PyBEP: An Open-Source Tool for Electrode Potential Determination from Battery OCV Measurements
Abstract
1. Introduction
- The functional dependence of OCPs as the function of the amount of intercalated lithium for both electrode materials (hereafter referred to as OCP curves).
- The stoichiometric ranges of lithium within which both electrodes are cycled (hereafter referred to as stoichiometry ranges).
- Select appropriate OCP curves from the database corresponding to the chemical composition of both electrodes.
- Calculate stoichiometric cycling ranges for both electrodes.
- Calculate incremental capacity (, i.e., the inverse of the OCV derivative with respect to the charge) of both electrodes for refining results.
2. Methods
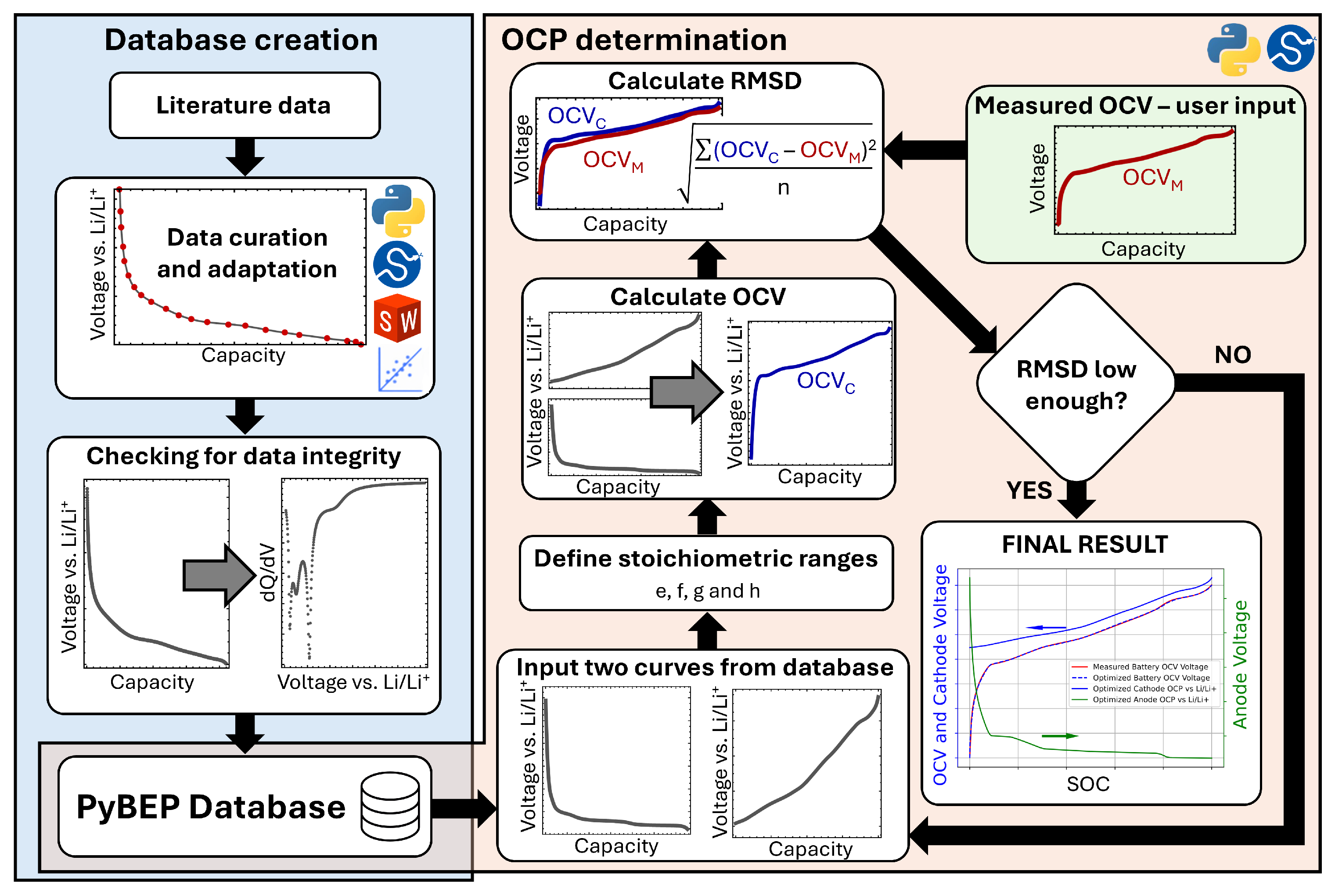
2.1. Database Creation
2.1.1. Literature Data
2.1.2. Data Curation and Adaptation
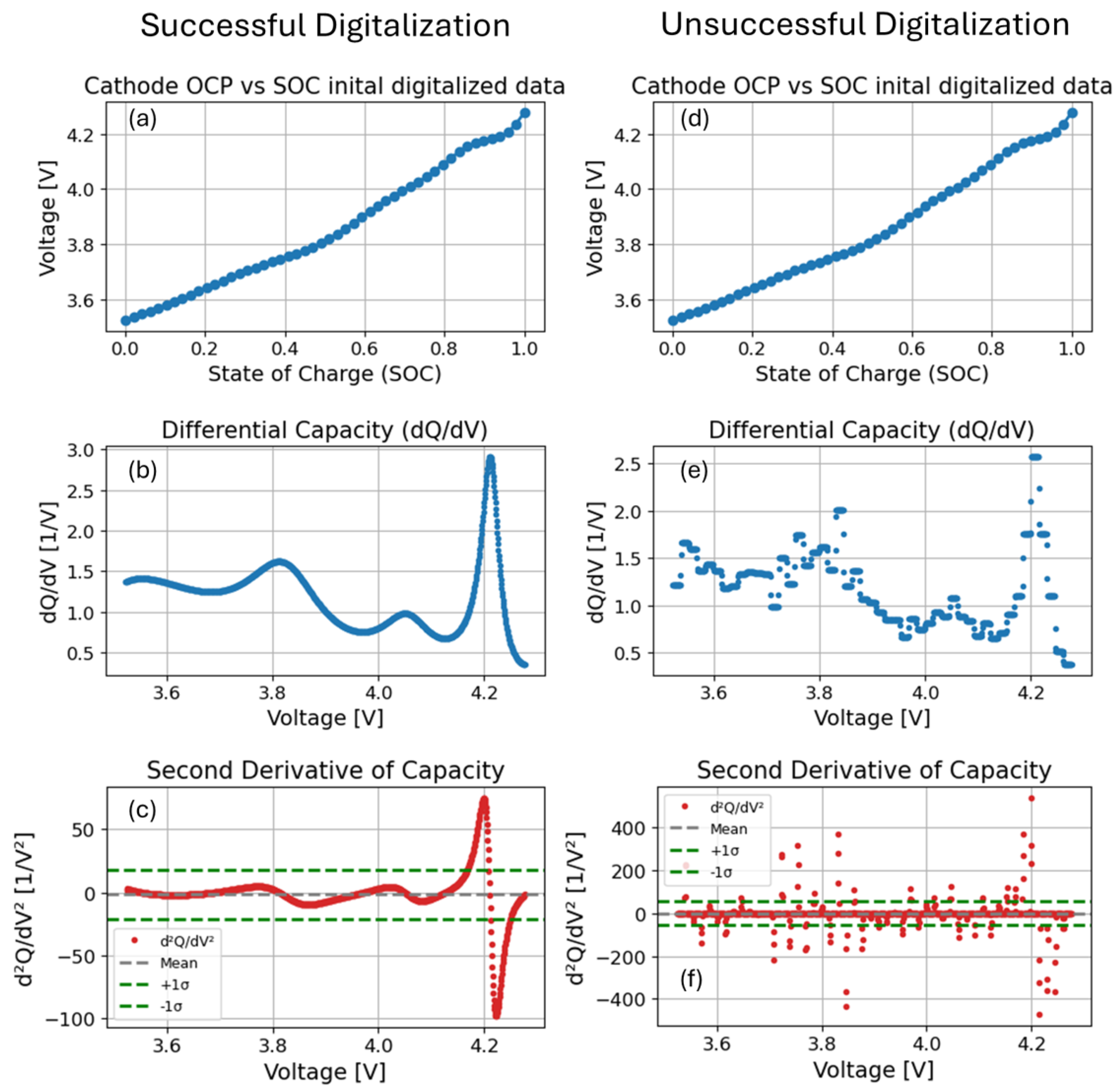
2.1.3. Data Integrity Check
2.1.4. PyBEP Database
2.2. Automated Selection of Open-Circuit Potential Curves and Calculation of Stoichiometric Ranges
2.2.1. Selecting Both OCP Curves from the Database
2.2.2. Determination of the Stoichiometric Ranges
2.2.3. OCV Calculation
2.2.4. RMSD Calculation
2.2.5. Convergence Criterion
2.3. OCV Measurements
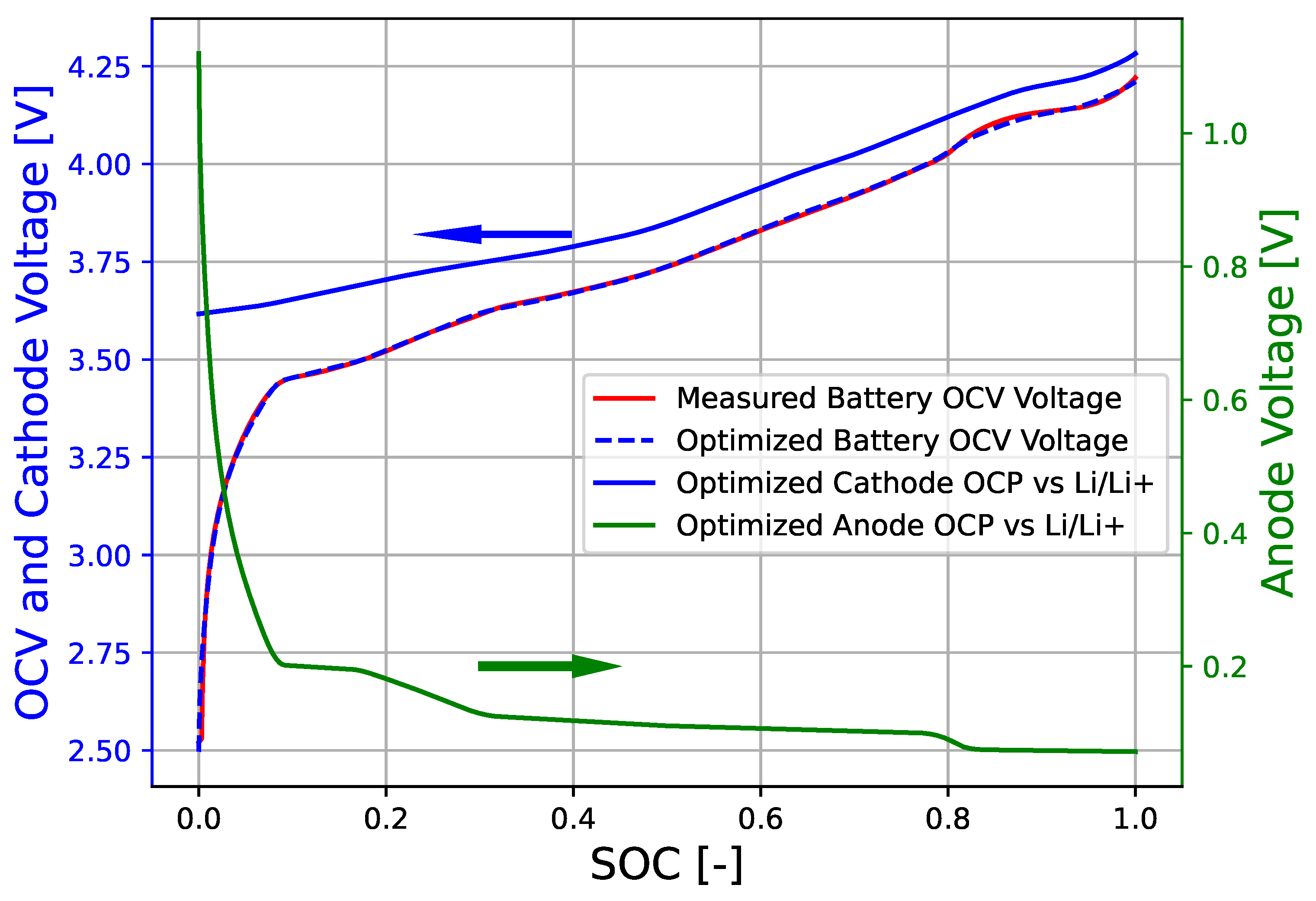
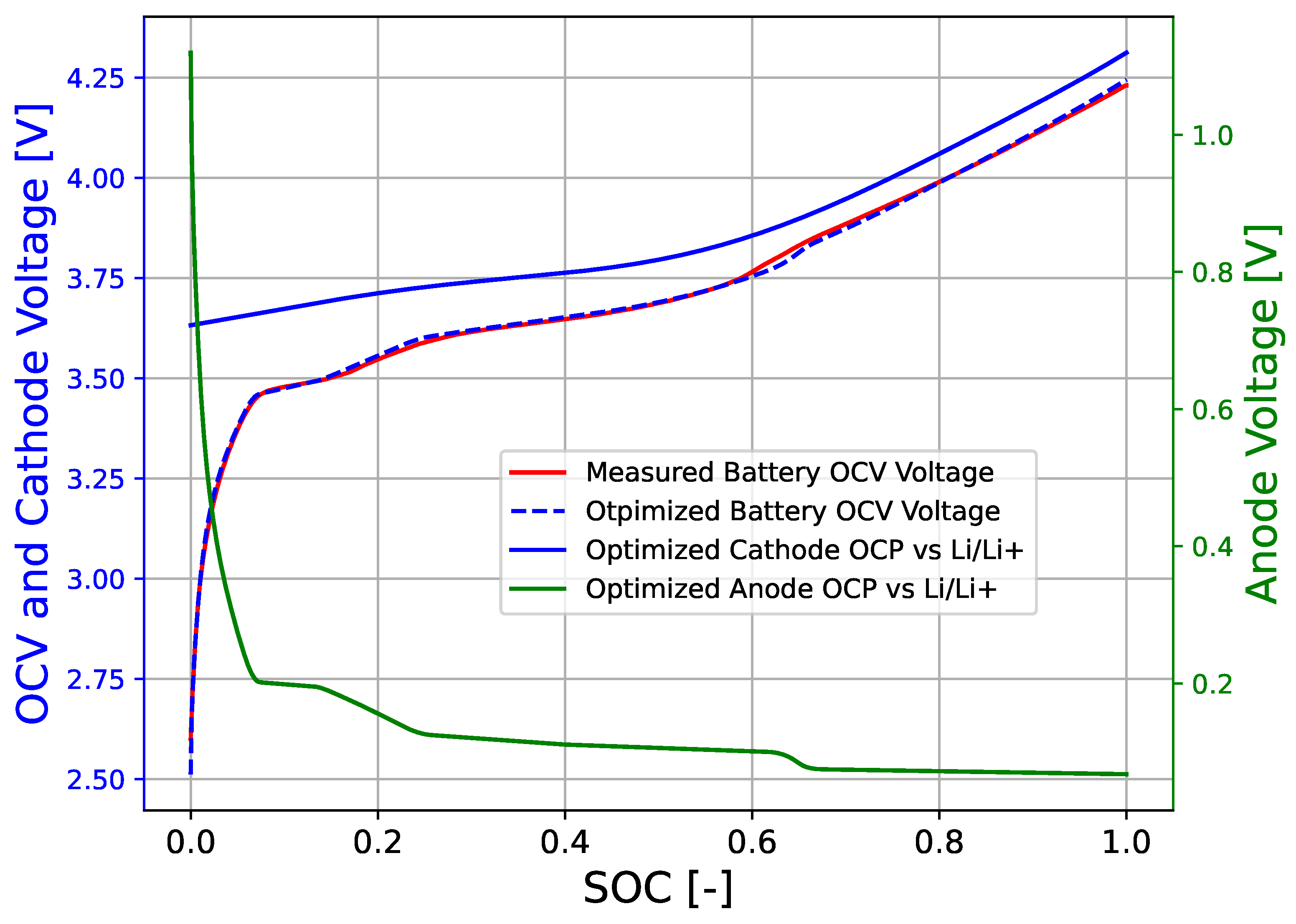
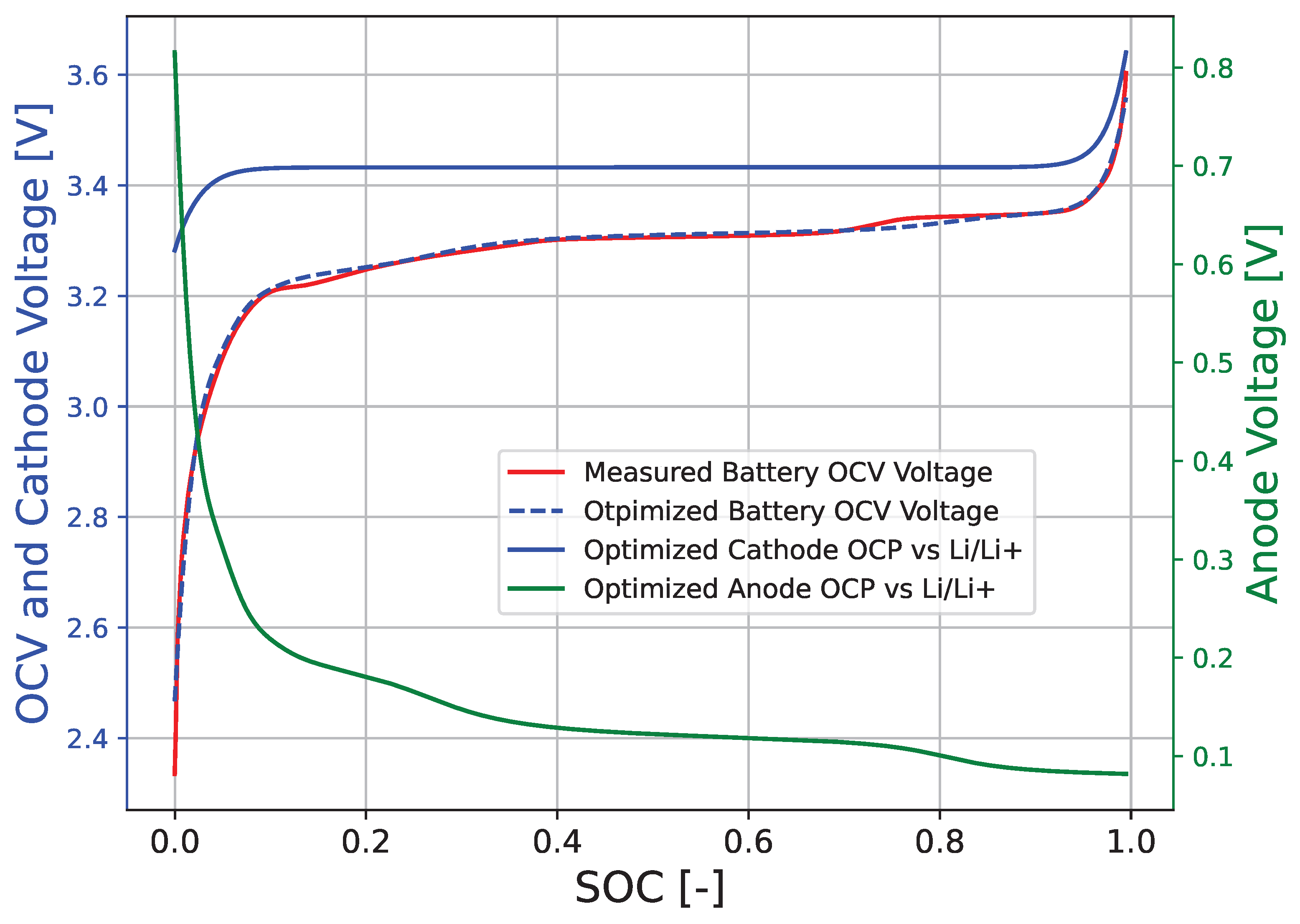
3. Results
4. Conclusions
- Determination of electrode chemical composition.
- Calculation of electrode stoichiometric ranges.
- Analysis of the dependence of electrode open-circuit potential on SOC.
- Enhancement of low-accuracy measurements of battery OCV.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Yu, B.; Li, Z.; Zhao, Y. Density functional theory for battery materials. Energy Environ. Mater. 2019, 2, 264–279. [Google Scholar] [CrossRef]
- Euchner, H.; Groß, A. Atomistic modeling of Li-and post-Li-ion batteries. Phys. Rev. Mater. 2022, 6, 040302. [Google Scholar] [CrossRef]
- Brosa Planella, F.; Ai, W.; Boyce, A.M.; Ghosh, A.; Korotkin, I.; Sahu, S.; Sulzer, V.; Timms, R.; Tranter, T.G.; Zyskin, M.; et al. A continuum of physics-based lithium-ion battery models reviewed. Prog. Energy 2022, 4, 042003. [Google Scholar] [CrossRef]
- Grazioli, D.; Magri, M.; Salvadori, A. Computational modeling of Li-ion batteries. Comput. Mech. 2016, 58, 889–909. [Google Scholar] [CrossRef]
- Katrašnik, T.; Mele, I.; Zelič, K. Multi-scale modelling of Lithium-ion batteries: From transport phenomena to the outbreak of thermal runaway. Energy Convers. Manag. 2021, 236, 114036. [Google Scholar] [CrossRef]
- Rosewater, D.M.; Copp, D.A.; Nguyen, T.A.; Byrne, R.H.; Santoso, S. Battery energy storage models for optimal control. IEEE Access 2019, 7, 178357–178391. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, Y.; Pan, Z.; Lu, Q. Review on the battery model and SOC estimation method. Processes 2021, 9, 1685. [Google Scholar] [CrossRef]
- Zelič, K.; Katrašnik, T.; Gaberšček, M. Derivation of transmission line model from the concentrated solution theory (CST) for porous electrodes. J. Electrochem. Soc. 2021, 168, 070543. [Google Scholar] [CrossRef]
- Mele, I.; Zelič, K.; Firm, M.; Moškon, J.; Gaberšček, M.; Katrašnik, T. Enhanced Porous Electrode Theory Based Electrochemical Model for Higher Fidelity Modelling and Deciphering of the EIS Spectra. J. Electrochem. Soc. 2024, 171, 080537. [Google Scholar] [CrossRef]
- Marshall, J.; Gastol, D.; Sommerville, R.; Middleton, B.; Goodship, V.; Kendrick, E. Disassembly of Li ion cells—Characterization and safety considerations of a recycling scheme. Metals 2020, 10, 773. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Balasingam, B.; Pattipati, K. Experimental data on open circuit voltage characterization for Li-ion batteries. Data Brief 2021, 36, 107071. [Google Scholar] [CrossRef]
- Pattipati, B.; Balasingam, B.; Avvari, G.; Pattipati, K.R.; Bar-Shalom, Y. Open circuit voltage characterization of lithium-ion batteries. J. Power Sources 2014, 269, 317–333. [Google Scholar] [CrossRef]
- Lu, D.; Trimboli, M.S.; Fan, G.; Zhang, R.; Plett, G.L. Implementation of a physics-based model for half-cell open-circuit potential and full-cell open-circuit voltage estimates: Part II. Processing full-cell data. J. Electrochem. Soc. 2021, 168, 070533. [Google Scholar] [CrossRef]
- Sulzer, V.; Marquis, S.G.; Timms, R.; Robinson, M.; Chapman, S.J. Python Battery Mathematical Modelling (PyBaMM). J. Open Res. Softw. 2021, 9, 14. [Google Scholar] [CrossRef]
- Klein, M. Battsimpy. 2017. Available online: https://github.com/matthewpklein/battsimpy (accessed on 28 July 2025).
- Li, S.; Rawat, S.K.; Zhu, T.; Offer, G.J.; Marinescu, M. Python-based Equivalent Circuit Network (PyECN) Model-ling Framework for Lithium-ion Batteries: Next generation open-source battery modelling framework for Lithium-ion batteries. engrXiv 2023. [Google Scholar] [CrossRef]
- Dhoot, R.; Timms, R.; Please, C. PyBaMM EIS: Efficient Linear Algebra Methods to Determine Li-ion Battery Behaviour. Available online: https://github.com/pybamm-team/pybamm-eis (accessed on 28 July 2025).
- Wang, F.; Zhai, Z.; Zhao, Z.; Di, Y.; Chen, X. Physics-informed neural network for lithium-ion battery degradation stable modeling and prognosis. Nat. Commun. 2024, 15, 4332. [Google Scholar] [CrossRef]
- Shi, Z. MambaLithium: Selective state space model for remaining-useful-life, state-of-health, and state-of-charge estimation of lithium-ion batteries. arXiv 2024, arXiv:2403.05430. [Google Scholar]
- Berliner, M.D.; Cogswell, D.A.; Bazant, M.Z.; Braatz, R.D. Methods—PETLION: Open-Source Software for Millisecond-Scale Porous Electrode Theory-Based Lithium-Ion Battery Simulations. J. Electrochem. Soc. 2021, 168, 090504. [Google Scholar] [CrossRef]
- Ai, W.; Liu, Y. JuBat: A Julia-based framework for battery modelling using finite element method. SoftwareX 2024, 27, 101760. [Google Scholar] [CrossRef]
- Planden, B.; Lukow, K.; Henshall, P.; Collier, G.; Morrey, D. A Computationally Informed Realisation Algorithm for Lithium-Ion Batteries Implemented with LiiBRA.jl. J. Energy Storage 2022, 55, 105637. [Google Scholar] [CrossRef]
- Planden, B.; Courtier, N.; Robinson, M.; Khetarpal, A.; Planella, F.B.; Howey, D. PyBOP: A Python package for battery model optimisation and parameterisation. arXiv 2024, arXiv:2412.15859. [Google Scholar]
- Wang, A.; O’Kane, S.; Planella, F.B.; Le Houx, J.; O’Regan, K.; Zyskin, M.; Edge, J.; Monroe, C.; Cooper, S.; Howey, D.A.; et al. Review of parameterisation and a novel database (LiionDB) for continuum Li-ion battery models. Prog. Energy 2022, 4, 032004. [Google Scholar] [CrossRef]
- COMSOL Multiphysics®, COMSOL AB, Stockholm, Sweden. Available online: https://www.comsol.com/ (accessed on 28 July 2025).
- AVL Cruise M. Available online: https://www.avl.com/en/simulation-solutions/software-offering/simulation-tools-a-z/avl-cruise-m (accessed on 28 July 2025).
- Dubarry, M.; Berecibar, M.; Devie, A.; Anseán, D.; Omar, N.; Villarreal, I. State of health battery estimator enabling degradation diagnosis: Model and algorithm description. J. Power Sources 2017, 360, 59–69. [Google Scholar] [CrossRef]
- Wang, A. LiionDB. 2022. Available online: https://github.com/ndrewwang/liiondb (accessed on 28 July 2025).
- Wang, A.; O’Kane, S.; Planella, F.B.; Houx, J.L.; O’Regan, K.; Zyskin, M.; Edge, J.; Monroe, C.; Cooper, S.; Howey, D.; et al. Parameterising continuum level Li-ion battery models & the LiionDB database. arXiv 2021, arXiv:2110.09879. [Google Scholar]
- Jiang, F.; Peng, P. Elucidating the performance limitations of lithium-ion batteries due to species and charge transport through five characteristic parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef] [PubMed]
- Dose, W.M.; Xu, C.; Grey, C.P.; De Volder, M.F. Effect of anode slippage on cathode cutoff potential and degradation mechanisms in Ni-rich Li-ion batteries. Cell Rep. Phys. Sci. 2020, 1, 100253. [Google Scholar] [CrossRef]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified electrochemical and thermal model of LiFePO4-graphite Li-ion batteries for fast charge applications. J. Electrochem. Soc. 2012, 159, A1508. [Google Scholar] [CrossRef]
- Wu, S.L.; Zhang, W.; Song, X.; Shukla, A.K.; Liu, G.; Battaglia, V.; Srinivasan, V. High rate capability of Li (Ni1/3Mn1/3Co1/3)O2 electrode for Li-ion batteries. J. Electrochem. Soc. 2012, 159, A438. [Google Scholar] [CrossRef]
- Verma, A.; Smith, K.; Santhanagopalan, S.; Abraham, D.; Yao, K.P.; Mukherjee, P.P. Galvanostatic intermittent titration and performance based analysis of LiNi0.5Co0.2Mn0.3O2 cathode. J. Electrochem. Soc. 2017, 164, A3380. [Google Scholar] [CrossRef]
- Chaouachi, O.; Réty, J.M.; Génies, S.; Chandesris, M.; Bultel, Y. Experimental and theoretical investigation of Li-ion battery active materials properties: Application to a graphite/Ni0.6Mn0.2Co0.2O2 system. Electrochim. Acta 2021, 366, 137428. [Google Scholar] [CrossRef]
- Price, K.V. Differential evolution: A fast and simple numerical optimizer. In Proceedings of the IEEE North American Fuzzy Information Processing, Berkeley, CA, USA, 19–22 June 1996; pp. 524–527. [Google Scholar]
- Song, X.; Yang, F.; Wang, D.; Tsui, K.L. Combined CNN-LSTM network for state-of-charge estimation of lithium-ion batteries. IEEE Access 2019, 7, 88894–88902. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pišek, J.; Katrašnik, T.; Zelič, K. PyBEP: An Open-Source Tool for Electrode Potential Determination from Battery OCV Measurements. Batteries 2025, 11, 295. https://doi.org/10.3390/batteries11080295
Pišek J, Katrašnik T, Zelič K. PyBEP: An Open-Source Tool for Electrode Potential Determination from Battery OCV Measurements. Batteries. 2025; 11(8):295. https://doi.org/10.3390/batteries11080295
Chicago/Turabian StylePišek, Jon, Tomaž Katrašnik, and Klemen Zelič. 2025. "PyBEP: An Open-Source Tool for Electrode Potential Determination from Battery OCV Measurements" Batteries 11, no. 8: 295. https://doi.org/10.3390/batteries11080295
APA StylePišek, J., Katrašnik, T., & Zelič, K. (2025). PyBEP: An Open-Source Tool for Electrode Potential Determination from Battery OCV Measurements. Batteries, 11(8), 295. https://doi.org/10.3390/batteries11080295







