Influence of Pulse Duration on Cutting-Edge Quality and Electrochemical Performance of Lithium Metal Anodes
Abstract
1. Introduction
2. State-of-the-Art Electrochemical Characterization of Lithium Metal Anodes
3. Materials and Methods
3.1. Used Material and Test Sample Geometry
3.2. Laser System and Laser Parameters
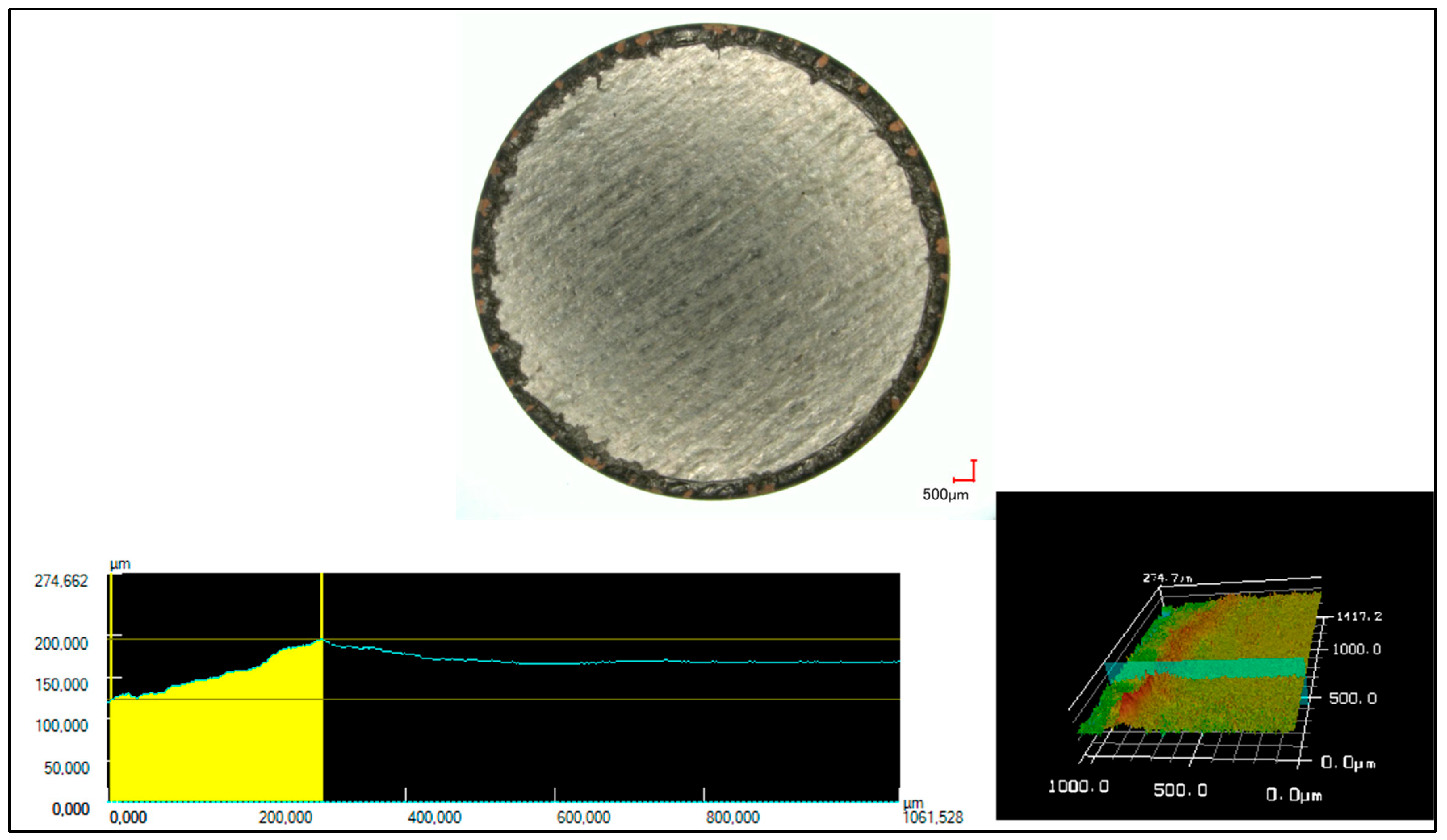
3.3. Analysis of Cutting-Edge Characteristics
3.4. Electrochemical Setup
4. Results and Discussion
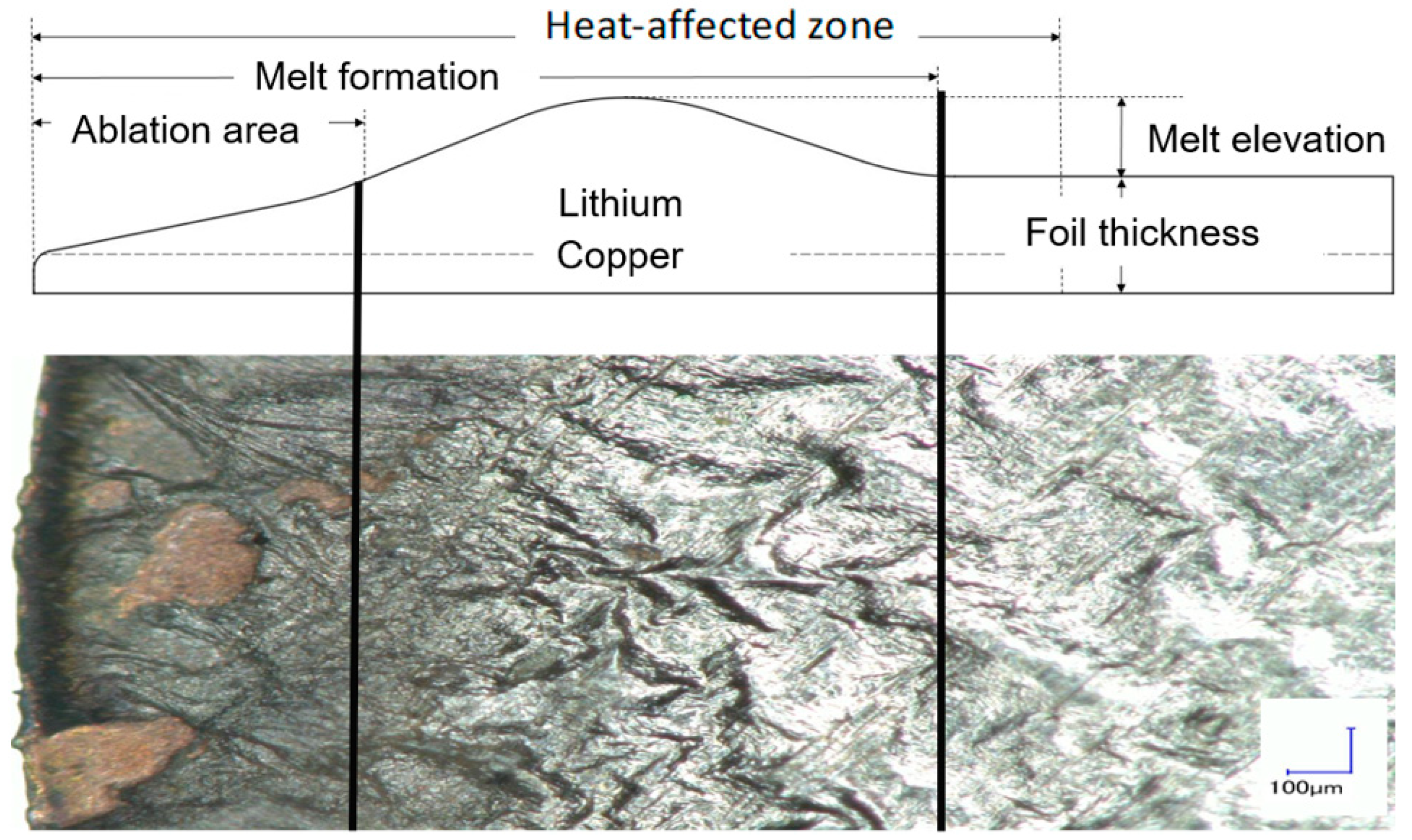
4.1. Characterization of the Cutting-Edge Characteristics
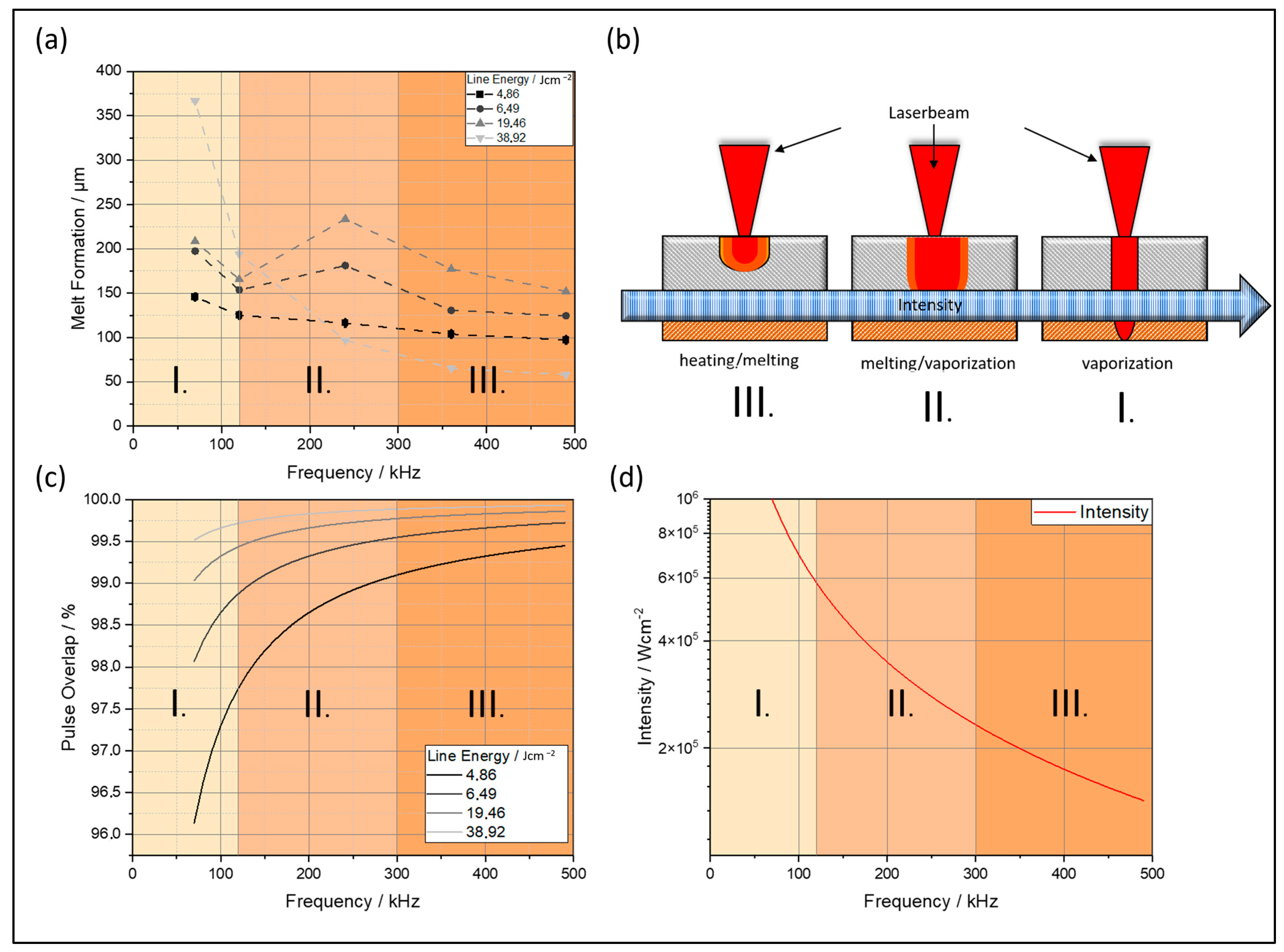
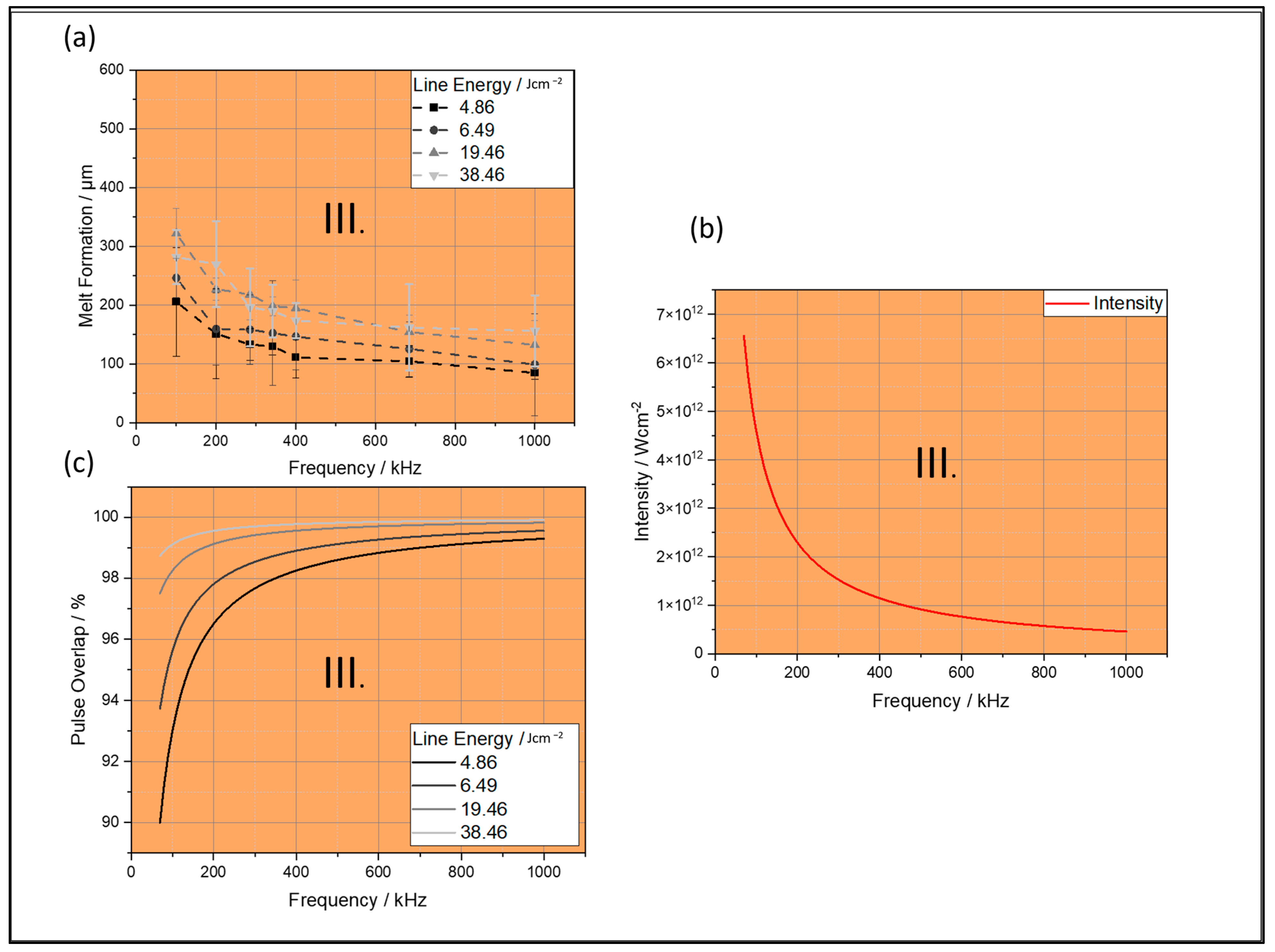
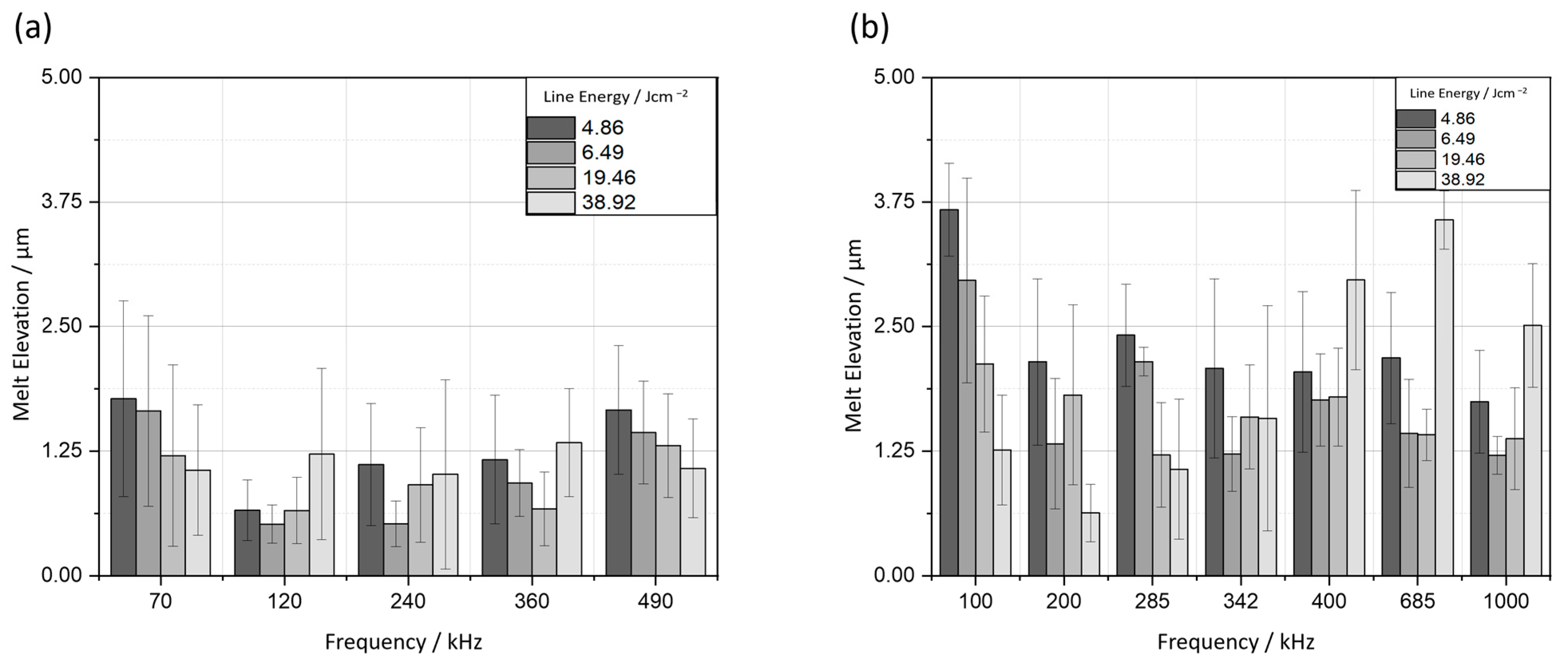
4.1.1. Melt Formation
4.1.2. Melt Elevation
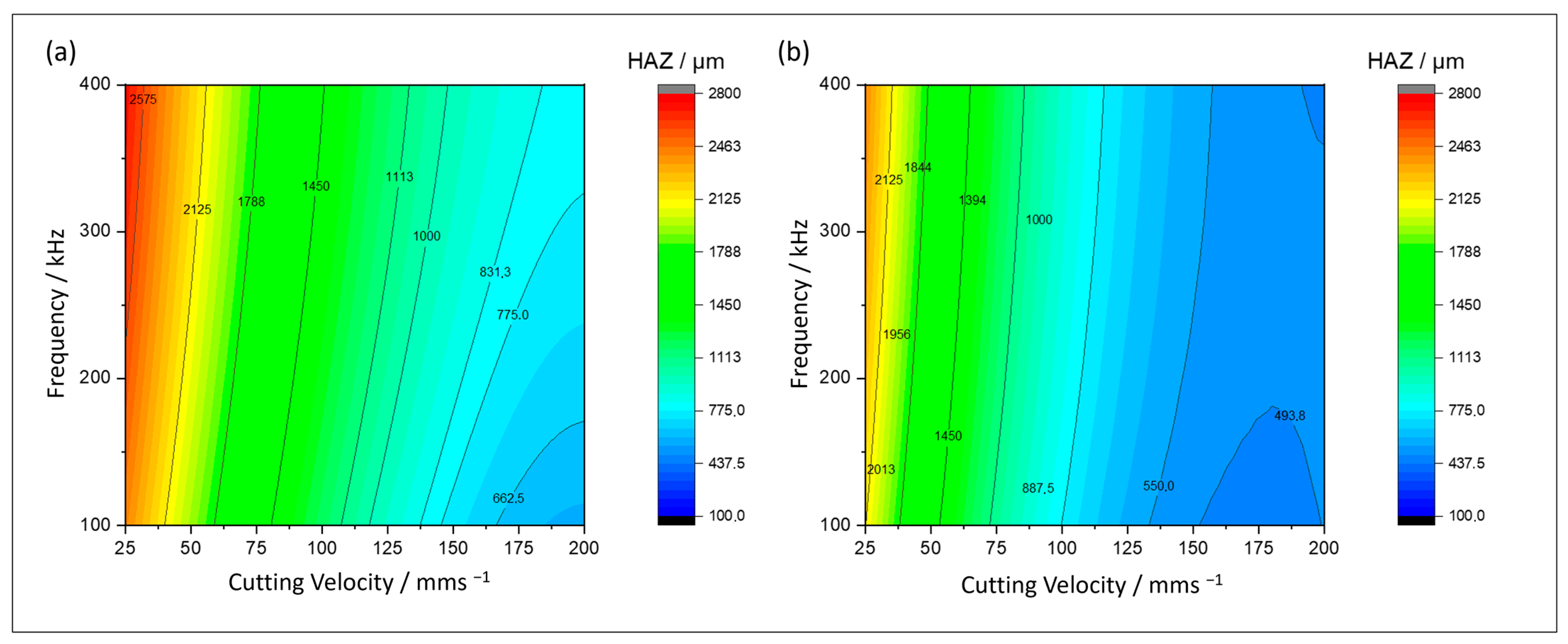
4.1.3. Heat-Affected Zone
4.2. Electrochemical Performance of Laser-Cut Lithium Metal Anodes
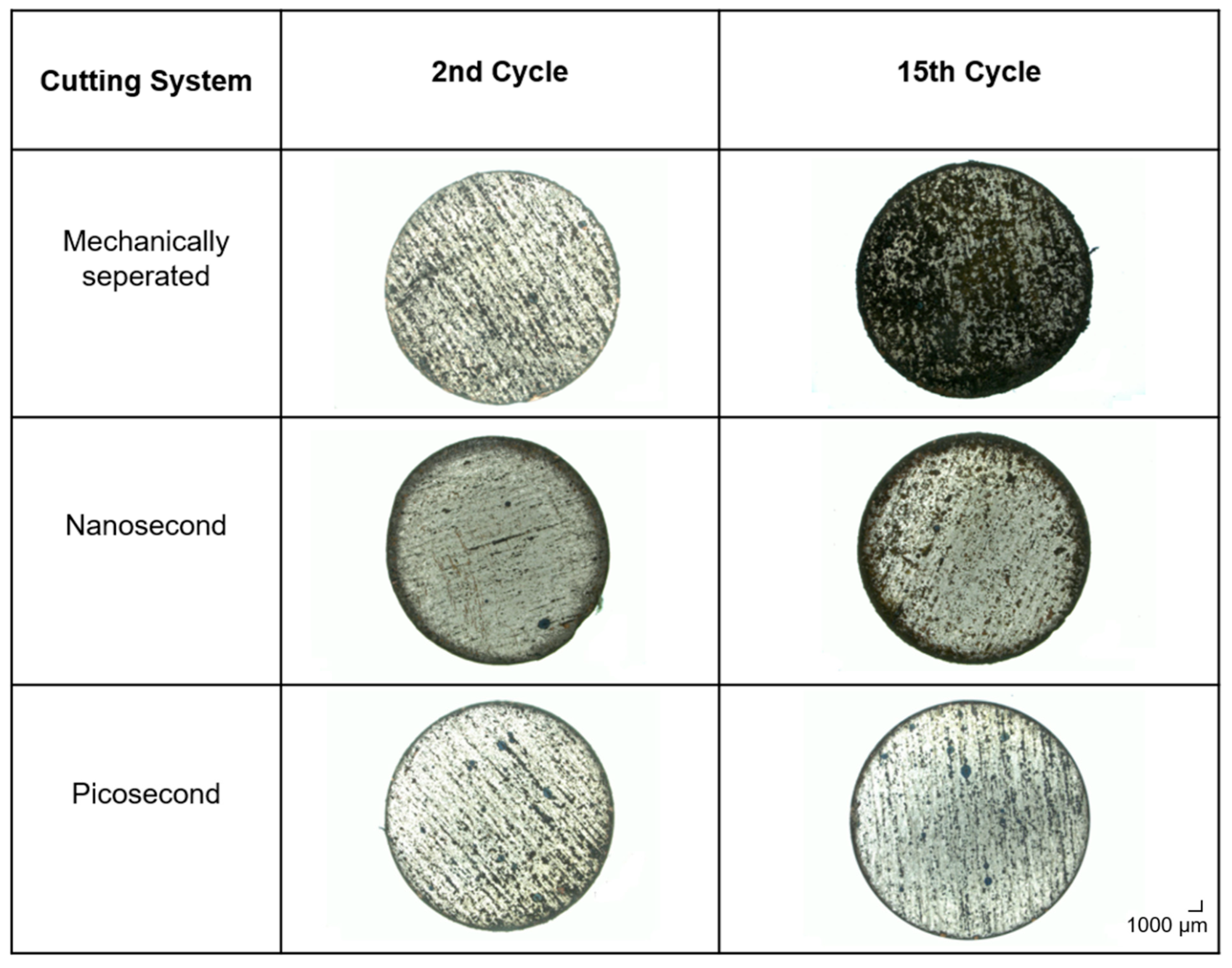
4.2.1. Melt Formation
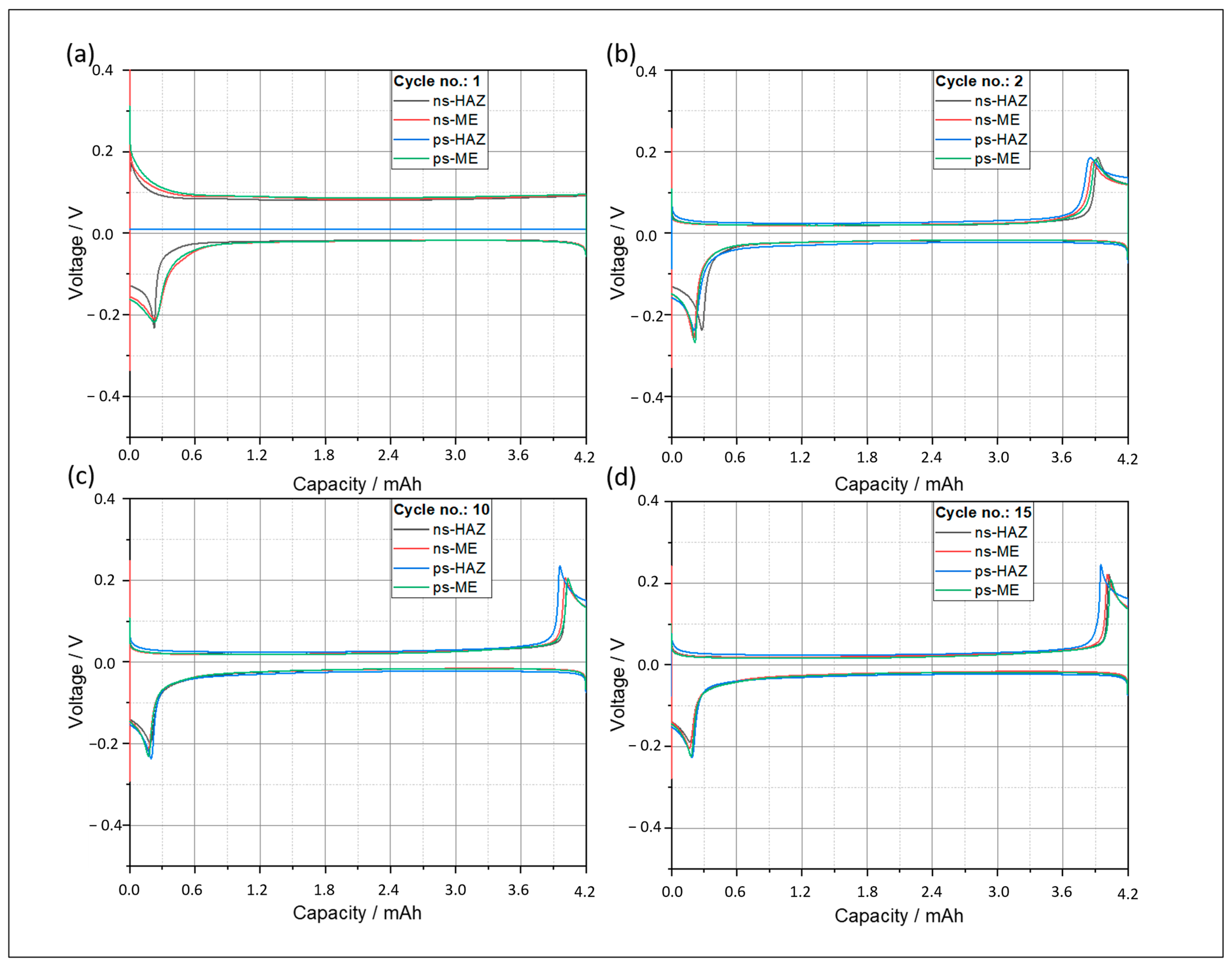
4.2.2. Influence of Cutting-Edge Characteristics on Electrochemical Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Hong, H.; Che Mohamad, N.A.R.; Chae, K.; Marques Mota, F.; Kim, D.H. The lithium metal anode in Li–S batteries: Challenges and recent progress. J. Mater. Chem. A 2021, 9, 10012–10038. [Google Scholar] [CrossRef]
- Chen, Q.; Geng, K.; Sieradzki, K. Prospects for Dendrite-Free Cycling of Li Metal Batteries. J. Electrochem. Soc. 2015, 162, A2004–A2007. [Google Scholar] [CrossRef]
- Jansen, T.; Blass, D.; Hartwig, S.; Dilger, K. Processing of Advanced Battery Materials—Laser Cutting of Pure Lithium Metal Foils. Batteries 2018, 4, 37. [Google Scholar] [CrossRef]
- Jansen, T.; Kandula, M.; Hartwig, S.; Hoffmann, L.; Haselrieder, W.; Dilger, K. Influence of Laser-Generated Cutting Edges on the Electrical Performance of Large Lithium-Ion Pouch Cells. Batteries 2019, 5, 73. [Google Scholar] [CrossRef]
- Sundaram, S.K.; Mazur, E. Inducing and probing non-thermal transitions in semiconductors using femtosecond laser pulses. Nat. Mater. 2003, 70, 691–697. [Google Scholar] [CrossRef]
- Schmidt, L.O.; Wehbe, H.; Kandula, M.W.; Dilger, K. (Eds.) Laserstrahlschneiden von Lithium-Metall Folien für den Gebrauch in All-Solid-State Batterie Systemen. In Proceedings of the 4th Symposium Materialtechnik, Clausthal, Germany, 25–26 February 2021; Shaker Verlag: Düren, Germany, 2021. [Google Scholar]
- Chichkov, B.N.; Momma, C.; Nolte, S.; Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Liu, X.; Du, D.; Mourou, G. Laser Ablation and Micromachining with Ultrashort Laser Pulses. IEEE J. Quantum Electron. 1997, 33, 1706–1716. [Google Scholar] [CrossRef]
- Leitz, K.-H.; Redlingshöfer, B.; Reg, Y.; Otto, A.; Schmidt, M. Metal Ablation with Short and Ultrashort Laser Pulses. Phys. Procedia 2011, 12, 230–238. [Google Scholar] [CrossRef]
- Bliedtner, J.; Müller, H.; Barz, A. (Eds.) Lasermaterialbearbeitung: Grundlagen—Verfahren—Anwendungen—Beispiele; Carl Hanser Verlag: Munich, Germany, 2013; ISBN 978-3-446-42168-4. [Google Scholar]
- Miotello, A.; Kelly, R. Laser-induced phase explosion: New physical problems when a condensed phase approaches the thermodynamic critical temperature. Appl. Phys. A 1999, 69, S67–S73. [Google Scholar] [CrossRef]
- Lu, Q.; Mao, S.S.; Mao, X.; Russo, R.E. Delayed phase explosion during high-power nanosecond laser ablation of silicon. Appl. Phys. Lett. 2002, 80, 3072–3074. [Google Scholar] [CrossRef]
- Mannion, P.; Magee, J.; Coyne, E.; O’Connor, G.; Glynn, T. The effect of damage accumulation behaviour on ablation thresholds and damage morphology in ultrafast laser micro-machining of common metals in air. Appl. Surf. Sci. 2004, 233, 275–287. [Google Scholar] [CrossRef]
- Orojloo, P.H.; Dib, S.A.; Kriegler, J.; Ballmes, H.; Zaeh, M.F.; Demir, A.G. Nanosecond- and picosecond-pulsed laser cutting of lithium metal anodes with copper current collector. Int. J. Adv. Manuf. Technol. 2025, 139, 657–671. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wood, K.N.; Kazyak, E.; LePage, W.S.; Davis, A.L.; Sanchez, A.J.; Dasgupta, N.P. Dead lithium: Mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 2017, 5, 11671–11681. [Google Scholar] [CrossRef]
- Bieker, G.; Winter, M.; Bieker, P. Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode. Phys. Chem. Chem. Phys. 2015, 17, 8670–8679. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862–2867. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Wood, K.N.; Kazyak, E.; Chadwick, A.F.; Chen, K.-H.; Zhang, J.-G.; Thornton, K.; Dasgupta, N.P. Dendrites and Pits: Untangling the Complex Behavior of Lithium Metal Anodes through Operando Video Microscopy. ACS Cent. Sci. 2016, 2, 790–801. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanism of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Rosso, M.; Brissot, C.; Teyssot, A.; Dollé, M.; Sannier, L.; Tarascon, J.-M.; Bouchet, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Stark, J.K.; Ding, Y.; Kohl, P.A. Dendrite-Free Electrodeposition and Reoxidation of Lithium-Sodium Alloy for Metal-Anode Battery. J. Electrochem. Soc. 2011, 158, A1100. [Google Scholar] [CrossRef]
- Howlett, P.C.; Brack, N.; Hollenkamp, A.F.; Forsyth, M.; MacFarlane, D.R. Characterization of the Lithium Surface in N-Methyl-N-alkylpyrrolidinium Bis(trifluoromethanesulfonyl)amide Room-Temperature Ionic Liquid Electrolytes. J. Electrochem. Soc. 2006, 153, A595–A606. [Google Scholar] [CrossRef]
- Sano, H.; Sakaebe, H.; Matsumoto, H. Observation of electrodeposited lithium by optical microscope in room temperature ionic liquid-based electrolyte. J. Power Sources 2011, 196, 6663–6669. [Google Scholar] [CrossRef]
- Sano, H.; Sakaebe, H.; Senoh, H.; Matsumoto, H. Effect of Current Density on Morphology of Lithium Electrodeposited in Ionic Liquid-Based Electrolytes. J. Electrochem. Soc. 2014, 161, A1236–A1240. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Yann Liaw, B.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Steiger, J.; Kramer, D.; Mönig, R. Mechanisms of dendritic growth investigated by in situ light microscopy during electrodeposition and dissolution of lithium. J. Power Sources 2014, 261, 112–119. [Google Scholar] [CrossRef]
- Steiger, J.; Richter, G.; Wenk, M.; Kramer, D.; Mönig, R. Comparison of the growth of lithium filaments and dendrites under different conditions. Electrochem. Commun. 2015, 50, 11–14. [Google Scholar] [CrossRef]
- Yoshimatsu, I.; Hirai, T.; Yamaki, J. Lithium Electrode Morphology during Cycling in Lithium Cells. J. Electrochem. Soc. 1988, 135, 2422–2427. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Teller, H.; Dan, P. Factors Which Limit the Cycle Life of Rechargeable Lithium (Metal) Batteries. J. Electrochem. Soc. 2000, 147, 1274. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Chen, W.; Lin, Q.; Ren, S.; Su, Y.; Tong, R.; Zhang, Y. Dendrite growth and inhibition in all-solid-state lithium metal batteries: In situ optical observation. J. Mater. Chem. A 2024, 12, 3575–3579. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, X.; Huang, S.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Long-Term Stable Cycling of Dendrite-Free Lithium Metal Batteries Using ZIF-90@PP Composite Separator. Nanomaterials 2024, 14, 975. [Google Scholar] [CrossRef]
- Oh, H.-S.; Lee, R.-H.; Lee, J.-K.; Yoon, J.-R.; Kim, H.-K.; Lee, S.-H. Attaining improved cycling durability and engineering a dendrite-free lithium metal anode. Mater. Adv. 2024, 5, 8294–8303. [Google Scholar] [CrossRef]
- Jansen, T.; Kandula, M.W.; Blass, D.; Hartwig, S.; Haselrieder, W.; Dilger, K. Evaluation of the Separation Process for the Production of Electrode Sheets. Energy Technol. 2020, 8, 1900519. [Google Scholar] [CrossRef]
- Hügel, H. Strahlwerkzeug Laser: Eine Einführung: Teubner-Studienbücher: Maschinenbau; Springer Fachmedien Wiesbaden GmbH: Wiesbaden, Germany, 1992; ISBN 978-3-519-06134-2. [Google Scholar]
- Marla, D.; Bhandarkar, U.V.; Joshi, S.S. A model of laser ablation with temperature-dependent material properties, vaporization, phase explosion and plasma shielding. Appl. Phys. A 2014, 116, 273–285. [Google Scholar] [CrossRef]
- Schnell, G.; Duenow, U.; Seitz, H. Effect of Laser Pulse Overlap and Scanning Line Overlap on Femtosecond Laser-Structured Ti6Al4V Surfaces. Materials 2020, 13, 969. [Google Scholar] [CrossRef]
- de Levie, R. The influence of surface roughness of solid electrodes on electrochemical measurements. Electrochim. Acta 1965, 10, 113–130. [Google Scholar] [CrossRef]
- Lorenz, P.; Ehrhardt, M.; Lotnyk, A.; Griebel, J.; Zimmer, K.; Zajadacz, J.; Himmerlich, M.; Bez, E.; Taborelli, M.; Rosenow, S.; et al. Cleaning of laser-induced periodic surface structures on copper by gentle wet chemical processing. Appl. Surf. Sci. 2025, 679, 161115. [Google Scholar] [CrossRef]
- Jeppson, D.W.; Ballif, J.L.; Yuan, W.W.; Chou, B.E. Lithium Literature Review: Lithium’s Properties and Interactions; Hanford Engineering Development Lab.: Richland, WA, USA, 1978. [Google Scholar]
- Irvine, W.R. The Reactions of Lithium with Nitrogen and Water Vapour; University of British Columbia: Vancouver, BC, Canada, 1961. [Google Scholar]
- Schmidt, L.O.; Wehbe, H.; Hartwig, S.; Kandula, M.W. Investigation of the Laser Material Interaction of Lithium Copper Foils Under Different Process Gases for All-Solid-State Batteries. Batteries 2025, 11, 195. [Google Scholar] [CrossRef]
- Lutey, A.H.; Fortunato, A.; Carmignato, S.; Ascari, A.; Liverani, E.; Guerrini, G. Quality and Productivity Considerations for Laser Cutting of LiFePO4 and LiNiMnCoO2 Battery Electrodes. Procedia CIRP 2016, 42, 433–438. [Google Scholar] [CrossRef]
- Marcicki, J.; Conlisk, A.T.; Rizzoni, G. A lithium-ion battery model including electrical double layer effects. J. Power Sources 2014, 251, 157–169. [Google Scholar] [CrossRef]
- Chang, H.J.; Ilott, A.J.; Trease, N.M.; Mohammadi, M.; Jerschow, A.; Grey, C.P. Correlating Microstructural Lithium Metal Growth with Electrolyte Salt Depletion in Lithium Batteries Using 7Li MRI. J. Am. Chem. Soc. 2015, 137, 15209–15216. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R.A. Determination of the Kinetic Parameters of Mixed-Conduction Electrodes and Application to the System Li3Sb. J. Electrochem. Soc. 1977, 124, 1569–1578. [Google Scholar] [CrossRef]
- Wood, K.N.; Noked, M.; Dasgupta, N.P. Lithium Metal Anodes: Toward an Improved Understanding of Coupled Morphological, Electrochemical, and Mechanical Behavior. ACS Energy Lett. 2017, 2, 664–672. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Mei, D.; Engelhard, M.H.; Cartmell, S.S.; Polzin, B.J.; Wang, C.; Zhang, J.-G.; Xu, W. Highly Stable Operation of Lithium Metal Batteries Enabled by the Formation of a Transient High-Concentration Electrolyte Layer. Adv. Energy Mater. 2016, 6, 1502151. [Google Scholar] [CrossRef]

| Nanosecond Laser System | Picosecond Laser System | |
|---|---|---|
| Model | G4 Pulsed Fiber Laser, SPI Lasers | Coherent HyperRapid 50, Coherent |
| Wavelength [nm] | 1062 ± 2 | 1062 ± 2 |
| Pulse duration [s] | 240 × 10−9 | 8.6 × 10−12 |
| Average power Pavg [W] | 72 ± 2 | 60 ± 2 |
| Spot diameter dspot [µm] | 74 ± 3 | 44 ± 1 |
| Frequencies PRF [kHz] | 70–490 | 100–1000 |
| Cutting velocities vc [mms−1] | 25–200 | 39–308 |
| Cutting System | Heat-Affected Zone [µm] | Melt Elevation [µm] |
|---|---|---|
| Nanosecond | 636 ± 48 | 1.21 ± 0.21 |
| Picosecond | 511 ± 32 | 2.08 ± 0.25 |
| Mechanical | 0 | 0 |
| Laser System | Sample | Heat-Affected Zone [µm] | Melt Elevation [µm] |
|---|---|---|---|
| Nanosecond | ns-HAZ | 2028 ± 48 | 0.87 ± 0.05 |
| Nanosecond | ns-ME | 511 ± 34 | 4.02 ± 0.51 |
| Picosecond | ps-HAZ | 2222 ± 61 | 0.66 ± 0.20 |
| Picosecond | ps-ME | 457 ± 12 | 3.67 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, L.O.; Wehbe, H.; Hartwig, S.; Kandula, M.W. Influence of Pulse Duration on Cutting-Edge Quality and Electrochemical Performance of Lithium Metal Anodes. Batteries 2025, 11, 286. https://doi.org/10.3390/batteries11080286
Schmidt LO, Wehbe H, Hartwig S, Kandula MW. Influence of Pulse Duration on Cutting-Edge Quality and Electrochemical Performance of Lithium Metal Anodes. Batteries. 2025; 11(8):286. https://doi.org/10.3390/batteries11080286
Chicago/Turabian StyleSchmidt, Lars O., Houssin Wehbe, Sven Hartwig, and Maja W. Kandula. 2025. "Influence of Pulse Duration on Cutting-Edge Quality and Electrochemical Performance of Lithium Metal Anodes" Batteries 11, no. 8: 286. https://doi.org/10.3390/batteries11080286
APA StyleSchmidt, L. O., Wehbe, H., Hartwig, S., & Kandula, M. W. (2025). Influence of Pulse Duration on Cutting-Edge Quality and Electrochemical Performance of Lithium Metal Anodes. Batteries, 11(8), 286. https://doi.org/10.3390/batteries11080286






