Numerical Study of the Effects of Heat Loss and Solid Thermal Conductivity on Syngas Production for Fuel Cells
Abstract
:1. Introduction
2. Mathematical Model
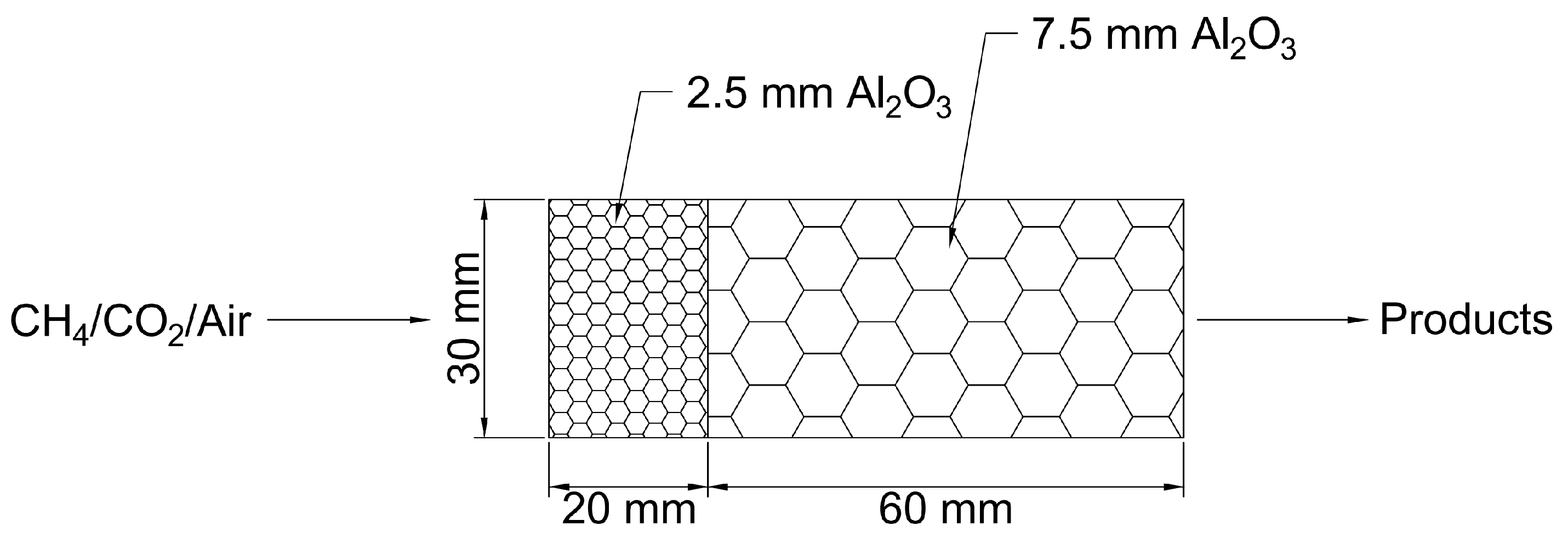
2.1. Model Description
2.2. Governing Equation
- Gas radiation is ignored;
- Porous media are isotropic and have an inert optical thickness;
- The gas in porous media is an ideal gas and the flow in porous media is laminar;
- A heat loss coefficient is used to account for the heat loss to the surroundings.
2.3. Boundary Conditions
2.4. Parameter Definition
2.5. Chemical Mechanism
2.6. Meshing and Solution Strategy
3. Results and Analysis
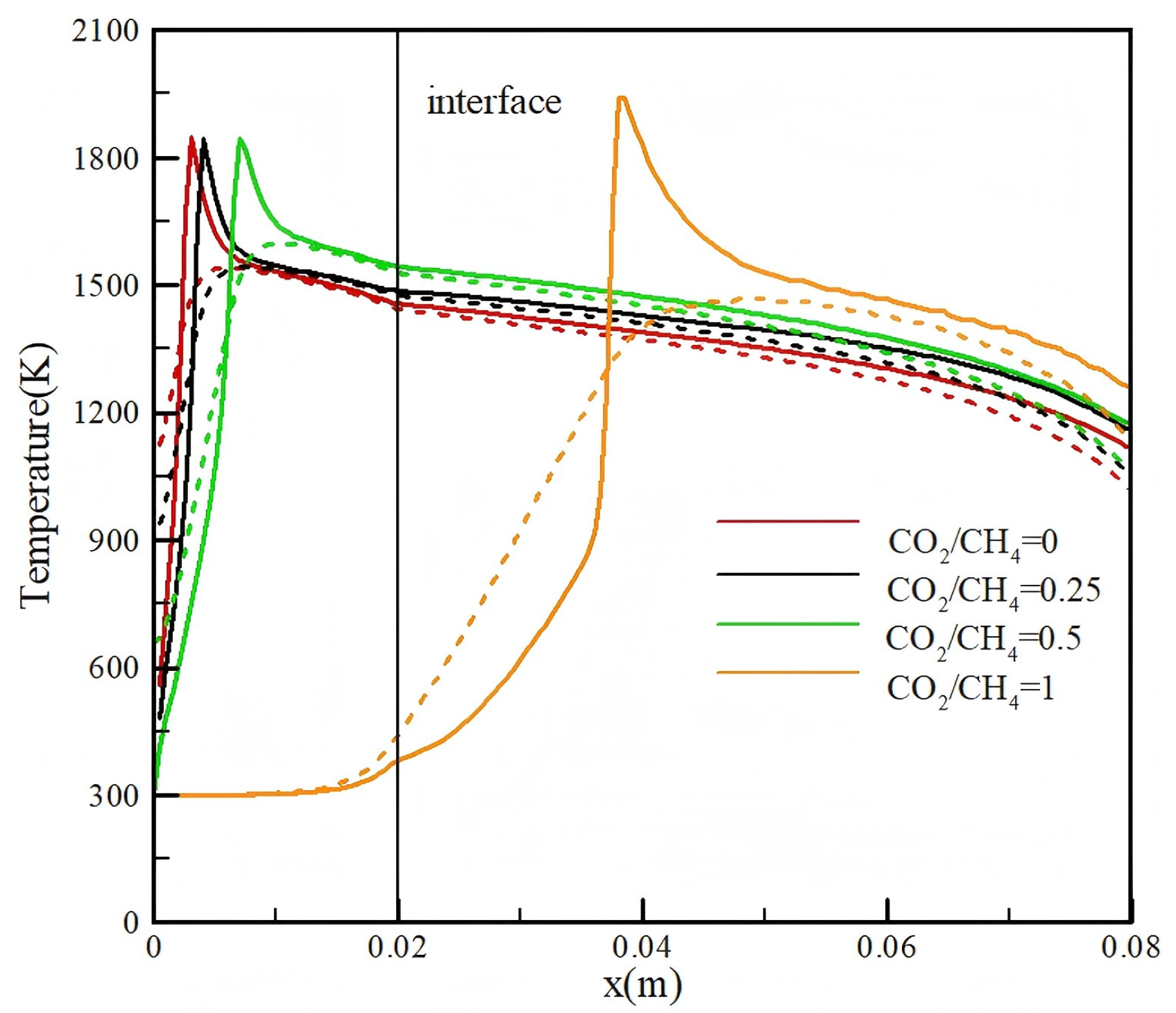
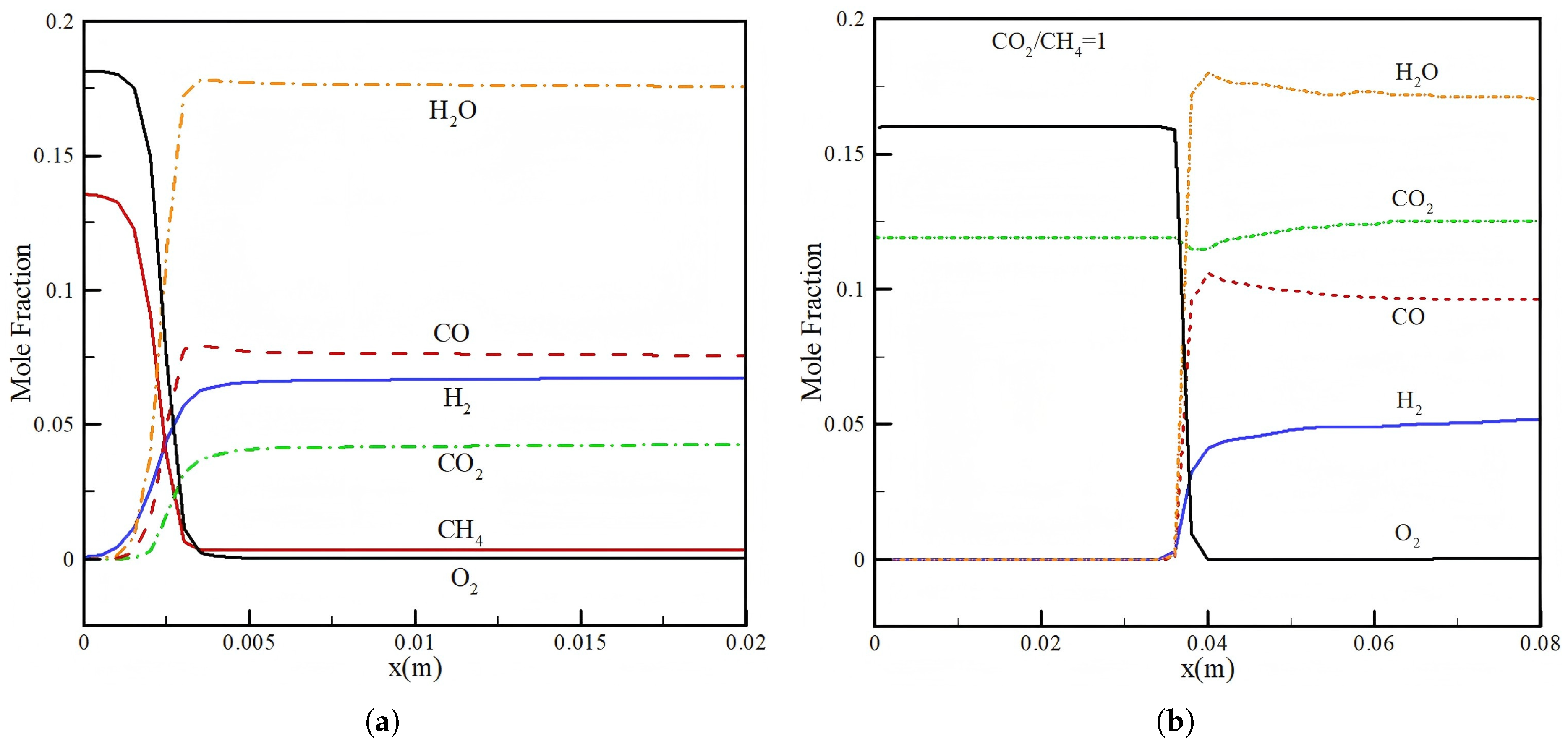
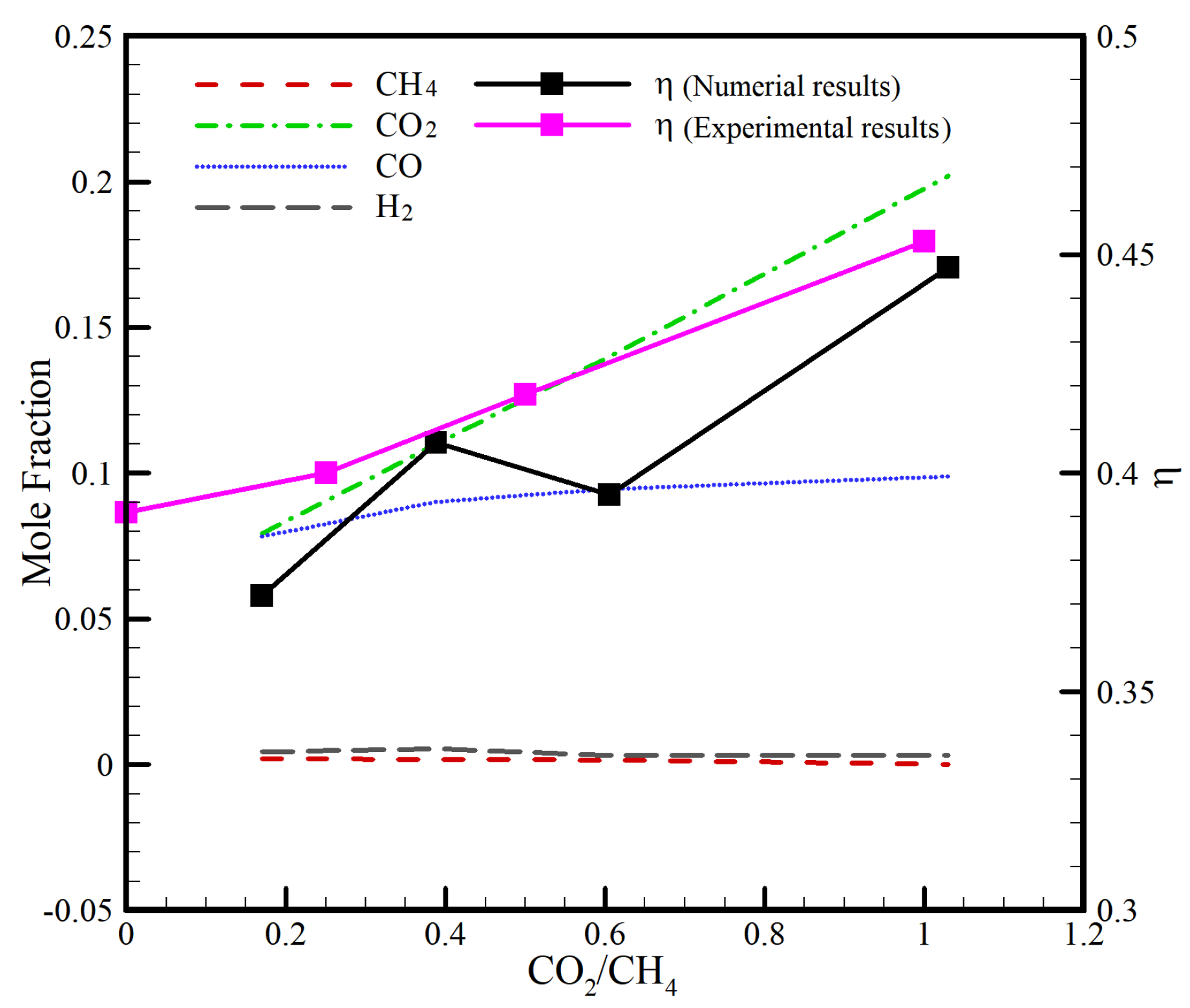
3.1. Effect of CO2 Injection
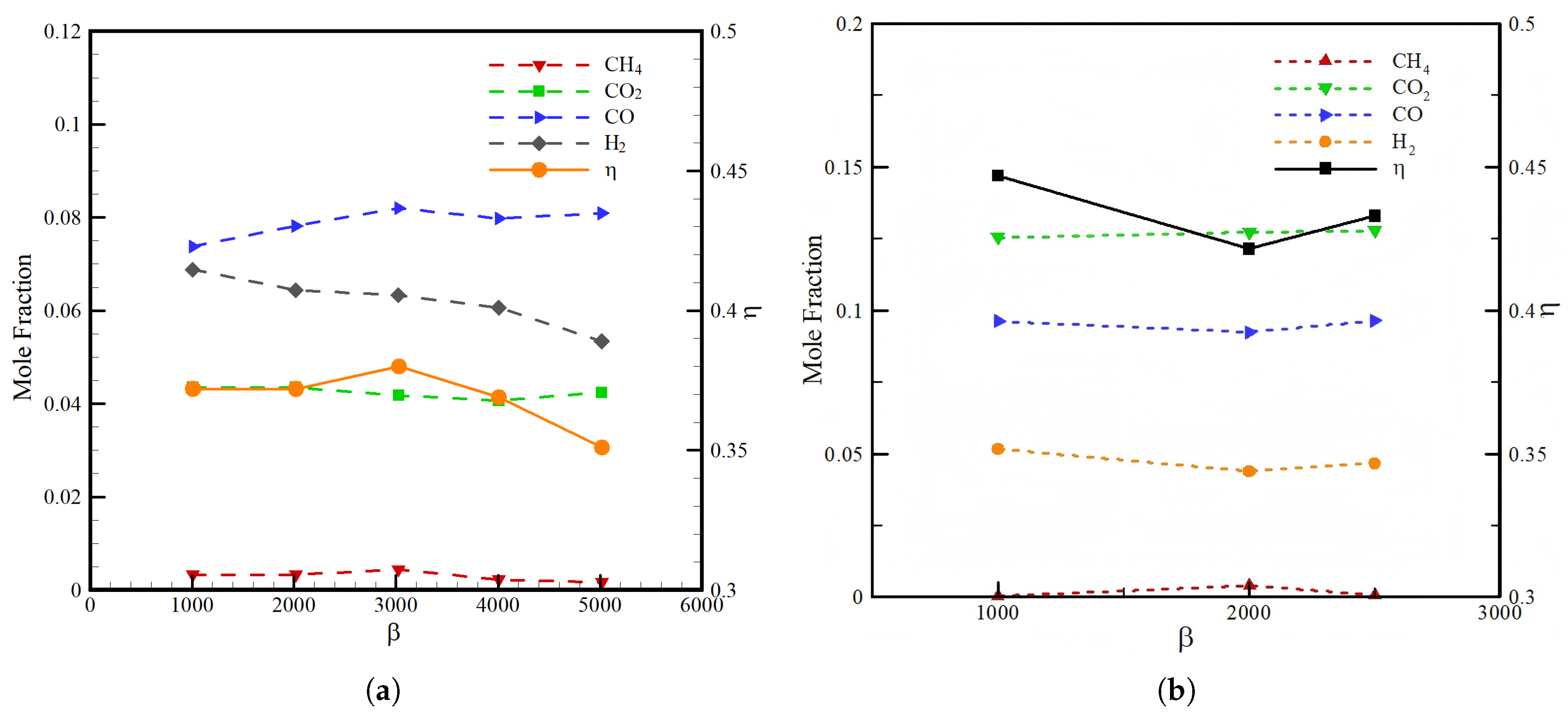
3.2. Effect of Heat Loss
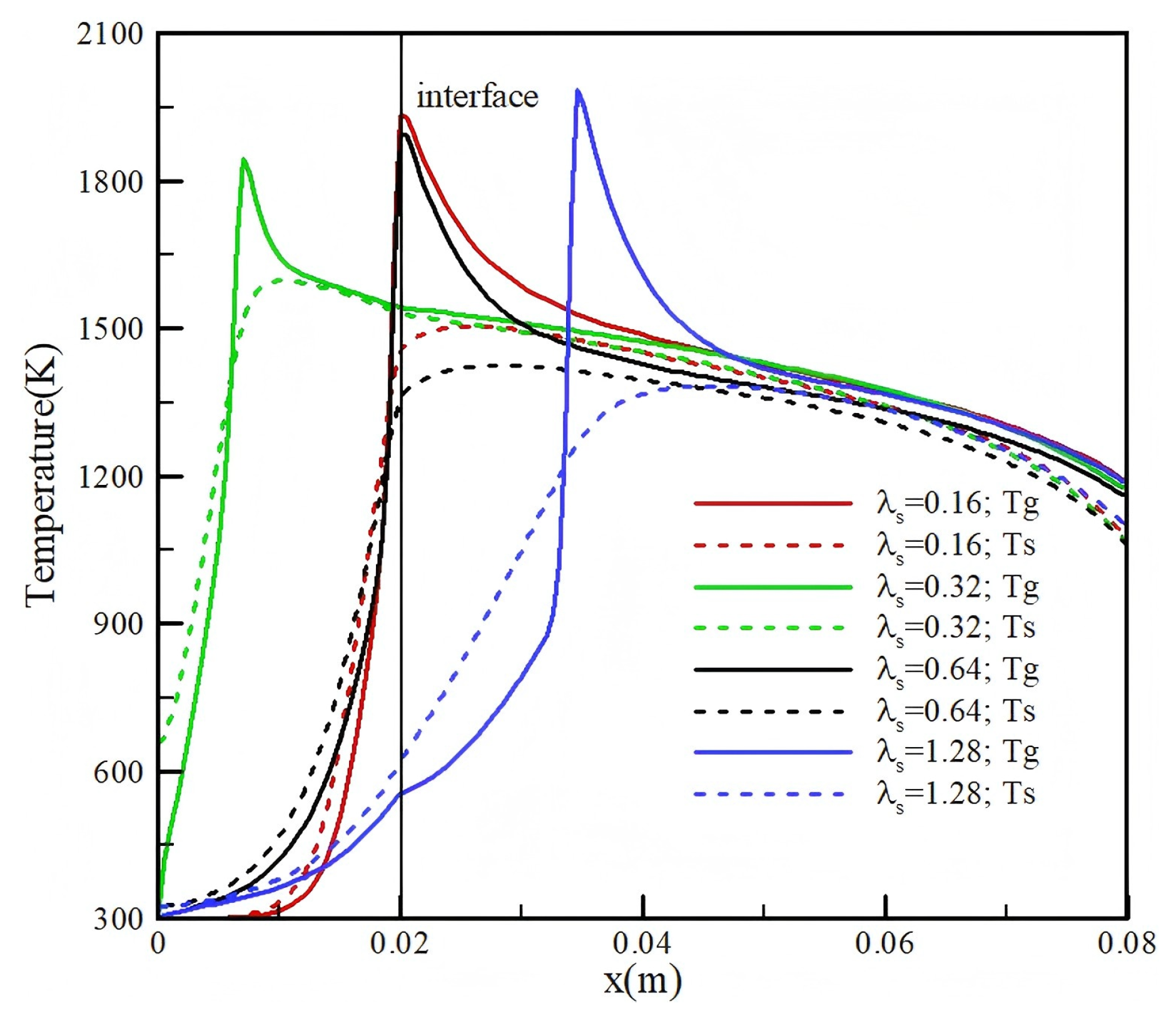
3.3. Effect of Thermal Conductivity
4. Conclusions
- Injection of CO2 into methane/air mixture can improve the conversion efficiency. As the CO2/CH4 ratio increases from 0 to 1, the conversion efficiency of the methane/air mixture improves from 37.1% to 44.7%;
- The conversion efficiency decreases from 0.15 to 0.12 when the heat loss coefficient is increased from 1000 to 2000 ;
- The thermal conductivity of porous media primarily affects the temperature of the solid and the conversion efficiency. When the thermal conductivity is increased from 0.32 to 0.64 for a molar ratio of CO2/CH4 = 0.5, the conversion efficiency increases from 39.5% to 42.9%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Definition |
| specific heat capacity, J·kg−1·K−1 | |
| d | the diameter of the ball, mm |
| the molar enthalpy of component i, J·mol−1 | |
| the volume convective heat transfer coefficient | |
| M | mole fraction |
| Nusselt number | |
| P | pressure, Pa |
| Prandtl number | |
| Reynolds number | |
| T | temperature, K |
| u | gas velocity, m·s−1 |
| molecular weight of the k component | |
| mass fraction of component k | |
| the reaction rate of the k component | |
| heat loss coefficient, W·m−3·K−1 | |
| porosity | |
| conversion efficiency | |
| effective thermal conductivity, W·m−1·K−1 | |
| radiative thermal conductivity, W·m−1·K−1 | |
| thermal conductivity for solid, W·m−1·K−1 | |
| density, kg·m−3 | |
| Stefan–Boltzmann constant, W·m−2·K−4 | |
| surface emissivity of porous media |
References
- Al-Hamamre, Z.; Al-Zoubi, A. The use of inert porous media based reactors for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1971–1986. [Google Scholar] [CrossRef]
- Toledo, M.G.; Utria, K.S.; González, F.A.; Zuñiga, J.P.; Saveliev, A.V. Hybrid filtration combustion of natural gas and coal. Int. J. Hydrogen Energy 2012, 37, 6942–6948. [Google Scholar] [CrossRef]
- Dhamrat, R.S.; Ellzey, J.L. Numerical and experimental study of the conversion of methane to hydrogen in a porous media reactor. Combust. Flame 2006, 144, 698–709. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, H.; Shi, Y.; Cao, T.; Cai, N.; Ye, X.; Wang, S. Power and heat co-generation by micro-tubular flame fuel cell on a porous media burner. Energy 2016, 109, 117–123. [Google Scholar] [CrossRef]
- Smith, C.H.; Leahey, D.M.; Miller, L.E.; Ellzey, J.L. Conversion of wet ethanol to syngas via filtration combustion: An experimental and computational investigation. Proc. Combust. Inst. 2011, 33, 3317–3324. [Google Scholar] [CrossRef]
- Dorofeenko, S.O.; Polianczyk, E.V. Conversion of hydrocarbon gases to synthesis gas in a reversed-flow filtration combustion reactor. Chem. Eng. J. 2016, 292, 183–189. [Google Scholar] [CrossRef]
- Fan, L.; Li, C.; Aravind, P.V.; Cai, W.; Han, M.; Brandon, N. Methane reforming in solid oxide fuel cells: Challenges and strategies. J. Power Sources 2022, 538, 231573. [Google Scholar] [CrossRef]
- Fukuhara, C.; Yamamoto, K.; Makiyama, Y.; Kawasaki, W.; Watanabe, R. A metal-honeycomb-type structured catalyst for steam reforming of methane: Effect of preparation condition change on reforming performance. Appl. Catal. A 2015, 492, 190–200. [Google Scholar] [CrossRef]
- Bingue, J.P.; Saveliev, A.V.; Fridman, A.A.; Kennedy, L.A. Hydrogen production in ultra-rich filtration combustion of methane and hydrogen sulfide. Int. J. Hydrogen Energy 2002, 27, 643–649. [Google Scholar] [CrossRef]
- Toledo, M.; Gracia, F.; Caro, S.; Gómez, J.; Jovicic, V. Hydrocarbons conversion to syngas in inert porous media combustion. Int. J. Hydrogen Energy 2016, 41, 5857–5864. [Google Scholar] [CrossRef]
- Toledo, M.; Bubnovich, V.; Saveliev, A.; Kennedy, L. Hydrogen production in ultrarich combustion of hydrocarbon fuels in porous media. Int. J. Hydrogen Energy 2009, 34, 1818–1827. [Google Scholar] [CrossRef]
- Araya, R.; Araus, K.; Utria, K.; Toledo, M. Optimization of hydrogen production by filtration combustion of natural gas by water addition. Int. J. Hydrogen Energy 2014, 39, 7338–7345. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Wang, Y.Q.; Shi, Y.X.; Cai, N.S. Experimental study for partial oxidation reforming of CH4/CO2 with rich combustion in a porous media burner. J. Eng. Thermophys. 2017, 38, 2269–2275. [Google Scholar]
- Zeng, H.; Wang, Y.; Shi, Y.; Ni, M.; Cai, N. Syngas production from CO2/CH4 rich combustion in a porous media burner: Experimental characterization and elementary reaction model. Fuel 2017, 199, 413–419. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Suo, S.; Xie, M.; Bai, M. Simulation of propane-air premixed combustion process in randomly packed beds. Appl. Therm. Eng. 2018, 141, 153–163. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, H.; Wu, D.; Wang, J.; Xie, M.Z.; Bai, M. Pore-scale simulation of hydrogen–air premixed combustion process in randomly packed beds. Energy Fuels 2017, 31, 12791–12803. [Google Scholar] [CrossRef]
- Shi, J.; Mao, M.; Li, H.; Liu, Y.; Liu, Y.; Deng, Y. Influence of chemical kinetics on predictions of performance of syngas production from fuel-rich combustion of CO2/CH4 mixture in a two-layer burner. Front. Chem. 2020, 7, 902. [Google Scholar] [CrossRef]
- Shi, J.; Mao, M.; Li, H.; Liu, Y.; Sun, Y. Pore-level study of syngas production from fuel-rich partial oxidation in a simplified two-Layer burner. Front. Chem. 2019, 7, 793. [Google Scholar] [CrossRef]
- Shi, J.; Mao, M.; Li, H.; Liu, Y.; Lv, J. A pore level study of syngas production in two-layer burner formed by staggered arrangement of particles. Int. J. Hydrogen Energy 2020, 45, 2331–2340. [Google Scholar] [CrossRef]
- Zheng, C.; Cheng, L.; Cen, K.; Bingue, J.P.; Saveliev, A. Partial oxidation of methane in a reciprocal flow porous burner with an external heat source. Int. J. Hydrogen Energy 2012, 37, 4119–4126. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; AlOtaibi, B.; Almutairi, G.; Minakshi, M.; Cheng, C.K.; Vo, D.-V.N. Degradation behaviors of solid oxide fuel cell stacks in steady-state and cycling conditions. Energy Fuels 2020, 34, 14864–14873. [Google Scholar] [CrossRef]
- Bowman, C.T.; Hanson, R.K.; Davidson, D.F.; Gardiner, W.C.; Lissianski, J.V.; Smith, G.P.; Golden, D.M.; Frenklach, M.; Goldenberg, M. GRI-Mech 3.0 Chemical Kinetic Mechanism. Combustion Research Group at Berkeley. 2009. Available online: http://combustion.berkeley.edu/gri-mech/new21/version12/text12.html (accessed on 6 September 2023).
- Kee, R.J.; Dixon, G.L.; Warnatz, J.; Coltrin, M.E.; Miller, J.A. A Fortran Computer Code Package for the Evaluation of Gas-Phase Multicomponent Transport Properties; Report SAND86-8246; Sandia National Laboratories: Albuquerque, NM, USA, 1986. [Google Scholar]
- Barra, A.J.; Diepvens, G.; Ellzey, J.L.; Henneke, M.R. Numerical study of the effects of material properties on flame stabilization in a porous burner. Combust. Flame 2003, 134, 369–379. [Google Scholar] [CrossRef]
- Contarin, F.; Saveliev, A.; Fridman, A.; Kennedy, L.A. Reciprocal flow filtration combustor with embedded heat exchangers: Numerical study. Int. J. Heat Mass Transf. 2003, 46, 949–961. [Google Scholar] [CrossRef]

| (W/m · K) | (%) | ||||
|---|---|---|---|---|---|
| 0.16 | 0.0867 | 0.0584 | 0.0894 | 0.0031 | 41.7 |
| 0.32 | 0.0838 | 0.0515 | 0.0885 | 0.0024 | 39.5 |
| 0.64 | 0.0894 | 0.0634 | 0.0870 | 0.0019 | 42.9 |
| 1.28 | 0.0857 | 0.0612 | 0.0897 | 0.0031 | 42.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, M.; Li, Z.; Wang, Z.; Zhang, X.; Shi, J.; Kong, X.; Lv, J. Numerical Study of the Effects of Heat Loss and Solid Thermal Conductivity on Syngas Production for Fuel Cells. Batteries 2025, 11, 187. https://doi.org/10.3390/batteries11050187
Wang X, Yu M, Li Z, Wang Z, Zhang X, Shi J, Kong X, Lv J. Numerical Study of the Effects of Heat Loss and Solid Thermal Conductivity on Syngas Production for Fuel Cells. Batteries. 2025; 11(5):187. https://doi.org/10.3390/batteries11050187
Chicago/Turabian StyleWang, Xiaolong, Mengmeng Yu, Zunmin Li, Zhen Wang, Xiuxia Zhang, Junrui Shi, Xiangjin Kong, and Jinsheng Lv. 2025. "Numerical Study of the Effects of Heat Loss and Solid Thermal Conductivity on Syngas Production for Fuel Cells" Batteries 11, no. 5: 187. https://doi.org/10.3390/batteries11050187
APA StyleWang, X., Yu, M., Li, Z., Wang, Z., Zhang, X., Shi, J., Kong, X., & Lv, J. (2025). Numerical Study of the Effects of Heat Loss and Solid Thermal Conductivity on Syngas Production for Fuel Cells. Batteries, 11(5), 187. https://doi.org/10.3390/batteries11050187







