Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions
Abstract
1. Introduction
2. TR Triggering Model
2.1. Mathematical Characterization of Trigger Conditions for TR
2.2. Prediction of TR Behavior
2.2.1. TR Trigger Time
2.2.2. Loss of Quality Due to Thermal Runaway Mass
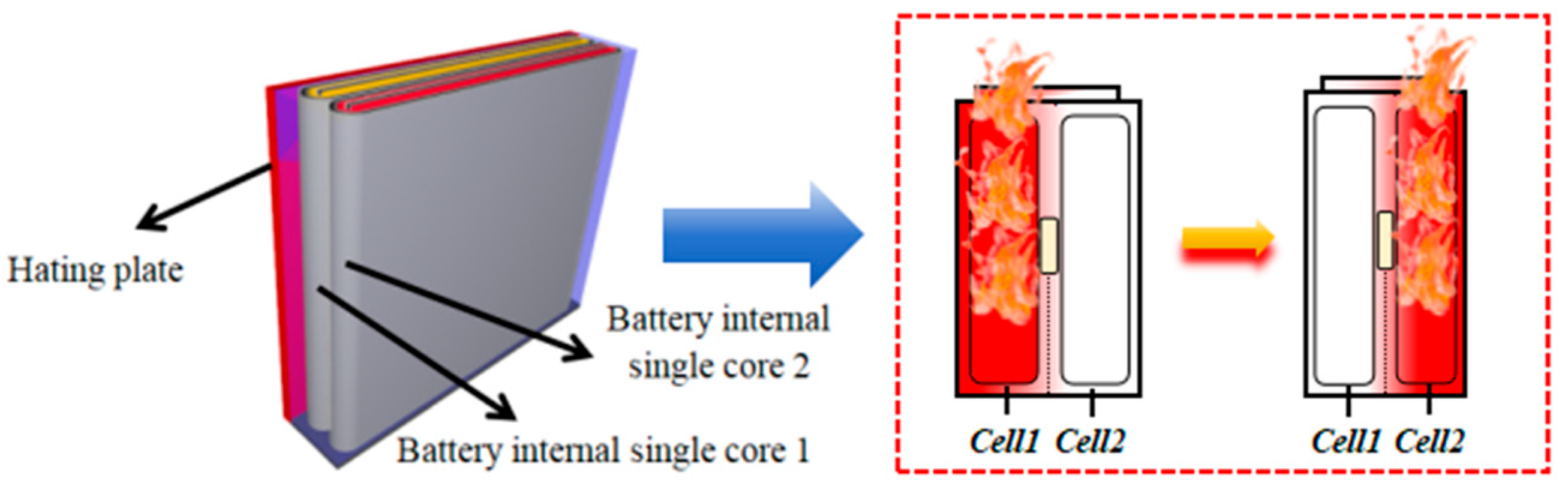
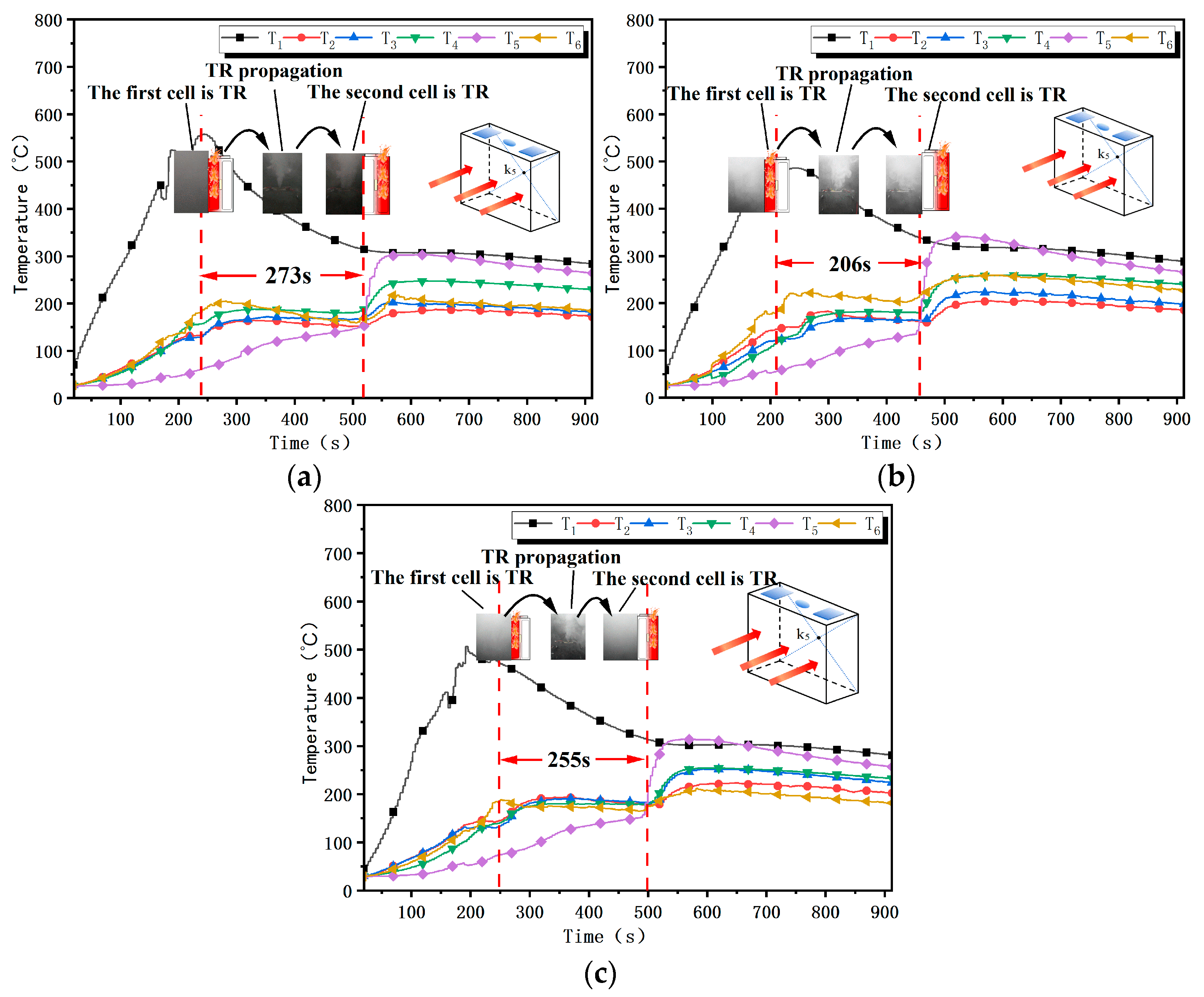
2.2.3. Battery Cell TR Interval
3. Experimental Designs
3.1. Battery Information
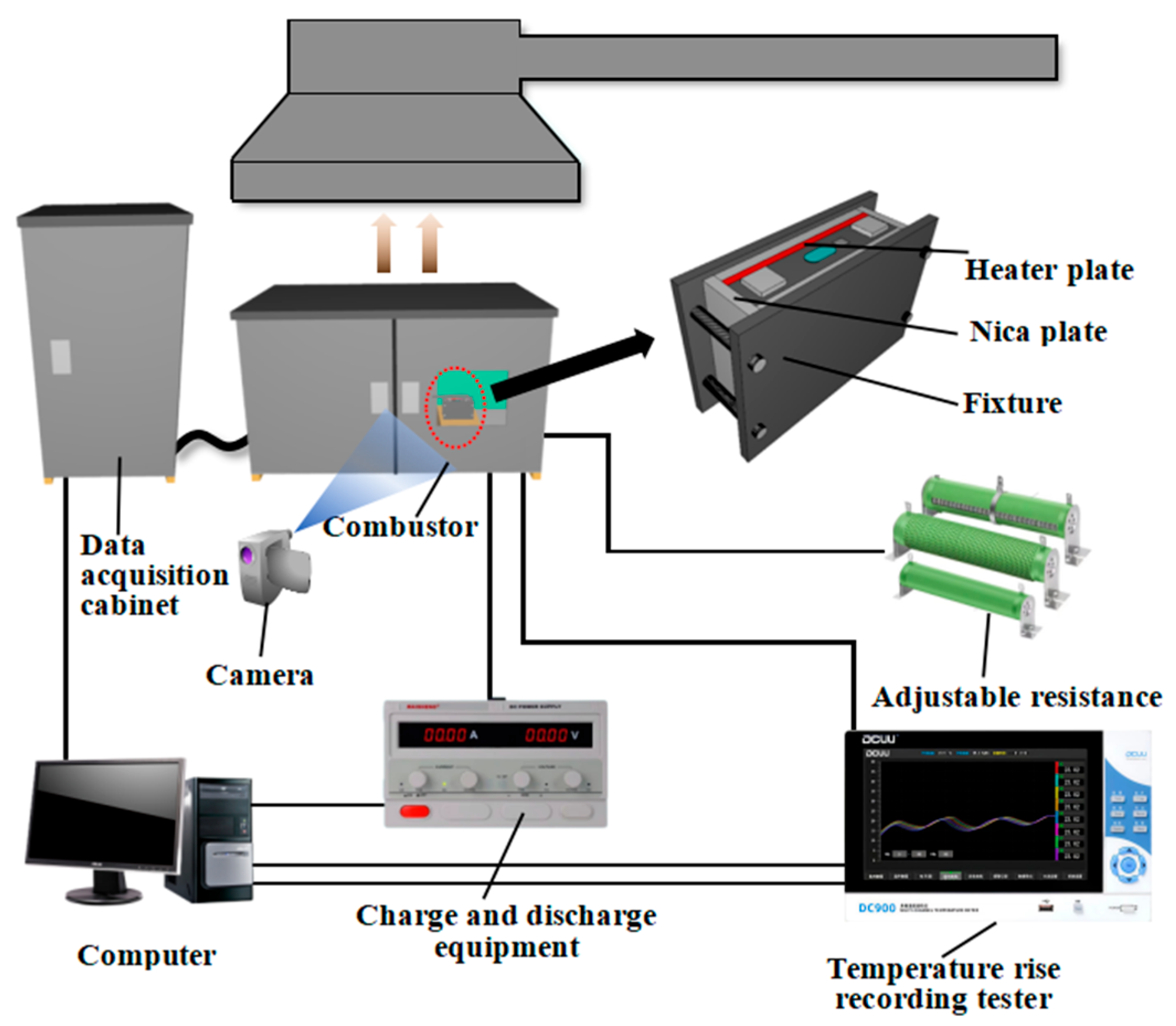
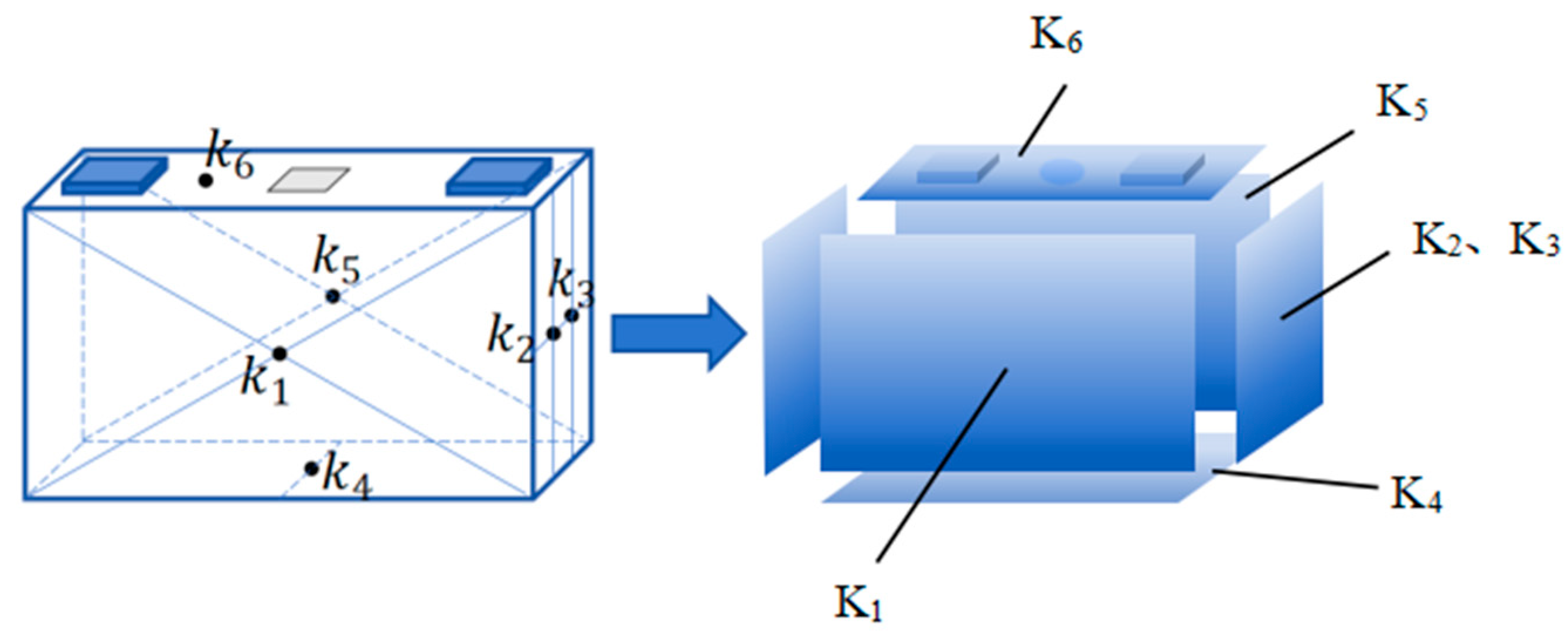
3.2. Experimental Setup
4. Results and Discussion
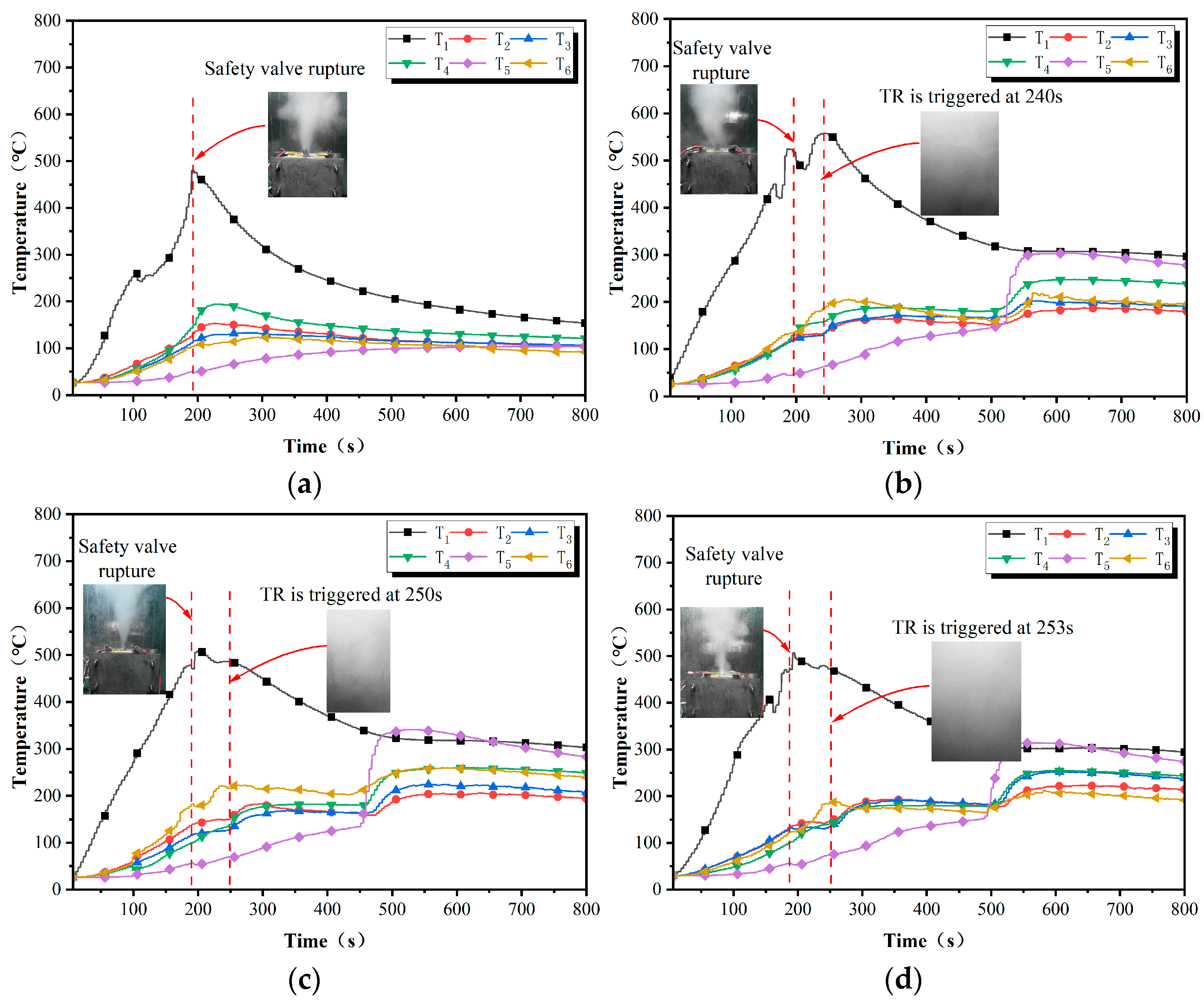
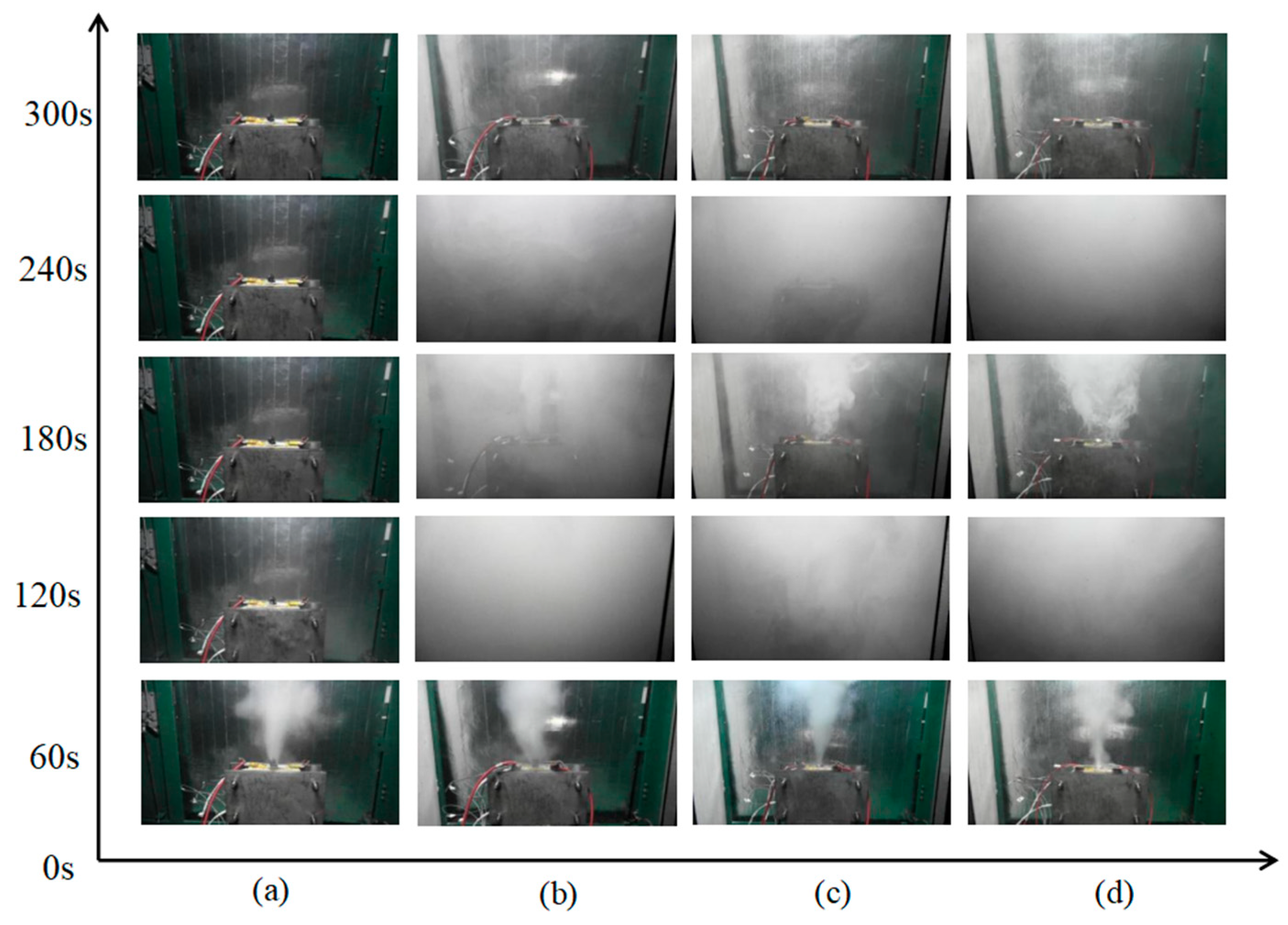
4.1. TR Trigger Time
4.2. Loss of Quality Due to Thermal Runaway
4.3. Battery Cell TR Interval
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Li, X.; Wang, Q.; Duan, Q.; Li, Y.; Li, L.; Wang, Q. Experimental investigation on thermal runaway propagation of large format lithium ion battery modules with two cathodes. Int. J. Heat Mass Transfer. 2021, 172, 121077. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large format lithium-ion pouch cells with Li (Ni0.6Co0.2Mn0.2) O2 cathode for electric vehicles: Thermal runaway features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; Qu, Z.; Zhang, J. Recent progress in lithium-ion battery thermal management for a wide range of temperature and abuse conditions. Int. J. Hydro. Energy 2022, 47, 9428–9459. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.E. Abuse by External Heating, Overcharging and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, X.; Zhou, X.; Yang, L.; Cao, B. A comprehensive study on the impact of heating position on thermal runaway of prismatic lithium-ion batteries. J. Power Sources 2022, 520, 230919. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Dongxu, O.; Cao, S.; Wang, Z.; Wang, J. A Simplified Analysis to Predict the Fire Hazard of Primary Lithium Battery. Appl. Sci. 2018, 8, 2329. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J. Internal short circuit in Li-ion cells. J. Power Sources 2009, 191, 568–574. [Google Scholar] [CrossRef]
- Hu, J.; Tang, X.; Zhu, X.; Liu, T.; Wang, X. Suppression of thermal runaway induced by thermal abuse in large-capacity lithium-ion batteries with water mist. Energy 2024, 286, 129669. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Zhai, H.; Wang, Q. Experimental investigation on the characteristics of thermal runaway and its propagation of large-format lithium ion batteries under overcharging and overheating conditions. Energy 2021, 233, 121103. [Google Scholar] [CrossRef]
- Finegan, D.; Tjaden, B.; Heenan, T.; Jervis, R.; Michiel, M.; Alexander, R.; Gareth, H.; Dan, J.; Paul, R. Tracking Internal Temperature and Structural Dynamics during Nail Penetration of Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A3285. [Google Scholar] [CrossRef]
- Ping, P.; Wang, Q.; Huang, P.; Sun, J.; Chen, C. Thermal behavior analysis of lithium-ion battery at elevated temperature using deconvolution method. Appl. Energy 2014, 129, 261–273. [Google Scholar] [CrossRef]
- Dai, Y.; Panahi, A. Thermal runaway process in lithium-ion batteries: A review. Next Energy 2025, 6, 100186. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, K.; Wang, Z.; Tong, X.; Guo, L. Theoretical analysis of lithium-ion battery failure characteristics under different states of charge. Fire Mater. 2018, 42, 680–686. [Google Scholar] [CrossRef]
- Huang, Z.; Duan, Q.; Li, J.; Yang, F.; Sun, J.; Wang, Q. Experimental and numerical investigation of heating power effect on thermal runaway propagation within large-format lithium iron phosphate battery. J. Energy Storage 2025, 109, 115098. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, Y.; Duan, Q.; Qin, P.; Sun, J.; Wang, Q. Heating position effect on internal thermal runaway propagation in large-format lithium iron phosphate battery. Appl. Energy 2022, 325, 119778. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; He, H. An Online Adaptive Internal Short Circuit Detection Method of Lithium-Ion Battery. Automot. Innov. 2021, 4, 93–102. [Google Scholar] [CrossRef]
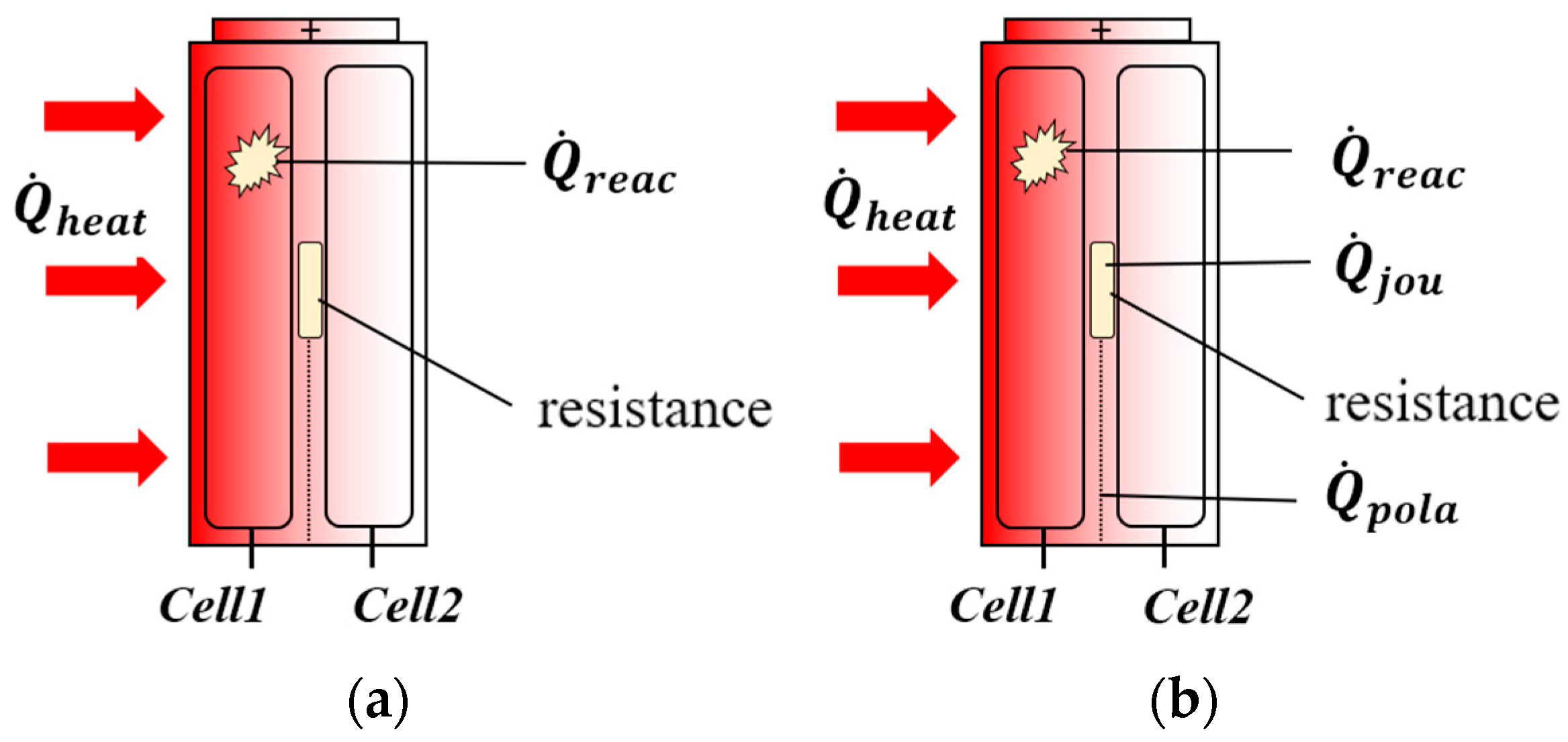
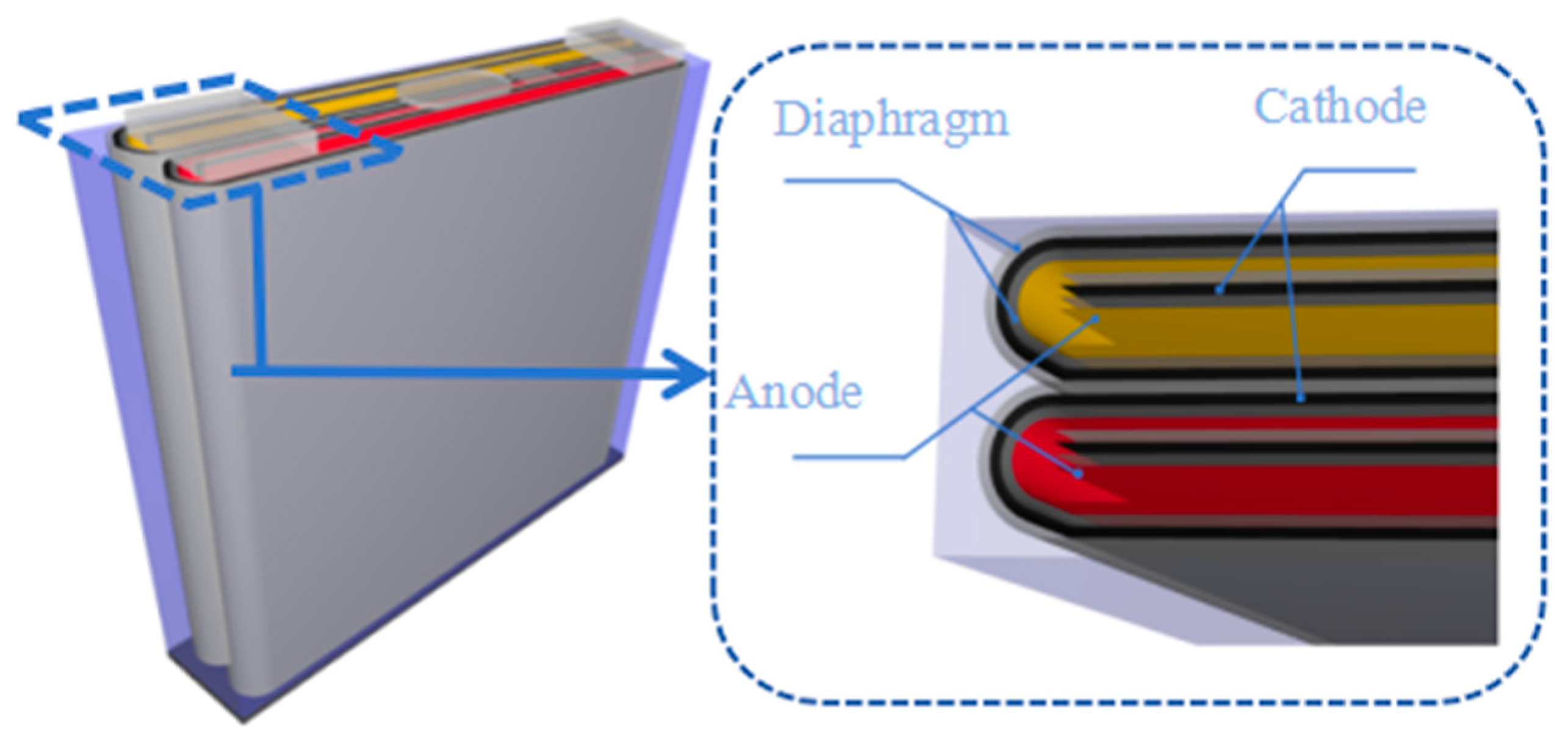

| Type | Structural Characteristics | Typical Applications |
|---|---|---|
| Single-layer jelly-roll | Axially spirally wound single continuous electrode separator tape with non-interconnected layers (parallel/series-free) | Cylindrical Cell |
| Dual-parallel jelly-roll architecture | Dual independent electrode tape with parallel winding and bipolar tab symmetric distribution enabling current path parallelization | High-Power Prismatic Battery (Power Battery) |
| Multi-tab segmented winding | Multi-tab segmented welding on monolithic electrode tape with distributed current collection for internal resistance minimization | Fast-Charging Pouch Cell |
| Shaped winding topology | Asymmetric winding path (e.g., elliptical/polygonal) for optimized electrode active material distribution | Customized Energy Storage Battery for Aerospace Applications |
| Parameter | Value |
|---|---|
| Cathode material | LiFePO4 (LFP) |
| Anode material | C |
| Rated capacity | 50.0 Ah |
| Operating voltage | 3.2 V~3.6 V |
| Operating temperature | −20 °C~65 °C |
| Battery size | 148 mm × 127 mm × 27 mm |
| Mass | 1035 ± 100 g |
| Serial Number | Battery Status | Heating Position |
|---|---|---|
| 1 | Constant-current charging | Front surface |
| 2 | Constant-voltage charging | |
| 3 | Discharged state | |
| 4 | Open state |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, D.; Li, X.; Sun, T.; Li, Q. Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions. Batteries 2025, 11, 196. https://doi.org/10.3390/batteries11050196
Li J, Li D, Li X, Sun T, Li Q. Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions. Batteries. 2025; 11(5):196. https://doi.org/10.3390/batteries11050196
Chicago/Turabian StyleLi, Jinmei, Dong Li, Xin Li, Ting Sun, and Qiang Li. 2025. "Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions" Batteries 11, no. 5: 196. https://doi.org/10.3390/batteries11050196
APA StyleLi, J., Li, D., Li, X., Sun, T., & Li, Q. (2025). Investigation of Thermal Runaway in Prismatic Batteries with Dual-Parallel Jelly-Roll Architecture Under Thermal Abuse Conditions. Batteries, 11(5), 196. https://doi.org/10.3390/batteries11050196





