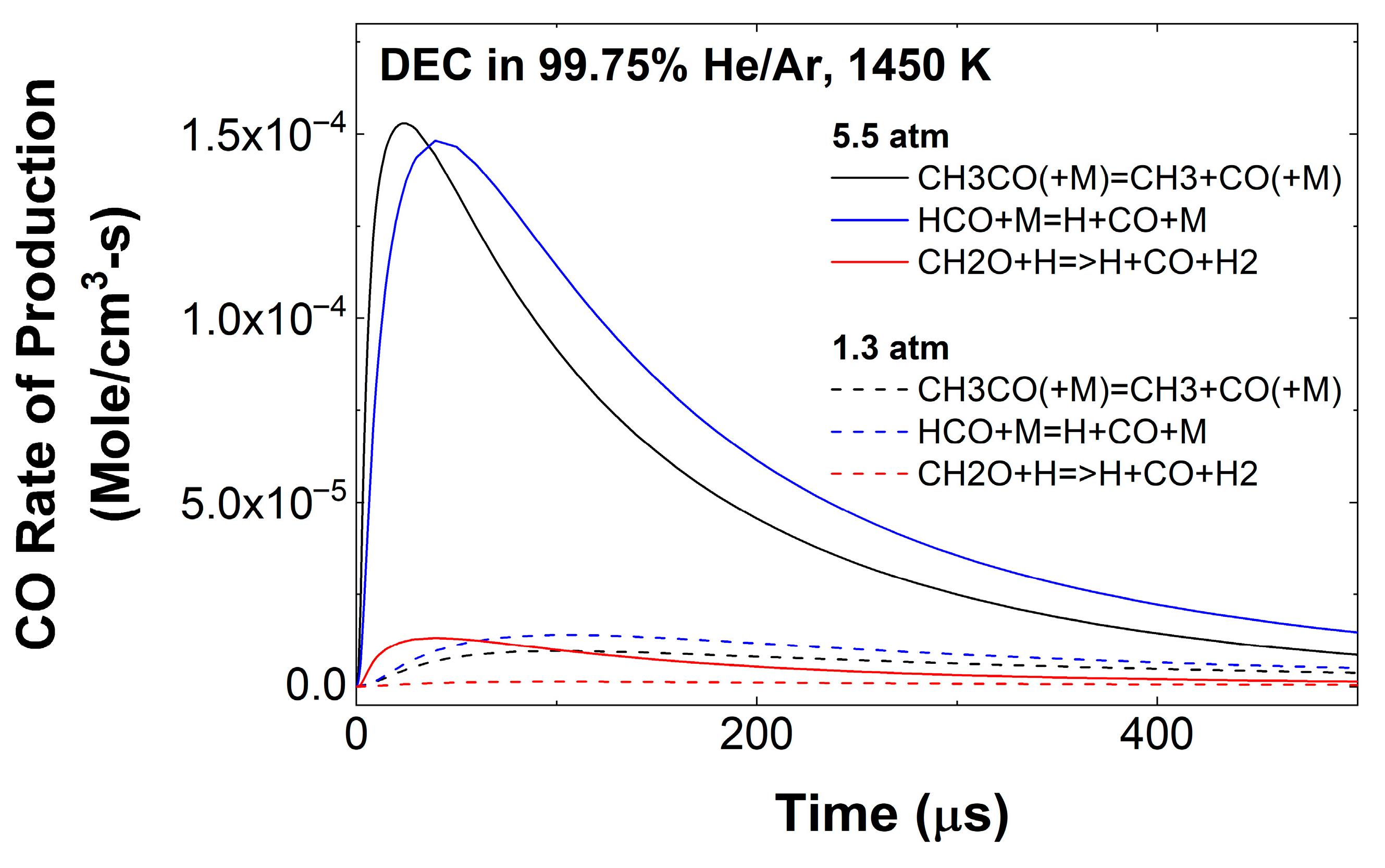
3.1. DEC Pyrolysis
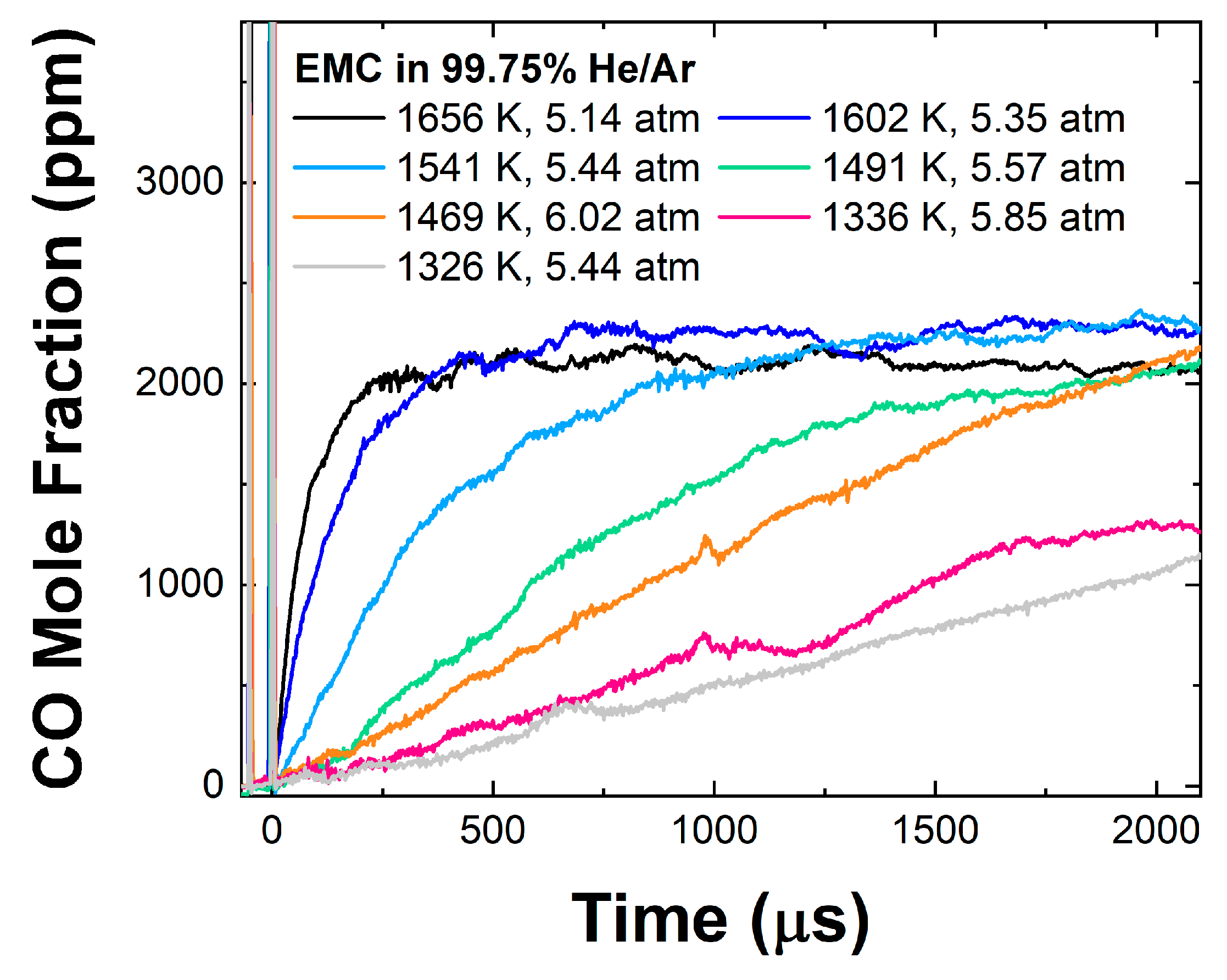
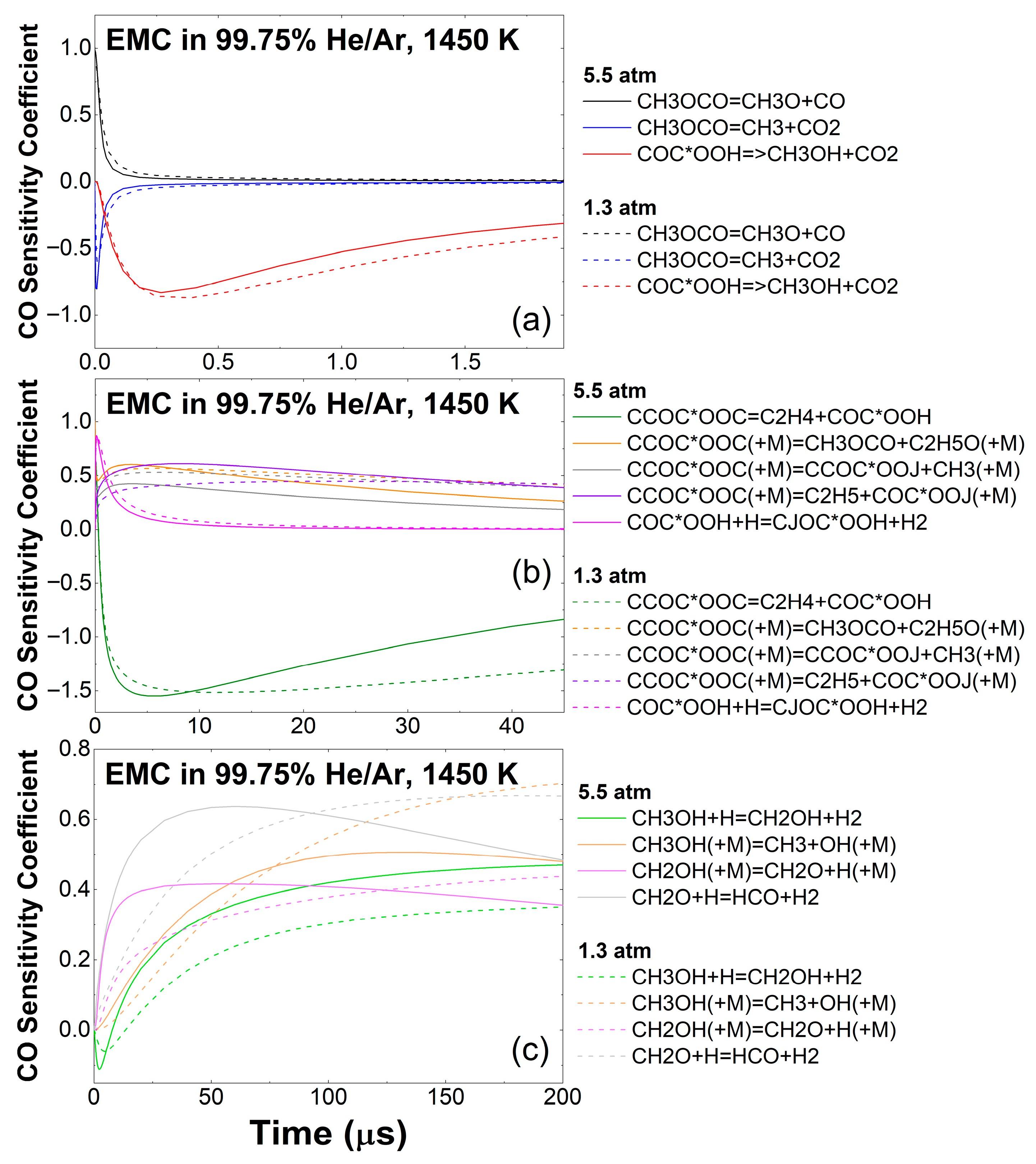
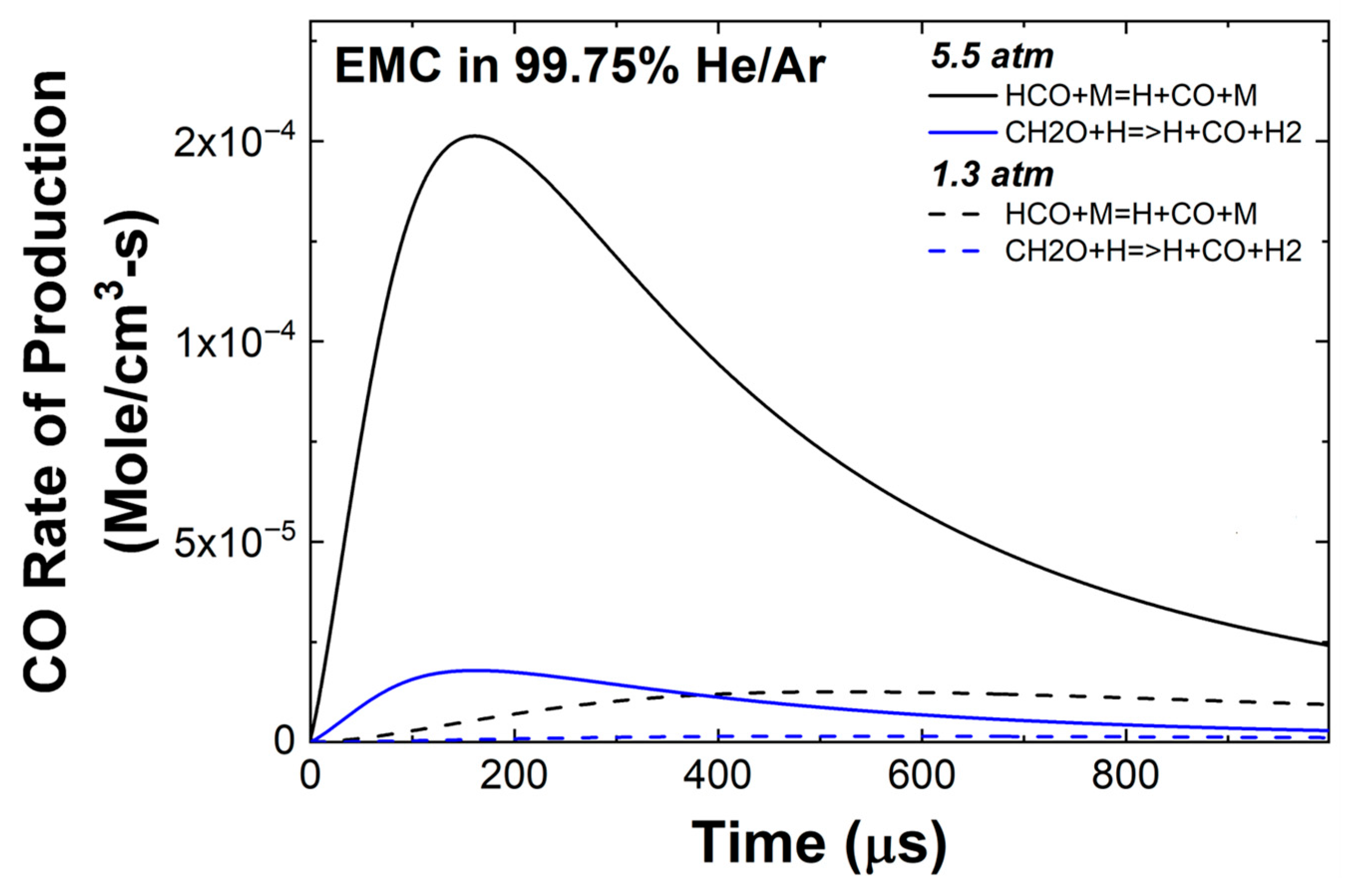
3.2. EMC Pyrolysis
3.3. Effect of Pressure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altenburg, T.; Corrocher, N.; Malerba, F. China’s leapfrogging in electromobility. A story of green transformation driving catch-up and competitive advantage. Technol. Forecast. Soc. Change 2022, 183, 121914. [Google Scholar] [CrossRef]
- Haghani, M.; Sprei, F.; Kazemzadeh, K.; Shahhoseini, Z.; Aghaei, J. Trends in electric vehicles research. Transp. Res. Part D Transp. Environ. 2023, 123, 103881. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.P.; Orendorff, C.J. How Electrolytes Influence Battery Safety. Electrochem. Soc. Interface 2012, 21, 45. [Google Scholar] [CrossRef]
- Mejame, P.P.M.; Jung, D.-Y.; Lee, H.; Lee, D.S.; Lim, S.-R. Effect of technological developments for smartphone lithium battery on metal-derived resource depletion and toxicity potentials. Resour. Conserv. Recycl. 2020, 158, 104797. [Google Scholar] [CrossRef]
- Mathieu, O.; Grégoire, C.M.; Turner, M.A.; Mohr, D.J.; Alturaifi, S.A.; Thomas, J.C.; Petersen, E.L. Experimental Investigation of the Combustion Properties of an Average Thermal Runaway Gas Mixture from Li-Ion Batteries. Energy Fuels 2022, 36, 3247–3258. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Shen, Z.; Yu, Q.; Xiong, R.; Shen, W. Towards a safer lithium-ion batteries: A critical review on cause, characteristics, warning and disposal strategy for thermal runaway. Adv. Appl. Energy 2023, 11, 100146. [Google Scholar] [CrossRef]
- Terazono, A.; Oguchi, M.; Akiyama, H.; Tomozawa, H.; Hagiwara, T.; Nakayama, J. Ignition and fire-related incidents caused by lithium-ion batteries in waste treatment facilities in Japan and countermeasures. Resour. Conserv. Recycl. 2024, 202, 107398. [Google Scholar] [CrossRef]
- Grégoire, C.; Cooper, S.; Khan-Ghauri, M.; Alturaifi, S.; Petersen, E.; Mathieu, O. Pyrolysis Study of Dimethyl Carbonate, Diethyl Carbonate, and Ethyl Methyl Carbonate using Shock-Tube Spectroscopic CO Measurements and Chemical Kinetics Investigation. Combust. Flame 2023, 249, 112594. [Google Scholar] [CrossRef]
- Nakamura, H.; Curran, H.J.; Polo Córdoba, A.; Pitz, W.J.; Dagaut, P.; Togbé, C.; Sarathy, S.M.; Mehl, M.; Agudelo, J.R.; Bustamante, F. An experimental and modeling study of diethyl carbonate oxidation. Combust. Flame 2015, 162, 1395–1405. [Google Scholar] [CrossRef]
- Shahla, R.; Togbé, C.; Thion, S.; Timothée, R.; Lailliau, M.; Halter, F.; Chauveau, C.; Dayma, G.; Dagaut, P. Burning velocities and jet-stirred reactor oxidation of diethyl carbonate. Proc. Combust. Inst. 2017, 36, 553–560. [Google Scholar] [CrossRef]
- Sela, P.; Zhang, Y.; Herzler, J.; Fikri, M.; Schulz, C.; Peukert, S. Pyrolysis of diethyl carbonate: Shock-tube and flow-reactor measurements and modeling. Proc. Combust. Inst. 2021, 38, 987–996. [Google Scholar] [CrossRef]
- Sun, W.; Huang, C.; Tao, T.; Zhang, F.; Li, W.; Hansen, N.; Yang, B. Exploring the high-temperature kinetics of diethyl carbonate (DEC) under pyrolysis and flame conditions. Combust. Flame 2017, 181, 71–81. [Google Scholar] [CrossRef]
- Kanayama, K.; Takahashi, S.; Morikura, S.; Nakamura, H.; Tezuka, T.; Maruta, K. Study on oxidation and pyrolysis of carbonate esters using a micro flow reactor with a controlled temperature profile. Part I: Reactivities of dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. Combust. Flame 2022, 237, 111810. [Google Scholar] [CrossRef]
- Takahashi, S.; Kanayama, K.; Morikura, S.; Nakamura, H.; Tezuka, T.; Maruta, K. Study on oxidation and pyrolysis of carbonate esters using a micro flow reactor with a controlled temperature profile. Part II: Chemical kinetic modeling of ethyl methyl carbonate. Combust. Flame 2022, 238, 111878. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Wu, Y.; Tang, C. Experimental and modeling study on ignition kinetics of ethyl methyl carbonate. Combust. Flame 2024, 261, 113318. [Google Scholar] [CrossRef]
- Feng, G.; Yang, Q.; Liu, Z.; Jiang, Z.; Zhao, C.; Wang, K.; Fuentes, A.; Chen, D.; He, X. Study of combustion characteristics of linear carbonates (DMC/DEC/EMC) and cyclic carbonate (EC): Laminar burning velocity and chemical reaction kinetics modeling. Fuel 2024, 363, 130881. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, L.; Wang, J.; Shao, X.; Wang, X.; Luo, Q.; Li, Y.; Tang, S. Laminar Combustion Characteristics of Spherically Propagating Ethyl Methyl Carbonate Flames. Energ. Fuels 2024, 38, 10156–10167. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, L.; Wang, X.; Tang, S.; Li, Y.; Xu, M.; Luo, Q. Investigation on the intrinsic instabilities of ethyl methyl carbonate flames. Fuel 2024, 367, 131526. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, L.; Wang, J.; Jia, H.; Wang, X.; Zhang, S.; Wang, D.; Lu, J. Investigation of chemical kinetic models for electrolyte solvent vapors released from thermal runaway lithium-ion batteries. J. Energy Storage 2025, 107, 114932. [Google Scholar] [CrossRef]
- Hu, E.; Chen, Y.; Zhang, Z.; Pan, L.; Li, Q.; Cheng, Y.; Huang, Z. Experimental and kinetic study on ignition delay times of dimethyl carbonate at high temperature. Fuel 2015, 140, 626–632. [Google Scholar] [CrossRef]
- Alexandrino, K.; Alzueta, M.U.; Curran, H.J. An experimental and modeling study of the ignition of dimethyl carbonate in shock tubes and rapid compression machine. Combust. Flame 2018, 188, 212–226. [Google Scholar] [CrossRef]
- Sun, W.; Yang, B.; Hansen, N.; Westbrook, C.K.; Zhang, F.; Wang, G.; Moshammer, K.; Law, C.K. An experimental and kinetic modeling study on dimethyl carbonate (DMC) pyrolysis and combustion. Combust. Flame 2016, 164, 224–238. [Google Scholar] [CrossRef]
- Peukert, S.L.; Sivaramakrishnan, R.; Michael, J.V. High Temperature Shock Tube and Theoretical Studies on the Thermal Decomposition of Dimethyl Carbonate and Its Bimolecular Reactions with H and D-Atoms. J. Phys. Chem. A 2013, 117, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.P.; Grégoire, C.M.; Mohr, D.J.; Mathieu, O.; Alturaifi, S.A.; Petersen, E.L. An Experimental Kinetics Study of Isopropanol Pyrolysis and Oxidation behind Reflected Shock Waves. Energies 2021, 14, 6808. [Google Scholar] [CrossRef]
- Petersen, E.L.; Rickard, M.J.A.; Crofton, M.W.; Abbey, E.D.; Traum, M.J.; Kalitan, D.M. A facility for gas- and condensed-phase measurements behind shock waves. Meas. Sci. Technol. 2005, 16, 1716. [Google Scholar] [CrossRef]
- Lipkowicz, J.T.; Nativel, D.; Cooper, S.; Wlokas, I.; Fikri, M.; Petersen, E.; Schulz, C.; Kempf, A.M. Numerical Investigation of Remote Ignition in Shock Tubes. Flow Turbul. Combust. 2021, 106, 471–498. [Google Scholar] [CrossRef]
- Spearrin, R.M.; Goldenstein, C.S.; Jeffries, J.B.; Hanson, R.K. Quantum cascade laser absorption sensor for carbon monoxide in high-pressure gases using wavelength modulation spectroscopy. Appl. Opt. 2014, 53, 1938–1946. [Google Scholar] [CrossRef]
- Grégoire, C.M.; Mathieu, O.; Petersen, E.L. High-Temperature Line Strengths with He- and Ar-Broadening Coefficients of the P(20) line in the 1 ← 0 band of Carbon Monoxide. Appl. Phys. B 2023, 129, 187. [Google Scholar] [CrossRef]
- Hanson, R.K.; Spearrin, R.M.; Goldenstein, C.S. Spectroscopy and Optical Diagnostics for Gases. In Spectroscopy and Optical Diagnostics for Gases; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Rothman, L.S.; Gordon, I.E.; Barber, R.J.; Dothe, H.; Gamache, R.R.; Goldman, A.; Perevalov, V.I.; Tashkun, S.A.; Tennyson, J. HITEMP, the high-temperature molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2139–2150. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, J.; Huang, G.; Guo, Y.; Duan, C. Simple empirical analytical approximation to the Voigt profile. J. Opt. Soc. Am. B 2001, 18, 666–672. [Google Scholar] [CrossRef]
- Mulvihill, C.R. H2O Laser Absorption and OH* Chemiluminescence Measurements of H2-NO2 Oxidation in a Shock Tube. Ph.D. Thesis, Mechanical Engineering, Texas A&M University, College Station, TX, USA, 2019. [Google Scholar]
- Cooper, S.P.; Grégoire, C.M.; Almarzooq, Y.M.; Petersen, E.L.; Mathieu, O. Experimental Kinetics Study on Diethyl Carbonate Oxidation. Fuel 2023, 4, 243–260. [Google Scholar] [CrossRef]
- Grégoire, C.; Almarzooq, Y.M.; Petersen, E.; Mathieu, O. Experimental and Modeling Study of the Combustion of Ethyl Methyl Carbonate, a Battery Electrolyte. Combust. Flame 2024, 260, 113225. [Google Scholar] [CrossRef]
- Khan-Ghauri, M.; Grégoire, C.M.; Kanayama, K.; Diévart, P.; Takahashi, S.; Tezuka, T.; Almarzooq, Y.M.; Nakamura, H.; Catoire, L.; Maruta, K.; et al. Experimental and Detailed Kinetics Modeling Study of Bis(2,2,2-trifluoroethyl) Carbonate, a Fire Suppressant for Lithium-Ion Battery. Energ. Fuels 2025, 39, 4893–4908. [Google Scholar] [CrossRef]
- Grégoire, C.M.; Almarzooq, Y.M.; Khan-Ghauri, M.; Diévart, P.; Catoire, L.; Petersen, E.L.; Mathieu, O. Enhancing lithium-ion battery safety: Investigating the flame-retardant efficacy of bis(2,2,2-trifluoroethyl) carbonate during ethyl methyl carbonate combustion. Proc. Combust. Inst. 2024, 40, 105559. [Google Scholar] [CrossRef]

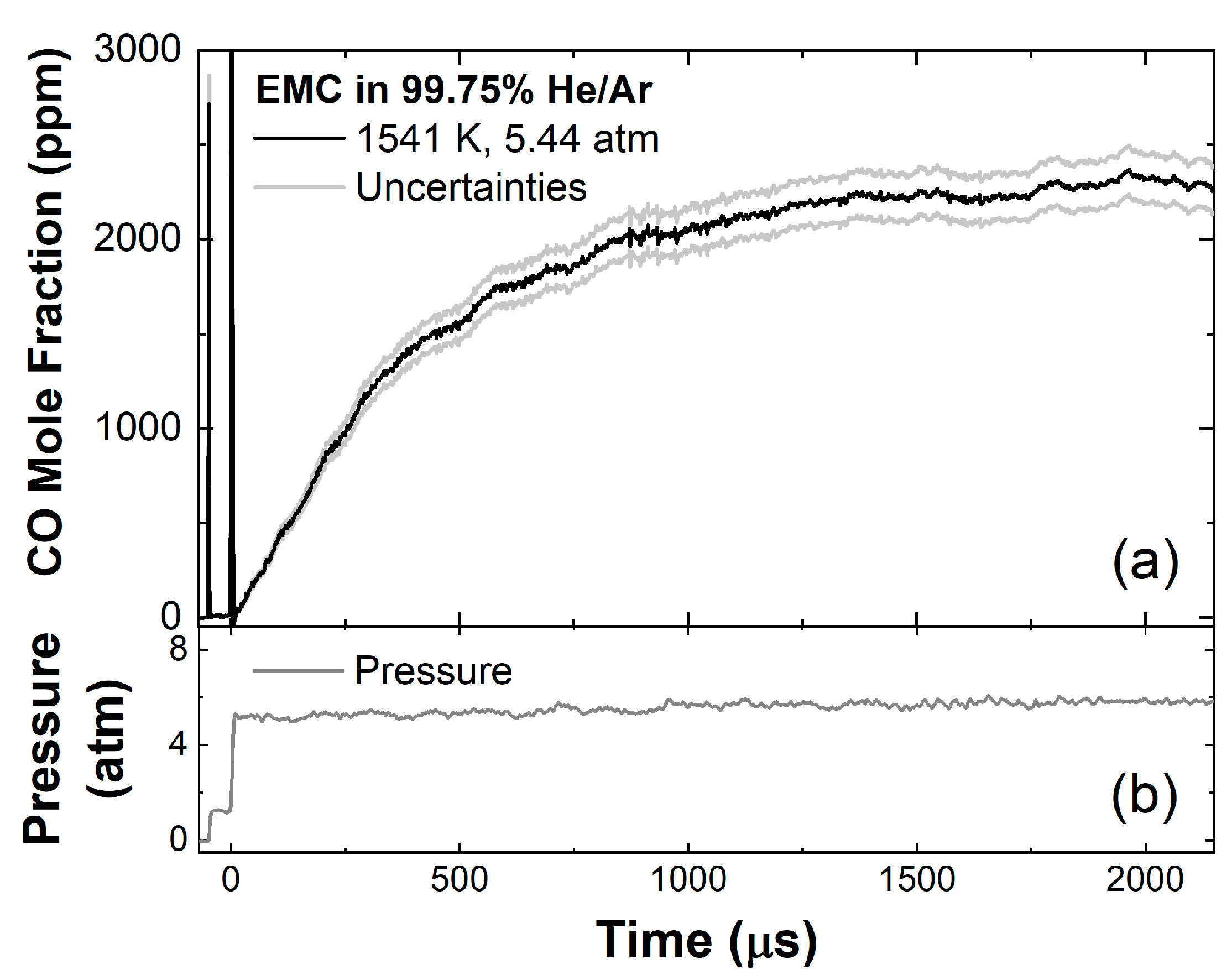



| T5 (K) | P5 (atm) | ||||
|---|---|---|---|---|---|
| 0.0025 | 0 | 0.2 | 0.7975 | 1219–1459 | 5.06–6.06 |
| 0 | 0.0025 | 0.2 | 0.7975 | 1326–1656 | 5.14–6.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grégoire, C.M.; Petersen, E.L.; Mathieu, O. Experimental and Numerical Study of the Impact of Pressure During the Pyrolysis of Diethyl Carbonate and Ethyl Methyl Carbonate. Batteries 2025, 11, 303. https://doi.org/10.3390/batteries11080303
Grégoire CM, Petersen EL, Mathieu O. Experimental and Numerical Study of the Impact of Pressure During the Pyrolysis of Diethyl Carbonate and Ethyl Methyl Carbonate. Batteries. 2025; 11(8):303. https://doi.org/10.3390/batteries11080303
Chicago/Turabian StyleGrégoire, Claire M., Eric L. Petersen, and Olivier Mathieu. 2025. "Experimental and Numerical Study of the Impact of Pressure During the Pyrolysis of Diethyl Carbonate and Ethyl Methyl Carbonate" Batteries 11, no. 8: 303. https://doi.org/10.3390/batteries11080303
APA StyleGrégoire, C. M., Petersen, E. L., & Mathieu, O. (2025). Experimental and Numerical Study of the Impact of Pressure During the Pyrolysis of Diethyl Carbonate and Ethyl Methyl Carbonate. Batteries, 11(8), 303. https://doi.org/10.3390/batteries11080303








