Challenges and Issues Facing Ultrafast-Charging Lithium-Ion Batteries
Abstract
1. Introduction
2. Overview of Ultrafast LIBs
| Strategies | Approaches | Mechanism | Schematics |
|---|---|---|---|
| Nanostructuring | Nanoparticles, nanotubes, nanosheets, etc. | Shorten diffusion length, increase surface area, and enhance ion and electron transportation rates |  |
| Surface modification | Carbon coating and metallic coating | Enhance the electronic and ionic conductivity to increase rate capability |  |
| Dopant manipulation | Homogenous/heterogeneous metal ions | Improve ionic conductivity due to deficiency or excess in electrons |  |
| Electrode engineering | Minimizing the strain effect and electrostatic interaction | Increase Li-ion diffusivity by reducing the activation energy |  |
| Hybrid composite design | Synthesizing composite materials by conducting additives | Enhancing the electronic and ionic transport rates; conducting materials act as electron pathways and reduce lithium-ion diffusion length |  |
3. Material Designs for Ultrafast-Charging Lithium-Ion Batteries
3.1. Anode Materials
| Anode Types | Materials | Specifications | Mechanism | Structure |
|---|---|---|---|---|
| Intercalation | Carbon materials, TiOx, NbOx | Capacity: <400 mAh/g C-rate: 2C–10C Cycle life: >500 Volume expansion: <10% | Layered structure with rapid Li-ion intercalation |  |
| Conversion | Metal oxides (Fe2O3, Co3O4) | Capacity: 500–1000 mAh/g C-rate: 2–5C Cycle life: ~200–500 cycles Volume expansion: 10–200% | Conversion reaction; forms new phases upon fast charging |  |
| Alloy | Silicon-based materials | Capacity: 1000–4200 mAh/g C-rate: 2–10C Cycle life: ~200–500 cycles Volume expansion: 100–300% | Alloy formation; significant expansion on lithiation |  |
3.2. Cathode Materials
| Cathode Types | Materials | Specifications | Mechanism | Structure |
|---|---|---|---|---|
| Layered oxides | LiCoO2 (LCO), LiNiCoMnO2 (NMC) | Capacity: 150–250 mAh/g C-rate: Up to 10C Cycle life: 500–2000 cycles Thermal stability: <200 °C | Layered structure; fast reversible Li-ion extraction |  |
| Spinel oxides | LiMn2O4, LiNiMnCoO2 | Capacity: <150 mAh/g C-rate: Up to 10C Cycle life: 500–2000 cycles Thermal stability: <250 °C | Three-dimensional spinel structure; fast diffusion of Li-ions | 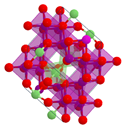 |
| Polyanion compounds | LiFePO4, Li3V2(PO4)3 | Capacity: 150–170 mAh/g C-rate: Up to 5C Cycle life: 2500 cycles Thermal stability: <300 °C | Olivine structure; stable under fast charging |  |
| Disordered rock salt oxides (DRXs) | α-LiFeO2 structure, LiFeSO4F transition | Capacity: >300 mAh/g C-rate: Up to 5C Cycle life: 500–2000 cycles Thermal stability: ~200–300 °C | Disordered rock salt structure with random Li and transition metal positioning; enables high-rate lithium diffusion |  |
| Conversion cathodes | FeF3, FeF2 | Capacity: 200–300 mAh/g C-rate: Up to 5C Cycle life: 500–1000 cycles Thermal stability: <150 °C | Reversible electrochemical conversion reaction providing high specific capacity and energy density | 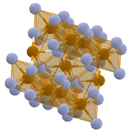 |
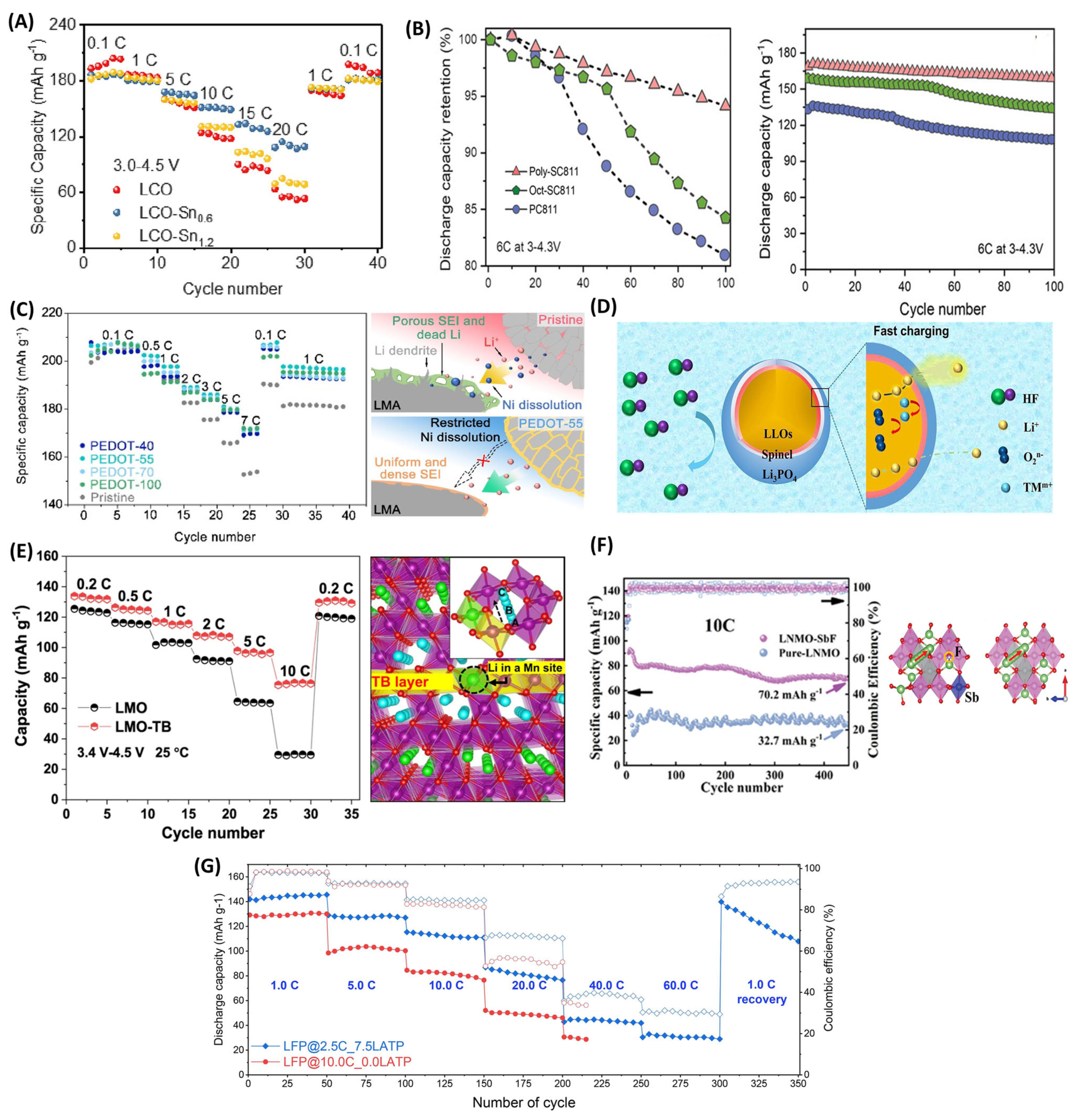
3.3. Electrolytes and Additives

3.4. Separator
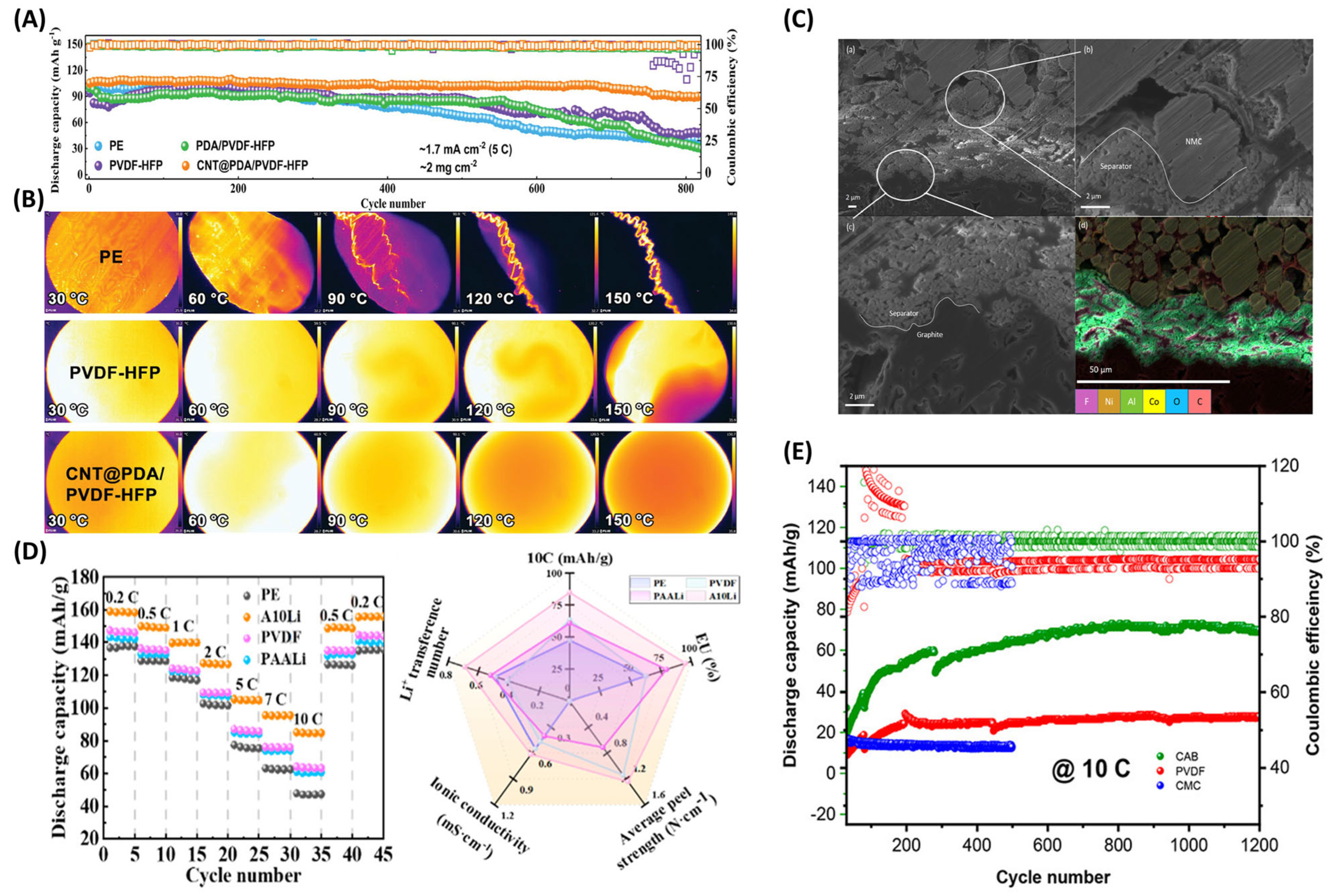
3.5. Binders
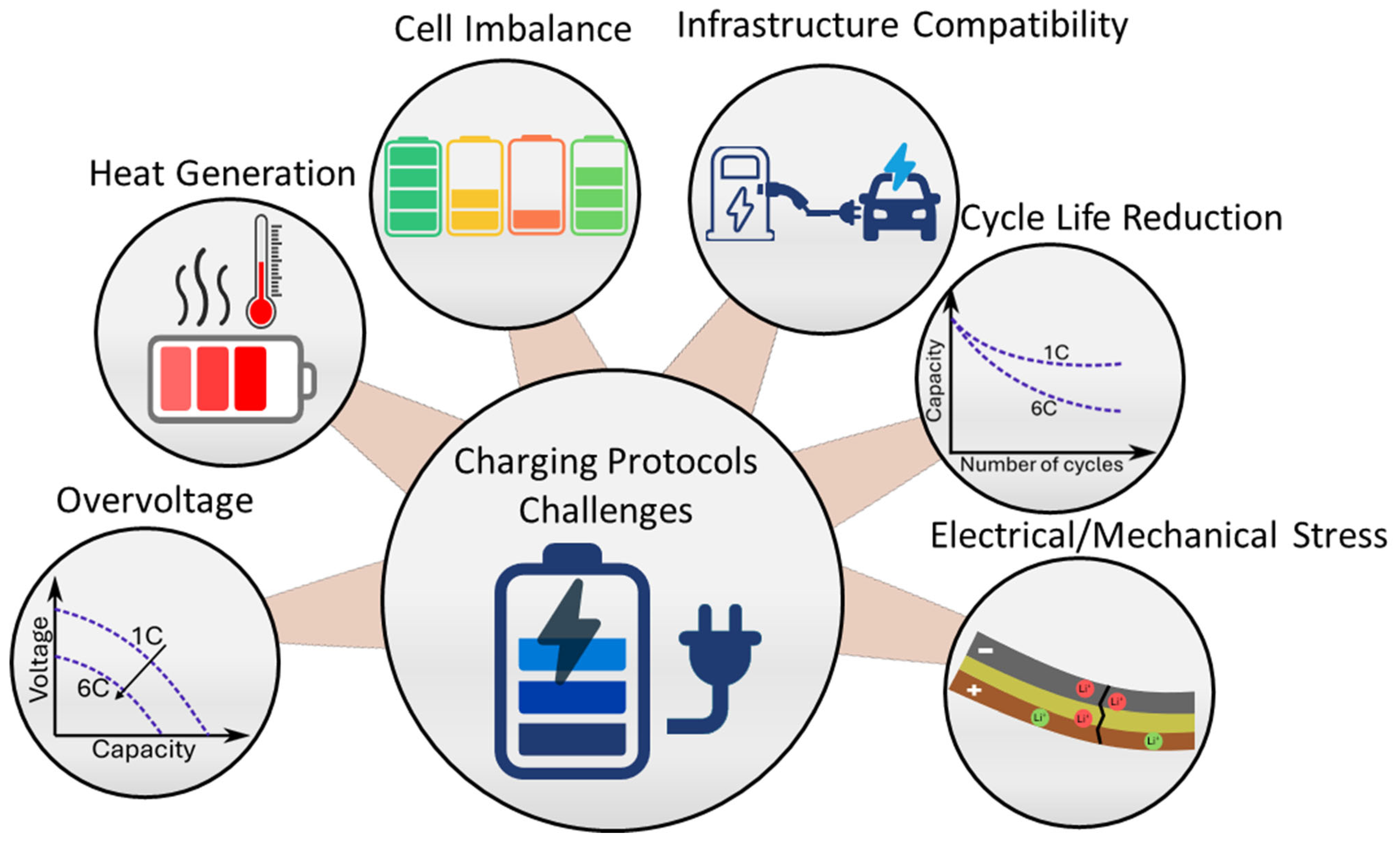
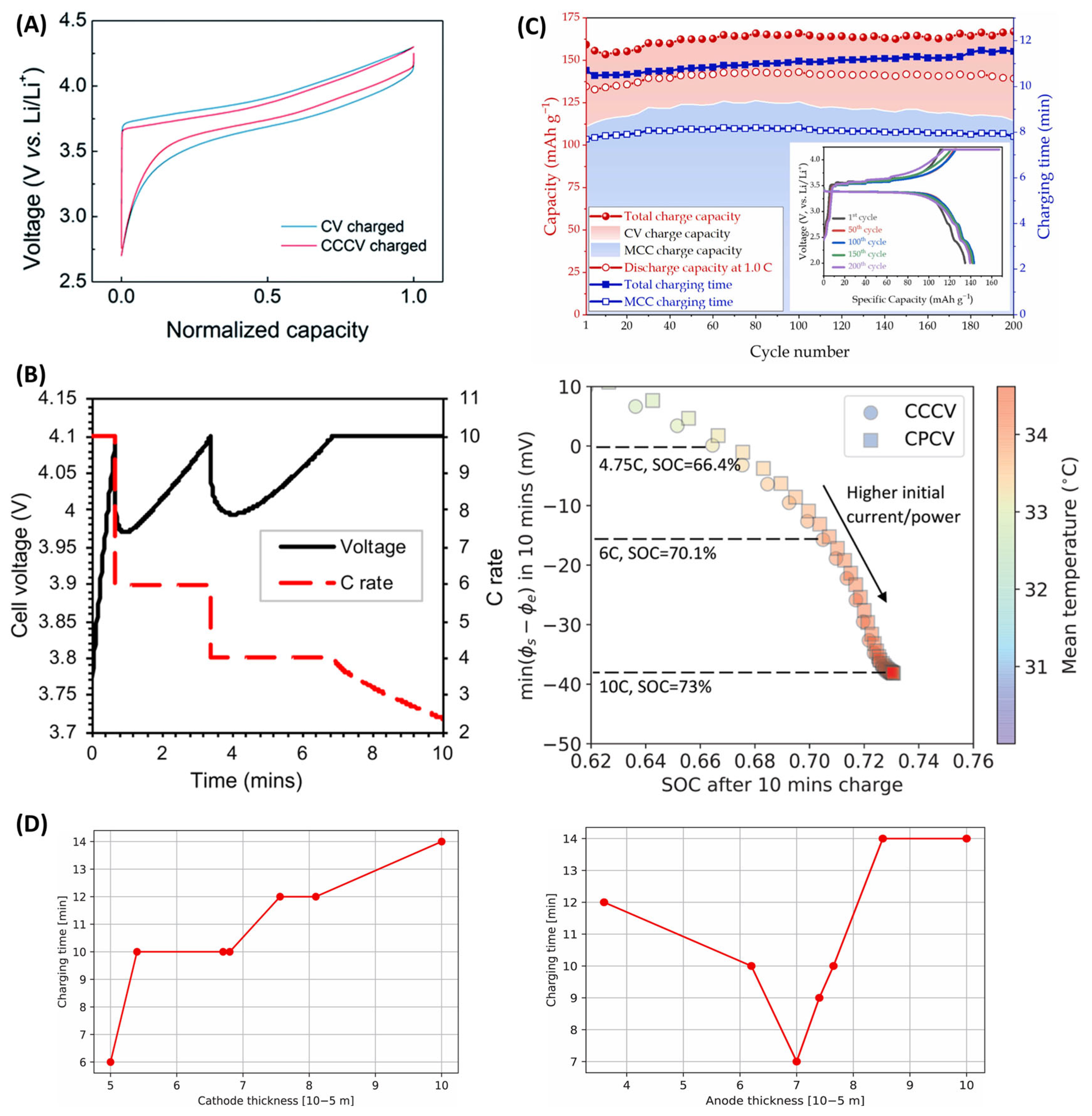
4. Charging Protocols
4.1. CC-CV
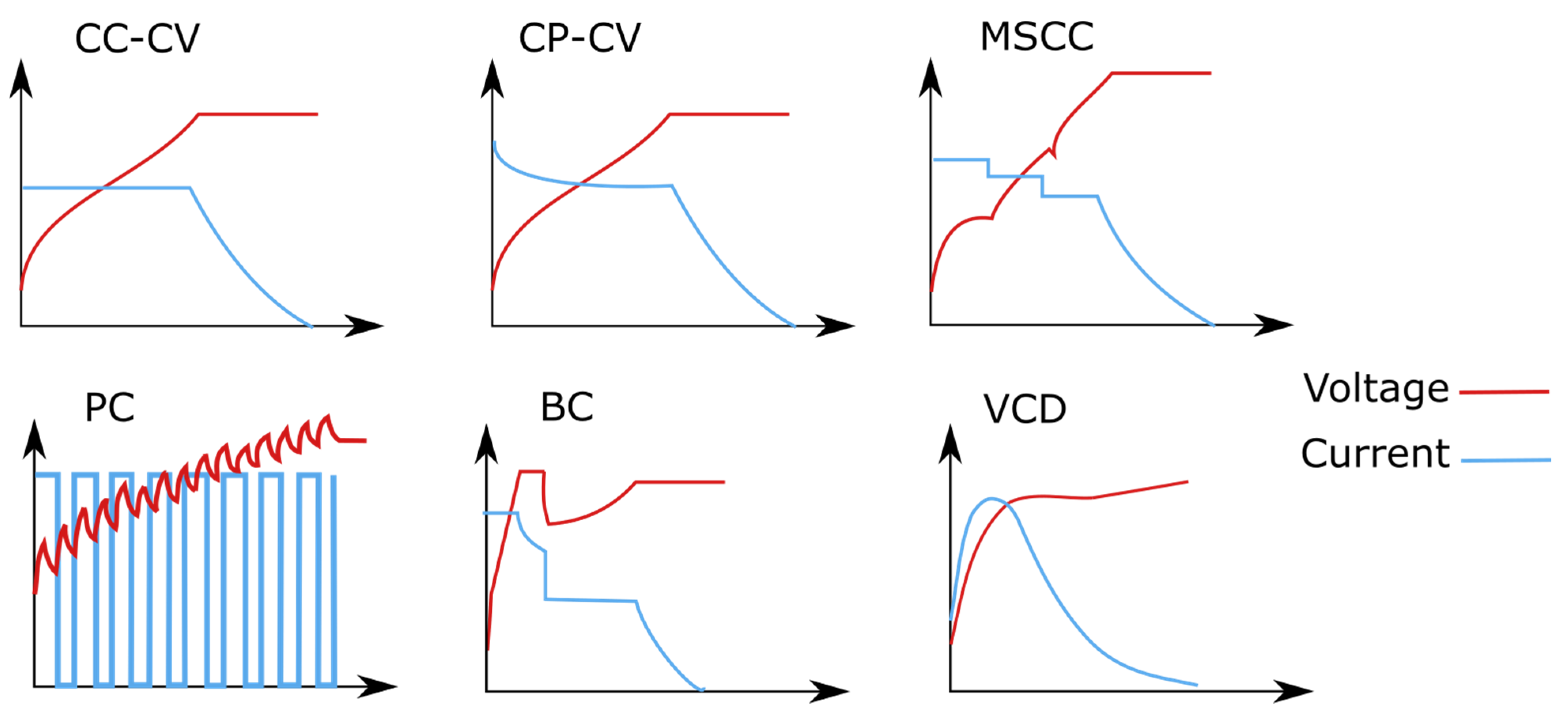
4.2. MSCC
4.3. PC
4.4. BC
5. Safety
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Definition |
| UFC | Ultra-fast charging |
| FC | Fast charging |
| XFC | Extremely fast charging |
| EV | Electric vehicle |
| BEV | Battery electric vehicle |
| LIB | Lithium-ion battery |
| CAGR | Compound annual growth rate |
| LFP | Lithium iron phosphate (LiFePO4) |
| LVO | Lithium vanadium oxide (Li3V2O5) |
| LTO | Lithium titanium oxide (Li4Ti5O12) |
| NMC | Lithium nickel manganese cobalt oxide (LiNiCoMnO2) |
| LCO | Lithium cobalt oxide (LiCoO2) |
| LMO | Lithium manganese oxide (LiMn2O4) |
| SEI | Solid electrolyte interface |
| CAM | Cathode active material |
| CEI | Cathode electrolyte interface |
| ICE | Initial coulombic efficiency |
| SOC | State of charge |
| HCE | High-oncentration electrolyte |
| LHCE | Localized high-concentration electrolyte |
| SSE | Solid-state electrolyte |
| CSE | Controlled solvation electrolyte |
| EC | Ethylene carbonate |
| DEC | Diethyl carbonate |
| DMC | Dimethyl carbonate |
| PE | Polyethylene |
| PP | Polypropylene |
| PAN | Polyacrylonitrile |
| PVDF | Polyvinylidene fluoride |
| PET | Polyethylene terephthalate |
| SBR | Styrene-butadiene rubber |
| CMC | Carboxymethyl cellulose |
| AM | Additive manufacturing |
| CCCV | Constant current–constant voltage |
| MSCC | Multi-stage constant current |
| VCD | Varying current decay |
| BC | Boost charging |
| PC | Pulse charging |
| BMS | Battery management systems |
References
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Gül, T.; Pales, A.F.; Connelly, E. Global EV Outlook 2024 Moving towards increased affordability. Electr. Veh. Intitiative 2024, 79. [Google Scholar]
- Lithium-ion Battery Market Size, Share, Industry Insights & Demand Forecast Report, 2032. Available online: https://www.fortunebusinessinsights.com/industry-reports/lithium-ion-battery-market-100123 (accessed on 1 March 2025).
- Rezaei, M.; Nekahi, A.; Feyzi, E.; Anil Kumar, M.R.; Nanda, J.; Zaghib, K. Advancing the circular economy by driving sustainable urban mining of end-of-life batteries and technological advancements. Energy Storage Mater. 2025, 75, 104035. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Suarez, C.; Martinez, W. Fast and Ultra-Fast Charging for Battery Electric Vehicles—A Review. In Proceedings of the 2019 IEEE Energy Conversion Congress and Exposition, ECCE 2019, Baltimore, MS, USA, 29 September–3 October 2019. [Google Scholar]
- Mateen, S.; Amir, M.; Haque, A.; Bakhsh, F.I. Ultra-fast charging of electric vehicles: A review of power electronics converter, grid stability and optimal battery consideration in multi-energy systems. Sustain. Energy Grids Netw. 2023, 35, 101112. [Google Scholar] [CrossRef]
- Yuan, N.; Yu, Z.; Zhang, Y.; Chang, H.; Kang, H. Review of Electric Vehicle Ultra-Fast DC Charging Station. In Proceedings of the 2022 7th Asia Conference on Power and Electrical Engineering, ACPEE 2022, Online, 16–17 April 2022. [Google Scholar]
- Bac, U.; Erdem, M. Optimization of electric vehicle recharge schedule and routing problem with time windows and partial recharge: A comparative study for an urban logistics fleet. Sustain. Cities Soc. 2021, 70, 102883. [Google Scholar] [CrossRef]
- Charging|Tesla Support. Available online: https://www.tesla.com/support/charging (accessed on 1 March 2025).
- Second-gen GAC Aion V All-Electric SUV launched in China. Available online: https://carnewschina.com/2024/07/24/second-gen-gac-aion-v-all-electric-suv-launched-with-750-km-range-price-starts-at-17800-usd/ (accessed on 23 May 2025).
- DESTEN Launches Ultra-Fast Charging Lithium Iron-Phosphate Battery, The First LFP Cell Capable of Charging in 6 Minutes—Desten. Available online: https://desten.com/desten-launches-ultra-fast-charging-lithium-iron-phosphate-battery-the-first-lfp-cell-capable-of-charging-in-6-minutes/ (accessed on 1 March 2025).
- Qu, J.G.; Jiang, Z.Y.; Zhang, J.F. Investigation on lithium-ion battery degradation induced by combined effect of current rate and operating temperature during fast charging. J. Energy Storage 2022, 52, 104811. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium-Ion Batteries with High Rate Capabilities. ACS Sustain. Chem. Eng. 2017, 5, 2799–2816. [Google Scholar] [CrossRef]
- Deb, N.; Singh, R.; Brooks, R.R.; Bai, K. A review of extremely fast charging stations for electric vehicles. Energies 2021, 14, 7566. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Hasan, M.F.; Chen, C.-F.; Shaffer, C.E.; Mukherjee, P.P. Analysis of the Implications of Rapid Charging on Lithium-Ion Battery Performance. J. Electrochem. Soc. 2015, 162, 7. [Google Scholar] [CrossRef]
- Yang, X.G.; Liu, T.; Wang, C.Y. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Zeng, Y.; Chalise, D.; Lubner, S.D.; Kaur, S.; Prasher, R.S. A review of thermal physics and management inside lithium-ion batteries for high energy density and fast charging. Energy Storage Mater. 2021, 41, 264–288. [Google Scholar] [CrossRef]
- Bandara, T.G.T.A.; Viera, J.C.; González, M. The next generation of fast charging methods for Lithium-ion batteries: The natural current-absorption methods. Renew. Sustain. Energy Rev. 2022, 162, 112338. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, H.; Ran, F. Fast-charging cathode materials for lithium & sodium ion batteries. Mater. Today 2023, 63, 360–379. [Google Scholar] [CrossRef]
- Huang, Y.X.; Wu, F.; Chen, R.J. Thermodynamic analysis and kinetic optimization of high-energy batteries based on multi-electron reactions. Natl. Sci. Rev. 2020, 7, 1367–1386. [Google Scholar] [CrossRef]
- Miao, J. Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem 2023, 4, 156–180. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Wang, S.; Liang, S. Ion migration and defect effect of electrode materials in multivalent-ion batteries. Prog. Mater. Sci. 2022, 125, 100911. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, D.; Xu, Y.; Liu, S.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Wang, Z.; Wu, Y.; et al. A Review on Electrode Materials of Fast-Charging Lithium-Ion Batteries. Chem. Rec. 2022, 22, e202200127. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Lima da Silva, A.; Müller, I.L. Operation of solid oxide fuel cells on glycerol fuel: A thermodynamic analysis using the Gibbs free energy minimization approach. J. Power Sources 2010, 195, 5637–5644. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Y.; Mao, B.; Wang, Y.; Yang, Z.; Meng, T.; Wang, X.; Cao, M. Strain-regulated Gibbs free energy enables reversible redox chemistry of chalcogenides for sodium ion batteries. Nat. Commun. 2022, 13, 5588. [Google Scholar] [CrossRef]
- Yu, F.; Huang, T.; Zhang, P.; Tao, Y.; Cui, F.Z.; Xie, Q.; Yao, S.; Wang, F. Design and synthesis of electrode materials with both battery-type and capacitive charge storage. Energy Storage Mater. 2019, 22, 235–255. [Google Scholar] [CrossRef]
- Barai, A.; Uddin, K.; Widanage, W.D.; McGordon, A.; Jennings, P. A study of the influence of measurement timescale on internal resistance characterisation methodologies for lithium-ion cells. Sci. Rep. 2018, 8, 21. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Pant, L.M.; Bui, J.C.; Crothers, A.R.; Ehlinger, V.M.; Fornaciari, J.C.; Liu, J.; Weber, A.Z. Method—Practices and Pitfalls in Voltage Breakdown Analysis of Electrochemical Energy-Conversion Systems. J. Electrochem. Soc. 2021, 168, 074503. [Google Scholar] [CrossRef]
- Perez, N. Kinetics of Activation Polarization. In Electrochemistry and Corrosion Science; Springer: Boston, MA, USA, 2016. [Google Scholar]
- Sebastian, S.S.; Dong, B.; Zerrin, T.; Pena, P.A.; Akhavi, A.S.; Li, Y.; Ozkan, C.S.; Ozkan, M. Adaptive fast charging methodology for commercial Li-ion batteries based on the internal resistance spectrum. Energy Storage 2020, 2, e141. [Google Scholar] [CrossRef]
- Wan, S.; Ma, W.; Xiao, Y.; Chen, S. High-Voltage and Fast-Charge Electrolytes for Lithium-Ion Batteries. Batter. Supercaps 2022, 5, e202200368. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Belharouak, I. Enabling fast charging of high energy density Li-ion cells with high lithium ion transport electrolytes. Electrochem. Commun. 2019, 103, 109–113. [Google Scholar] [CrossRef]
- Kang, J.; Koo, B.; Kang, S.; Lee, H. Physicochemical nature of polarization components limiting the fast operation of Li-ion batteries. Chem. Phys. Rev. 2021, 2, 041307. [Google Scholar] [CrossRef]
- Allagui, A.; Benaoum, H.; Wang, C. Deformed Butler-Volmer Models for Convex Semilogarithmic Current-Overpotential Profiles of Li-ion Batteries. J. Phys. Chem. C 2022, 126, 3029–3036. [Google Scholar] [CrossRef]
- Rüetschi, P. Overvoltage and Catalysis. J. Electrochem. Soc. 1959, 106, 819. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.; Eikerling, M. The rate-determining term of electrocatalytic reactions with first-order kinetics. Electrochim. Acta 2021, 393, 139019. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. eTransportation 2022, 11, 100145. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent Insights into Rate Performance Limitations of Li-ion Batteries. Batter. Supercaps 2021, 4, 268–285. [Google Scholar] [CrossRef]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.; Wang, C.; Koratkar, N. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Ravikumar, B.; Mynam, M.; Rai, B. Effect of Salt Concentration on Properties of Lithium Ion Battery Electrolytes: A Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 8173–8181. [Google Scholar] [CrossRef]
- Camacho-Forero, L.E.; Smith, T.W.; Balbuena, P.B. Effects of high and low salt concentration in electrolytes at lithium-metal anode surfaces. J. Phys. Chem. C 2017, 121, 182–194. [Google Scholar] [CrossRef]
- Gao, N.; Kim, S.; Chinnam, P.; Dufek, E.J.; Colclasure, A.M.; Jansen, A.; Son, S.B.; Bloom, I.; Dunlop, A.; Trask, S.; et al. Methodologies for Design, Characterization and Testing of Electrolytes that Enable Extreme Fast Charging of Lithium-ion Cells. Energy Storage Mater. 2022, 44, 296–312. [Google Scholar] [CrossRef]
- Smiatek, J.; Heuer, A.; Winter, M. Properties of Ion Complexes and Their Impact on Charge Transport in Organic Solvent-Based Electrolyte Solutions for Lithium Batteries: Insights from a Theoretical Perspective. Batteries 2018, 4, 62. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Zhang, Z.; Li, Y.; Yang, S.; He, X. Insight Understanding of External Pressure on Lithium Plating in Commercial Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2406966. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Evans, M.; Dickerson, C.; Jansen, A.N.; Polzin, B.J.; Dunlop, A.R.; Trask, S.E.; Jackman, R.; Bloom, I.; et al. Extreme Fast Charge Challenges for Lithium-Ion Battery: Variability and Positive Electrode Issues. J. Electrochem. Soc. 2019, 166, A1926–A1938. [Google Scholar] [CrossRef]
- Li, G. Regulating Mass Transport Behavior for High-Performance Lithium Metal Batteries and Fast-Charging Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002891. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, J.; Yang, H.; Yang, A.; Cui, C.; Zhang, Y.; Li, X.; Gui, S.; Wei, Y.; Feng, X.; et al. Visualizing fast interlayer anisotropic lithium diffusion via single crystal microbattery. Matter 2022, 5, 4015–4028. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, C.; Huang, J.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Small 2019, 15, e1805389. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, J.; Yang, L.; Qu, M.; Qi, D.; Zhang, K.H.L. Wide Bandgap Oxide Semiconductors: From Materials Physics to Optoelectronic Devices. Adv. Mater. 2021, 33, 2006230. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.; Chou, S.; Chen, J. Recent Developments on and Prospects for Electrode Materials with Hierarchical Structures for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Chen, J. Surface and Interface Engineering of Electrode Materials for Lithium-Ion Batteries. Adv. Mater. 2015, 27, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Taneja, Y.; Dube, D.; Singh, R. Recent advances in elemental doping and simulation techniques: Improving structural, photophysical and electronic properties of titanium dioxide. J. Mater. Chem. C 2024, 12, 14774–14808. [Google Scholar] [CrossRef]
- Feng, H.-P.; Tang, L.; Zeng, G.-M.; Tang, J.; Deng, Y.-C.; Yan, M.; Liu, Y.-N.; Zhou, Y.-Y.; Ren, X.-Y.; Chen, S. Carbon-based core–shell nanostructured materials for electrochemical energy storage. J. Mater. Chem. A 2018, 6, 7310–7337. [Google Scholar] [CrossRef]
- Jiang, L.-B.; Yuan, X.-Z.; Liang, J.; Zhang, J.; Wang, H.; Zeng, G.-M. Nanostructured core-shell electrode materials for electrochemical capacitors. J. Power Sources 2016, 331, 408–425. [Google Scholar] [CrossRef]
- Wen, L.; Li, F.; Cheng, H. Carbon Nanotubes and Graphene for Flexible Electrochemical Energy Storage: From Materials to Devices. Adv. Mater. 2016, 28, 4306–4337. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.-M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium-Sulfur Batteries. Adv. Mater. 2019, 31, 1800863. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Gao, C. Superstructured Assembly of Nanocarbons: Fullerenes, Nanotubes, and Graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, K.; Li, Y.; Chen, J.; Wang, H.; Li, L.; Li, H.; Ren, X.; Ouyang, X.; Liu, J.; et al. Minimize the Electrode Concentration Polarization for High-Power Lithium Batteries. Adv. Funct. Mater. 2024, 34, 2410926. [Google Scholar] [CrossRef]
- Zhang, T.; Ran, F. Design Strategies of 3D Carbon-Based Electrodes for Charge/Ion Transport in Lithium Ion Battery and Sodium Ion Battery. Adv. Funct. Mater. 2021, 31, 2010041. [Google Scholar] [CrossRef]
- Ji, W.; Qu, H.; Zhang, X.; Zheng, D.; Qu, D. Electrode Architecture Design to Promote Charge-Transport Kinetics in High-Loading and High-Energy Lithium-Based Batteries. Small Methods 2021, 5, 2100518. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Zhao, P.; Wang, X. Fast charging optimization for lithium-ion batteries based on dynamic programming algorithm and electrochemical-thermal-capacity fade coupled model. J. Power Sources 2019, 438, 227015. [Google Scholar] [CrossRef]
- Makeen, P.; Ghali, H.A.; Memon, S. A Review of Various Fast Charging Power and Thermal Protocols for Electric Vehicles Represented by Lithium-Ion Battery Systems. Futur. Transp. 2022, 2, 281–299. [Google Scholar] [CrossRef]
- He, J.; Meng, J.; Huang, Y. Challenges and recent progress in fast-charging lithium-ion battery materials. J. Power Sources 2023, 570, 232965. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Tao, L.; Meng, J.; Xu, X.; Liu, F.; Wang, X. Electrode and Electrolyte Design Strategies Toward Fast-Charging Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2409097. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Cai, W.; Yao, Y.-X.; Zhu, G.-L.; Yan, C.; Jiang, L.-L.; He, C.; Huang, J.-Q.; Zhang, Q. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806–3833. [Google Scholar] [CrossRef]
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent advances in cathode materials for rechargeable lithium–sulfur batteries. Nanoscale 2019, 11, 15418–15439. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.; Yoon, W. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. Engl. 2019, 59, 2578–2605. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- Li, L.; Duan, Y. Engineering Polymer-Based Porous Membrane for Sustainable Lithium-Ion Battery Separators. Polymers 2023, 15, 3690. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Bijoy, T.K. A Comprehensive Review of Current and Emerging Binder Technologies for Energy Storage Applications. ACS Appl. Energy Mater. 2023, 6, 11773–11794. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Liu, R.; Li, X.; Wu, Y.; Ran, F. “Fast-Charging” Anode Materials for Lithium-Ion Batteries from Perspective of Ion Diffusion in Crystal Structure. ACS Nano 2024, 18, 2611–2648. [Google Scholar] [CrossRef]
- Zhao, H.; Bo, X.; Xu, H.; Wang, L.; Daoud, W.A.; He, X. Advancing lithium-ion battery anodes towards a sustainable future: Approaches to achieve high specific capacity, rapid charging, and improved safety. Energy Storage Mater. 2024, 72, 103696. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, challenge and perspective of graphite-based anode materials for lithium batteries: A review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Nikgoftar, K.; Reddy, A.K.M.R.; Reddy, M.V.; Zaghib, K. Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries. Batteries 2025, 11, 123. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef]
- Li, W.; Ning, Q.; Xi, X.; Hou, B.; Guo, J.; Yang, Y.; Chen, B.; Wu, X. Highly Improved Cycling Stability of Anion De-/Intercalation in the Graphite Cathode for Dual-Ion Batteries. Adv. Mater. 2019, 31, e1804766. [Google Scholar] [CrossRef]
- Ding, X.; Zhou, Q.; Li, X.; Xiong, X. Fast-charging anodes for lithium ion batteries: Progress and challenges. Chem. Commun. 2024, 60, 2472–2488. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, B.; Zhang, Y.; Chen, Z.; Wang, X.; Zhan, R.; Ou, Y.; Wang, W.; Liu, X.; Duan, X.; et al. Fast-charging capability of graphite-based lithium-ion batteries enabled by Li3P-based crystalline solid–electrolyte interphase. Nat. Energy 2023, 8, 1365–1374. [Google Scholar] [CrossRef]
- Chen, K.; Goel, V.; Namkoong, M.J.; Wied, M.; Müller, S.; Wood, V.; Sakamoto, J.; Thornton, K.; Dasgupta, N.P. Enabling 6C Fast Charging of Li-Ion Batteries with Graphite/Hard Carbon Hybrid Anodes. Adv. Energy Mater. 2021, 11, 2003336. [Google Scholar] [CrossRef]
- Du, P.; Fan, X.; Zhang, B.; Cao, L.; Ren, J.; Ou, X.; Guo, X.; Liu, Q. The lithiophobic-to-lithiophilic transition on the graphite towards ultrafast-charging and long-cycling lithium-ion batteries. Energy Storage Mater. 2022, 50, 648–657. [Google Scholar] [CrossRef]
- Du, P.; Zhang, B.; Cao, L.; Ou, X. Designed graphite with an activated edge for fast-charging lithium-ion storage properties. Chem. Commun. 2022, 58, 7372–7375. [Google Scholar] [CrossRef]
- Mao, L.; Zhao, X.; Cheng, Q.; Yang, G.; Liao, F.; Chen, L.; He, P.; Chen, S. Recent advances and perspectives of two-dimensional Ti-based electrodes for electrochemical energy storage. Sustain. Energy Fuels 2021, 5, 5061–5113. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Li, M.; Wang, X.; Bai, S.; Shi, Y.; Tian, J.; Shan, Z.; Meng, Y.S.; Liu, P.; et al. Nanosheet-assembled hierarchical Li4Ti5O12 microspheres for high-volumetric-density and high-rate Li-ion battery anode. Energy Storage Mater. 2019, 21, 361–371. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Ma, G.; Zheng, X. Enhanced rate capability of Li4Ti5O12 anode material by a photo-assisted sol–gel route for lithium-ion batteries. Electrochem. Commun. 2021, 131, 107119. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, S.; Tang, X.; Wang, H.; Zhang, M.; Liu, F.; Yao, N.; Ma, Y. Enhanced Li-ion migration behavior in Li3V2O5 rock-salt anode via stepwise lattice tailoring. Energy Storage Mater. 2023, 54, 284–293. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Lu, J.; Pei, C.; Li, T.; Xiao, T.; Ni, S. Neural-network design of Li3VO4/NC fibers toward superior high-rate Li-ion storage. J. Mater. Chem. A 2021, 9, 24002–24011. [Google Scholar] [CrossRef]
- Feyzi, E.; R, A.K.M.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A comprehensive review of silicon anodes for high-energy lithium-ion batteries: Challenges, latest developments, and perspectives. Next Energy 2024, 5, 100176. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Wang, Y.; Lv, L.; Feng, H.; Gu, S.; Zheng, H. Decorating silicon surface by an electroactive covalent organic framework to develop high-performance lithium-ion batteries. Energy Storage Mater. 2024, 66, 103207. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hwang, J.-Y.; Shin, H.-J.; Jeong, M.-G.; Chung, K.Y.; Sun, Y.-K.; Jung, H.-G. Nano/Microstructured Silicon–Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2019, 20, 625–635. [Google Scholar] [CrossRef]
- Sahu, S.R.; Rikka, V.R.; Haridoss, P.; Chatterjee, A.; Gopalan, R.; Prakash, R. A Novel α-MoO3/Single-Walled Carbon Nanohorns Composite as High-Performance Anode Material for Fast-Charging Lithium-Ion Battery. Adv. Energy Mater. 2020, 10, 2001627. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, T.; Zhu, X.; Zhao, Y.; He, H.; Hung, C.-T.; Zhang, X.; Chen, Y.; Tang, X.; Wang, J.; et al. Inorganic-organic competitive coating strategy derived uniform hollow gradient-structured ferroferric oxide-carbon nanospheres for ultra-fast and long-term lithium-ion battery. Nat. Commun. 2021, 12, 2973. [Google Scholar] [CrossRef]
- Bouguern, M.D.; Reddy, A.K.M.R.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries 2024, 10, 39. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Wei, H.; Huang, Y.; Tang, L.; Wang, Z.; Yan, C.; Mao, J.; Dai, K.; Wu, Q.; et al. Fundamentals of Ion-Exchange Synthesis and Its Implications in Layered Oxide Cathodes: Recent Advances and Perspective. Adv. Energy Mater. 2023, 13, 2300125. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Ni, Y.; Zhang, K.; Cheng, F.; Chen, J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater. Today 2021, 43, 132–165. [Google Scholar] [CrossRef]
- Xu, E.; Sun, X.; Lyv, W.; Li, F.; Li, R.; Cai, W.; Wu, H.; Wu, K.; Zhang, Y. Optimizing the Electrochemical Performance of Olivine LiMnxFe1–xPO4 Cathode Materials: Ongoing Progresses and Challenges. Ind. Eng. Chem. Res. 2024, 63, 9631–9660. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Z.; Lu, Y.; Xie, W.; Yan, Z.; Chen, J. Insights into Cation Migration and Intermixing in Advanced Cathode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2402068. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, X.; Sun, Y.; Xu, Y.; Chang, B.; Liu, C.; Cao, A.; Wan, L. Precise Surface Engineering of Cathode Materials for Improved Stability of Lithium-Ion Batteries. Small 2019, 15, e1901019. [Google Scholar] [CrossRef]
- Yang, L.; Yang, K.; Zheng, J.; Xu, K.; Amine, K.; Pan, F. Harnessing the surface structure to enable high-performance cathode materials for lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 4667–4680. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Yang, Z. A review on cathode materials for advanced lithium ion batteries: Microstructure designs and performance regulations. Nanotechnology 2020, 31, 012001. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Yin, F.; Liu, P.; Cloetens, P.; Liu, Y.; Lin, F.; Zhao, K. Heterogeneous damage in Li-ion batteries: Experimental analysis and theoretical modeling. J. Mech. Phys. Solids 2019, 129, 160–183. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, R.; Zhang, K.; Lee, S.; Mu, L.; Liu, P.; Waters, C.K.; Spence, S.; Xu, Z.; Wei, C.; et al. Quantification of Heterogeneous Degradation in Li-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900674. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Shen, L.; Liu, J.; Wang, Z.; Xu, S.; Law, H.M.; Ciucci, F. Fast-Charging Solid-State Li Batteries: Materials, Strategies, and Prospects. Adv. Mater. 2024, e2417796. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, Z.; Zhu, Y.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; Yu, G. Multiscale Understanding and Architecture Design of High Energy/Power Lithium-Ion Battery Electrodes. Adv. Energy Mater. 2021, 11, 2000808. [Google Scholar] [CrossRef]
- Zhang, S.S. Challenges and Strategies for Fast Charge of Li-Ion Batteries. ChemElectroChem 2020, 7, 3569–3577. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Chen, X.; Yang, Q.; Mu, D.; Li, L.; Wu, F.; Chen, R. In-depth understanding of the deterioration mechanism and modification engineering of high energy density Ni-rich layered lithium transition-metal oxide cathode for lithium-ion batteries. Chem. Eng. J. 2023, 465, 142946. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Daali, A.; Amine, R.; Chen, Z.; Amine, K. Challenges and Strategies to Advance High-Energy Nickel-Rich Layered Lithium Transition Metal Oxide Cathodes for Harsh Operation. Adv. Funct. Mater. 2020, 30, 2004748. [Google Scholar] [CrossRef]
- Bi, Z.; Yi, Z.; Zhang, L.; Wang, G.; Zhang, A.; Liao, S.; Zhao, Q.; Peng, Z.; Song, L.; Wang, Y.; et al. Ultrathin dense LiF coverage coupled with a near-surface gradient fluorination lattice enables fast-charging long-life 4.6 V LiCoO2. Energy Environ. Sci. 2024, 17, 2765–2775. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Chen, G.; Lu, M.; Nevar, A.A.; Dudko, N.; Shi, L.; Huang, L.; Tarasenko, N.V.; Zhang, D. Sn, S co-doped LiCoO2 with low lithium ion diffusion energy barrier and high passivation surface for fast charging lithium ion batteries. Chem. Eng. J. 2023, 468, 143585. [Google Scholar] [CrossRef]
- Jamil, S.; Wang, G.; Fasehullah, M.; Xu, M. Challenges and prospects of nickel-rich layered oxide cathode material. J. Alloy. Compd. 2022, 909, 164727. [Google Scholar] [CrossRef]
- Li, D.; Liu, W.; Liang, W.; Xu, R. Degradation mechanisms and modification strategies of nickel-rich NCM cathode in lithium-ion batteries. Mater. Res. Express 2024, 11, 012006. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, T.; McShane, E.; McCloskey, B.D.; Chen, G. Single-Crystal LiNixMnyCo1−x−yO2 Cathodes for Extreme Fast Charging. Small 2022, 18, 2105833. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, C.S.; Song, H.W.; Chang, S.-J.; Kim, H.; Park, J.; Hu, S.; Zhao, K.; Lee, S. Ultrahigh active material content and highly stable Ni-rich cathode leveraged by oxidative chemical vapor deposition. Energy Storage Mater. 2022, 48, 1–11. [Google Scholar] [CrossRef]
- Kou, P.; Zhang, Z.; Wang, Z.; Zheng, R.; Liu, Y.; Lv, F.; Xu, N. Opportunities and Challenges of Layered Lithium-Rich Manganese-Based Cathode Materials for High Energy Density Lithium-Ion Batteries. Energy Fuels 2023, 37, 18243–18265. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, Z.; Ren, P.; Zhang, B.; Chen, Z.; Guo, Z.; Ren, F.; Sun, Z.; Liu, S.; Song, P.; et al. Recent Advances in Oxygen Redox Activity of Lithium-Rich Manganese-Based Layered Oxides Cathode Materials: Mechanism, Challenges and Strategies. Adv. Energy Mater. 2024, 14, 2402061. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Zhang, H.; Zhang, S.; Lai, Y.; Zhang, Z. Constructing stable surface structures enabling fast charging for Li-rich layered oxide cathodes. Chem. Eng. J. 2022, 427, 132036. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Wu, T.; Zhang, J.; Wang, P.; Zhang, L.; Yang, C.; Peng, C.; Lu, H. A Bifunctional-Modulated Conformal Li/Mn-Rich Layered Cathode for Fast-Charging, High Volumetric Density and Durable Li-Ion Full Cells. Nano-Micro Lett. 2021, 13, 118. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Huang, Z.; Yang, J.; Liu, F.; Chu, M.; Liu, T.; Wang, C.; Zhu, W.; Li, S.; et al. Twin boundary defect engineering improves lithium-ion diffusion for fast-charging spinel cathode materials. Nat. Commun. 2021, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hai, F.; Chen, W.; Yi, Y.; Guo, J.; Xue, W.; Tang, W.; Li, M. Improving Fast-Charging Capability of High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode under Long-Term Cyclability through Co-Doping Strategy. Small Methods 2024, 8, e2301759. [Google Scholar] [CrossRef]
- Kang, T.; Meng, Y.; Liu, X. Optimizing lithium-ion diffusion in LiFePO4: The impact of Ti4+ doping on high-rate capability and electrochemical stability. Ionics 2025, 31, 2419–2428. [Google Scholar] [CrossRef]
- Nekahi, A.; M.R., A.K.; Li, X.; Deng, S.; Zaghib, K. Sustainable LiFePO4 and LiMnxFe1−xPO4 (x = 0.1–1) cathode materials for lithium-ion batteries: A systematic review from mine to chassis. Mater. Sci. Eng. R Rep. 2024, 159, 100797. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, J.; Lee, Y. A hybrid Carbon–Li1.3Al0.3Ti1.7(PO4)3 conductive coating for high current rate LiFePO4 cathode material. Chem. Eng. J. 2023, 461, 141750. [Google Scholar] [CrossRef]
- Okada, K.; Kimura, I.; Machida, K. High rate capability by sulfur-doping into LiFePO4 matrix. RSC Adv. 2018, 8, 5848–5853. [Google Scholar] [CrossRef]
- Shi, M.; Li, R.; Liu, Y. In situ preparation of LiFePO4/C with unique copolymer carbon resource for superior performance lithium-ion batteries. J. Alloy. Compd. 2021, 854, 157162. [Google Scholar] [CrossRef]
- Logan, E.; Dahn, J. Electrolyte Design for Fast-Charging Li-Ion Batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium—Ion Batteries; Springer Nature: Singapore, 2022; Volume 5, ISBN 0123456789. [Google Scholar]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Research progress on electrolytes for fast-charging lithium-ion batteries. Chin. Chem. Lett. 2023, 34, 107122. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Cheng, S.; Xie, J. Fast-charging of lithium-ion batteries: A review of electrolyte design aspects. Batter. Energy 2023, 2, 20230018. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Bolloju, S.; Vangapally, N.; Elias, Y.; Luski, S.; Wu, N.-L.; Aurbach, D. Electrolyte additives for Li-ion batteries: Classification by elements. Prog. Mater. Sci. 2025, 147, 101349. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Jin, J.; Wu, M.; Yuan, H.; Zhang, S.; Davey, K.; Guo, Z.; Wen, Z. Electrolyte Design for Lithium-Ion Batteries for Extreme Temperature Applications. Adv. Mater. 2024, 36, e2308484. [Google Scholar] [CrossRef]
- Cao, C.; Zhong, Y.; Shao, Z. Electrolyte Engineering for Safer Lithium-Ion Batteries: A Review. Chin. J. Chem. 2023, 41, 1119–1141. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, Y.; Wang, B.; Wang, H.; Zhang, L. Crucial Roles of Ethyl Methyl Carbonate in Lithium-Ion and Dual-Ion Batteries: A Review. Langmuir 2024, 40, 11353–11370. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Electrode–electrolyte interfaces in lithium-based batteries. Energy Environ. Sci. 2018, 11, 527–543. [Google Scholar] [CrossRef]
- Yan, C.; Xu, R.; Xiao, Y.; Ding, J.; Xu, L.; Li, B.; Huang, J. Toward Critical Electrode/Electrolyte Interfaces in Rechargeable Batteries. Adv. Funct. Mater. 2020, 30, 1909887. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Yang, S.-J.; Yan, C.; Huang, J.-Q. Electrolyte inhomogeneity induced lithium plating in fast charging lithium-ion batteries. J. Energy Chem. 2022, 73, 394–399. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, K.; Jiang, S.; Xu, X.; Zheng, J.; Yin, J.; Gao, Y. Recent Progress on Multifunctional Electrolyte Additives for High-Energy-Density Li Batteries–A Review. ChemElectroChem 2024, 11, e202300702. [Google Scholar] [CrossRef]
- Taneja, N.; Kumar, A.; Gupta, P.; Gupta, M.; Singh, P.; Bharti; Agrawal, N.; Bocchetta, P.; Kumar, Y. Advancements in liquid and solid electrolytes for their utilization in electrochemical systems. J. Energy Storage 2022, 56, 105950. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Guan, X.; Cao, F.; Li, C.; Xu, J. Lithium dendrite-free and fast-charging for high voltage nickel-rich lithium metal batteries enabled by bifunctional sulfone-containing electrolyte additives. J. Power Sources 2020, 452, 227833. [Google Scholar] [CrossRef]
- Dong, L.; Liu, Y.; Wen, K.; Chen, D.; Rao, D.; Liu, J.; Yuan, B.; Dong, Y.; Wu, Z.; Liang, Y.; et al. High-Polarity Fluoroalkyl Ether Electrolyte Enables Solvation-Free Li+ Transfer for High-Rate Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104699. [Google Scholar] [CrossRef]
- Aruchamy, K.; Ramasundaram, S.; Divya, S.; Chandran, M.; Yun, K.; Oh, T.H. Gel Polymer Electrolytes: Advancing Solid-State Batteries for High-Performance Applications. Gels 2023, 9, 585. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, S.; Zhang, D.; Ma, J.; Zheng, H.; Zheng, M.; Lv, R.; Yu, K.; Wu, J.; Wang, X.; et al. Nonflammable, localized high-concentration electrolyte towards a high-safety lithium metal battery. Energy Storage Mater. 2022, 52, 355–364. [Google Scholar] [CrossRef]
- Carrillo-Bohórquez, O.; Kumar, R.; Kuroda, D.G. The Challenge of Characterizing High-Concentration Electrolytes at the Molecular Level: A Perspective. J. Phys. Chem. C 2024, 128, 18695–18710. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, K.; Zhou, S.; Liu, Z.; Guan, X.; Mo, D. Nonflammable Localized High-Concentration Electrolytes with Long-Term Cycling Stability for High-Performance Li Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 48694–48704. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Chen, J.; Huguet, P.; Peng, Z.; Deabate, S. Solvent–Diluent Interaction-Mediated Solvation Structure of Localized High-Concentration Electrolytes. ACS Appl. Mater. Interfaces 2022, 14, 4211–4219. [Google Scholar] [CrossRef]
- Lin, S.; Hua, H.; Lai, P.; Zhao, J. A Multifunctional Dual-Salt Localized High-Concentration Electrolyte for Fast Dynamic High-Voltage Lithium Battery in Wide Temperature Range. Adv. Energy Mater. 2021, 11, 2101775. [Google Scholar] [CrossRef]
- Xiao, P.; Yun, X.; Chen, Y.; Guo, X.; Gao, P.; Zhou, G.; Zheng, C. Insights into the solvation chemistry in liquid electrolytes for lithium-based rechargeable batteries. Chem. Soc. Rev. 2023, 52, 5255–5316. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Souza, R.; Moreira, A.; Moita, A. An overview of the ionic liquids and their hybrids operating in electrochemical cells and capacitors. Ionics 2024, 30, 4343–4385. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Peng, J.; Ou, M.; Wei, P.; Fang, C.; Li, Q.; Huang, J.; Han, J.; Huang, Y. A High Rate and Stable Hybrid Li/Na-Ion Battery Based on a Hydrated Molten Inorganic Salt Electrolyte. Small 2021, 17, 2101650. [Google Scholar] [CrossRef]
- Kautz, D.J.; Cao, X.; Gao, P.; Matthews, B.E.; Xu, Y.; Han, K.S.; Omenya, F.; Engelhard, M.H.; Jia, H.; Wang, C.; et al. Designing Electrolytes With Controlled Solvation Structure for Fast-Charging Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 18–21. [Google Scholar] [CrossRef]
- Huang, X.; Li, R.; Sun, C.; Zhang, H.; Zhang, S.; Lv, L.; Huang, Y.; Fan, L.; Chen, L.; Noked, M.; et al. Solvent-Assisted Hopping Mechanism Enables Ultrafast Charging of Lithium-Ion Batteries. ACS Energy Lett. 2022, 7, 3947–3957. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Su, Q.; Wu, M.; Que, X.; Liu, H. High-Power Bipolar Solid-State Batteries Enabled by In-Situ-Formed Ionogels for Vehicle Applications. ACS Appl. Mater. Interfaces 2022, 14, 5402–5413. [Google Scholar] [CrossRef]
- Niitani, K.; Ushiroda, S.; Kuwata, H.; Ohata, H.N.; Shimo, Y.; Hozumi, M.; Matsunaga, T.; Nakanishi, S. Hard Carbon Anode with a Sodium Carborane Electrolyte for Fast-Charging All-Solid-State Sodium-Ion Batteries. ACS Energy Lett. 2022, 7, 145–149. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Hu, Q.; Guo, S.; Yu, L.; Li, Q.; Liu, Q.; Hu, X. Safer Lithium-Ion Batteries from the Separator Aspect: Development and Future Perspectives. Energy Environ. Mater. 2021, 4, 336–362. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841. [Google Scholar] [CrossRef]
- Tong, B.; Li, X. Towards separator safety of lithium-ion batteries: A review. Mater. Chem. Front. 2023, 8, 309–340. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Feng, C.; Chen, D.; Han, J.; He, W. Recent Development in Separators for High-Temperature Lithium-Ion Batteries. Small 2019, 15, e1901689. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.L.; Abidin, M.N.Z.; Ma’amor, A.; Hashim, N.A.; Hashim, M.L.H. Polyethylene terephthalate membrane: A review of fabrication techniques, separation processes, and modifications. Sep. Purif. Technol. 2025, 354, 129343. [Google Scholar] [CrossRef]
- Mokhtari, F.; Samadi, A.; Rashed, A.O.; Li, X.; Razal, J.M.; Kong, L.; Varley, R.J.; Zhao, S. Recent progress in electrospun polyvinylidene fluoride (PVDF)-based nanofibers for sustainable energy and environmental applications. Prog. Mater. Sci. 2025, 148, 101376. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Ma, M.; Orhan, A.; Feng, L.; de Beer, M.P.; Troksa, A.L.; Hwee, N.; Heo, T.W.; Biener, J.; Wan, J.; Ye, J. 3D Printed Nanoporous Separators Based on Polymerization-Induced Phase Separation for Fast-Charging, High Cycling Stability Li-Ion Batteries. ACS Appl. Eng. Mater. 2024, 2, 2245–2254. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, Y.; Zeng, C.; Jin, H. Recent progress of advanced separators for Li-ion batteries. J. Mater. Sci. 2024, 59, 12154–12176. [Google Scholar] [CrossRef]
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite Separators for Robust High Rate Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Parekh, M.H.; Gray, F.S.; Fleetwood, J.; Adams, T.; Tomar, V.; Pol, V.G.; Mukherjee, P.P. The Role of Separator Thermal Stability in Safety Characteristics of Lithium-ion Batteries. J. Electrochem. Soc. 2022, 169, 090521. [Google Scholar] [CrossRef]
- Hashmi, M.M.; Arif, N.A.; Ali, S.M.; Khan, M.B.; Singh, M.P.; Khan, Z.H. Recent progress in separators for rechargeable batteries. Horizons From Nat. Nanomater. 2022, 417–498. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Q.; Zhen, C.; Niu, Y.; Han, Y.; Zeng, G.; Chen, D.; Feng, C.; Chen, N.; Lv, W.; et al. Recent progress in flame-retardant separators for safe lithium-ion batteries. Energy Storage Mater. 2021, 37, 628–647. [Google Scholar] [CrossRef]
- Yuan, B.; Feng, Y.; Qiu, X.; He, Y.; Dong, L.; Zhong, S.; Liu, J.; Liang, Y.; Liu, Y.; Xie, H.; et al. A Safe Separator with Heat-Dispersing Channels for High-Rate Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2308929. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Zhu, Y.-J.; Wu, J.; Yu, H.-P.; Xie, S.; Li, D.; Wang, Z.; Li, H. Reversible Li plating regulation on graphite anode through a barium sulfate nanofibers-based dielectric separator for fast charging and high-safety lithium-ion battery. J. Energy Chem. 2025, 101, 511–523. [Google Scholar] [CrossRef]
- Frankenberger, M.; Singh, M.; Dinter, A.; Jankowksy, S.; Schmidt, A.; Pettinger, K.-H. Laminated Lithium Ion Batteries with improved fast charging capability. J. Electroanal. Chem. 2019, 837, 151–158. [Google Scholar] [CrossRef]
- Dang, N.; Mao, J.; Mao, Y.; Yi, W.; Li, D.; Cheng, T.; He, L.; Deng, J.; Zhao, Z.; Zhao, T.; et al. Functionalized Polyethylene Separators with Efficient Li-Ion Transport Rate for Fast-Charging Li-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 17, 2169–2179. [Google Scholar] [CrossRef]
- Pradhan, A.; Badam, R.; Miyairi, R.; Takamori, N.; Matsumi, N. Extreme Fast Charging Capability in Graphite Anode via a Lithium Borate Type Biobased Polymer as Aqueous Polyelectrolyte Binder. ACS Mater. Lett. 2023, 5, 413–420. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Luo, M.; Rodrigues, M.-T.F.; Trask, S.E.; Abraham, D.P. Fast charging of Li-ion cells: Effect of separator membranes and mapping of “safe lines” to avoid Li plating. J. Power Sources 2022, 549, 232086. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Si, J.; Chen, F.; Cao, K.; Chen, C. Bifunctional composite separator with redistributor and anion absorber for dendrites-free and fast-charging lithium metal batteries. Chem. Eng. J. 2022, 430, 132971. [Google Scholar] [CrossRef]
- Makki, M.; Lee, C.W.; Ayoub, G. Stress Distribution Inside a Lithium-Ion Battery Cell during Fast Charging and Its Effect on Degradation of Separator. Batteries 2023, 9, 502. [Google Scholar] [CrossRef]
- Srivastava, M.; M. R., A.K.; Zaghib, K. Binders for Li-Ion Battery Technologies and Beyond: A Comprehensive Review. Batteries 2024, 10, 268. [Google Scholar] [CrossRef]
- Dou, W.; Zheng, M.; Zhang, W.; Liu, T.; Wang, F.; Wan, G.; Liu, Y.; Tao, X. Review on the Binders for Sustainable High-Energy-Density Lithium Ion Batteries: Status, Solutions, and Prospects. Adv. Funct. Mater. 2023, 33, 2305161. [Google Scholar] [CrossRef]
- He, Q.; Ning, J.; Chen, H.; Jiang, Z.; Wang, J.; Chen, D.; Zhao, C.; Liu, Z.; Perepichka, I.F.; Meng, H.; et al. Achievements, challenges, and perspectives in the design of polymer binders for advanced lithium-ion batteries. Chem. Soc. Rev. 2024, 53, 7091–7157. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.; Kim, H.; Kim, J.; Jin, H.-J. Polymeric Binder Design for Sustainable Lithium-Ion Battery Chemistry. Polymers 2024, 16, 254. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent technology development in solvent-free electrode fabrication for lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 183, 113515. [Google Scholar] [CrossRef]
- Liang, X.; Ahmad, N.; Zhang, B.; Zeng, C.; Cao, X.; Dong, Q.; Yang, W. Research progress of robust binders with superior mechanical properties for high-performance silicon-based lithium-ion batteries. Mater. Chem. Front. 2024, 8, 1480–1512. [Google Scholar] [CrossRef]
- Qin, T.; Yang, H.; Li, Q.; Yu, X.; Li, H. Design of functional binders for high-specific-energy lithium-ion batteries: From molecular structure to electrode properties. Ind. Chem. Mater. 2024, 2, 191–225. [Google Scholar] [CrossRef]
- Lee, S.; Koo, H.; Kang, H.S.; Oh, K.-H.; Nam, K.W. Advances in Polymer Binder Materials for Lithium-Ion Battery Electrodes and Separators. Polymers 2023, 15, 4477. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Rao, L.; Cho, H.; Brooks, C.J.; Sayre, J.R.; Kim, J.-H. Advancing Extreme Fast-Charging Capabilities of LiFePO4 Using Conductive Polymer Coatings and Lithium Polyacrylate Binder. ACS Appl. Energy Mater. 2024, 7, 7131–7139. [Google Scholar] [CrossRef]
- Usseglio-Viretta, F.L.E.; Colclasure, A.M.; Dunlop, A.R.; Trask, S.E.; Jansen, A.N.; Abraham, D.P.; Rodrigues, M.-T.F.; Dufek, E.J.; Tanim, T.R.; Chinnam, P.R.; et al. Carbon-Binder Weight Loading Optimization for Improved Lithium-Ion Battery Rate Capability. J. Electrochem. Soc. 2022, 169, 070519. [Google Scholar] [CrossRef]
- Scheck, V.; Memm, M.; Hölzle, M.; Wohlfahrt-Mehrens, M. Improving the Fast Charging Capability of Lithium-Ion Battery Graphite Anodes by Implementing an Alternative Binder System. J. Electrochem. Soc. 2023, 170, 120514. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Guan, Z.; Zhao, Q.; Sun, N.; Xu, B. A Flexible Si@C Electrode with Excellent Stability Employing an MXene as a Multifunctional Binder for Lithium-Ion Batteries. ChemSusChem 2020, 13, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, C.; Zhou, Y.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. The application of Al2O3 in separators and solid electrolytes of lithium-ion battery: A review. Energy Storage Mater. 2024, 71, 103575. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Z.; Kang, Y.; Zhou, Y.; Li, Y.; He, X.; Wang, L.; Mai, W.; Wang, X.; Zhou, G.; et al. Rational design of functional binder systems for high-energy lithium-based rechargeable batteries. Energy Storage Mater. 2021, 35, 353–377. [Google Scholar] [CrossRef]
- Lin, C.; Tang, A.; Mu, H.; Wang, W.; Wang, C. Aging Mechanisms of Electrode Materials in Lithium-Ion Batteries for Electric Vehicles. J. Chem. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Koo, J.K.; Choi, H.; Seo, J.K.; Hwang, S.M.; Lee, J.; Kim, Y.-J. Microstructure engineering of nickel-rich oxide/carbon composite cathodes for fast charging of lithium-ion batteries. Ceram. Int. 2022, 48, 31859–31865. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Z.; Knoll, A.C. Charging Optimization for Li-Ion Battery in Electric Vehicles: A Review. IEEE Trans. Transp. Electrification 2022, 8, 3068–3089. [Google Scholar] [CrossRef]
- Xie, W.; Liu, X.; He, R.; Li, Y.; Gao, X.; Li, X.; Peng, Z.; Feng, S.; Feng, X.; Yang, S. Challenges and opportunities toward fast-charging of lithium-ion batteries. J. Energy Storage 2020, 32, 101837. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Cheng, Q.; Guo, B.; Yang, J. Classification and Review of the Charging Strategies for Commercial Lithium-Ion Batteries. IEEE Access 2019, 7, 43511–43524. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Charging protocols for lithium-ion batteries and their impact on cycle life—An experimental study with different 18650 high-power cells. J. Energy Storage 2016, 6, 125–141. [Google Scholar] [CrossRef]
- Song, J.; Liu, Z.; Knehr, K.W.; Kubal, J.J.; Kim, H.-K.; Dees, D.W.; Nelson, P.A.; Ahmed, S. Pathways towards managing cost and degradation risk of fast charging cells with electrical and thermal controls. Energy Environ. Sci. 2021, 14, 6564–6573. [Google Scholar] [CrossRef]
- Dufek, E.J.; Abraham, D.P.; Bloom, I.; Chen, B.-R.; Chinnam, P.R.; Colclasure, A.M.; Gering, K.L.; Keyser, M.; Kim, S.; Mai, W.; et al. Developing extreme fast charge battery protocols–A review spanning materials to systems. J. Power Sources 2022, 526, 231129. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Schneider, J.; Frank, A.; Wildfeuer, L.; Lin, X.; Jossen, A.; Lienkamp, M. Review of fast charging strategies for lithium-ion battery systems and their applicability for battery electric vehicles. J. Energy Storage 2021, 44, 103306. [Google Scholar] [CrossRef]
- Attia, P.M.; Grover, A.; Jin, N.; Severson, K.A.; Markov, T.M.; Liao, Y.-H.; Chen, M.H.; Cheong, B.; Perkins, N.; Yang, Z.; et al. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature 2020, 578, 397–402. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Study of the charging process of a LiCoO2-based Li-ion battery. J. Power Sources 2006, 160, 1349–1354. [Google Scholar] [CrossRef]
- Sabarimuthu, M.; Senthilnathan, N.; Kamalesh, M.S. Multi-stage constant current–constant voltage under constant temperature (MSCC-CV-CT) charging technique for lithium-ion batteries in light weight electric vehicles (EVs). Electr. Eng. 2023, 105, 4289–4309. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Lee, C.-K. A Study and Implementation of Inductive Power Transfer System Using Hybrid Control Strategy for CC-CV Battery Charging. Sustainability 2023, 15, 3606. [Google Scholar] [CrossRef]
- Hemavathi, S.; Shinisha, A. A study on trends and developments in electric vehicle charging technologies. J. Energy Storage 2022, 52, 105013. [Google Scholar] [CrossRef]
- Ghaeminezhad, N.; Monfared, M. Charging control strategies for lithium-ion battery packs: Review and recent developments. IET Power Electron. 2022, 15, 349–367. [Google Scholar] [CrossRef]
- Sikha, G.; Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Comparison of the capacity fade of Sony US 18650 cells charged with different protocols. J. Power Sources 2003, 122, 67–76. [Google Scholar] [CrossRef]
- Guo, Z.; Liaw, B.Y.; Qiu, X.; Gao, L.; Zhang, C. Optimal charging method for lithium ion batteries using a universal voltage protocol accommodating aging. J. Power Sources 2015, 274, 957–964. [Google Scholar] [CrossRef]
- Tahir, M.U.; Sangwongwanich, A.; Stroe, D.I.; Blaabjerg, F. The Effect of Multi-Stage Constant Current Charging on Lithium-ion Battery’s Performance. In Proceedings of the CPE-POWERENG 2023—17th IEEE International Conference on Compatibility, Power Electronics and Power Engineering, Tallinn, Estonia, 14–16 June 2023. [Google Scholar]
- Zhang, C.; Jiang, J.; Gao, Y.; Zhang, W.; Liu, Q.; Hu, X. Charging optimization in lithium-ion batteries based on temperature rise and charge time. Appl. Energy 2017, 194, 569–577. [Google Scholar] [CrossRef]
- Nambisan, P.; Saha, P.; Khanra, M. Real-time optimal fast charging of Li-ion batteries with varying temperature and charging behaviour constraints. J. Energy Storage 2021, 41, 102918. [Google Scholar] [CrossRef]
- Goncharova, I.; Yazami, R. An Optimized CCCV Protocol for Fast Charging Lithium Ion Batteries. Int. J. Eng. Sci. Invent. 2019, 8, 57–64. [Google Scholar]
- Ku, K.; Son, S.-B.; Gim, J.; Park, J.; Liang, Y.; Stark, A.; Lee, E.; Libera, J. Understanding the constant-voltage fast-charging process using a high-rate Ni-rich cathode material for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 288–295. [Google Scholar] [CrossRef]
- Mai, W.; Colclasure, A.M.; Smith, K. Model-Instructed Design of Novel Charging Protocols for the Extreme Fast Charging of Lithium-Ion Batteries Without Lithium Plating. J. Electrochem. Soc. 2020, 167, 080517. [Google Scholar] [CrossRef]
- Liu, Z.; Li, K.; Zhang, W.; Xie, Y.; Liu, J.; Sun, L.; Zan, J. Research on safe charging strategy of lithium-ion battery based on three-electrode equivalent circuit model. J. Energy Storage 2023, 72, 108563. [Google Scholar] [CrossRef]
- Galuppini, G.; Berliner, M.D.; Lian, H.; Zhuang, D.; Bazant, M.Z.; Braatz, R.D. Efficient computation of safe, fast charging protocols for multiphase lithium-ion batteries: A lithium iron phosphate case study. J. Power Sources 2023, 580, 233272. [Google Scholar] [CrossRef]
- Our Charging Technology—XTAR. Available online: https://www.xtar.cc/our-charging-technology (accessed on 1 March 2025).
- Han, S. Optimal Charging Current Protocol with Multi-Stage Constant Current Using Dandelion Optimizer for Time-Domain Modeled Lithium-Ion Batteries. Appl. Sci. 2024, 14, 11320. [Google Scholar] [CrossRef]
- Tahir, M.U.; Sangwongwanich, A.; Stroe, D.-I.; Blaabjerg, F. Overview of multi-stage charging strategies for Li-ion batteries. J. Energy Chem. 2023, 84, 228–241. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, J.; Lee, Y. Control of Li-ion transport through a combination of conductive coating and charging protocol for a stable and extremely fast charging cathode. J. Alloy. Compd. 2024, 988, 174263. [Google Scholar] [CrossRef]
- Chen, G.-J.; Liu, Y.-H.; Wang, S.-C.; Luo, Y.-F.; Yang, Z.-Z. Searching for the optimal current pattern based on grey wolf optimizer and equivalent circuit model of Li-ion batteries. J. Energy Storage 2021, 33, 101933. [Google Scholar] [CrossRef]
- Anseán, D.; González, M.; Viera, J.; García, V.; Blanco, C.; Valledor, M. Fast charging technique for high power lithium iron phosphate batteries: A cycle life analysis. J. Power Sources 2013, 239, 9–15. [Google Scholar] [CrossRef]
- An, F.; Zhang, R.; Wei, Z.; Li, P. Multi-stage constant-current charging protocol for a high-energy-density pouch cell based on a 622NCM/graphite system. RSC Adv. 2019, 9, 21498–21506. [Google Scholar] [CrossRef]
- Ha, Y.J.; Roh, S.; Park, B.K.; Kim, M.; Jeong, D.H.; Kim, H.; Kim, I.; Jo, Y.N.; Choi, J.W.; Kim, K.J. Innovative Fast-Charging Protocol Incorporating Proactive Lithium-Plating Suppression Pulses for Lithium-Ion Batteries: Protocol Design, Validation, and Post-Mortem Analysis. Adv. Energy Mater. 2024, 14, 2402032. [Google Scholar] [CrossRef]
- Mathieu, R.; Briat, O.; Gyan, P.; Vinassa, J.-M. Fast charging for electric vehicles applications: Numerical optimization of a multi-stage charging protocol for lithium-ion battery and impact on cycle life. J. Energy Storage 2021, 40, 102756. [Google Scholar] [CrossRef]
- Liu, K.; Zou, C.; Li, K.; Wik, T. Charging Pattern Optimization for Lithium-Ion Batteries With an Electrothermal-Aging Model. IEEE Trans. Ind. Inform. 2018, 14, 5463–5474. [Google Scholar] [CrossRef]
- El Ouazzani, H.; El Hassani, I.; Barka, N.; Masrour, T. MSCC-DRL: Multi-Stage constant current based on deep reinforcement learning for fast charging of lithium ion battery. J. Energy Storage 2024, 75, 109695. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Exner, M.; Huang, X.; Li, Y.; Liu, Y.; Wang, H.; Kowal, J.; Zhang, Q.; Kristensen, P.K.; et al. Unravelling the Mechanism of Pulse Current Charging for Enhancing the Stability of Commercial LiNi0.5Mn0.3Co0.2O2/Graphite Lithium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2400190. [Google Scholar] [CrossRef]
- Wu, B.; Yufit, V.; Marinescu, M.; Offer, G.J.; Martinez-Botas, R.F.; Brandon, N.P. Coupled thermal–electrochemical modelling of uneven heat generation in lithium-ion battery packs. J. Power Sources 2013, 243, 544–554. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; A Kohl, P. The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J. Power Sources 2001, 102, 302–309. [Google Scholar] [CrossRef]
- Adejare, A.A.; Okemakinde, F.E.; Tingbari, V.M.; Lee, J.; Kim, J. Comparative Analysis of Charging Protocol for Degradation Reduction and Remaining-Useful-Life Enhancement of a Lithium-Ion Battery. Energy Technol. 2024, 12, 2400584. [Google Scholar] [CrossRef]
- Monem, M.A.; Trad, K.; Omar, N.; Hegazy, O.; Mantels, B.; Mulder, G.; Van den Bossche, P.; Van Mierlo, J. Lithium-ion batteries: Evaluation study of different charging methodologies based on aging process. Appl. Energy 2015, 152, 143–155. [Google Scholar] [CrossRef]
- Abdel-Monem, M.; Trad, K.; Omar, N.; Hegazy, O.; Bossche, P.V.D.; Van Mierlo, J. Influence analysis of static and dynamic fast-charging current profiles on ageing performance of commercial lithium-ion batteries. Energy 2017, 120, 179–191. [Google Scholar] [CrossRef]
- Aryanfar, A.; Brooks, D.; Merinov, B.V.; Goddard, W.A., III; Colussi, A.J.; Hoffmann, M.R. Dynamics of Lithium Dendrite Growth and Inhibition: Pulse Charging Experiments and Monte Carlo Calculations. J. Phys. Chem. Lett. 2014, 5, 1721–1726. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Advanced Integrated Fast-Charging Protocol for Lithium-Ion Batteries by Considering Degradation. ACS Sustain. Chem. Eng. 2024, 12, 6786–6796. [Google Scholar] [CrossRef]
- Zhou, H.; Alujjage, A.S.; Terese, M.; Fear, C.; Joshi, T.; Rikka, V.R.; Jeevarajan, J.A.; Mukherjee, P.P. Effect of fast charging on degradation and safety characteristics of lithium-ion batteries with LiFePO4 cathodes. Appl. Energy 2025, 377, 124465. [Google Scholar] [CrossRef]
- Purushothaman, B.K.; Landau, U. Rapid Charging of Lithium-Ion Batteries Using Pulsed Currents. J. Electrochem. Soc. 2006, 153, A533. [Google Scholar] [CrossRef]
- Bandara, T.T.A.; Ansean, D.; Viera, J.; Sanchez, L.; Gonzalez, M. Fast Charging Protocols based on Pulse-Modulation with Varying Relaxation for Electric Vehicle Li-ion cells. In Proceedings of the 2020 IEEE Vehicle Power and Propulsion Conference (VPPC), Online, 18 November–16 December 2020; pp. 1–6. [Google Scholar]
- Charge–TAE Power Solutions. Available online: https://power-solutions.tae.com/charge (accessed on 1 March 2025).
- Lo Franco, F.; Ricco, M.; Mandrioli, R.; Viatkin, A.; Grandi, G. Current Pulse Generation Methods for Li-ion Battery Chargers. In Proceedings of the—2020 2nd IEEE International Conference on Industrial Electronics for Sustainable Energy Systems, IESES 2020, Cagliari, Italy, 1–3 September 2020. [Google Scholar]
- Notten, P.; Veld, J.O.H.; van Beek, J. Boostcharging Li-ion batteries: A challenging new charging concept. J. Power Sources 2005, 145, 89–94. [Google Scholar] [CrossRef]
- Chavan, S.; Venkateswarlu, B.; Salman, M.; Liu, J.; Pawar, P.; Joo, S.W.; Choi, G.S.; Kim, S.C. Thermal management strategies for lithium-ion batteries in electric vehicles: Fundamentals, recent advances, thermal models, and cooling techniques. Int. J. Heat Mass Transf. 2024, 232, 125918. [Google Scholar] [CrossRef]
- Kumar, R.R.; Bharatiraja, C.; Udhayakumar, K.; Devakirubakaran, S.; Sekar, K.S.; Mihet-Popa, L. Advances in Batteries, Battery Modeling, Battery Management System, Battery Thermal Management, SOC, SOH, and Charge/Discharge Characteristics in EV Applications. IEEE Access 2023, 11, 105761–105809. [Google Scholar] [CrossRef]
- Song, M.; Choe, S.-Y. Fast and safe charging method suppressing side reaction and lithium deposition reaction in lithium ion battery. J. Power Sources 2019, 436, 226835. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Tanim, T.R.; Kim, S.; Colclasure, A.M.; Yang, Z.; Gering, K.; Weddle, P.J.; Evans, M.; Dufek, E.J.; Lin, Y.; Wen, J.; et al. Rational designs to enable 10-min fast charging and long cycle life in lithium-ion batteries. J. Power Sources 2023, 582, 233519. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Q.; Wang, S.; Song, Y.; Shi, B.; He, J. Aging and post-aging thermal safety of lithium-ion batteries under complex operating conditions: A comprehensive review. J. Power Sources 2024, 623, 235453. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Cavalheiro, G.M.; Du, X.; Liu, B.; Du, Z.; Zhang, G. In Situ Measurement of Lithium-Ion Cell Internal Temperatures during Extreme Fast Charging. J. Electrochem. Soc. 2019, 166, A3254–A3259. [Google Scholar] [CrossRef]
- Mathieu, R.; Briat, O.; Gyan, P.; Vinassa, J.-M. Comparison of the impact of fast charging on the cycle life of three lithium-ion cells under several parameters of charge protocol and temperatures. Appl. Energy 2021, 283, 116344. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Gao, Y.; Zhang, W.; Liu, Q.; Hu, X. Polarization Based Charging Time and Temperature Rise Optimization for Lithium-ion Batteries. Energy Procedia 2016, 88, 675–681. [Google Scholar] [CrossRef][Green Version]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to avoid dendrite formation in metal batteries: Innovative strategies for dendrite suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Yang, Z.; Charalambous, H.; Lin, Y.; Trask, S.E.; Yu, L.; Wen, J.; Jansen, A.; Tsai, Y.; Wiaderek, K.M.; Ren, Y.; et al. Extreme fast charge aging: Correlation between electrode scale and heterogeneous degradation in Ni-rich layered cathodes. J. Power Sources 2022, 521, 230961. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Feng, X.; Ren, D.; Li, Y.; Hou, J.; Wu, Y.; Du, J.; Lu, L.; Ouyang, M. Battery eruption triggered by plated lithium on an anode during thermal runaway after fast charging. Energy 2022, 239, 122097. [Google Scholar] [CrossRef]
- Boroujeni, S.M.; Fill, A.; Ridder, A.; Birke, K.P. Influence of Temperature and Electrolyte Composition on the Performance of Lithium Metal Anodes. Batteries 2021, 7, 67. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Lu, L.; Ouyang, M.; Zheng, S.; Li, J.; He, X. An electrochemical-thermal coupled overcharge-to-thermal-runaway model for lithium ion battery. J. Power Sources 2017, 364, 328–340. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, X.; Chen, S.; Zhu, J.; Han, G.; Tang, X.; Hua, W.; Dai, H.; Ye, J. Comprehensive Investigation of a Slight Overcharge on Degradation and Thermal Runaway Behavior of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 35054–35068. [Google Scholar] [CrossRef]
- Hu, J.; Liu, T.; Wang, X.; Wang, Z.; Wu, L. Investigation on thermal runaway of 18,650 lithium ion battery under thermal abuse coupled with charging. J. Energy Storage 2022, 51, 104482. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y.; Wongwises, S. Lessons from the Electric Vehicle Crashworthiness Leading to Battery Fire. Energies 2021, 14, 4802. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Xiao, Y.; Wang, D.; Gao, Z.; Huang, Z.; Ren, S.; Jiang, N.; Wu, S. Advancements in the safety of Lithium-Ion Battery: The Trigger, consequence and mitigation method of thermal runaway. Chem. Eng. J. 2024, 481, 148450. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Strategies to Solve Lithium Battery Thermal Runaway: From Mechanism to Modification. Electrochem. Energy Rev. 2021, 4, 633–679. [Google Scholar] [CrossRef]
- Ji, C.; Liu, D.; Liu, Y.; Wang, S.; Wang, Y.; Zhang, Z.; Wang, B. Effect of low temperature and high-rate cyclic aging on thermal characteristics and safety of lithium-ion batteries. Process. Saf. Environ. Prot. 2024, 188, 1514–1526. [Google Scholar] [CrossRef]
- Lei, D.; Wang, Y.; Fu, J.; Zhu, X.; Shi, J.; Wang, Y. Electrochemical-thermal analysis of large-sized lithium-ion batteries: Influence of cell thickness and cooling strategy in charging. Energy 2024, 307, 132629. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, Y.; Xie, B.; Shi, A.; Ning, Y.; Lou, S.; Yin, G. Challenges and Opportunities for Fast-Charging Batteries. J. Phys. Chem. C 2023, 127, 15021–15034. [Google Scholar] [CrossRef]
- Pesaran, A.A. Battery thermal models for hybrid vehicle simulations. J. Power Sources 2002, 110, 377–382. [Google Scholar] [CrossRef]
- Greco, A.; Jiang, X.; Cao, D. An investigation of lithium-ion battery thermal management using paraffin/porous-graphite-matrix composite. J. Power Sources 2015, 278, 50–68. [Google Scholar] [CrossRef]
- Duan, X.; Wang, H.; Jia, Y.; Wang, L.; Liu, B.; Xu, J. A multiphysics understanding of internal short circuit mechanisms in lithium-ion batteries upon mechanical stress abuse. Energy Storage Mater. 2022, 45, 667–679. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Oh, J.; Lee, S.; Kim, H.; Ryu, J.; Gil, B.; Lee, J.; Kim, M. Overcharge-Induced Phase Heterogeneity and Resultant Twin-Like Layer Deformation in Lithium Cobalt Oxide Cathode for Lithium-Ion Batteries. Adv. Sci. 2022, 9, e2203639. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, M.; Li, Y.; Feng, X.; Zhang, K.; Bai, Y.; Wang, X.; Wu, C. High-Mass-Loading Electrodes for Advanced Secondary Batteries and Supercapacitors. Electrochem. Energy Rev. 2021, 4, 382–446. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2015, 163, A138–A149. [Google Scholar] [CrossRef]
- Tian, X.; Yi, Y.; Fang, B.; Yang, P.; Wang, T.; Liu, P.; Qu, L.; Li, M.; Zhang, S. Design Strategies of Safe Electrolytes for Preventing Thermal Runaway in Lithium Ion Batteries. Chem. Mater. 2020, 32, 9821–9848. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Xue, J.; Hu, D.; Xu, J. A Mechanistic and Quantitative Understanding of the Interactions between SiO and Graphite Particles. Adv. Energy Mater. 2022, 13, 2202584. [Google Scholar] [CrossRef]
- Liu, T.; Ge, S.; Yang, X.-G.; Wang, C.-Y. Effect of thermal environments on fast charging Li-ion batteries. J. Power Sources 2021, 511, 230466. [Google Scholar] [CrossRef]
- Odom, S.A.; Ergun, S.; Poudel, P.P.; Parkin, S.R. A fast, inexpensive method for predicting overcharge performance in lithium-ion batteries. Energy Environ. Sci. 2013, 7, 760–767. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, Y.; Liu, X.; Luo, R.; Liu, Y.; Ma, C. Thermal performance and structural optimization of a hybrid thermal management system based on MHPA/PCM/liquid cooling for lithium-ion battery. Appl. Therm. Eng. 2023, 235, 121341. [Google Scholar] [CrossRef]
- Yeganehdoust, F.; Reddy, A.K.M.R.; Zaghib, K. Cell Architecture Design for Fast-Charging Lithium-Ion Batteries in Electric Vehicles. Batteries 2025, 11, 20. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Xiong, X.; Zhu, J.; Gao, Q.; Wang, H.; Wu, H. Novel concept design of low energy hybrid battery thermal management system using PCM and multistage Tesla valve liquid cooling. Appl. Therm. Eng. 2022, 220, 119680. [Google Scholar] [CrossRef]
- Murali, G.; Sravya, G.; Jaya, J.; Vamsi, V.N. A review on hybrid thermal management of battery packs and it’s cooling performance by enhanced PCM. Renew. Sustain. Energy Rev. 2021, 150, 111513. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal runaway propagation model for designing a safer battery pack with 25 Ah LiNi Co Mn O2 large format lithium ion battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, Z.; Du, J.; Guo, H.; Lu, L.; Ouyang, M. External Liquid Cooling Method for Lithium-Ion Battery Modules Under Ultra-Fast Charging. IEEE Trans. Ind. Appl. 2022, 58, 7658–7667. [Google Scholar] [CrossRef]
- Newsroom|Hyundai Mobis. Available online: https://www.mobis.com/en/aboutus/press.do?category=press&idx=6000 (accessed on 1 March 2025).
- Battery Systems for Electric Vehicles. Available online: https://www.carrar.net/ (accessed on 1 March 2025).
- MAHLE Develops New Battery Cooling System for Faster Charging of Electric Cars—MAHLE Newsroom. Available online: https://newsroom.mahle.com/press/en/press-releases/mahle-develops-new-battery-cooling-system-for-faster-charging-of-electric-cars-85120 (accessed on 1 March 2025).
- Zeng, Y.; Zhang, B.; Fu, Y.; Shen, F.; Zheng, Q.; Chalise, D.; Miao, R.; Kaur, S.; Lubner, S.D.; Tucker, M.C.; et al. Extreme fast charging of batteries using thermal switching and self-heating. arXiv 2022, arXiv:2205.06762. [Google Scholar]
- Zeng, Y.; Zhang, B.; Fu, Y.; Shen, F.; Zheng, Q.; Chalise, D.; Miao, R.; Kaur, S.; Lubner, S.D.; Tucker, M.C.; et al. Extreme fast charging of commercial Li-ion batteries via combined thermal switching and self-heating approaches. Nat. Commun. 2023, 14, 3229. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Liu, T.; Gao, Y.; Ge, S.; Leng, Y.; Wang, D.; Wang, C.-Y. Asymmetric Temperature Modulation for Extreme Fast Charging of Lithium-Ion Batteries. Joule 2019, 3, 3002–3019. [Google Scholar] [CrossRef]
- Hyun, G.; Cao, S.; Ham, Y.; Youn, D.-Y.; Kim, I.-D.; Chen, X.; Jeon, S. Three-Dimensional, Submicron Porous Electrode with a Density Gradient to Enhance Charge Carrier Transport. ACS Nano 2022, 16, 9762–9771. [Google Scholar] [CrossRef]
- He, J.; Liao, Y.; Hu, Q.; Zeng, Z.; Yi, L.; Wang, Y.; Lu, H.; Pan, M. Investigation of polyimide as an anode material for lithium-ion battery and its thermal safety behavior. Ionics 2020, 26, 3343–3350. [Google Scholar] [CrossRef]
- Son, D.-K.; Kim, J.; Raj, M.R.; Lee, G. Elucidating the structural redox behaviors of nanostructured expanded graphite anodes toward fast-charging and high-performance lithium-ion batteries. Carbon 2021, 175, 187–201. [Google Scholar] [CrossRef]
- Lee, Y.; Jeghan, S.M.N.; Lee, G. Boost charging lithium-ion battery using expanded graphite anode with enhanced performance. Mater. Lett. 2021, 299, 130077. [Google Scholar] [CrossRef]
- Dendrite Prevention in EV Batteries. Available online: https://xray.greyb.com/ev-battery/preventing-dendrite-formation-in-lithium-metal-batteries (accessed on 1 March 2025).
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2019, 3, 1–42. [Google Scholar] [CrossRef]
- Kim, D.S.; Chung, D.J.; Bae, J.; Jeong, G.; Kim, H. Surface engineering of graphite anode material with black TiO2-x for fast chargeable lithium ion battery. Electrochim. Acta 2017, 258, 336–342. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 2018, 3, 310–316. [Google Scholar] [CrossRef]
- Wang, F.; Ke, X.; Shen, K.; Zhu, L.; Yuan, C. A Critical Review on Materials and Fabrications of Thermally Stable Separators for Lithium-Ion Batteries. Adv. Mater. Technol. 2021, 7, 2100772. [Google Scholar] [CrossRef]
- Lv, P.; Huo, T.; Gao, S.; Li, P.; Li, P.; Guo, Z.; Shi, J.; Zhang, D.; Xie, S.; He, Y.; et al. Sponge-like Nanoporous SiO2-Based Polyethylene Separators for Safe Fast-Charging Lithium-Ion Batteries. ACS Appl. Nano Mater. 2024, 7, 28553–28562. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.; Lin, Y.; Shi, H.; Xie, S.; Sun, Q.; Chen, X.; He, Y.; Tang, C. Polyethylene ceramic separators with semi-interpenetrating polymer network boosting fast-charging cycle capacity retention and safety for lithium-ion batteries. J. Power Sources 2023, 570, 233022. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Li, C.; Shen, X.; Peng, L.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A Modified Ceramic-Coating Separator with High-Temperature Stability for Lithium-Ion Battery. Polymers 2017, 9, 159. [Google Scholar] [CrossRef]
- Yan, S.; Chen, X.; Zhou, P.; Wang, P.; Zhou, H.; Zhang, W.; Xia, Y.; Liu, K. Regulating the growth of lithium dendrite by coating an ultra-thin layer of gold on separator for improving the fast-charging ability of graphite anode. J. Energy Chem. 2022, 67, 467–473. [Google Scholar] [CrossRef]
- Rafiz, K.; Harika, N.; Lin, J.Y. Electrode-supported high-tortuosity zeolite separator enabling fast-charging and dendrite-free lithium-ion/metal batteries. Electrochim. Acta 2023, 468, 143129. [Google Scholar] [CrossRef]
- Yadav, P.; Thakur, P.; Maity, D.; Narayanan, T.N. High Rate, Dendrite Free Lithium Metal Batteries of Extended Cyclability via a Scalable Separator Modification Approach. Small 2023, 20, e2308344. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kim, S.-M.; Song, J.H.; Yim, T.; Woo, S.-G.; Lee, K.-W.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sources 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Tanim, T.R.; Yang, Z.; Finegan, D.P.; Chinnam, P.R.; Lin, Y.; Weddle, P.J.; Bloom, I.; Colclasure, A.M.; Dufek, E.J.; Wen, J.; et al. A Comprehensive Understanding of the Aging Effects of Extreme Fast Charging on High Ni NMC Cathode. Adv. Energy Mater. 2022, 12, 2103712. [Google Scholar] [CrossRef]
- Mai, W.; Usseglio-Viretta, F.L.; Colclasure, A.M.; Smith, K. Enabling fast charging of lithium-ion batteries through secondary-/dual- pore network: Part II - numerical model. Electrochimica Acta 2020, 341, 136034. [Google Scholar] [CrossRef]
- Li, B.; Chao, Y.; Li, M.; Xiao, Y.; Li, R.; Yang, K.; Cui, X.; Xu, G.; Li, L.; Yang, C.; et al. A Review of Solid Electrolyte Interphase (SEI) and Dendrite Formation in Lithium Batteries. Electrochem. Energy Rev. 2023, 6, 7. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Zhang, X.-Q.; Wang, Y.-N.; Li, J.-L.; Zheng, Z.; Huang, J.-Q. Understanding the transport mechanism of lithium ions in solid-electrolyte interphase in lithium metal batteries with liquid electrolytes. Mater. Today 2024, 77, 39–65. [Google Scholar] [CrossRef]
- Lim, J.J.N.; Lim, G.J.H.; Cai, Y.; Chua, R.; Guo, Y.; Yan, Y.; Srinivasan, M. Electrolyte designs for safer lithium-ion and lithium-metal batteries. J. Mater. Chem. A 2023, 11, 22688–22717. [Google Scholar] [CrossRef]
- Badwekar, K.; Dingari, N.N.; Mynam, M.; Rai, B. Role of lithium salt in reducing the internal heating of a lithium ion battery during fast charging. J. Appl. Electrochem. 2022, 52, 941–951. [Google Scholar] [CrossRef]
- Seo, J.; Lee, G.; Hur, J.; Sung, M.; Seo, J.; Kim, D. Mechanically Interlocked Polymer Electrolyte with Built-In Fast Molecular Shuttles for All-Solid-State Lithium Batteries. Adv. Energy Mater. 2021, 11, 2102583. [Google Scholar] [CrossRef]
- Li, Y.; An, Y.; Tian, Y.; Fei, H.; Xiong, S.; Qian, Y.; Feng, J. Stable and Safe Lithium Metal Batteries with Ni-Rich Cathodes Enabled by a High Efficiency Flame Retardant Additive. J. Electrochem. Soc. 2019, 166, A2736–A2740. [Google Scholar] [CrossRef]
- Guo, H.-L.; Sun, H.; Jiang, Z.-L.; Hu, J.-Y.; Luo, C.-S.; Gao, M.-Y.; Cheng, J.-Y.; Shi, W.-K.; Zhou, H.-J.; Sun, S.-G. Asymmetric Structure Design of Electrolytes with Flexibility and Lithium Dendrite-Suppression Ability for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46783–46791. [Google Scholar] [CrossRef] [PubMed]
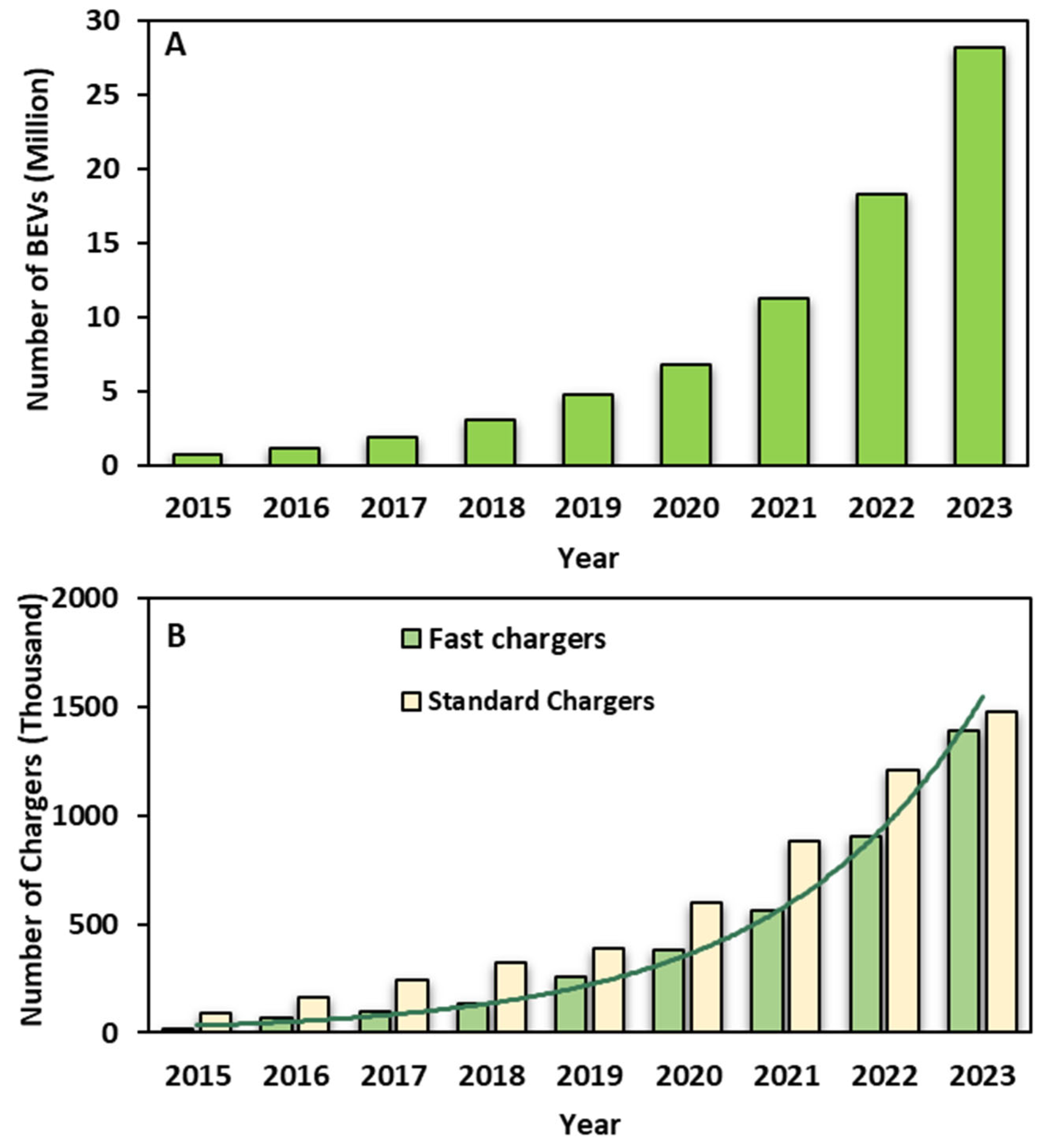


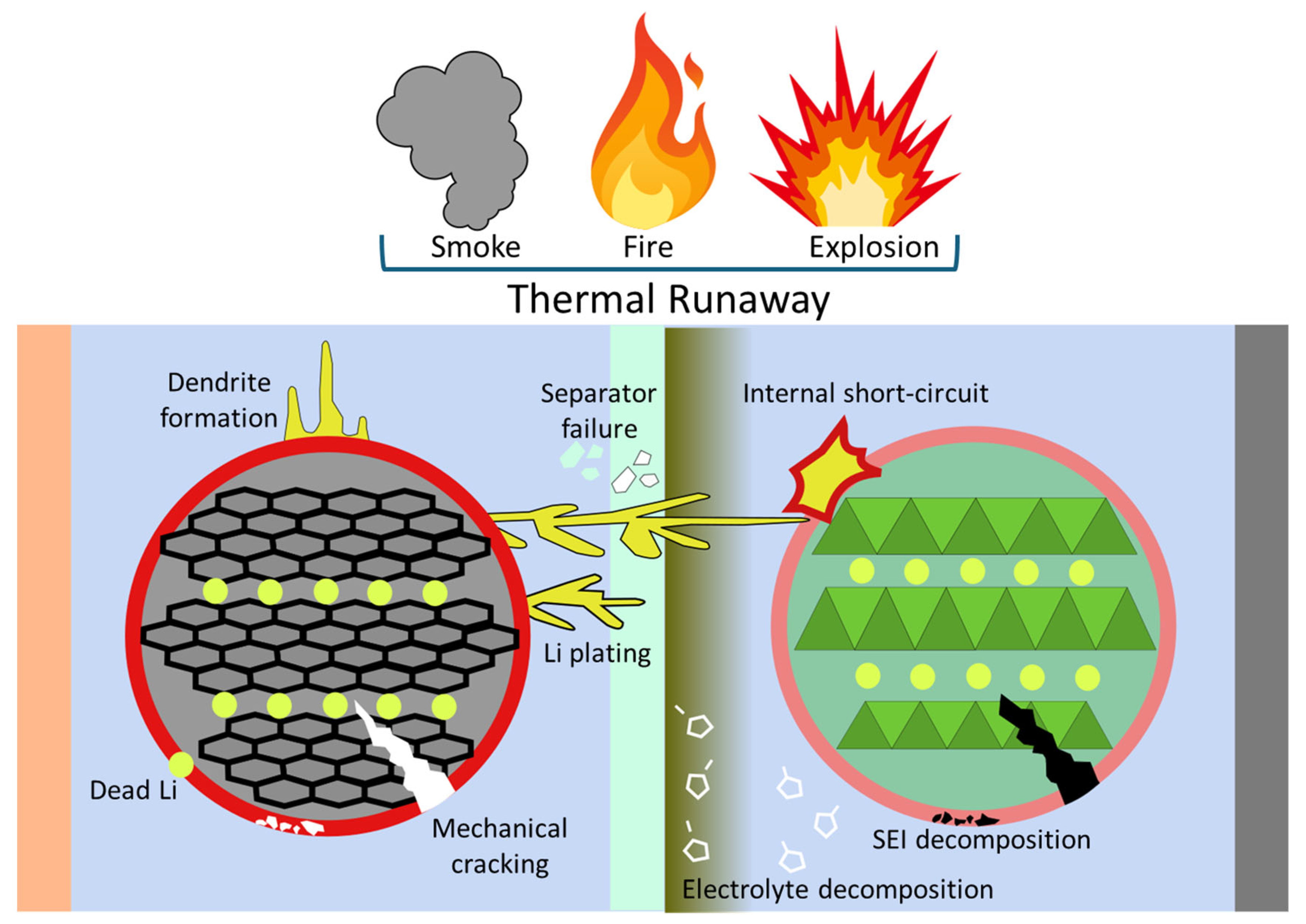
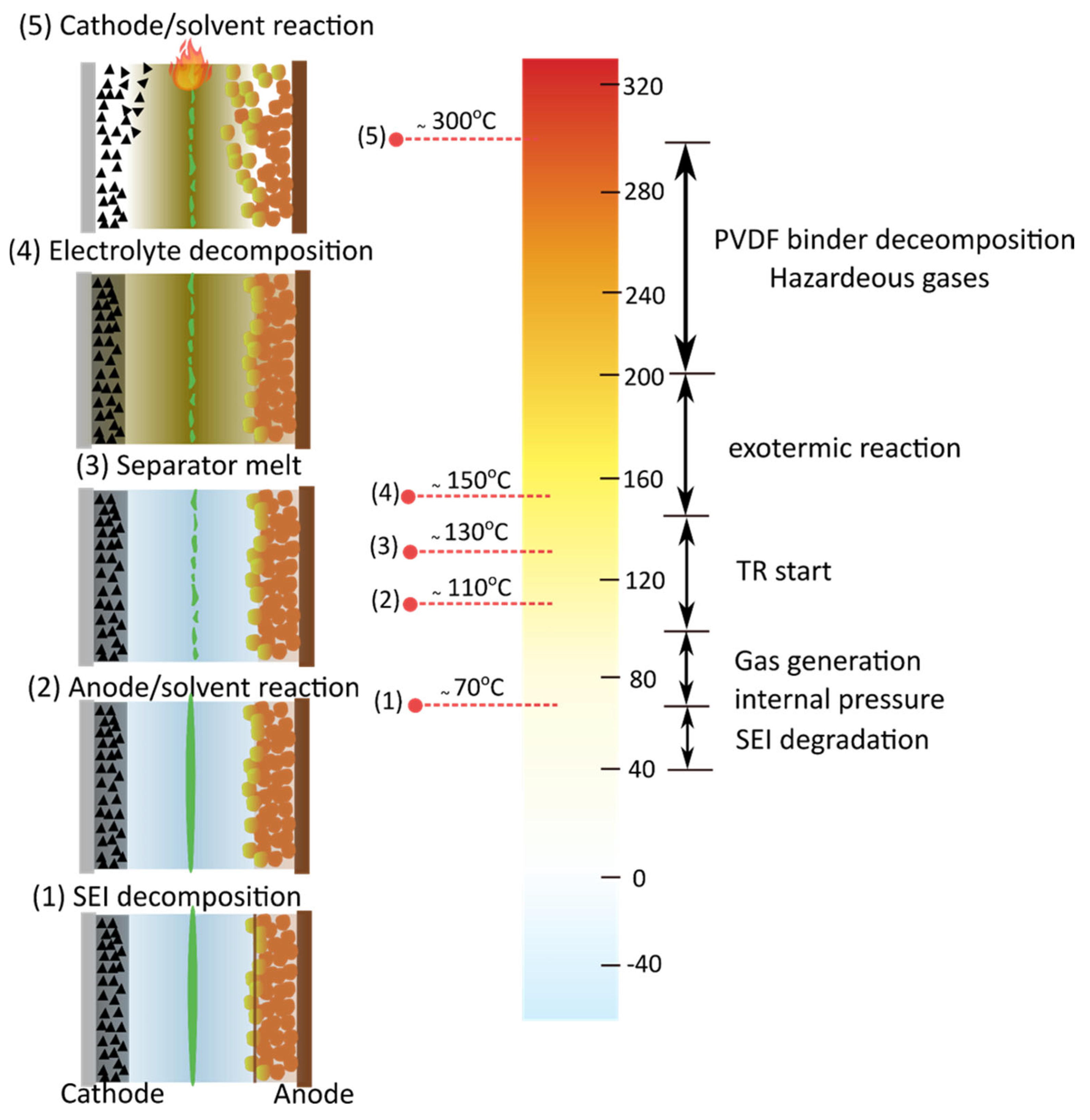
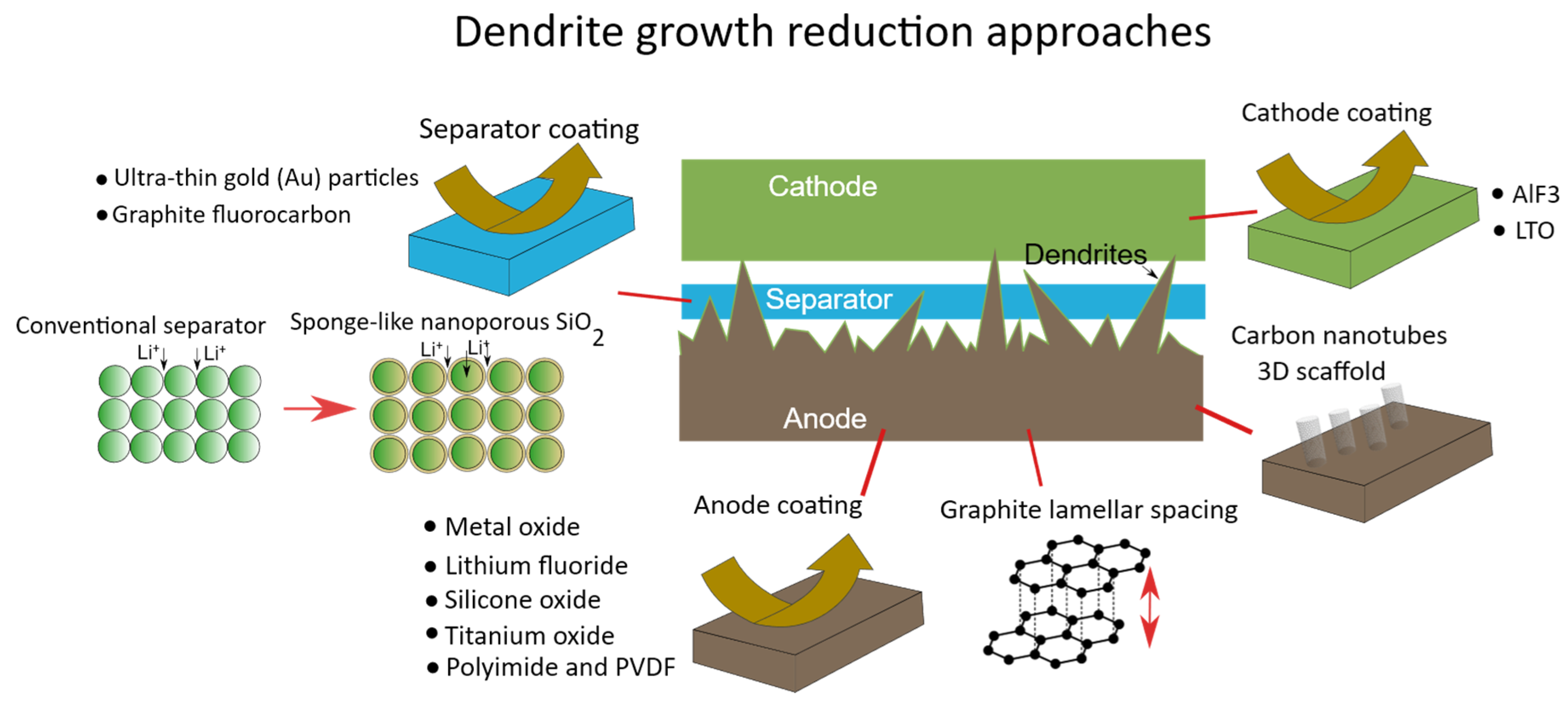
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghili Mehrizi, A.; Yeganehdoust, F.; Madikere Raghunatha Reddy, A.K.; Zaghib, K. Challenges and Issues Facing Ultrafast-Charging Lithium-Ion Batteries. Batteries 2025, 11, 209. https://doi.org/10.3390/batteries11060209
Aghili Mehrizi A, Yeganehdoust F, Madikere Raghunatha Reddy AK, Zaghib K. Challenges and Issues Facing Ultrafast-Charging Lithium-Ion Batteries. Batteries. 2025; 11(6):209. https://doi.org/10.3390/batteries11060209
Chicago/Turabian StyleAghili Mehrizi, Amirreza, Firoozeh Yeganehdoust, Anil Kumar Madikere Raghunatha Reddy, and Karim Zaghib. 2025. "Challenges and Issues Facing Ultrafast-Charging Lithium-Ion Batteries" Batteries 11, no. 6: 209. https://doi.org/10.3390/batteries11060209
APA StyleAghili Mehrizi, A., Yeganehdoust, F., Madikere Raghunatha Reddy, A. K., & Zaghib, K. (2025). Challenges and Issues Facing Ultrafast-Charging Lithium-Ion Batteries. Batteries, 11(6), 209. https://doi.org/10.3390/batteries11060209








