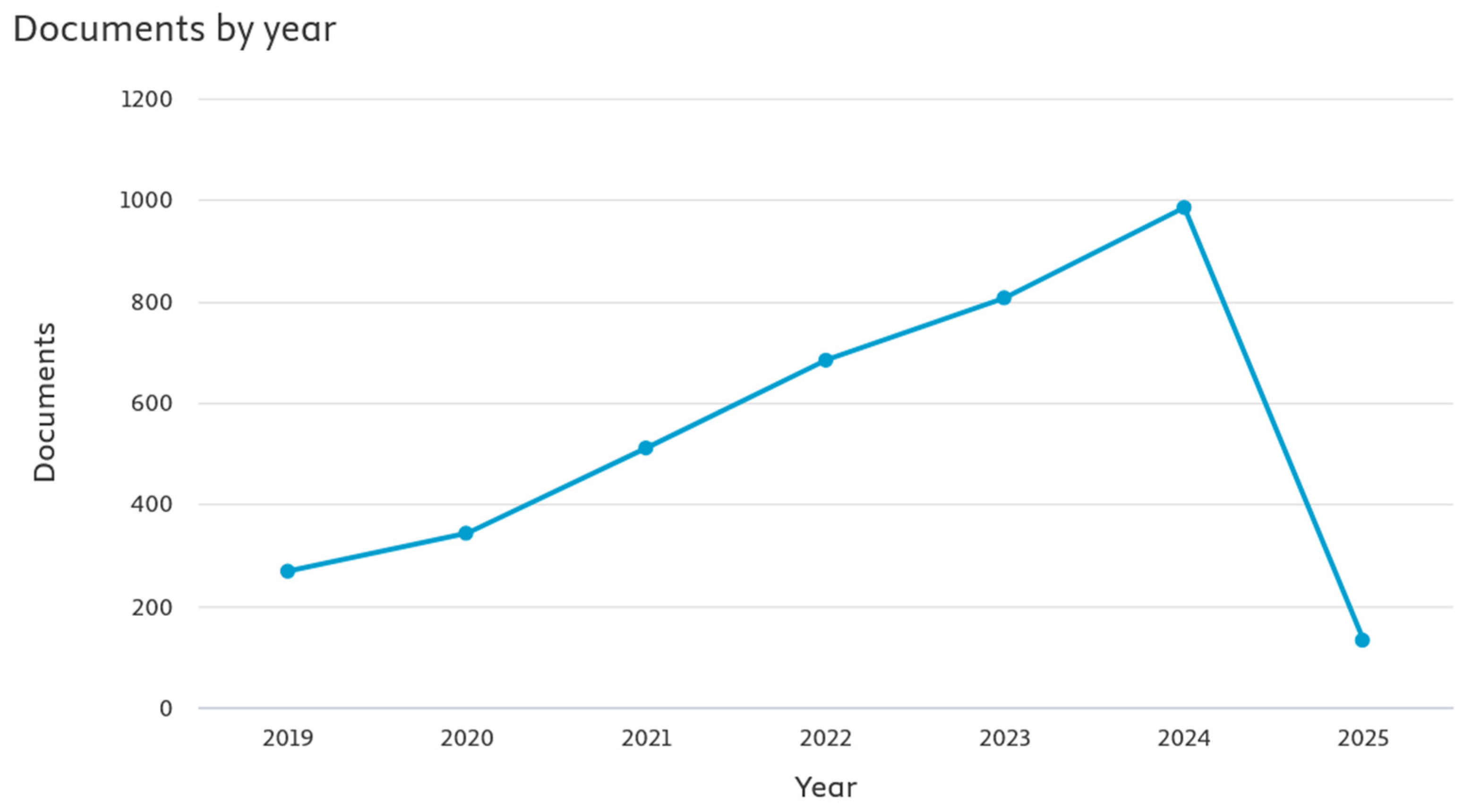
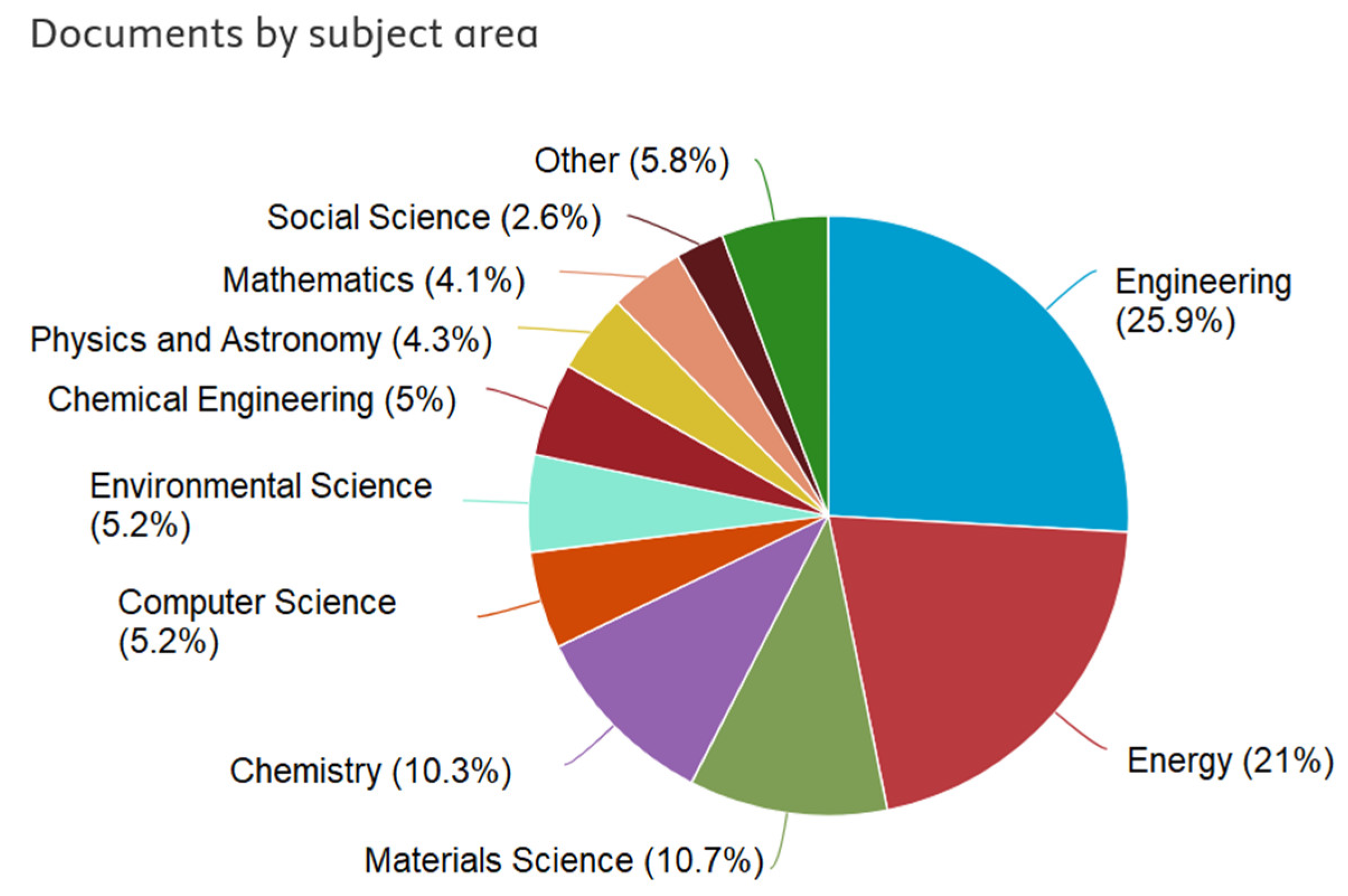
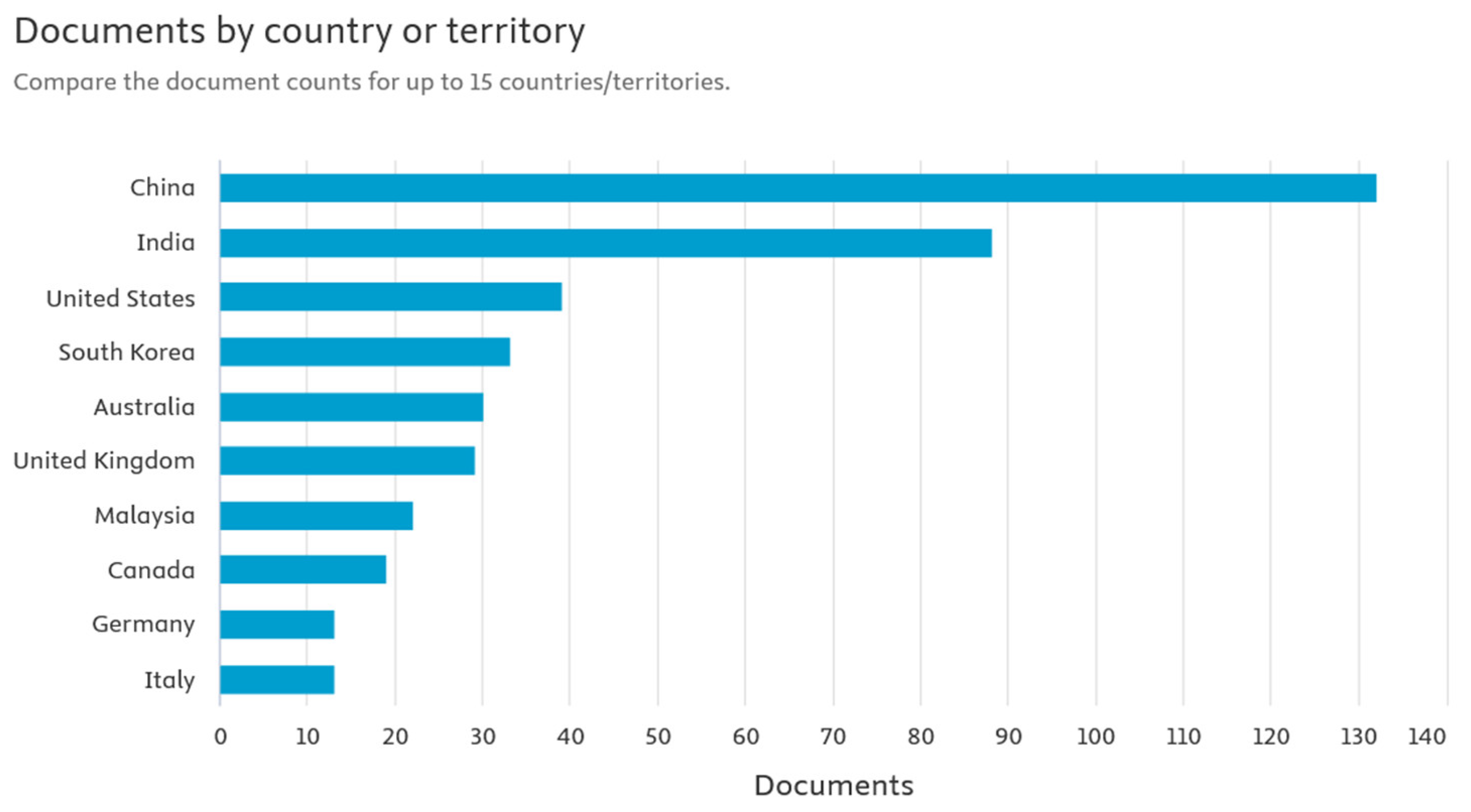
Powering the Future Smart Mobility: A European Perspective on Battery Storage
Abstract
1. Introduction
2. The European Legislative Framework
- RePowerEU: This initiative seeks to make Europe energy independent from fossil fuels by 2027, prioritizing clean technologies like battery energy storage.
- Fit for 55 Package: This package aims to reduce greenhouse gas emissions by 55% by 2030 and accelerate the electrification of various sectors, increasing battery demand.
- Net-Zero Industrial Act (NZIA): This act aims to increase clean-tech industrial capacity, including battery manufacturing.
- Critical Raw Materials Act (CRMA): This act enhances the collection and recycling of waste products to secure the supply of critical raw materials for batteries.
- EU Battery Regulation: This regulation promotes the circular economy, resource efficiency, and sustainability of batteries throughout their lifecycle. Key points in the Battery Regulation are the following:
- ∘
- Mandatory sustainability and safety requirements for the placing of batteries on the European market, including restrictions on certain substances, carbon footprint requirements, performance and durability requirements, etc.;
- ∘
- Recycled content requirements;
- ∘
- Traceability through labelling, marking, and information requirements, notably with the creation of the digital battery passport;
- ∘
- Mandatory implementation of due diligence policies;
- ∘
- Extended producer responsibility;
- ∘
- Targets for the collection of waste batteries, and provisions regarding the treatment, reuse, and recycling of batteries, notably materials recovery targets;
- ∘
- Green public procurement.
3. State-of-the-Art of EV Battery Technologies
3.1. Lead–Acid Batteries
3.2. Nickel-Based Technologies
3.3. Lithium Batteries
3.4. Sodium-Based Technologies
3.5. EV Batteries Requirements
| Generation | TRL | Anode | Cathode | Electrolyte |
|---|---|---|---|---|
| 1 | 9 | Carbon/Graphite | LFP, NCA, LCO | Organic liquid |
| 2a | 9 | Carbon/Graphite | NMC111 | Organic liquid |
| 2b | 9 | Carbon/Graphite | NMC532, NMC622 | Organic liquid |
| 3a | 9 | C/Si (5–10% Si) | NMC622, NMC811 | Organic liquid |
| 3b | 5–9 | Si/C (>10% Si) | HE-NMC | Organic liquid |
| 3b | 4 | Si/C (>10% Si) | HV-LNMO | Organic liquid |
| 3a | 5–6 1 | Si/C (>10%) | LCO, NMC, LMO, NCA | Solid state |
| 4b | 5–6 2 | Li metal | LCO, NMC, LMO, NCA | Solid state |
| 4 | 4 | Li metal | Li2-S | Solid state |
| 5 | 4 | Li metal | O2 | Different possibilities |
| - | 9 | Hard-carbon | PBA 3 | Organic liquid |
| - | 4 | Na-metal, Tin alloys | layered oxides, polyanion compounds | Different possibilities |
3.6. Life Duration
- Loss of Lithium Inventory (LLI): This mode includes mechanisms that reduce the amount of cyclable lithium available for transport between the electrodes.
- Loss of Active Material (LAM): This encompasses mechanisms that lead to a decrease in the material available for electrochemical activity. LAM is often further divided into losses at the anode and losses at the cathode.
- Conductivity Loss (CL): Also known as impedance change, this mode groups the mechanisms that affect the kinetics of the cell.
| Type of Electrode | Degradation Mechanisms | Mitigation Strategies | Source |
|---|---|---|---|
| Ni-rich layered cathode | Microcracks, lithium–nickel hybridization and irreversible phase transitions, anisotropic lattice deformation, and surface degradation | Elemental doping, coating modification, electrolyte modification, construction of radial concentration gradients in polycrystalline secondary particles, fabrication of rod-shaped primary particles, single-crystal high-nickel cathodes. | [90,91,92,93] |
| Lithium-rich manganese oxide cathode | Irreversible oxygen loss, structural degradation of the material, particle fragmentation, and transition metal migration | Surface coating, ion doping, component regulation, single crystal structures. | [94,95] |
| Li-metal anode | Dendrite growth | Coating artificial protective films, surface morphology control, high electrolyte concentration, electrolyte additives. | [85] |
3.7. Fast Charge
3.7.1. Electrode
3.7.2. Electrolytes
3.7.3. Battery Engineering
3.8. Energy Density
3.8.1. Anode
| Composition | Gravimetric Capacity (mAh/g) | V vs. Li/Li+ | Volume Change | Reference |
|---|---|---|---|---|
| C (graphite) | 372 | 0.3 V | 10% | [194,228] |
| Li4Ti5O12 | 175 | 0.87 V | 1% | [194,199] |
| Li22Si5 (Li4.4Si) | 4200 | 0.1 V | 310% | [210,211] |
| Li15Si4 | 3570 | 50–60 mV | 280% | [179] |
| Porous carbon–iron oxide (PC–Fe3O4) | 926 | 0.8V | 200% | [222,223,224,229] |
3.8.2. Cathode
3.9. Safety
3.10. Current Trends and Future Developments in EV Batteries Research
3.10.1. Solid State LIBs
- (1)
- Room temperature conductivity ≥10−4 S cm−1;
- (2)
- Electronic insulation <10−10 S cm−1 (Li+ migration number is approximately 1);
- (3)
- Wide electrochemical window (>5.5 V vs. Li/Li+);
- (4)
- Good compatibility with the selected electrode material;
- (5)
- Good thermal stability and mechanical properties, wet environment resistance;
- (6)
- Low cost and low environmental impact raw materials;
- (7)
- A simple synthesis method.
3.10.2. Sodium-Ion Batteries
| Cathode | Anode | Specific Energy [Wh/kg] | Specific Capacity [mAh/g] | Source |
|---|---|---|---|---|
| Na0.612K0.056MnO | Presodiated HC | 314.4 | 230.6 | [327] |
| NaNi0.5Mn0.5-γSnγO2 | HC | 335–270 with γ = 0–0.5 | - | [328] |
| NMO-HTS | HC | 248 | 180 | [329] |
| Na3V1.8(CrMnFenAl)0.2(PO4)3 | HC | 202 | - | [344] |
| NaxMnFe(CN)6 | TiO2 | 111 | 120 | [334] |
| PBA | HC | 100–130 | - | [335] |
| Na[Cu1/9Ni2/9Fe1/3Mn1/3]O2 | Anode Free | 200 | - | [325] |
3.10.3. Potassium-Ion Batteries
3.10.4. Magnesium-Ion Batteries
3.10.5. Lithium–Sulfur Batteries
3.10.6. Zinc-Based Batteries
4. The Sustainability Issue
- Minimizing the carbon footprint across the entire value and production chain;
- Ensuring ethical and responsible raw material acquisition;
- Fostering a circular design that incorporates recycled content and enables reuse, repurposing, remanufacturing, and final recycling;
- Providing transparent communication and tracking of performance and material/chemical contents for end users and supply chain stakeholders.
4.1. Life Cycle Emissions
4.2. Recycling
- Access to detailed battery composition and chemistry information eliminates costly sampling procedures, allowing for more efficient and lower-cost sorting while minimizing contamination risks. Sampling costs could potentially decrease by 50% to 80%.
- A detailed dismantling manual can reduce disassembly time and costs of battery packs by 20–40%.
- Additionally, dismantling manuals can facilitate the automation of parts of the dismantling process, particularly for heavy and hazardous operations, resulting in a further 20–30% reduction in dismantling costs.
- Optimizing the recycling treatment process and potentially reducing material and processing costs by 10–20% could be achieved through a homogenous battery recycling feedstock. This consistent input, pre-processed to eliminate unwanted materials, would streamline the feed-in process (batch sequencing) and allow for finer control over process parameters.
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 3D-NTC | Three-dimensional nitrogen-doped turbostratic carbon |
| AFNB | Anode free sodium batteries |
| APC | All phenyl complex |
| BEV | Battery electric vehicle |
| BMS | Battery management system |
| BOL | Begin of life |
| BTMS | Battery thermal management system |
| CA | Cyanoacrylate |
| CS | Carbon sphere |
| CSE | Composite solid electrolytes |
| EOL | End of life |
| EUCAR | European Council for Automotive R&D |
| EV | Electric vehicle |
| FEC | Fluoroethylene carbonate |
| HC | Hard carbon |
| HCF | Hexacyanoferrates |
| HE-NMC | High-energy lithium nickel manganese cobalt oxide |
| HLM | High lithium manganese oxide |
| HTS | High temperature thermal shock |
| HV-LNMO | High-voltage lithium nickel manganese oxide |
| ISE | Inorganic solid electrolyte |
| KMgHCF | Potassium magnesium hexacyanoferrates |
| K2TP | Dipotassium terephthalate |
| LCO | Lithium cobalt oxides |
| Li+ | Lithium ion |
| LIB | Lithium-ion battery |
| LiS | Lithium–sulfur |
| LMO | Lithium manganese oxide |
| LFP | Lithium iron phosphate |
| LLZO | Li7La3Zr2O12 |
| LTO | Lithium titanate oxide |
| MACC | Magnesium Aluminum Chloride Complex |
| MIB | Magnesium ion battery |
| MF | Muffle furnace-sintered |
| MOF | Metal–organic framework |
| MVOH | Mg0.75V10O24·4H2O |
| NaNiCl | Sodium–nickel chloride |
| NaS | Sodium–sulfur |
| NASICON | Sodium superionic conductors |
| NaxMnFe(CN)6 | Sodium manganese hexacyanoferrate |
| NCA | Lithium nickel cobalt aluminum oxide |
| Ni-Cd | Nickel–cadmium |
| Ni–Fe | Nickel–iron |
| Ni-H2 | Nickel–hydrogen |
| Ni–MH | Nickel–metal hydride |
| Ni–Zn | Nickel–zinc |
| NMB | Sodium metal batteries |
| NMC | Lithium nickel manganese cobalt oxide |
| NMO | Sodium manganese oxide |
| NVP | Na3V2(PO4)3 |
| OEM | Original equipment manufacturer |
| OLE | Organic liquid electrolyte |
| PAN | Polyacrylonitrile |
| PBA | Prussian blue analog |
| PEGMEA | Poly-ethylene glycol methyl ether acrylate |
| PEO | Poly-ethylene oxide |
| PHEV | Plug-in hybrid electric vehicle |
| PIB | Potassium ion battery |
| PTCDA | Perylenetetracarboxylic dianhydride |
| PMMA | Poly-methyl methacrylate |
| PVDF | Poly-vinylidene fluoride |
| PVDF-HFP | Poly-vinylidene fluoride-hexafluoropropylene |
| rGO | Reduced graphene oxide |
| SC | Single crystal |
| SE | Solid electrolyte |
| SEI | Solid electrolyte interphase |
| SN | Succinonitrile |
| SHE | Standard hydrogen electrode |
| SIB | Sodium-ion battery |
| SOC | State of charge |
| SOH | State of health |
| SPE | solid polymer electrolyte |
| SSB | Solid state battery |
| SSE | Solid state electrolyte |
| TiO2 | Titanium dioxide |
| tLi+ | Lithium transference number |
| TM | Transition metal |
| TMC | Transition metal chalcogenides |
| TMO | Transition metal oxide |
| TRL | Technological readiness level |
| USABC | United States Advanced Battery Consortium |
| ZB | Zinc-based battery |
Appendix A
Appendix A.1. Review Methods
| Additional Criteria | Safety | Life OR Lifespan OR Cyclelife | Cost OR Market | Energy OR Power | Sustainability |
|---|---|---|---|---|---|
| No. of articles from 2019 | 883 | 984 | 1331 | 3039 | 256 |
| No. of articles from 2023 | 487 | 536 | 672 | 1550 | 178 |
Appendix A.2. Search Results

References
- European Environment Agency. Transport and Environment Report 2022: Digitalisation in the Mobility System: Challenges and Opportunities; Publications Office: Luxembourg, 2022.
- Bielewski, M.A.P.; Bobba, S.; Kronberga, A.; Georgakaki, A.; Letout, S.; Kuokkanen, A.; Mountraki, A.; Ince, E.; Shtjefni, D.; Joanny, G.; et al. Clean Energy Technology Observatory: Batteries for Energy Storage in the European Union—2022 Status Report on Technology Development, Trends, Value Chains and Markets; Joint Research Centre: Luxemburg, 2022. [Google Scholar]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- International Energy Agency World Energy Outlook Special Report Batteries and Secure Energy Transitions; International Energy Agency (IEA): Paris, France, 2024.
- Bielewski, M.; Pfrang, A.; Quintero Pulido, D.; Bobba, S.; Schade, B.; Georgakaki, A.; Letout, S.; Mountraki, A.; Ince, E. Clean Energy Technology Observatory Battery Technology-KJ0124064ENN; Joint Research Centre: Luxemburg, 2024. [Google Scholar]
- Fleischmann, J.; Hanicke, M.; Horetsky, E.; Ibrahim, D.; Jautelat, S.; Linder, M.; Schaufuss, P.; Torscht, L.; van de Rijt, A. Battery 2030: Resilient, Sustainable, and Circular; McKinsey & Company: New York, NY, USA, 2023. [Google Scholar]
- IEA. EV Battery Supply Chain Sustainability; International Energy Agency (IEA): Paris, France, 2024.
- Bloomberg NEF. Lithium-Ion Battery Pack Prices See Largest Drop Since 2017, Falling to $115 per Kilowatt-Hour: Bloomberg NEF. Available online: https://about.bnef.com/blog/lithium-ion-battery-pack-prices-see-largest-drop-since-2017-falling-to-115-per-kilowatt-hour-bloombergnef/ (accessed on 3 March 2025).
- Orangi, S.; Manjong, N.; Clos, D.P.; Usai, L.; Burheim, O.S.; Strømman, A.H. Historical and Prospective Lithium-Ion Battery Cost Trajectories from a Bottom-up Production Modeling Perspective. J. Energy Storage 2024, 76, 109800. [Google Scholar] [CrossRef]
- Houache, M.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Paul Lienert For EV Batteries, Lithium Iron Phosphate Narrows the Gap with Nickel, Cobalt. Available online: https://www.reuters.com/business/autos-transportation/ev-batteries-lithium-iron-phosphate-narrows-gap-with-nickel-cobalt-2023-06-22/ (accessed on 5 February 2025).
- Timo Möller, C.C.; Azevedo, M.; Campagnol, N.; Kinzel, Y. The Battery Chemistries Powering the Future of Electric Vehicles; McKinsey & Company: New York, NY, USA, 2024. [Google Scholar]
- Lu, Y.; Zhu, T. Status and Prospects of Lithium Iron Phosphate Manufacturing in the Lithium Battery Industry. MRS Commun. 2024, 14, 888–899. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, F.; Glossmann, T.; Burnett, C.; Liu, Z. From Laboratory Innovations to Materials Manufacturing for Lithium-Based Batteries. Nat. Energy 2023, 8, 329–339. [Google Scholar] [CrossRef]
- Ghasemi Yeklangi, A.; Ghafari, A.; Asgari Sima, F.; Akbari, S. Advancing Lithium-Ion Battery Manufacturing: Novel Technologies and Emerging Trends. J. Appl. Electrochem. 2024, 54, 2653–2682. [Google Scholar] [CrossRef]
- Fonseca, N.; Thummalapalli, S.V.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Patil, D.; Thippanna, V.; Ramanathan, A.; Xu, W.; Guo, S.; et al. 3D Printing-Enabled Design and Manufacturing Strategies for Batteries: A Review. Small 2023, 19, e2302718. [Google Scholar] [CrossRef]
- Maksimovna Vakhrusheva, D.; Xu, J. Model-Driven Manufacturing of High-Energy-Density Batteries: A Review. Batter. Supercaps 2024, 8, e202400539. [Google Scholar] [CrossRef]
- Gutwald, B.; Baumann, N.; Funk, F.; Reichenstein, T.; Albayrak, B.; Franke, J. Sustainable Manufacturing Practices: A Systematic Analysis and Guideline for Assessing the Industrial Product Carbon Footprint. In Proceedings of the 2024 1st International Conference on Production Technologies and Systems for E-Mobility, EPTS 2024, Bamberg, Germany, 5–6 June 2024. [Google Scholar]
- Wang, Y.; Kang, X.; Chen, Z. A Survey of Digital Twin Techniques in Smart Manufacturing and Management of Energy Applications. Green Energy Intell. Transp. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Ralls, A.M.; Leong, K.; Clayton, J.; Fuelling, P.; Mercer, C.; Navarro, V.; Menezes, P.L. The Role of Lithium-Ion Batteries in the Growing Trend of Electric Vehicles. Materials 2023, 16, 6063. [Google Scholar] [CrossRef]
- Knehr, K.; Kubal, J.; Ahmed, S. Estimated Cost of EV Batteries 2019–2024. Chemical Sciences and Engineering division of Argonne National Laboratory. 2024. Available online: https://www.anl.gov/cse/reference/estimated-cost-of-ev-batteries (accessed on 31 March 2025).
- Hertel, D.; Bräunig, G.; Thürer, M. Towards a Green Electromobility Transition: A Systematic Review of the State of the Art on Electric Vehicle Battery Systems Disassembly. J. Manuf. Syst. 2024, 74, 387–396. [Google Scholar] [CrossRef]
- Kaarlela, T.; Villagrossi, E.; Rastegarpanah, A.; San-Miguel-Tello, A.; Pitkäaho, T. Robotised Disassembly of Electric Vehicle Batteries: A Systematic Literature Review. J. Manuf. Syst. 2024, 74, 901–921. [Google Scholar] [CrossRef]
- Li, W.; Peng, Y.; Zhu, Y.; Pham, D.T.; Nee, A.Y.C.; Ong, S.K. End-of-Life Electric Vehicle Battery Disassembly Enabled by Intelligent and Human-Robot Collaboration Technologies: A Review. Robot. Comput. -Integr. Manuf. 2024, 89, 102758. [Google Scholar] [CrossRef]
- Neri, A.; Butturi, M.A.; Gamberini, R. Sustainable Management of Electric Vehicle Battery Remanufacturing: A Systematic Literature Review and Future Directions. J. Manuf. Syst. 2024, 77, 859–874. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Y.; Kong, L.; Yi, M.; Song, J.; Hao, D.; Zhou, X.; Zhang, Z.; Yan, J. Manufacturing Processes and Recycling Technology of Automotive Lithium-Ion Battery: A Review. J. Energy Storage 2023, 67, 107533. [Google Scholar] [CrossRef]
- Siddiqi, S. Sodium-Ion Batteries 2024–2034: Technology, Players, Markets, and Forecasts. IDTechEX. 2025. Available online: https://www.idtechex.com/en/research-report/sodium-ion-batteries/978 (accessed on 27 February 2025).
- Sanchez, J. BYD Breaks Ground on World’s Largest Sodium-Ion Battery Plant. Available online: https://www.drivencarguide.co.nz/news/byd-breaks-ground-on-worlds-largest-sodium-ion-battery-plant/ (accessed on 26 February 2025).
- Farasis The World’s First EV Powered by Farasis Energy’s Sodium-Ion Batteries Rolls off the Assembly Line. Available online: https://www.farasis-energy.com/en/the-worlds-first-ev-powered-by-farasis-energys-sodium-ion-batteries-rolls-off-the-assembly-line/ (accessed on 26 February 2025).
- Prnewswire Yadea Launches Sodium Battery Electric Two-Wheelers, Leading a Revolution in the Electric Mobility Industry. Available online: https://www.prnewswire.com/news-releases/yadea-launches-sodium-battery-electric-two-wheelers-leading-a-revolution-in-the-electric-mobility-industry-302345708.html#:~:text=8%2C%202025%20%2FPRNewswire%2F%20%2D%2D,unveiling%20its%20latest%20electric%20two%2D (accessed on 26 February 2025).
- Ragonnaud, G. Powering the EU’s Future: Strengthening the EU Battery Industry; European Parliamentary Research Service: Luxembourg, 2025. [Google Scholar]
- Battery2030+ Roadmap. Available online: https://battery2030.eu/research/roadmap/ (accessed on 21 February 2025).
- Shah, R.; Mittal, V.; Precilla, A.M. Challenges and Advancements in All-Solid-State Battery Technology for Electric Vehicles. J 2024, 7, 204–217. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2024; IEA: Paris, France, 2024.
- European Parliament. Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC; European Parliament: Strasbourg, France, 2023.
- GB 38031-2025 Electric Vehicles Traction Battery Safety Requirements (English Version). Available online: https://www.codeofchina.com/standard/GB38031-2025.html (accessed on 4 April 2025).
- H.R. 2024—Mercury-Containing and Rechargeable Battery Management Act. Available online: https://www.congress.gov/bill/104th-congress/house-bill/2024 (accessed on 20 February 2025).
- Inflation Reduction Act of 2022. Available online: https://www.irs.gov/inflation-reduction-act-of-2022 (accessed on 5 April 2025).
- Garche, J.; Moseley, P.T.; Karden, E. Lead–Acid Batteries for Hybrid Electric Vehicles and Battery Electric Vehicles. In Advances in Battery Technologies for Electric Vehicles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 75–101. ISBN 978-1-78242-377-5. [Google Scholar]
- Saakes, M.; Woortmeijer, R.; Schmal, D. Bipolar Lead–Acid Battery for Hybrid Vehicles. J. Power Sources 2005, 144, 536–545. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, Y.; Tao, X.; Lin, Z.; Lu, J. Bipolar Electrodes for Next-Generation Rechargeable Batteries. Adv. Sci. 2020, 7, 2001207. [Google Scholar] [CrossRef]
- Battery Council International New Study Confirms U.S.’ Most Recycled Consumer Product—Lead Batteries—Maintains Remarkable Milestone: 99% Recycling Rate. Available online: https://batterycouncil.org/news/press-release/new-study-confirms-lead-batteries-maintain-remarkable-99-recycling-rate/ (accessed on 14 February 2025).
- EUROBAT TF Innovation. Technical Annex to the EUROBAT White Paper Battery Innovation Roadmap V3.0; EUROBAT: Brussels, Belgium, 2024. [Google Scholar]
- Tsais, P.-J.; Chan, L.I. Nickel-Based Batteries: Materials and Chemistry. In Electricity Transmission, Distribution and Storage Systems; Elsevier: Amsterdam, The Netherlands, 2013; pp. 309–397. ISBN 978-1-84569-784-6. [Google Scholar]
- Nei, J.; Wang, M. Hydrogen Storage Alloy Development for Wide Operating Temperature Nickel-Metal Hydride Battery Applications. Int. J. Hydrog. Energy 2024, 49, 19–38. [Google Scholar] [CrossRef]
- Arya, S.; Verma, S. Nickel-Metal Hydride (Ni-MH) Batteries. In Rechargeable Batteries; Wiley: Hoboken, NJ, USA, 2020; pp. 131–175. [Google Scholar]
- International Energy Agency (IEA). Global EV Outlook 2023; International Energy Agency (IEA): Paris, France, 2023.
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N. Maximizing Energy Density of Lithium-Ion Batteries for Electric Vehicles: A Critical Review. Energy Rep. 2023, 9, 11–21. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Argyrou, M.C.; Christodoulides, P.; Kalogirou, S.A. Energy Storage for Electricity Generation and Related Processes: Technologies Appraisal and Grid Scale Applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Moseley, P.T.; Rand, D.A.J. High-Temperature Sodium Batteries for Energy Storage. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 253–268. ISBN 978-0-444-62616-5. [Google Scholar]
- Veneri, O.; Capasso, C.; Patalano, S. Experimental Study on the Performance of a ZEBRA Battery Based Propulsion System for Urban Commercial Vehicles. Appl. Energy 2017, 185, 2005–2018. [Google Scholar] [CrossRef]
- Nikiforidis, G.; Van De Sanden Ac, M.C.M.; Tsampas, M.N. High and Intermediate Temperature Sodium-Sulfur Batteries for Energy Storage: Development, Challenges and Perspectives. RSC Adv. 2019, 9, 5649–5673. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, X.; Chen, R.; Ye, K.; Lipton, J.; Maclean, S.A.; Wang, H.; Taylor, A.D.; Weng, G.M. Recent Advances in Rocking Chair Batteries and Beyond. Energy Storage Mater. 2023, 60, 102820. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Dorau, F.A.; Sommer, A.; Koloch, J.; Röß-Ohlenroth, R.; Schreiber, M.; Neuner, M.; Abo Gamra, K.; Lin, Y.; Schöberl, J.; Bilfinger, P.; et al. Comprehensive Analysis of Commercial Sodium-Ion Batteries: Structural and Electrochemical Insights. J. Electrochem. Soc. 2024, 171, 090521. [Google Scholar] [CrossRef]
- Rehm, M.; Fischer, M.; Gomez, M.R.; Schütte, M.; Sauer, D.U.; Jossen, A. Comparing the Electrical Performance of Commercial Sodium-Ion and Lithium-Iron-Phosphate Batteries. J. Power Sources 2025, 633, 236290. [Google Scholar] [CrossRef]
- Yao, K.; Xu, S.; Yang, Y.; Zheng, Y.; Zuraqi, K.; Yang, D.; Liu, J.; Mani, U.; Rui, X. Designing Sodium Alloys for Dendrite-free Sodium-metal Batteries. Inf. Funct. Mater. 2024, 1, 242–263. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From Lithium to Sodium: Cell Chemistry of Room Temperature Sodium–Air and Sodium–Sulfur Batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Burd, J.T.J.; Moore, E.A.; Ezzat, H.; Kirchain, R.; Roth, R. Improvements in Electric Vehicle Battery Technology Influence Vehicle Lightweighting and Material Substitution Decisions. Appl. Energy 2021, 283, 116269. [Google Scholar] [CrossRef]
- Jannesar Niri, A.; Poelzer, G.A.; Zhang, S.E.; Rosenkranz, J.; Pettersson, M.; Ghorbani, Y. Sustainability Challenges throughout the Electric Vehicle Battery Value Chain. Renew. Sustain. Energy Rev. 2024, 191, 114176. [Google Scholar] [CrossRef]
- Naseri, F.; Kazemi, Z.; Larsen, P.G.; Arefi, M.M.; Schaltz, E. Cyber-Physical Cloud Battery Management Systems: Review of Security Aspects. Batteries 2023, 9, 382. [Google Scholar] [CrossRef]
- Rahmani, P.; Chakraborty, S.; Mele, I.; Katrašnik, T.; Bernhard, S.; Pruefling, S.; Wilkins, S.; Hegazy, O. Driving the Future: A Comprehensive Review of Automotive Battery Management System Technologies, and Future Trends. J. Power Sources 2025, 629, 235827. [Google Scholar] [CrossRef]
- Batteries Europe Secretariat Research and Innovation Roadmap on Battery Technologies Powering Europe’s Green Revolution: Paving the Way to a More Resilient and Sustainable Battery Industry 2; Batteries Europe: Brussels, Belgium, 2023.
- Itani, K.; De Bernardinis, A. Review on New-Generation Batteries Technologies: Trends and Future Directions. Energies 2023, 16, 7530. [Google Scholar] [CrossRef]
- Tong, Z.; Zhu, X. A Patent Landscape Analysis on the High-Voltage Spinel LiNi0.5Mn1.5O4 for next-Generation Lithium-Ion Batteries. Next Energy 2024, 5, 100158. [Google Scholar] [CrossRef]
- König, A.; Nicoletti, L.; Schröder, D.; Wolff, S.; Waclaw, A.; Lienkamp, M. An Overview of Parameter and Cost for Battery Electric Vehicles. World Electr. Veh. J. 2021, 12, 21. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-Free, High-Nickel Layered Oxide Cathodes for Lithium-Ion Batteries: Progress, Challenges, and Perspectives. Energy Storage Mater. 2021, 34, 250–259. [Google Scholar] [CrossRef]
- Maisel, F.; Neef, C.; Marscheider-Weidemann, F.; Nissen, N.F. A Forecast on Future Raw Material Demand and Recycling Potential of Lithium-Ion Batteries in Electric Vehicles. Resour. Conserv. Recycl. 2023, 192, 106920. [Google Scholar] [CrossRef]
- Choix, B.; Uhlig, F. Sustainability Requirements for Batteries in the EU; European Economic and Social Committee: Brussels, Belgium, 2020. [Google Scholar]
- Duffner, F.; Wentker, M.; Greenwood, M.; Leker, J. Battery Cost Modeling: A Review and Directions for Future Research. Renew. Sustain. Energy Rev. 2020, 127, 109872. [Google Scholar] [CrossRef]
- Gutierrez, A.; He, M.; Yonemoto, B.T.; Yang, Z.; Wang, J.; Meyer, H.M.; Thackeray, M.M.; Croy, J.R. Advancing Lithium- and Manganese-Rich Cathodes through a Combined Electrolyte Additive/Surface Treatment Strategy. J. Electrochem. Soc. 2019, 166, A3896–A3907. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- EUCAR. Battery Requirements for Future Automotive Applications EG BEV&FCEV; EUCAR: Etterbeek, Belgium, 2019. [Google Scholar]
- USABC Goals for Advanced High-Performance Batteries for Electric Vehicle (EV) Applications; USCAR: Southfield, MI, USA, 2021.
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Hackmann, M.; Knörzer, H.; Pfeuffer, J.; Jeckel, P. Battery Aging in Practice: Analysis of over 7,000 Vehicles Provide Deep Insights into Battery Life and Vehicle Residual Value; P3 group GmbH: Stuttgart, Germany, 2024. [Google Scholar]
- European Commission. Joint Research Centre. Performance and Durability Requirements in the Batteries Regulation. Part 1, General Assessment and Data Basis; Publications Office: Luxembourg, 2024. [Google Scholar]
- Timilsina, L.; Badr, P.R.; Hoang, P.H.; Ozkan, G.; Papari, B.; Edrington, C.S. Battery Degradation in Electric and Hybrid Electric Vehicles: A Survey Study. IEEE Access 2023, 11, 42431–42462. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium Ion Battery Degradation: What You Need to Know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Menye, J.S.; Camara, M.-B.; Dakyo, B. Lithium Battery Degradation and Failure Mechanisms: A State-of-the-Art Review. Energies 2025, 18, 342. [Google Scholar] [CrossRef]
- Takenaka, N.; Bouibes, A.; Yamada, Y.; Nagaoka, M.; Yamada, A. Frontiers in Theoretical Analysis of Solid Electrolyte Interphase Formation Mechanism. Adv. Mater. 2021, 33, 2100574. [Google Scholar] [CrossRef]
- Zhao, J.; Lv, Z.; Li, D.; Feng, X.; Wang, Z.; Wu, Y.; Shi, D.; Fowler, M.; Burke, A.F. Battery Engineering Safety Technologies (BEST): M5 Framework of Mechanisms, Modes, Metrics, Modeling, and Mitigation. eTransportation 2024, 22, 100364. [Google Scholar] [CrossRef]
- Li, B.; Chao, Y.; Li, M.; Xiao, Y.; Li, R.; Yang, K.; Cui, X.; Xu, G.; Li, L.; Yang, C.; et al. A Review of Solid Electrolyte Interphase (SEI) and Dendrite Formation in Lithium Batteries. Electrochem. Energy Rev. 2023, 6, 7. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.; Kim, J.; Kim, J.; Park, J.-H.; Kim, K.-T.; Park, J.-H.; Kim, T.; Min, K. Data-Driven Methods for Predicting the State of Health, State of Charge, and Remaining Useful Life of Li-Ion Batteries: A Comprehensive Review. Int. J. Precis. Eng. Manuf. 2023, 24, 1281–1304. [Google Scholar] [CrossRef]
- Qu, X.; Shi, D.; Zhao, J.; Tran, M.-K.; Wang, Z.; Fowler, M.; Lian, Y.; Burke, A.F. Insights and Reviews on Battery Lifetime Prediction from Research to Practice. J. Energy Chem. 2024, 94, 716–739. [Google Scholar] [CrossRef]
- Rezaei Larijani, M.; Hedayati Kia, S.; Zolghadri, M.; El Hajjaji, A. Battery Characterization, Life Cycle, and Modeling. In Handbook of Power Electronics in Autonomous and Electric Vehicles; Elsevier: Amsterdam, The Netherlands, 2024; pp. 323–335. ISBN 978-0-323-99545-0. [Google Scholar]
- Yang, Y.; Zhao, L.; Zhang, Y.; Yang, Z.; Lai, W.-H.; Liang, Y.; Dou, S.-X.; Liu, M.; Wang, Y.-X. Challenges and Prospects of Low-Temperature Rechargeable Batteries: Electrolytes, Interfaces, and Electrodes. Adv. Sci. 2024, 11, e2410318. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cai, W.; Hu, Z.; Huang, Q.; Wang, A.; Zeng, Z.; Song, J.; Sun, Y.; Kong, Q.; Feng, W.; et al. Damage Mechanisms and Recent Research Advances in Ni-Rich Layered Cathode Materials for Lithium-Ion Batteries. Electron 2024, 2, e27. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, H.; Qin, N.; Wang, C.; Wang, L.; Lu, J. Single-Crystal Nickel-Rich Cathode Materials: Challenges and Strategies. Wuli Huaxue Xuebao/ Acta Phys. Chim. Sin. 2024, 40, 2308051. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Dose, W.; Dey, S.; Wang, X.; Wu, Y.; Li, D.; Ci, L. Microstructures of Layered Ni-Rich Cathodes for Lithium-Ion Batteries. Chem. Soc. Rev. 2024, 53, 4707–4740. [Google Scholar] [CrossRef]
- Xie, H.; Peng, H.; Jiang, D.; Xiao, Z.; Liu, X.; Liang, H.; Wu, M.; Liu, D.; Li, Y.; Sun, Y.; et al. Structures, Issues, and Optimization Strategies of Ni-Rich and Co-Low Cathode Materials for Lithium-Ion Battery. Chem. Eng. J. 2023, 470, 144051. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Liang, Y.; Chen, R.; Cao, Y. Modification of Lithium-Rich Manganese Oxide Materials: Coating, Doping and Single Crystallization. Batter. Supercaps 2024, 8, e202400443. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Tang, M.; Zhan, L.; Zhai, Y.; Chen, W.; Zhou, M.; Ji, Y.; Wang, P. A Review of High-Capacity Lithium-Rich Manganese-Based Cathode Materials for a New Generation of Lithium Batteries. Inorganica Chim. Acta 2024, 572, 122239. [Google Scholar] [CrossRef]
- Ding, S.; Li, Y.; Dai, H.; Wang, L.; He, X. Accurate Model Parameter Identification to Boost Precise Aging Prediction of Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2023, 13, 2301452. [Google Scholar] [CrossRef]
- Kumar, R. State Estimation of Lithium-Ion Battery for Electric Vehicle Application: Types, Design Methods, and Future Trends; Taylor&Francis: London, UK, 2024; ISBN 978-1-040-00910-9. [Google Scholar]
- Chidambaram, R.K.; Chatterjee, D.; Barman, B.; Das, P.P.; Taler, D.; Taler, J.; Sobota, T. Effect of Regenerative Braking on Battery Life. Energies 2023, 16, 5303. [Google Scholar] [CrossRef]
- Khan, A.; Naqvi, I.H.; Bhargava, C.; Lin, C.-P.; Boles, S.T.; Kong, L.; Pecht, M. Safety and Reliability Analysis of Lithium-Ion Batteries with Real-Time Health Monitoring. Renew. Sustain. Energy Rev. 2025, 212, 115408. [Google Scholar] [CrossRef]
- Soltani, M.; Vilsen, S.B.; Stroe, A.I.; Knap, V.; Stroe, D.I. Degradation Behaviour Analysis and End-of-Life Prediction of Lithium Titanate Oxide Batteries. J. Energy Storage 2023, 68, 107745. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Abdolrasol, M.G.M.; Ayob, A.; Lipu, M.S.H.; Ansari, S.; Kiong, T.S.; Saad, M.H.M.; Ustun, T.S.; Kalam, A. Advanced Data-Driven Fault Diagnosis in Lithium-Ion Battery Management Systems for Electric Vehicles: Progress, Challenges, and Future Perspectives. eTransportation 2024, 22, 100374. [Google Scholar] [CrossRef]
- Bashir, H.; Yaqoob, A.; Jawaid, I.; Khalid, W.; Javed, M.Y.; Sultan, W. A Review of Battery Management System and Modern State Estimation Approaches in Lithiumion Batteries for Electric Vehicle. In Proceedings of the 2022 5th International Conference on Energy Conservation and Efficiency, ICECE 2022—Proceedings, Lahore, Pakistan, 16–17 March 2022. [Google Scholar]
- Challoob, A.F.; Bin Rahmat, N.A.; A/L Ramachandaramurthy, V.K.; Humaidi, A.J. Energy and Battery Management Systems for Electrical Vehicles: A Comprehensive Review & Recommendations. Energy Explor. Exploit. 2024, 42, 341–372. [Google Scholar] [CrossRef]
- Hossain Lipu, M.S.; Ansari, S.; Miah, M.S.; Meraj, S.T.; Hasan, K.; Shihavuddin, A.S.M.; Hannan, M.A.; Muttaqi, K.M.; Hussain, A. Deep Learning Enabled State of Charge, State of Health and Remaining Useful Life Estimation for Smart Battery Management System: Methods, Implementations, Issues and Prospects. J. Energy Storage 2022, 55, 105752. [Google Scholar] [CrossRef]
- Yang, B.; Qian, Y.; Li, Q.; Chen, Q.; Wu, J.; Luo, E.; Xie, R.; Zheng, R.; Yan, Y.; Su, S.; et al. Critical Summary and Perspectives on State-of-Health of Lithium-Ion Battery. Renew. Sustain. Energy Rev. 2024, 190, 114077. [Google Scholar] [CrossRef]
- Park, J.; Jin, Y.; Kam, W.; Han, S. A Practical Semi-Empirical Model for Predicting the SoH of Lithium-Ion Battery: A Novel Perspective on Short-Term Rest. J. Energy Storage 2024, 96, 112659. [Google Scholar] [CrossRef]
- Vasta, E.; Scimone, T.; Nobile, G.; Eberhardt, O.; Dugo, D.; De Benedetti, M.M.; Lanuzza, L.; Scarcella, G.; Patanè, L.; Arena, P.; et al. Models for Battery Health Assessment: A Comparative Evaluation. Energies 2023, 16, 632. [Google Scholar] [CrossRef]
- Omakor, J.; Miah, M.S.; Chaoui, H. Battery Reliability Assessment in Electric Vehicles: A State-of-the-Art. IEEE Access 2024, 12, 77903–77931. [Google Scholar] [CrossRef]
- Vanem, E.; Salucci, C.B.; Bakdi, A.; Alnes, Ø.Å.S. Data-Driven State of Health Modelling—A Review of State of the Art and Reflections on Applications for Maritime Battery Systems. J. Energy Storage 2021, 43, 103158. [Google Scholar] [CrossRef]
- Ali, Z.M.; Calasan, M.; Gandoman, F.H.; Jurado, F.; Abdel Aleem, S.H.E. Review of Batteries Reliability in Electric Vehicle and E-Mobility Applications. Ain Shams Eng. J. 2024, 15, 102442. [Google Scholar] [CrossRef]
- Clemente, C.; Giovanni, C.; Ottorino, V.; Stanislao, P.; Ferdinando, V. Estimation of the State of Charge Health of Electric Vehicle Batteries Through Machine Learning Approach. In Proceedings of the Energy Proceedings, Doha, Qatar, 3–7 December 2023; Volume 45. [Google Scholar]
- Massaoudi, M.; Abu-Rub, H.; Ghrayeb, A. Advancing Lithium-Ion Battery Health Prognostics With Deep Learning: A Review and Case Study. IEEE Open J. Ind. Appl. 2024, 5, 43–62. [Google Scholar] [CrossRef]
- Cicconi, P.; Kumar, P. Design Approaches for Li-Ion Battery Packs: A Review. J. Energy Storage 2023, 73, 109197. [Google Scholar] [CrossRef]
- Zhong, D.; Xia, Z.; Zhu, Y.; Duan, J. Overview of Predictive Maintenance Based on Digital Twin Technology. Heliyon 2023, 9, e14534. [Google Scholar] [CrossRef]
- Alfaro-Viquez, D.; Zamora-Hernandez, M.; Fernandez-Vega, M.; Garcia-Rodriguez, J.; Azorin-Lopez, J. A Comprehensive Review of AI-Based Digital Twin Applications in Manufacturing: Integration Across Operator, Product, and Process Dimensions. Electronics 2025, 14, 646. [Google Scholar] [CrossRef]
- Mulpuri, S.K.; Sah, B.; Kumar, P. An Intelligent Battery Management System (BMS) with End-Edge-Cloud Connectivity—A Perspective. Sustain. Energy Fuels 2025, 9, 1142–1159. [Google Scholar] [CrossRef]
- Kreuzer, T.; Papapetrou, P.; Zdravkovic, J. Artificial Intelligence in Digital Twins—A Systematic Literature Review. Data Knowl. Eng. 2024, 151, 102304. [Google Scholar] [CrossRef]
- Es-haghi, M.S.; Anitescu, C.; Rabczuk, T. Methods for Enabling Real-Time Analysis in Digital Twins: A Literature Review. Comput. Struct. 2024, 297, 107342. [Google Scholar] [CrossRef]
- Cavus, M.; Dissanayake, D.; Bell, M. Next Generation of Electric Vehicles: AI-Driven Approaches for Predictive Maintenance and Battery Management. Energies 2025, 18, 1041. [Google Scholar] [CrossRef]
- Zhao, J.; Qu, X.; Wu, Y.; Fowler, M.; Burke, A.F. Artificial Intelligence-Driven Real-World Battery Diagnostics. Energy AI 2024, 18, 100419. [Google Scholar] [CrossRef]
- Iranshahi, K.; Brun, J.; Arnold, T.; Sergi, T.; Müller, U.C. Digital Twins: Recent Advances and Future Directions in Engineering Fields. Intell. Syst. Appl. 2025, 26, 200516. [Google Scholar] [CrossRef]
- Saeed, S.; Altamimi, S.A.; Alkayyal, N.A.; Alshehri, E.; Alabbad, D.A. Digital Transformation and Cybersecurity Challenges for Businesses Resilience: Issues and Recommendations. Sensors 2023, 23, 6666. [Google Scholar] [CrossRef]
- Nuroldayeva, G.; Serik, Y.; Adair, D.; Uzakbaiuly, B.; Bakenov, Z. State of Health Estimation Methods for Lithium-Ion Batteries. Int. J. Energy Res. 2023, 2023, 1–21. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Fu, L.; Zhen, D.; Gu, F.; Ball, A.D. A Review on Rapid State of Health Estimation of Lithium-Ion Batteries in Electric Vehicles. Sustain. Energy Technol. Assess. 2023, 60, 103457. [Google Scholar] [CrossRef]
- Che, Y.; Hu, X.; Lin, X.; Guo, J.; Teodorescu, R. Health Prognostics for Lithium-Ion Batteries: Mechanisms, Methods, and Prospects. Energy Environ. Sci. 2023, 16, 338–371. [Google Scholar] [CrossRef]
- Sedláčková, E.; Pražanová, A.; Plachý, Z.; Klusoňová, N.; Knap, V.; Dušek, K. Acoustic Emission Technique for Battery Health Monitoring: Comprehensive Literature Review. Batteries 2025, 11, 14. [Google Scholar] [CrossRef]
- Dini, P.; Colicelli, A.; Saponara, S. Review on Modeling and SOC/SOH Estimation of Batteries for Automotive Applications. Batteries 2024, 10, 34. [Google Scholar] [CrossRef]
- Gaga, A.; Tannouche, A.; Mehdaoui, Y.; El Hadadi, B. Methods for Estimating Lithium-Ion Battery State of Charge for Use in Electric Vehicles: A Review. Energy Harvest. Syst. 2022, 9, 211–225. [Google Scholar] [CrossRef]
- Anekal, L.; Samanta, A.; Williamson, S. Wide-Ranging Parameter Extraction of Lithium-Ion Batteries to Estimate State of Health Using Electrochemical Impedance Spectroscopy. In Proceedings of the 1st IEEE Industrial Electronics Society Annual On-Line Conference, ONCON 2022, kharagpur, India, 9–11 December 2022. [Google Scholar]
- Wang, W.; Zhang, Y.; Xie, B.; Huang, L.; Dong, S.; Xu, G.; Cui, G. Deciphering Advanced Sensors for Life and Safety Monitoring of Lithium Batteries. Adv. Energy Mater. 2024, 14, 2304173. [Google Scholar] [CrossRef]
- Cheng, A.; Xin, Y.; Wu, H.; Yang, L.; Deng, B. A Review of Sensor Applications in Electric Vehicle Thermal Management Systems. Energies 2023, 16, 5139. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zeng, W. Gas Sensing Technology as the Key to Safety Warning of Lithium-Ion Battery: Recent Advance. Sens. Actuators A Phys. 2024, 365, 114890. [Google Scholar] [CrossRef]
- Diao, W.; Jiang, J.; Zhang, C.; Liang, H.; Pecht, M. Energy State of Health Estimation for Battery Packs Based on the Degradation and Inconsistency. Energy Procedia 2017, 142, 3578–3583. [Google Scholar] [CrossRef]
- Fan, G.; Zhou, B.; Ye, S.; Shen, H.; Huo, D.; Zhang, X. Unveiling the Secrets behind Physics-Based Modeling of Lithium-Ion Battery Degradation and Its Key Applications. J. Energy Storage 2024, 102, 114086. [Google Scholar] [CrossRef]
- Jaime-Barquero, E.; Bekaert, E.; Olarte, J.; Zulueta, E.; Lopez-Guede, J.M. Artificial Intelligence Opportunities to Diagnose Degradation Modes for Safety Operation in Lithium Batteries. Batteries 2023, 9, 388. [Google Scholar] [CrossRef]
- Nigam, A.; Sharma, H.; Kumar, P.; Kumar, A. Modeling and Simulation Study for Power Management and Battery Degradation of Smart Electric Vehicles; Wiley Semiconductor: Hoboken, NJ, USA, 2024; ISBN 978-1-394-22504-0. [Google Scholar]
- Shi, J.; Zhang, H.; Yu, H.; Xu, Y.; Xu, S.; Sheng, L.; Feng, X.; Wang, X. Experimental Determinations of Thermophysical Parameters for Lithium-Ion Batteries: A Systematic Review. eTransportation 2024, 20, 100321. [Google Scholar] [CrossRef]
- Abramushkina, E.; Zhaksylyk, A.; Geury, T.; El Baghdadi, M.; Hegazy, O. A Thorough Review of Cooling Concepts and Thermal Management Techniques for Automotive Wbg Inverters: Topology, Technology and Integration Level. Energies 2021, 14, 4981. [Google Scholar] [CrossRef]
- Alkhedher, M.; Al Tahhan, A.B.; Yousaf, J.; Ghazal, M.; Shahbazian-Yassar, R.; Ramadan, M. Electrochemical and Thermal Modeling of Lithium-Ion Batteries: A Review of Coupled Approaches for Improved Thermal Performance and Safety Lithium-Ion Batteries. J. Energy Storage 2024, 86, 111172. [Google Scholar] [CrossRef]
- Behi, H.; Gandoman, F.H.; Karimi, D.; Hosen, M.D.S.; Behi, M.; Jaguemont, J.; Van Mierlo, J.; Berecibar, M. Thermal Management of the Li-Ion Batteries to Improve the Performance of the Electric Vehicles Applications; Wiley: Hoboken, NY, USA, 2022; ISBN 978-1-119-80107-8. [Google Scholar]
- Chavan, S.; Venkateswarlu, B.; Salman, M.; Liu, J.; Pawar, P.; Joo, S.W.; Choi, G.S.; Kim, S.C. Thermal Management Strategies for Lithium-Ion Batteries in Electric Vehicles: Fundamentals, Recent Advances, Thermal Models, and Cooling Techniques. Int. J. Heat Mass Transf. 2024, 232, 125918. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, L.; Yin, L.; Dang, C.; Ren, H.; Zhang, Z. A Review on Thermal Management of Li-Ion Battery: From Small-Scale Battery Module to Large-Scale Electrochemical Energy Storage Power Station. J. Therm. Sci. 2025, 34, 1–23. [Google Scholar] [CrossRef]
- Garud, K.S.; Tai, L.D.; Hwang, S.-G.; Nguyen, N.-H.; Lee, M.-Y. A Review of Advanced Cooling Strategies for Battery Thermal Management Systems in Electric Vehicles. Symmetry 2023, 15, 1322. [Google Scholar] [CrossRef]
- Khan, A.; Yaqub, S.; Ali, M.; Ahmad, A.W.; Nazir, H.; Khalid, H.A.; Iqbal, N.; Said, Z.; Sopian, K. A State-of-the-Art Review on Heating and Cooling of Lithium-Ion Batteries for Electric Vehicles. J. Energy Storage 2024, 76, 109852. [Google Scholar] [CrossRef]
- Rallabandi, S.; Issac Selvaraj, R.V. Advancements in Battery Cooling Techniques for Enhanced Performance and Safety in Electric Vehicles: A Comprehensive Review. Energy Technol. 2024, 12, 2301404. [Google Scholar] [CrossRef]
- Wu, C.; Sun, Y.; Tang, H.; Zhang, S.; Yuan, W.; Zhu, L.; Tang, Y. A Review on the Liquid Cooling Thermal Management System of Lithium-Ion Batteries. Appl. Energy 2024, 375, 124173. [Google Scholar] [CrossRef]
- Yang, B.; Ji, J.; Zhang, X.; Hua, W. Passive Cooling of Lithium-Ion Batteries Based on Flexible Phase Change Materials: Molecular Structure, Interactions and Mechanistic Aspects. J. Mol. Liq. 2023, 391, 123340. [Google Scholar] [CrossRef]
- He, J.; Meng, J.; Huang, Y. Challenges and Recent Progress in Fast-Charging Lithium-Ion Battery Materials. J. Power Sources 2023, 570, 232965. [Google Scholar] [CrossRef]
- Song, C.; Han, S.H.; Moon, H.; Choi, N.-S. Unlocking Fast-Charging Capabilities of Lithium-Ion Batteries through Liquid Electrolyte Engineering. EcoMat 2024, 6, e12476. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Qin, Y.; Gao, T. Enhancing the Charging Performance of Lithium-Ion Batteries by Reducing SEI and Charge Transfer Resistances. ACS Appl. Mater. Interfaces 2022, 14, 33004–33012. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Liu, R.; Li, X.; Wu, Y.; Ran, F. “Fast-Charging” Anode Materials for Lithium-Ion Batteries from Perspective of Ion Diffusion in Crystal Structure. ACS Nano 2024, 18, 2611–2648. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, J.; Gao, Q.; Zhang, W.; Cheng, X.; Song, C.; Li, G. Recent Advances in Fast-Charging Lithium-Ion Batteries: Mechanism, Materials, and Future Opportunities. Chem. Eng. J. 2025, 506, 159927. [Google Scholar] [CrossRef]
- Pegel, H.; Wycisk, D.; Sauer, D.U. Influence of Cell Dimensions and Housing Material on the Energy Density and Fast-Charging Performance of Tabless Cylindrical Lithium-Ion Cells. Energy Storage Mater. 2023, 60, 102796. [Google Scholar] [CrossRef]
- Pegel, H.; Wycisk, D.; Scheible, A.; Tendera, L.; Latz, A.; Sauer, D.U. Fast-Charging Performance and Optimal Thermal Management of Large-Format Full-Tab Cylindrical Lithium-Ion Cells under Varying Environmental Conditions. J. Power Sources 2023, 556, 232408. [Google Scholar] [CrossRef]
- Yeganehdoust, F.; Kumar, A.; Reddy, M.R.; Zaghib, K. Cell Architecture Design for Fast-Charging Lithium-Ion Batteries in Electric Vehicles. Batteries 2025, 11, 20. [Google Scholar] [CrossRef]
- Manthiram, A.; Song, B.; Li, W. A Perspective on Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Ahangari, M.; Zhou, M.; Luo, H. Review of Layered Transition Metal Oxide Materials for Cathodes in Sodium-Ion Batteries. Micromachines 2025, 16, 137. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, Z.; Yu, Y.; Liu, Y.; Li, J.; Kang, F. Surface Substitution of Polyanion to Improve Structure Stability and Electrochemical Properties of Lithium-Rich Layered Cathode Oxides. Appl. Surf. Sci. 2020, 512, 145741. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Xing, T.; Liu, H.; Liu, Z.; Li, Z.; Wu, M. Recent Progress in the Use of Polyanions as Solid Electrolytes. New Carbon Mater. 2022, 37, 358–370. [Google Scholar] [CrossRef]
- Huang, Q.; Turcheniuk, K.; Ren, X.; Magasinski, A.; Song, A.-Y.; Xiao, Y.; Kim, D.; Yushin, G. Cycle Stability of Conversion-Type Iron Fluoride Lithium Battery Cathode at Elevated Temperatures in Polymer Electrolyte Composites. Nat. Mater. 2019, 18, 1343–1349. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Fu, C.; Ding, Y.; Liu, G.; Cao, Y.; Chen, Z. Recent Advances in Conversion-Type Electrode Materials for Post Lithium-Ion Batteries. ACS Mater. Lett. 2021, 3, 956–977. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion Cathodes for Rechargeable Lithium and Lithium-Ion Batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Su, Y.; Chen, J.; Li, H.; Sun, H.; Yang, T.; Liu, Q.; Ichikawa, S.; Zhang, X.; Zhu, D.; Zhao, J.; et al. Enabling Long Cycle Life and High Rate Iron Difluoride Based Lithium Batteries by In Situ Cathode Surface Modification. Adv. Sci. 2022, 9, 2201419. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, J.; Kong, J.; Loh, X.J.; Wang, J.; Liu, Z. “Porous and Yet Dense” Electrodes for High-Volumetric-Performance Electrochemical Capacitors: Principles, Advances, and Challenges. Adv. Sci. 2022, 9, 2103953. [Google Scholar] [CrossRef] [PubMed]
- Saju, S.K.; Chattopadhyay, S.; Xu, J.; Alhashim, S.; Pramanik, A.; Ajayan, P.M. Hard Carbon Anode for Lithium-, Sodium-, and Potassium-Ion Batteries: Advancement and Future Perspective. Cell Rep. Phys. Sci. 2024, 5, 101851. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yu, W.; Xiao, R.; Huang, F.; Tian, H.; Wang, C.; Chen, X.; Shao, J. Scalable Fabrication of Turbostratic Graphene with High Density and High Ion Conductivity for Compact Capacitive Energy Storage. Matter 2023, 6, 4032–4049. [Google Scholar] [CrossRef]
- Miao, J. Review on Electrode Degradation at Fast Charging of Li-Ion and Li Metal Batteries from a Kinetic Perspective. Electrochem 2023, 4, 156–180. [Google Scholar] [CrossRef]
- Ezhyeh, Z.N.; Khodaei, M.; Torabi, F. Review on Doping Strategy in Li4Ti5O12 as an Anode Material for Lithium-Ion Batteries. Ceram. Int. 2023, 49, 7105–7141. [Google Scholar] [CrossRef]
- Khan, M.; Yan, S.; Ali, M.; Mahmood, F.; Zheng, Y.; Li, G.; Liu, J.; Song, X.; Wang, Y. Innovative Solutions for High-Performance Silicon Anodes in Lithium-Ion Batteries: Overcoming Challenges and Real-World Applications. Nano-Micro Lett. 2024, 16, 179. [Google Scholar] [CrossRef]
- Asamoah, G.A.; Korsah, M.; Jeyasundar, P.G.S.A.; Ahmed, M.; Lau, S.Y.; Danquah, M.K. Nanotechnology-Based Lithium-Ion Battery Energy Storage Systems. Sustainability 2024, 16, 9231. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Q.; Wang, J.; Li, H.; Wang, G.; Yang, J.; Song, Y.; Qin, Y.; Liu, L. Improved Cycling Performance of a Silicon Anode for Lithium Ion Batteries Using Carbon Nanocoils. RSC Adv. 2014, 4, 40812–40815. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, L.; Zhou, R.; Du, X.; Jiao, Z.; Zhang, B. High-Performance Silicon-Rich Microparticle Anodes for Lithium-Ion Batteries Enabled by Internal Stress Mitigation. Nano-Micro Lett. 2023, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zeng, Z.; Cheng, S.; Xie, J. Fast-charging of Lithium-ion Batteries: A Review of Electrolyte Design Aspects. Battery Energy 2023, 2, 20230018. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, L.; Jiang, L. Research on Performance Constraints and Electrolyte Optimization Strategies for Lithium-Ion Batteries at Low Temperatures. RSC Adv. 2025, 15, 7995–8018. [Google Scholar] [CrossRef]
- Wan, S.; Ma, W.; Wang, Y.; Xiao, Y.; Chen, S. Electrolytes Design for Extending the Temperature Adaptability of Lithium-Ion Batteries: From Fundamentals to Strategies. Adv. Mater. 2024, 36, 2311912. [Google Scholar] [CrossRef]
- Kianfar, M.; Ipakchi, H.; Mohajer, S.; Rasoulifard, M.H.; Seyed Dorraji, M.S.; Louaguef, D.B.; Azat, S.; Saeb, M.R.; Vahabi, H. Flame-Retardant Self-Healing Polymers: A Review. J. Polym. Sci. 2024. [CrossRef]
- Liu, W.; Jiang, Y.; Wang, N.; Fu, W. Recent Progress in Flame Retardant Technology of Battery: A Review. Resour. Chem. Mater. 2023, 2, 80–99. [Google Scholar] [CrossRef]
- Gao, Z.; Rao, S.; Zhang, T.; Li, W.; Yang, X.; Chen, Y.; Zheng, Y.; Ding, Y.; Dong, T.; Li, S. Design Strategies of Flame-Retardant Additives for Lithium Ion Electrolyte. J. Electrochem. Energy Convers. Storage 2022, 19, 030910. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Huang, Y.; Zhang, M.; Li, Z.; Wang, Y. Recent Progress on Garnet-Type Oxide Electrolytes for All-Solid-State Lithium-Ion Batteries. Ceram. Int. 2023, 49, 29375–29390. [Google Scholar] [CrossRef]
- Sarfraz, N.; Kanwal, N.; Ali, M.; Ali, K.; Hasnain, A.; Ashraf, M.; Ayaz, M.; Ifthikar, J.; Ali, S.; Hendi, A.; et al. Materials Advancements in Solid-State Inorganic Electrolytes for Highly Anticipated All Solid Li-Ion Batteries. Energy Storage Mater. 2024, 71, 103619. [Google Scholar] [CrossRef]
- Lain, M.J.; Kendrick, E. Understanding the Limitations of Lithium Ion Batteries at High Rates. J. Power Sources 2021, 493, 229690. [Google Scholar] [CrossRef]
- Zhou, H.; Alujjage, A.S.; Terese, M.; Fear, C.; Joshi, T.; Rikka, V.R.; Jeevarajan, J.A.; Mukherjee, P.P. Effect of Fast Charging on Degradation and Safety Characteristics of Lithium-Ion Batteries with LiFePO4 Cathodes. Appl. Energy 2025, 377, 124465. [Google Scholar] [CrossRef]
- Dai, N.; Long, J. Research on Fast-Charging Battery Thermal Management System Based on Refrigerant Direct Cooling. Sci Rep 2023, 13, 11707. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.D.; Garud, K.S.; Hwang, S.-G.; Lee, M.-Y. A Review on Advanced Battery Thermal Management Systems for Fast Charging in Electric Vehicles. Batteries 2024, 10, 372. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, I.; Thakur, A.K.; Yu, S.; Lau, K.T.; He, S.; Dong, K.; Chen, J.; Xiangrong, L.; Ahmad, M.; et al. Advancements in Battery Thermal Management System for Fast Charging/Discharging Applications. Energy Storage Mater. 2024, 65, 103144. [Google Scholar] [CrossRef]
- Wassiliadis, N.; Schneider, J.; Frank, A.; Wildfeuer, L.; Lin, X.; Jossen, A.; Lienkamp, M. Review of Fast Charging Strategies for Lithium-Ion Battery Systems and Their Applicability for Battery Electric Vehicles. J. Energy Storage 2021, 44, 103306. [Google Scholar] [CrossRef]
- Ji, G.; He, L.; Wu, T.; Cui, G. The Design of Fast Charging Strategy for Lithium-Ion Batteries and Intelligent Application: A Comprehensive Review. Appl. Energy 2025, 377, 124538. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Bai, Z.; Yang, N.; Guo, G.; Banjoko, O.S. Enhancing Fast Charging Performance of Lithium-Ion Batteries: The Role of Operating Temperature and Charging Rate. Electrochim. Acta 2025, 511, 145390. [Google Scholar] [CrossRef]
- Ghani, F.; An, K.; Lee, D. A Review on Design Parameters for the Full-Cell Lithium-Ion Batteries. Batteries 2024, 10, 340. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, N.; Lu, Y.; Zhang, Z.; Li, M.; Liu, J.; Song, W.; Zhao, Y.; Miao, Z. Strategies toward the Development of High-Energy-Density Lithium Batteries. J. Energy Storage 2024, 88, 111666. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Monika, A.K.; Patial, B.S. A Review on Recent Advances in Anode Materials in Lithium Ion Batteries. Mater. Today Electron. 2024, 7, 100089. [Google Scholar] [CrossRef]
- Yi, X.; Qi, G.; Liu, X.; Depcik, C.; Liu, L. Challenges and Strategies toward Anode Materials with Different Lithium Storage Mechanisms for Rechargeable Lithium Batteries. J. Energy Storage 2024, 95, 112480. [Google Scholar] [CrossRef]
- Joy, R.; Balakrishnan, N.T.M.; Das, A.; Shafeek, S.; Thakur, V.K.; Zaghib, K.; Jaffarali, J.F.M.; Reddy, M.V.V.; Raghavan, P. Graphene: Chemistry and Applications for Lithium-Ion Batteries. Electrochem 2022, 3, 143–183. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Yao, N.; Gao, Y.-C.; Yuan, Y.-H.; Gao, Y.-B.; Tang, C.; Zhang, Q. Advanced Carbon as Emerging Energy Materials in Lithium Batteries: A Theoretical Perspective. InfoMat 2025, e12653. [Google Scholar] [CrossRef]
- Dang, G.; Zhang, M.; Min, F.; Zhang, Y.; Zhang, B.; Zhang, Q.; Wang, J.; Zhou, Y.; Liu, W.; Xie, J.; et al. Lithium Titanate Battery System Enables Hybrid Electric Heavy-Duty Vehicles. J. Energy Storage 2023, 74, 109313. [Google Scholar] [CrossRef]
- Chen, H.; Chahbaz, A.; Yang, S.; Zhang, W.; Sauer, D.U.; Li, W. Thermodynamic and Kinetic Degradation of LTO Batteries: Impact of Different SOC Intervals and Discharge Voltages in Electric Train Applications. eTransportation 2024, 21, 100340. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.; Zhang, B.; Yang, Q.-H.; et al. Gassing in Li4Ti5O12-Based Batteries and Its Remedy. Sci. Rep. 2012, 2, 913. [Google Scholar] [CrossRef]
- Liu, J.; Bian, P.; Li, J.; Ji, W.; Hao, H.; Yu, A. Gassing Behavior of Lithium Titanate Based Lithium Ion Batteries with Different Types of Electrolytes. J. Power Sources 2015, 286, 380–387. [Google Scholar] [CrossRef]
- Cicconi, P.; Postacchini, L.; Pallotta, E.; Monteriù, A.; Prist, M.; Bevilacqua, M.; Germani, M. A Life Cycle Costing of Compacted Lithium Titanium Oxide Batteries for Industrial Applications. J. Power Sources 2019, 436, 226837. [Google Scholar] [CrossRef]
- Peng, C.; Liang, S.; Yu, Y.; Cao, L.; Yang, C.; Liu, X.; Guo, K.; Müller-Buschbaum, P.; Cheng, Y.; Wang, C. A Chronicle of Titanium Niobium Oxide Materials for High-performance Lithium-ion Batteries: From Laboratory to Industry. Carbon Neutralization 2024, 3, 1036–1091. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Saito, N.; Zhang, Z.; Yang, L.; Hirano, S.-I. Search for Stable Host Materials as Low-Voltage Anodes for Lithium-Ion Batteries: A Mini-Review. Energy Storage Mater. 2023, 55, 364–387. [Google Scholar] [CrossRef]
- Wen, X.; Ma, C.; Du, C.; Liu, J.; Zhang, X.; Qu, D.; Tang, Z. Enhanced Electrochemical Properties of Vanadium-Doped Titanium Niobate as a New Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2015, 186, 58–63. [Google Scholar] [CrossRef]
- Kumar Prajapati, A.; Bhatnagar, A. A Review on Anode Materials for Lithium/Sodium-Ion Batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Ayodhya, D. A Review of Recent Progress in 2D MXenes: Synthesis, Properties, and Applications. Diam. Relat. Mater. 2023, 132, 109634. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y.; Barsoum, M.W. Mxenes: A New Family of Two-Dimensional Materials and Its Application As Electrodes for Li and Na-Ion Batteries. Meet. Abstr. 2015, MA2015-01, 849. [Google Scholar] [CrossRef]
- Awan, H.T.A.; Abdah, M.A.A.M.; Mehar, M.; Walvekar, R.; Chaudhary, V.; Khalid, M.; Khosla, A. MXene-Polymer Hybrid Composites for Advanced Energy Storage: Insights into Supercapacitors and Batteries. J. Energy Storage 2024, 95, 112449. [Google Scholar] [CrossRef]
- Feyzi, E.; M R, A.K.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A Comprehensive Review of Silicon Anodes for High-Energy Lithium-Ion Batteries: Challenges, Latest Developments, and Perspectives. Next Energy 2024, 5, 100176. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-Ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Peter Holderith Silicon Anode EV Batteries Are The Real Deal, But It’s Complicated. Available online: https://insideevs.com/news/740255/silicon-anode-batteries-advantages-evs/ (accessed on 25 February 2025).
- Saidi, N.M.; Abdah, M.A.A.M.; Mustafa, M.N.; Walvekar, R.; Khalid, M.; Khosla, A. Advancements in Silicon Anodes for Enhanced Lithium-Ion Batteries Performance: Innovations Toward Next-Gen Superbatteries. Battery Energy 2025, e20240048. [Google Scholar] [CrossRef]
- Prachi Patel The Age of Silicon Is Here…for Batteries. Available online: https://spectrum.ieee.org/silicon-anode-battery (accessed on 24 February 2025).
- Saddique, J.; Wu, M.; Ali, W.; Xu, X.; Jiang, Z.-G.; Tong, L.; Zheng, H.; Hu, W. Opportunities and Challenges of Nano Si/C Composites in Lithium Ion Battery: A Mini Review. J. Alloys Compd. 2024, 978, 173507. [Google Scholar] [CrossRef]
- Choi, M.; Lee, E.; Sung, J.; Kim, N.; Ko, M. Comparison of Commercial Silicon-Based Anode Materials for the Design of a High-Energy Lithium-Ion Battery. Nano Res. 2024, 17, 5270–5277. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Hu, K.; Xu, B.; Wang, N.; Bai, Z.; Xu, X.; Yang, J. Nanostructure Designing and Hybridizing of High-Capacity Silicon-Based Anode for Lithium-Ion Batteries. Prog. Nat. Sci. Mater. Int. 2023, 33, 16–36. [Google Scholar] [CrossRef]
- Hossain, M.A.M.; Tiong, S.K.; Hannan, M.A.; Ker, P.J.; Fattah, I.M.R.; Mahlia, T.M.I. Recent Advances in Silicon Nanomaterials for Lithium-Ion Batteries: Synthesis Approaches, Emerging Trends, Challenges, and Opportunities. Sustain. Mater. Technol. 2024, 40, e00964. [Google Scholar] [CrossRef]
- Toki, G.F.I.; Hossain, M.K.; Rehman, W.U.; Manj, R.Z.A.; Wang, L.; Yang, J. Recent Progress and Challenges in Silicon-Based Anode Materials for Lithium-Ion Batteries. Ind. Chem. Mater. 2024, 2, 226–269. [Google Scholar] [CrossRef]
- Amardeep, A.; Freschi, D.J.; Wang, J.; Liu, J. Fundamentals, Preparation, and Mechanism Understanding of Li/Na/Mg-Sn Alloy Anodes for Liquid and Solid-State Lithium Batteries and Beyond. Nano Res. 2023, 16, 8191–8218. [Google Scholar] [CrossRef]
- Guo, S.; Feng, Y.; Wang, L.; Jiang, Y.; Yu, Y.; Hu, X. Architectural Engineering Achieves High-Performance Alloying Anodes for Lithium and Sodium Ion Batteries. Small 2021, 17, 2005248. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Wang, X. The Anode Materials for Lithium-Ion and Sodium-Ion Batteries Based on Conversion Reactions: A Review. ChemElectroChem 2023, 10, e202201151. [Google Scholar] [CrossRef]
- Ming, J.; Kwak, W.J.; Youn, S.J.; Ming, H.; Hassoun, J.; Sun, Y. Lithiation of an Iron Oxide-Based Anode for Stable, High-Capacity Lithium-Ion Batteries of Porous Carbon–Fe3O4/Li[Ni0.59Co0.16Mn0.25]O2. Energy Technol. 2014, 2, 778–785. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, R.; Mu, L.; Xu, S. Fe3O4 Anodes for Lithium Batteries: Production Techniques and General Applications. Comptes Rendus Chim. 2019, 22, 96–102. [Google Scholar] [CrossRef]
- Li, Q.; Han, N.; Chai, J.; Zhang, W.; Du, J.; Tian, H.; Liu, H.; Wang, G.; Tang, B. Strategies to Improve Metal-Organic Frameworks and Their Derived Oxides as Lithium Storage Anode Materials. Energy 2023, 282, 128378. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, B.; Li, C.; Zhu, H.; Liu, G. Review of Progress in the Application of Polytetrafluoroethylene-Based Battery Separators. ACS Appl. Mater. Interfaces 2024, 16, 63109–63128. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.U.; Cai, G.; Chen, Z. Metal-Organic Frameworks for the Enhancement of Lithium-Based Batteries: A Mini Review on Emerging Functional Designs. Adv. Sci. 2024, 11, 2305280. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A. Low Voltage Anode Materials for Lithium-Ion Batteries. Energy Storage Mater. 2017, 7, 157–180. [Google Scholar] [CrossRef]
- Qian, X.; Xu, Q.; Hang, T.; Shanmugam, S.; Li, M. Electrochemical Deposition of Fe3O4 Nanoparticles and Flower-like Hierarchical Porous Nanoflakes on 3D Cu-Cone Arrays for Rechargeable Lithium Battery Anodes. Mater. Des. 2017, 121, 321–334. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Effective Upcycling of NMC 111 to NMC 622 Cathodes by Hydrothermal Relithiation and Ni-Enriching Annealing. Next Energy 2024, 4, 100122. [Google Scholar] [CrossRef]
- Evro, S.; Ajumobi, A.; Mayon, D.; Tomomewo, O.S. Navigating Battery Choices: A Comparative Study of Lithium Iron Phosphate and Nickel Manganese Cobalt Battery Technologies. Future Batter. 2024, 4, 100007. [Google Scholar] [CrossRef]
- Celadon, A.; Sun, H.; Sun, S.; Zhang, G. Review Batteries for Electric Vehicles: Technical Advancements, Environmental Challenges, and Market Perspectives. Susmat 2024, 4, e234. [Google Scholar] [CrossRef]
- EVDB Which Cars Have LFP Batteries? Available online: https://evdb.nz/ev-battery (accessed on 14 February 2025).
- Zeekr The New Zeekr 5.5C EV Batteries Can Charge at the Fastest Rate in the World. Available online: https://www.zeekrlife.com/global/posts/the-new-zeekr-5-5c-ev-batteries-can-charge-at-the-fastest-rate-in-the-world (accessed on 14 February 2025).
- Mark Andrews General Motors to Produce Ultra-Fast 6C Charging EV in China. Available online: https://carnewschina.com/2024/09/26/general-motors-to-produce-ultra-fast-6c-charging-ev-in-china/ (accessed on 14 February 2025).
- Mark Andrews Geely’s Aegis Short Blade LFP Battery—Full Details Including Extreme Safety. Available online: https://carnewschina.com/2024/06/28/geelys-aegis-short-blade-lfp-battery-full-details-including-extreme-safety/ (accessed on 14 February 2025).
- Green Car Congress, “Gotion High-Tech Launches New L600 LMFP Astroinno Battery; Single-Cell Energy Density of 240Wh/kg”. Available online: https://www.greencarcongress.com/2023/05/20230524-astroinno.html (accessed on 20 February 2025).
- Hasan, S.; Islam, M.S.; Bashar, S.A.; Tamzid, A.A.N.; Hossain, R.B.; Haque, M.A.; Faishal, R. Beyond Lithium-Ion: The Promise and Pitfalls of BYD’s Blade Batteries for Electric Vehicles. E3S Web Conf. 2023, 469, 00005. [Google Scholar] [CrossRef]
- Hasselwander, S.; Meyer, M.; Österle, I. Techno-Economic Analysis of Different Battery Cell Chemistries for the Passenger Vehicle Market. Batteries 2023, 9, 379. [Google Scholar] [CrossRef]
- Waseem, M.; Lakshmi, G.S.; Ahmad, M.; Suhaib, M. Energy Storage Technology and Its Impact in Electric Vehicle: Current Progress and Future Outlook. Next Energy 2025, 6, 100202. [Google Scholar] [CrossRef]
- Almadani, M.M.M.; Emmanuel Oni, O.; Mary Longe, O.; Olatomiwa, L. Comparison of Battery Chemistries for Electric Vehicle Applications. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Pretoria, South Africa, 23 April 2024; IEOM Society International: Johannesburg, South Africa; Pretoria, South Africa, 2024. [Google Scholar]
- Sharma, S.; Panwar, A.K.; Tripathi, M.M. Storage Technologies for Electric Vehicles. J. Traffic Transp. Eng. (Engl. Ed.) 2020, 7, 340–361. [Google Scholar] [CrossRef]
- Takami, N.; Inagaki, H.; Tatebayashi, Y.; Saruwatari, H.; Honda, K.; Egusa, S. High-Power and Long-Life Lithium-Ion Batteries Using Lithium Titanium Oxide Anode for Automotive and Stationary Power Applications. J. Power Sources 2013, 244, 469–475. [Google Scholar] [CrossRef]
- Vishnumurthy, K.A.; Girish, K.H. A Comprehensive Review of Battery Technology for E-Mobility. J. Indian Chem. Soc. 2021, 98, 100173. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An Overview of Modification Strategies to Improve LiNi0.8Co0.1Mn0.1O2 (NCM811) Cathode Performance for Automotive Lithium-Ion Batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Dasari, H.A.; Rammohan, A. Evaluating Fault Detection Strategies for Lithium-Ion Batteries in Electric Vehicles. Eng. Res. Express 2024, 6, 032302. [Google Scholar] [CrossRef]
- Karmakar, A.; Zhou, H.; Vishnugopi, B.S.; Mukherjee, P.P. Li-Ion Battery Safety: A Perspective on Hierarchy of Scales. Annu. Rev. Heat Transf. 2023, 26, 11–68. [Google Scholar] [CrossRef]
- Jia, Z.; Jin, K.; Mei, W.; Qin, P.; Jinhua, S.; Wang, Q. Advances and Perspectives in Fire Safety of Lithium-Ion Battery Energy Storage Systems. eTransportation 2025, 24, 100390. [Google Scholar] [CrossRef]
- Comanescu, C. Ensuring Safety and Reliability: An Overview of Lithium-Ion Battery Service Assessment. Batteries 2025, 11, 6. [Google Scholar] [CrossRef]
- Menale, C.; Mancino, A.N.; Vitiello, F.; Sglavo, V.; Vellucci, F.; Caiazzo, L.; Bubbico, R. Analysis of the Thermal Runaway Mitigation Performances of Dielectric Fluids During Overcharge Abuse Tests of Lithium-Ion Cells with Lithium Titanate Oxide Anodes. WEVJ 2024, 15, 554. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A Review of Thermal Runaway Prevention and Mitigation Strategies for Lithium-Ion Batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and Explosion Characteristics of Vent Gas from Lithium-Ion Batteries after Thermal Runaway: A Comparative Study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]
- Shan, T.; Zhang, P.; Wang, Z.; Zhu, X. Insights into Extreme Thermal Runaway Scenarios of Lithium-Ion Batteries Fire and Explosion: A Critical Review. J. Energy Storage 2024, 88, 111532. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, H.; Dong, X.; Hai, B. Research Progress on Thermal Runaway Warning Methods and Fire Extinguishing Technologies for Lithium-Ion Batteries. WEVJ 2025, 16, 81. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Wang, H.; Li, H.; Bao, H.; Zhao, Z.; Liu, B. Mechanical Issues of Lithium-Ion Batteries in Road Traffic Conditions: A Review. Thin-Walled Struct. 2024, 201, 111985. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, X.; Tran, M.-K.; Fowler, M.; Ouyang, M.; Burke, A.F. Battery Safety: Fault Diagnosis from Laboratory to Real World. J. Power Sources 2024, 598, 234111. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, F.; Gao, Z.; Liu, M.; Wang, J.; Kou, Z.; Lin, Y.; Li, Y.; Gao, L.; Chen, Y.; et al. Review of Mechanical Abuse Related Thermal Runaway Models of Lithium-Ion Batteries at Different Scales. J. Energy Storage 2023, 64, 107145. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Q.; Wang, S.; Song, Y.; Shi, B.; He, J. Aging and Post-Aging Thermal Safety of Lithium-Ion Batteries under Complex Operating Conditions: A Comprehensive Review. J. Power Sources 2024, 623, 235453. [Google Scholar] [CrossRef]
- Cui, Z.; Manthiram, A. Thermal Stability and Outgassing Behaviors of High-Nickel Cathodes in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202307243. [Google Scholar] [CrossRef]
- Liang, T.; Cheng, D.; Chen, J.; Wu, X.; Xiong, H.; Yu, S.; Zhang, Z.; Liu, H.; Liu, S.; Song, X. Evolution from Passive to Active Components in Lithium Metal and Lithium-Ion Batteries Separators. Mater. Today Energy 2024, 45, 101684. [Google Scholar] [CrossRef]
- Ha, J.; Lee, J.; Kim, Y.-T.; Choi, J. Effective Approaches to Preventing Dendrite Growth in Lithium Metal Anodes: A Review. Appl. Chem. Eng. 2023, 34, 365–382. [Google Scholar] [CrossRef]
- Mishra, R.; Anne, M.; Das, S.; Chavva, T.; Shelke, M.V.; Pol, V.G. Glory of Fire Retardants in Li-Ion Batteries: Could They Be Intrinsically Safer? Adv. Sustain. Syst. 2024, 8, 2400273. [Google Scholar] [CrossRef]
- Yun, S.; Liang, X.; Xi, J.; Liao, L.; Cui, S.; Chen, L.; Li, S.; Hu, Q. Electrolytes for High-Safety Lithium-Ion Batteries at Low Temperature: A Review. Polymers 2024, 16, 2661. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, C.; Wang, F.; Manthiram, A. Navigating Thermal Stability Intricacies of High-Nickel Cathodes for High-Energy Lithium Batteries. Nat. Energy 2025, 10, 490–501. [Google Scholar] [CrossRef]
- Yerkinbekova, Y.; Kumarov, A.; Tatykayev, B.; Mentbayeva, A.; Repo, E.; Laakso, E. Ni-Rich Cathode Materials with Concentration Gradients for High-Energy and Safe Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2025, 626, 235686. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, D.; Xu, Y.; Chen, L.; Zhang, X.; Ma, Q. Safety Issues of Layered Nickel-Based Cathode Materials for Lithium-Ion Batteries: Origin, Strategies and Prospects. Batteries 2023, 9, 156. [Google Scholar] [CrossRef]
- Bin Abu Sofian, A.D.A.; Imaduddin, I.S.; Majid, S.R.; Kurniawan, T.A.; Chew, K.W.; Lay, C.-H.; Show, P.L. Nickel-Rich Nickel–Cobalt–Manganese and Nickel–Cobalt–Aluminum Cathodes in Lithium-Ion Batteries: Pathways for Performance Optimization. J. Clean. Prod. 2024, 435, 140324. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Yuan, F.; Lyu, M.; Yang, P.; Zhang, L.; Zhou, M.; Wang, L.; Zhang, S.; Wang, L. Ni-Rich Cathode Materials for Stable High-Energy Lithium-Ion Batteries. Nano. Energy 2024, 126, 109620. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A Review of Lithium Ion Battery Failure Mechanisms and Fire Prevention Strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Dai, Y.; Panahi, A. Thermal Runaway Process in Lithium-Ion Batteries: A Review. Next Energy 2025, 6, 100186. [Google Scholar] [CrossRef]
- Shen, R.; Quan, Y.; McIntosh, J.D.; Salem, A.; Wang, Q. Fire Safety of Battery Electric Vehicles: Hazard Identification, Detection, and Mitigation. SAE Int. J. Electrified Veh. 2024, 13, 279–294. [Google Scholar] [CrossRef]
- Choudhary, A.; Fatima, S.; Panigrahi, B.K. State-of-the-Art Technologies in Fault Diagnosis of Electric Vehicles: A Component-Based Review. IEEE Trans. Transp. Electrif. 2023, 9, 2324–2347. [Google Scholar] [CrossRef]
- He, H.; Sun, F.; Wang, Z.; Lin, C.; Zhang, C.; Xiong, R.; Deng, J.; Zhu, X.; Xie, P.; Zhang, S.; et al. China’s Battery Electric Vehicles Lead the World: Achievements in Technology System Architecture and Technological Breakthroughs. Green Energy Intell. Transp. 2022, 1, 100020. [Google Scholar] [CrossRef]
- European Parliament. Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on Batteries and Accumulators and Waste Batteries and Accumulators and Repealing Directive 91/157/EEC; European Parliament and the Council: Brussels, Belgium, 2018.
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and Possibilities for Aqueous Battery Systems. Commun. Mater. 2023, 4, 37. [Google Scholar] [CrossRef]
- Guan, S.; Peng, Q.; Guo, X.; Zheng, Y.; Liao, E.; Sun, S.; Shin, K.; Liu, B.; Zhou, X.; Zou, C.; et al. A Review of the Advances and Prospects of Aqueous Dual-Ion Batteries. Chem. Eng. J. 2024, 493, 152864. [Google Scholar] [CrossRef]
- Khan, T.; Garg, A.K.; Gupta, A.; Madan, A.K.; Jain, P.K. Comprehensive Review on Latest Advances on Rechargeable Batteries. J. Energy Storage 2023, 57, 106204. [Google Scholar] [CrossRef]
- Heo, J.J.; Ryu, J. A Critical Review of Ultrafast Charging Dual-Ion Batteries. Korean J. Chem. Eng. 2024. [CrossRef]
- Nzereogu, P.U.; Oyesanya, A.; Ogba, S.N.; Ayanwunmi, S.O.; Sobajo, M.S.; Chimsunum, V.C.; Ayanwunmi, V.O.; Amoo, M.O.; Adefemi, O.T.; Chukwudi, C.C. Solid-State Lithium-Ion Battery Electrolytes: Revolutionizing Energy Density and Safety. Hybrid Adv. 2025, 8, 100339. [Google Scholar] [CrossRef]
- Koech, A.K.; Mwandila, G.; Mulolani, F. A Review of Improvements on Electric Vehicle Battery. Heliyon 2024, 10, e34806. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, M.; Versaci, D.; Francia, C.; Bodoardo, S.; Amici, J. Enhancing the Safety and Stability of Lithium Metal Batteries through the Use of Composite Ionogels. Electrochim. Acta 2023, 463, 142857. [Google Scholar] [CrossRef]
- Umair, M.; Zhou, S.; Li, W.; Rana, H.T.H.; Yang, J.; Cheng, L.; Li, M.; Yu, S.; Wei, J. Oxide Solid Electrolytes in Solid-State Batteries. Batter. Amp. Supercaps 2024, e202400667. [Google Scholar] [CrossRef]
- Rufino Júnior, C.A.; Sanseverino, E.R.; Gallo, P.; Amaral, M.M.; Koch, D.; Kotak, Y.; Diel, S.; Walter, G.; Schweiger, H.-G.; Zanin, H. Unraveling the Degradation Mechanisms of Lithium-Ion Batteries. Energies 2024, 17, 3372. [Google Scholar] [CrossRef]
- Ai, S.; Wu, X.; Wang, J.; Li, X.; Hao, X.; Meng, Y. Research Progress on Solid-State Electrolytes in Solid-State Lithium Batteries: Classification, Ionic Conductive Mechanism, Interfacial Challenges. Nanomaterials 2024, 14, 1773. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, W.; Su, S.; Jiang, X.; Bando, Y.; Zhang, B.; Ma, Z.; Wang, X. Advances of Solid Polymer Electrolytes with High-Voltage Stability. Next Mater. 2025, 7, 100364. [Google Scholar] [CrossRef]
- Joshi, A.; Mishra, D.K.; Singh, R.; Zhang, J.; Ding, Y. A Comprehensive Review of Solid-State Batteries. Appl. Energy 2025, 386, 125546. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Zhu, J.; Hao, S.-M.; Qin, X.; Fan, L.-Z.; Zhang, L.; Zhou, W. Multi-Layered Electrolytes for Solid-State Lithium Batteries. Next Energy 2023, 1, 100042. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, T.; Zhao, B.; Shen, F.; Jin, H.; Han, X. Recent Advances in Organic-Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Energy Storage Mater. 2021, 34, 388–416. [Google Scholar] [CrossRef]
- Sashmitha, K.; Rani, M.U. A Comprehensive Review of Polymer Electrolyte for Lithium-Ion Battery. Polym. Bull. 2023, 80, 89–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Du, J.; Zhang, X.; Chen, A.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Huang, X.; He, X. Lithium Bis(Trifluoromethanesulfonyl)Imide (LiTFSI): A Prominent Lithium Salt in Lithium-Ion Battery Electrolytes—Fundamentals, Progress, and Future Perspectives. Adv. Funct. Mater. 2024, 34, 2408319. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Xin, M.; Chen, S.; Xu, P.; Li, D.; Liu, J.; Wang, Y.; Xie, H.; Sun, X.; et al. Ultra-Thin and High-Voltage-Stable Bi-Phasic Solid Polymer Electrolytes for High-Energy-Density Li Metal Batteries. Nano. Energy 2024, 119, 109054. [Google Scholar] [CrossRef]
- Pereira, J.; Souza, R.; Moreira, A.; Moita, A. An Overview of the Ionic Liquids and Their Hybrids Operating in Electrochemical Cells and Capacitors. Ionics 2024, 30, 4343–4385. [Google Scholar] [CrossRef]
- Inada, R.; Yasuda, S.; Hosokawa, H.; Saito, M.; Tojo, T.; Sakurai, Y. Formation and Stability of Interface between Garnet-Type Ta-Doped Li7La3Zr2O12 Solid Electrolyte and Lithium Metal Electrode. Batteries 2018, 4, 26. [Google Scholar] [CrossRef]
- Ahmad, H.; Kubra, K.T.; Butt, A.; Nisar, U.; Iftikhar, F.J.; Ali, G. Recent Progress, Challenges, and Perspectives in the Development of Solid-State Electrolytes for Sodium Batteries. J. Power Sources 2023, 581, 233518. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Wang, X.; Zhou, A.; Yang, Z. Research Progress and Application Prospect of Solid-State Electrolytes in Commercial Lithium-Ion Power Batteries. Energy Storage Mater. 2021, 35, 70–87. [Google Scholar] [CrossRef]
- Lannelongue, P.; Lindberg, S.; Gonzalo, E.; Golov, A.; Bonilla, F.; Lopez Del Amo, J.M.; Marchandier, T.; Tron, A.; Carrasco, J.; López-Aranguren, P. Stable Cycling of Halide Solid State Electrolyte Enabled by a Dynamic Layered Solid Electrolyte Interphase between Li Metal and Li3YCl4Br2. Energy Storage Mater. 2024, 72, 103733. [Google Scholar] [CrossRef]
- He, B.; Zhang, F.; Xin, Y.; Xu, C.; Hu, X.; Wu, X.; Yang, Y.; Tian, H. Halogen Chemistry of Solid Electrolytes in All-Solid-State Batteries. Nat. Rev. Chem. 2023, 7, 826–842. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Su, W.; Hwang, B.J. Halide Solid-State Electrolytes: Stability and Application for High Voltage All-Solid-State Li Batteries. Adv. Energy Mater. 2023, 13, 2202854. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Fu, C.; Yin, G.; Wang, L.; Wu, Y.; Huo, H. Advancements and Challenges in Organic–Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries. Nano-Micro Lett. 2025, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, C.; Zhang, W.; Xia, Y.; Huang, H.; Gan, Y.; He, X.; Xia, X.; Tao, X.; Zhang, J. A Critical Review on Composite Solid Electrolytes for Lithium Batteries: Design Strategies and Interface Engineering. J. Energy Chem. 2023, 84, 189–209. [Google Scholar] [CrossRef]
- Polymer Electrolytes and Their Composites for Energy Storage/Conversion Devices; Emerging materials and technologies; Sharma, A.L., Arya, A., Gaur, A., Eds.; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-003-20866-2. [Google Scholar]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium Solid-State Batteries: State-of-the-Art and Challenges for Materials, Interfaces and Processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Zhang, Q.; Yan, L.; Wang, T.-B.; Hou, P.-Y. A Review of Interfaces within Solid-State Electrolytes: Fundamentals, Issues and Advancements. Chem. Eng. J. 2022, 437, 135179. [Google Scholar] [CrossRef]
- Hun, Q.; Lan, L.; Lu, X.; Hu, Q.; Liang, X.; Guo, Y.; Wang, Y. Bilayer Heterostructure Electrolytes Were Prepared by a UV-Curing Process for High Temperature Lithium-Ion Batteries. Polymers 2024, 16, 2972. [Google Scholar] [CrossRef]
- Scheller, M.; Durdel, A.; Frank, A.; Jossen, A. Model-Based Performance Evaluation of Hybrid Solid-State Batteries: Impact of Laser-Ablated Geometrical Structures. Batteries 2024, 10, 392. [Google Scholar] [CrossRef]
- Burazer, S.; Popović, J. Mechanochemical Synthesis of Solid-State Electrolytes. Inorganics 2024, 12, 54. [Google Scholar] [CrossRef]
- Van Der Ven, A.; McMeeking, R.M.; Clément, R.J.; Garikipati, K. Ferroelastic Toughening: Can It Solve the Mechanics Challenges of Solid Electrolytes? Curr. Opin. Solid State Mater. Sci. 2023, 27, 101056. [Google Scholar] [CrossRef]
- Ning, Z.; Li, G.; Melvin, D.L.R.; Chen, Y.; Bu, J.; Spencer-Jolly, D.; Liu, J.; Hu, B.; Gao, X.; Perera, J.; et al. Dendrite Initiation and Propagation in Lithium Metal Solid-State Batteries. Nature 2023, 618, 287–293. [Google Scholar] [CrossRef]
- John Carey Solid-State Batteries Could Revolutionize EVs and More—If They Can Surmount Technical and Financial Hurdles. Available online: https://www.pnas.org/doi/10.1073/pnas.2425219121 (accessed on 5 February 2025).
- Biba, Jacob 7 New Battery Technologies to Watch. Available online: https://builtin.com/hardware/new-battery-technologies (accessed on 27 February 2025).
- Tan, A.K.X.; Paul, S. Beyond Lithium: Future Battery Technologies for Sustainable Energy Storage. Energies 2024, 17, 5768. [Google Scholar] [CrossRef]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Biemolt, J.; Jungbacker, P.; Van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond Lithium-Based Batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef]
- Qi, T.; Xiong, K.; Zhang, X. Research Progress of Carbon Materials in the Anodes of Sodium-Ion Batteries. J. Power Sources 2025, 626, 235721. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.S.; Liang, H.; Zhang, W.; Zhu, Y. Synthesis Strategies of Hard Carbon Anodes for Sodium-Ion Batteries. Mater. Rep. Energy 2024, 4, 100268. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.; Qiao, X.; Wang, R.; Hu, C.; Chen, T.; Niu, Y.; Zhong, B.; Wu, Z.; Guo, X. The Chance of Sodium Titanate Anode for the Practical Sodium-Ion Batteries. Chin. J. Chem. Eng. 2024, 72, 226–244. [Google Scholar] [CrossRef]
- Wei, C.; Qu, D.; Li, Q.; Sun, Z.; Song, Z.; Guan, H.; Niu, L. Recent Advances on Transition Metal Chalcogenide for Sodium-Ion Batteries. Batteries 2023, 9, 467. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Wishart, J.F.; Abraham, D.P. What Makes Fluoroethylene Carbonate Different? J. Phys. Chem. C 2015, 119, 14954–14964. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Zheng, Y.; Luo, Y.; Jiang, X.; Wang, Y.; Wang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; et al. Sodium-Ion Battery at Low Temperature: Challenges and Strategies. Nanomaterials 2024, 14, 1604. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, Y.; Sun, Q.; Zheng, Y.; Zhang, Y.; Zhang, M.; Zhang, D.; Zhang, Z.; Jiang, Y. Research Progress of Modified Metal Current Collectors in Sodium Metal Anodes. Chin. Chem. Lett. 2024, 36, 109997. [Google Scholar] [CrossRef]
- Chen, C.; Yao, W.; Tang, Y. Emerging Solutions to Enable the Efficient Use of Sodium Metal Anodes: Progress and Perspectives. Adv Funct Mater. 2024, 34, 2310833. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Weng, S.; Ding, F.; Qi, X.; Lu, J.; Li, Y.; Zhang, X.; Rong, X.; Lu, Y.; et al. Interfacial Engineering to Achieve an Energy Density of over 200 Wh Kg−1 in Sodium Batteries. Nat Energy 2022, 7, 511–519. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Ji, W.; Tapia-Ruiz, N.; Winter, M.; Li, J. Structural Evolution Mechanisms and Design Strategies of Layered Cathodes for Sodium-Ion Batteries. Next Energy 2025, 7, 100241. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhao, S.; Liu, Y.; Yang, Y.; Yu, H.; Lee, S.; Lee, G.-H.; Kang, Y.-M.; Liu, R.; et al. Tuning Local Chemistry of P2 Layered-Oxide Cathode for High Energy and Long Cycles of Sodium-Ion Battery. Nat Commun 2021, 12, 2256. [Google Scholar] [CrossRef]
- Sathiya, M.; Jacquet, Q.; Doublet, M.; Karakulina, O.M.; Hadermann, J.; Tarascon, J. A Chemical Approach to Raise Cell Voltage and Suppress Phase Transition in O3 Sodium Layered Oxide Electrodes. Adv. Energy Mater. 2018, 8, 1702599. [Google Scholar] [CrossRef]
- Zheng, B.; Qian, H.; Cheng, G.; Yuan, C.; Cheng, Y.; Wang, M.-S.; Liu, X.; Xiang, Y. Ultrafast Lattice Engineering for High Energy Density and High-Rate Sodium-Ion Layered Oxide Cathodes. Energy Storage Mater. 2025, 74, 103868. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Zhang, Z.; Tang, Y.; Jia, C.; Ji, X.; Wang, H. Understanding Crystal Structures, Ion Diffusion Mechanisms and Sodium Storage Behaviors of NASICON Materials. Energy Storage Mater. 2021, 34, 171–193. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Li, W.; Zhao, K.; Liu, M.; Wu, Q.; Yang, Y.; He, G.; Parkin, I.P.; Shearing, P.R.; et al. Sodium Superionic Conductors (NASICONs) as Cathode Materials for Sodium-Ion Batteries. Electrochem. Energy Rev. 2021, 4, 793–823. [Google Scholar] [CrossRef]
- Deng, L.; Yu, F.-D.; Xia, Y.; Jiang, Y.-S.; Sui, X.-L.; Zhao, L.; Meng, X.-H.; Que, L.-F.; Wang, Z.-B. Stabilizing Fluorine to Achieve High-Voltage and Ultra-Stable Na3V2(PO4)2F3 Cathode for Sodium Ion Batteries. Nano Energy 2021, 82, 105659. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhao, X.; He, L.; Liu, H.; Song, Y.; Sun, S.; Li, Q.; Xing, X.; Chen, J. A Novel NASICON-Type Na4 MnCr(PO4)3 Demonstrating the Energy Density Record of Phosphate Cathodes for Sodium-Ion Batteries. Adv. Mater. 2020, 32, 1906348. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Feng, P.; Zhou, M.; Wang, K.; Wang, Y.; Zaghib, K.; Jiang, K. High-Performance Manganese Hexacyanoferrate with Cubic Structure as Superior Cathode Material for Sodium-Ion Batteries. Adv Funct Mater. 2020, 30, 1908754. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The Scale-up and Commercialization of Nonaqueous Na-Ion Battery Technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Liu, X.; Yang, Z.; He, X.; Li, L.; Qiao, Y.; Chen, W.; Zeng, R.; Wang, Y.; et al. Organic Cathode Materials for Sodium-Ion Batteries: From Fundamental Research to Potential Commercial Application. Adv Funct Mater. 2022, 32, 2107718. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. 2014, 126, 6002–6006. [Google Scholar] [CrossRef]
- Chu, S.; Guo, S.; Zhou, H. Advanced Cobalt-Free Cathode Materials for Sodium-Ion Batteries. Chem. Soc. Rev. 2021, 50, 13189–13235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, J.; Guo, Z. Challenges and Future Perspectives on Sodium and Potassium Ion Batteries for Grid-Scale Energy Storage. Mater. Today 2021, 50, 400–417. [Google Scholar] [CrossRef]
- Meng, J.; Jia, G.; Yang, H.; Wang, M. Recent Advances for SEI of Hard Carbon Anode in Sodium-Ion Batteries: A Mini Review. Front. Chem. 2022, 10, 986541. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Zhang, X.-L.; Zhao, X.-X.; Zhao, Y.-Y.; Aravindan, V.; Liu, Y.-H.; Geng, H.; Wu, X.-L. Electrode/Electrolyte Additives for Practical Sodium-Ion Batteries: A Mini Review. Inorg. Chem. Front. 2023, 10, 37–48. [Google Scholar] [CrossRef]
- Yue, Y.; Jia, Z.; Li, Y.; Wen, Y.; Lei, Q.; Duan, Q.; Sun, J.; Wang, Q. Thermal Runaway Hazards Comparison between Sodium-Ion and Lithium-Ion Batteries Using Accelerating Rate Calorimetry. Process Saf. Environ. Prot. 2024, 189, 61–70. [Google Scholar] [CrossRef]
- Bhutia, P.T.; Grugeon, S.; El Mejdoubi, A.; Laruelle, S.; Marlair, G. Safety Aspects of Sodium-Ion Batteries: Prospective Analysis from First Generation Towards More Advanced Systems. Batteries 2024, 10, 370. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, G.; Lin, J.; Zhu, J.; Pan, J.; Fang, G.; Liang, S.; Cao, X. A Multicationic-Substituted Configurational Entropy-Enabled NASICON Cathode for High-Power Sodium-Ion Batteries. Nano Energy 2024, 128, 109812. [Google Scholar] [CrossRef]
- Liao, J.; Yuan, Z.; Hu, Q.; Sheng, X.; Song, L.; Xu, Y.; Du, Y.; Zhou, X. Heat-Resistant Carbon-Coated Potassium Magnesium Hexacyanoferrate Nanoplates for High-Performance Potassium-Ion Batteries. Angew Chem Int Ed 2024, 63, e202409145. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Sun, M.; Wang, W.; Chen, C.; Altunkaya, M.; Emwas, A.; Han, Y.; Schwingenschlögl, U.; Alshareef, H.N. Direct Pyrolysis of Supermolecules: An Ultrahigh Edge-Nitrogen Doping Strategy of Carbon Anodes for Potassium-Ion Batteries. Adv. Mater. 2020, 32, 2000732. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, B.; Jing, Z.; Chen, M.; Zhai, Y.; Kong, Z.; Iqbal, S.; Zeng, S.; Sun, X.; et al. Controllable Growth of Crystal Facets Enables Superb Cycling Stability of Anode Material for Potassium Ion Batteries. Adv Funct Mater. 2024, 34, 2406988. [Google Scholar] [CrossRef]
- Ji, B.; Yao, W.; Zheng, Y.; Kidkhunthod, P.; Zhou, X.; Tunmee, S.; Sattayaporn, S.; Cheng, H.-M.; He, H.; Tang, Y. A Fluoroxalate Cathode Material for Potassium-Ion Batteries with Ultra-Long Cyclability. Nat Commun 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current Status and Future Directions of Multivalent Metal-Ion Batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Shah, R.; Mittal, V.; Matsil, E.; Rosenkranz, A. Magnesium-Ion Batteries for Electric Vehicles: Current Trends and Future Perspectives. Adv. Mech. Eng. 2021, 13, 16878140211003398. [Google Scholar] [CrossRef]
- Dominko, R.; Bitenc, J.; Berthelot, R.; Gauthier, M.; Pagot, G.; Di Noto, V. Magnesium Batteries: Current Picture and Missing Pieces of the Puzzle. J. Power Sources 2020, 478, 229027. [Google Scholar] [CrossRef]
- Mizrahi, O.; Amir, N.; Pollak, E.; Chusid, O.; Marks, V.; Gottlieb, H.; Larush, L.; Zinigrad, E.; Aurbach, D. Electrolyte Solutions with a Wide Electrochemical Window for Rechargeable Magnesium Batteries. J. Electrochem. Soc. 2008, 155, A103. [Google Scholar] [CrossRef]
- Doe, R.E.; Han, R.; Hwang, J.; Gmitter, A.J.; Shterenberg, I.; Yoo, H.D.; Pour, N.; Aurbach, D. Novel, Electrolyte Solutions Comprising Fully Inorganic Salts with High Anodic Stability for Rechargeable Magnesium Batteries. Chem. Commun. 2014, 50, 243–245. [Google Scholar] [CrossRef]
- Liu, F.; Guoqin, C.; Jinjin, B.; Honghong, L.; Yan, Z.; Guosheng, S.; Aiguo, Z.; Li, Z.F.; Junhua, H. Recent Advances Based on Mg Anodes and Their Interfacial Modulation in Mg Batteries. J. of Magnesium and Alloys 2022, 10, 2699–2716. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, J.C.; Velázquez, J.M. Structure–Reactivity Relationships in Chevrel Phase Electrocatalysts for Small-Molecule Reduction Reactions. Curr. Opin. Electrochem. 2022, 34, 101002. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, B.; Li, S.; Lian, X.; Luo, Y.; Shen, X.; Xu, C.; Wang, J.; Pan, F. Recent Advances and Prospects of Chalcogenide Cathodes for Rechargeable Magnesium Batteries. Adv Funct Mater. 2024, 34, 2405586. [Google Scholar] [CrossRef]
- Shin, C.-H.; Lee, H.-Y.; Gyan-Barimah, C.; Yu, J.-H.; Yu, J.-S. Magnesium: Properties and Rich Chemistry for New Material Synthesis and Energy Applications. Chem. Soc. Rev. 2023, 52, 2145–2192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research Advances of Magnesium and Magnesium Alloys Worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Zhang, G.; Jiang, Y.; Yin, Y.; Xiong, F.; Li, Q.; Huang, D.; Zhang, Q.; Gu, L.; et al. Fast and Stable Mg2+ Intercalation in a High Voltage NaV2O2(PO4)2F/rGO Cathode Material for Magnesium-Ion Batteries. Sci. China Mater. 2020, 63, 1651–1662. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Wang, D.; Chen, A.; Li, X.; Huang, Z.; Liang, G.; Wang, Y.; Zhi, C. Tellurium: A High-Performance Cathode for Magnesium Ion Batteries Based on a Conversion Mechanism. ACS Nano. 2022, 16, 5349–5357. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, B.; Liu, H.; Tan, J.; Li, H.; Zhang, X.; Diao, J.; Yue, J.; Huang, G.; Wang, J.; et al. H2 O-Mg2+ Waltz-Like Shuttle Enables High-Capacity and Ultralong-Life Magnesium-Ion Batteries. Adv. Sci. 2024, 11, 2401005. [Google Scholar] [CrossRef]
- Han, W.; Li, Z.; Wang, R. Progress Study on the Development of Lithium-Sulfur Batteries. IOP Conf. Ser. Earth Environ. Sci. 2022, 1011, 012004. [Google Scholar] [CrossRef]
- Dörfler, S.; Althues, H.; Härtel, P.; Abendroth, T.; Schumm, B.; Kaskel, S. Challenges and Key Parameters of Lithium-Sulfur Batteries on Pouch Cell Level. Joule 2020, 4, 539–554. [Google Scholar] [CrossRef]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Seetha Lakshmi, K.C. Beyond Lithium-Ion: Emerging Frontiers in next-Generation Battery Technologies. Front. Batter. Electrochem. 2024, 3, 1377192. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Jia, S.; Zhao, Q.; Zheng, Q.; Ma, Y.; Ma, T.; Li, X. Recent Advances in Inhibiting Shuttle Effect of Polysulfide in Lithium-Sulfur Batteries. J. Energy Storage 2023, 72, 108372. [Google Scholar] [CrossRef]
- Sun, J.; Wang, T.; Gao, Y.; Pan, Z.; Hu, R.; Wang, J. Will lithium-sulfur Batteries Be the next beyond-lithium Ion Batteries and Even Much Better? InfoMat 2022, 4, e12359. [Google Scholar] [CrossRef]
- GELION Lithium-Sulfur Cell High Energy Density Milestone Achieved. Available online: https://gelion.com/news/lithium-sulfur-cell-high-energy-density-milestone-achieved/ (accessed on 5 March 2025).
- Ni, W. Perspectives on Advanced Lithium–Sulfur Batteries for Electric Vehicles and Grid-Scale Energy Storage. Nanomaterials 2024, 14, 990. [Google Scholar] [CrossRef]
- Oh, B.; Yoon, B.; Ahn, S.; Jang, J.; Lim, D.; Seo, I. High-Energy-Density Lithium–Sulfur Battery Based on a Lithium Polysulfide Catholyte and Carbon Nanofiber Cathode. Energies 2024, 17, 5258. [Google Scholar] [CrossRef]
- Leckie, T.J.; Robertson, S.D.; Brightman, E. Recent Advances in in Situ/Operando Characterization of Lithium–Sulfur Batteries. Energy Adv. 2024, 3, 2479–2502. [Google Scholar] [CrossRef]
- Sun, Y.-K. Emerging All-Solid-State Lithium–Sulfur Batteries: Holy Grails for Future Secondary Batteries. ACS Energy Lett. 2024, 9, 5092–5095. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, B.; Huang, S.; Han, D. Review on Multi-Functional Separator for Li-S Batteries. Sustain. Polym. Energy 2023, 1, 10003. [Google Scholar] [CrossRef]
- Ovc-Okene, D.; Shankar, L.S.; Vizintin, A.; Kun, R. Revitalizing Li–S Batteries: The Power of Electrolyte Additives. RSC Adv. 2025, 15, 5381–5404. [Google Scholar] [CrossRef]
- Bi, C.-X.; Hou, L.-P.; Li, Z.; Zhao, M.; Zhang, X.-Q.; Li, B.-Q.; Zhang, Q.; Huang, J.-Q. Protecting Lithium Metal Anodes in Lithium–Sulfur Batteries: A Review. Energy Mater Adv. 2023, 4, 0010. [Google Scholar] [CrossRef]
- Jan, W.; Khan, A.D.; Iftikhar, F.J.; Ali, G. Recent Advancements and Challenges in Deploying Lithium Sulfur Batteries as Economical Energy Storage Devices. J. Energy Storage 2023, 72, 108559. [Google Scholar] [CrossRef]
- Tiwari, S.; Yadav, V.; Poonia, A.K.; Pal, D. Exploring Advances in Sulfur Composite Cathodes for Lithium-Sulfur Batteries: A Comprehensive Review. J. Energy Storage 2024, 94, 112347. [Google Scholar] [CrossRef]
- Cai, D.; Zheng, F.; Li, Y.; Zhang, C.; Qin, Z.; Li, W.; Liu, Y.; Li, A.; Zhang, J. Design of Coatings for Sulfur-Based Cathode Materials in Lithium-Sulfur Batteries: A Review. Chem. Asian J. 2024, 19, e202400099. [Google Scholar] [CrossRef]
- Sandeep Rao, K.; Dutta Pathak, D.; Mandal, B.P.; Debnath, A.K.; Tyagi, A.K. Lithium–Sulfur Battery Performance Enhancement by Optimal Loading of Graphene Oxide on Separator with MXene/Reduced Graphene Oxide/Sulfur Cathode. Mater. Today Sustain. 2023, 23, 100414. [Google Scholar] [CrossRef]
- Innocenti, A.; Bresser, D.; Garche, J.; Passerini, S. A Critical Discussion of the Current Availability of Lithium and Zinc for Use in Batteries. Nat. Commun 2024, 15, 4068. [Google Scholar] [CrossRef]
- Mageto, T.; Bhoyate, S.D.; Mensah-Darkwa, K.; Kumar, A.; Gupta, R.K. Development of High-Performance Zinc-Ion Batteries: Issues, Mitigation Strategies, and Perspectives. J. Energy Storage 2023, 70, 108081. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.-H.; Kim, C.-H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; AlObaid, A.A.; Lee, S.-Y.; et al. A Review of Rechargeable Zinc–Air Batteries: Recent Progress and Future Perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef]
- Iqbal, A.; El-Kadri, O.M.; Hamdan, N.M. Insights into Rechargeable Zn-Air Batteries for Future Advancements in Energy Storing Technology. J. Energy Storage 2023, 62, 106926. [Google Scholar] [CrossRef]
- Tang, L.; Peng, H.; Kang, J.; Chen, H.; Zhang, M.; Liu, Y.; Kim, D.H.; Liu, Y.; Lin, Z. Zn-Based Batteries for Sustainable Energy Storage: Strategies and Mechanisms. Chem. Soc. Rev. 2024, 53, 4877–4925. [Google Scholar] [CrossRef]
- Lu, Q.; Zou, X.; Bu, Y.; An, L.; Wang, Y.; Shao, Z. What Matters in Engineering Next-Generation Rechargeable Zn-Air Batteries? Next Energy 2023, 1, 100025. [Google Scholar] [CrossRef]
- Hong, S.; Choi, Z.; Hwang, B.; Matic, A. Research Trends and Future Perspectives on Zn-Ion Batteries Using Ga-Based Liquid Metal Coatings on Zn Anodes. ACS Energy Lett. 2024, 9, 5421–5433. [Google Scholar] [CrossRef]
- Huang, R.-B.; Wang, M.-Y.; Xiong, J.-F.; Zhang, H.; Tian, J.-H.; Li, J.-F. Anode Optimization Strategies for Zinc–Air Batteries. eScience 2024, 100309. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Cheacharoen, R.; Olaru, S.; Kheawhom, S. Discharge Profile of a Zinc-Air Flow Battery at Various Electrolyte Flow Rates and Discharge Currents. Sci. Data 2020, 7, 196. [Google Scholar] [CrossRef]
- Gourley, S.W.D.; Brown, R.; Adams, B.D.; Higgins, D. Zinc-Ion Batteries for Stationary Energy Storage. Joule 2023, 7, 1415–1436. [Google Scholar] [CrossRef]
- Domenico Frattini Electrically Rechargeable Zinc-Air Batteries: One of the Most Sustainable Technologies of the Post-Lithium Era. Available online: https://cicenergigune.com/en/blog/electrically-rechargeable-zinc-air-batteries-the-most-sustainable-technologies (accessed on 3 February 2025).
- ECOS—European Environmental Citizens’ Organisation for Standardisation Europe Needs an Ambitious Regulatory Framework to Guarantee Sustainability of Batteries; ECOS: Brussels, Belgium, 2019.
- Rufino Júnior, C.A.; Riva Sanseverino, E.; Gallo, P.; Koch, D.; Diel, S.; Walter, G.; Trilla, L.; Ferreira, V.J.; Pérez, G.B.; Kotak, Y.; et al. Towards to Battery Digital Passport: Reviewing Regulations and Standards for Second-Life Batteries. Batteries 2024, 10, 115. [Google Scholar] [CrossRef]
- Vasileios, R.; Patricia, U. Implementing the EU Digital Battery Passport; CEPS: Brussels, Belgium, 2024. [Google Scholar]
- Bassi, A. Rules for the Calculation of the Carbon Footprint of Electric Vehicle Batteries (CFB-EV); Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- European Commission. Joint Research Centre. Clean Energy Technology Observatory, Batteries for Energy Storage in the European Union: Status Report on Technology Development, Trends, Value Chains and Markets: 2022; Publications Office: Luxembourg, 2022. [Google Scholar]
- JRC RMIS—Raw Materials Information System. Available online: https://rmis.jrc.ec.europa.eu/ (accessed on 7 March 2025).
- Battery Pass Consortium. Unlocking the Value of the EU Battery Passport; Systemiq, Amsterdam, Berlin, Jakarta, London, Munich, Paris and São Paulo. 2024. Available online: https://www.systemiq.earth/unlocking-the-value-of-the-eu-battery-passport/ (accessed on 31 March 2025).
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A Perspective on the Sustainability of Cathode Materials Used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Tan, J.; Keiding, J.K. The Cobalt and Lithium Global Supply Chains: Status, Risks and Recommendations. In MiMa Rapport; GEUS: Copenhagen, Denmark, 2023; Volume 2023. [Google Scholar] [CrossRef]
- Accardo, A.; Dotelli, G.; Musa, M.L.; Spessa, E. Life Cycle Assessment of an NMC Battery for Application to Electric Light-Duty Commercial Vehicles and Comparison with a Sodium-Nickel-Chloride Battery. Appl. Sci. 2021, 11, 1160. [Google Scholar] [CrossRef]
- Yudhistira, R.; Khatiwada, D.; Sanchez, F. A Comparative Life Cycle Assessment of Lithium-Ion and Lead-Acid Batteries for Grid Energy Storage. J. Clean. Prod. 2022, 358, 131999. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The Environmental Impact of Li-Ion Batteries and the Role of Key Parameters—A Review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 202100771. [Google Scholar] [CrossRef]
- Quan, J.; Zhao, S.; Song, D.; Wang, T.; He, W.; Li, G. Comparative Life Cycle Assessment of LFP and NCM Batteries Including the Secondary Use and Different Recycling Technologies. Sci. Total Environ. 2022, 819, 153105. [Google Scholar] [CrossRef] [PubMed]
- Meegoda, J.N.; Malladi, S.; Zayas, I.C. End-of-Life Management of Electric Vehicle Lithium-Ion Batteries in the United States. Clean Technol. 2022, 4, 1162–1174. [Google Scholar] [CrossRef]
- Reinhart, L.; Vrucak, D.; Woeste, R.; Lucas, H.; Rombach, E.; Friedrich, B.; Letmathe, P. Pyrometallurgical Recycling of Different Lithium-Ion Battery Cell Systems: Economic and Technical Analysis. J. Clean. Prod. 2023, 416, 137834. [Google Scholar] [CrossRef]
- Thomas, F.; Mahdi, L.; Lemaire, J.; Santos, D.M.F. Technological Advances and Market Developments of Solid-State Batteries: A Review. Materials 2024, 17, 239. [Google Scholar] [CrossRef]
- GLOBE NEWSWIRE Solid State Battery Market Size and Forecast 2020–2030: Market Poised for Strong Growth with Projected CAGR of Over 30% from 2023 to 2030. Available online: https://www.globenewswire.com/news-release/2024/12/10/2994815/28124/en/Solid-State-Battery-Market-Size-and-Forecast-2020-2030-Market-Poised-for-Strong-Growth-with-Projected-CAGR-of-Over-30-from-2023-to-2030.html (accessed on 17 April 2025).
- Nekahi, A.; Dorri, M.; Rezaei, M.; Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Zaghib, K. Comparative Issues of Metal-Ion Batteries toward Sustainable Energy Storage: Lithium vs. Sodium. Batteries 2024, 10, 279. [Google Scholar] [CrossRef]
- Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM BMBF Project Launched for Fast Industrial Implementation of Sodium-Ion Technology. Available online: https://www.ifam.fraunhofer.de/en/Press_Releases/bmbf-project-sodium-ion-technology.html (accessed on 17 April 2025).
- Endo, C.; Kaufmann, T.; Schmuch, R.; Thielmann, A. Unav Benchmarking International Battery Policies; Fraunhofer-Gesellschaft: München, Germany, 2024. [Google Scholar]
- Transport & Environment. An Industrial Blueprint for Batteries in Europe; Transport & Environment: Brussels, Belgium, 2024. [Google Scholar]

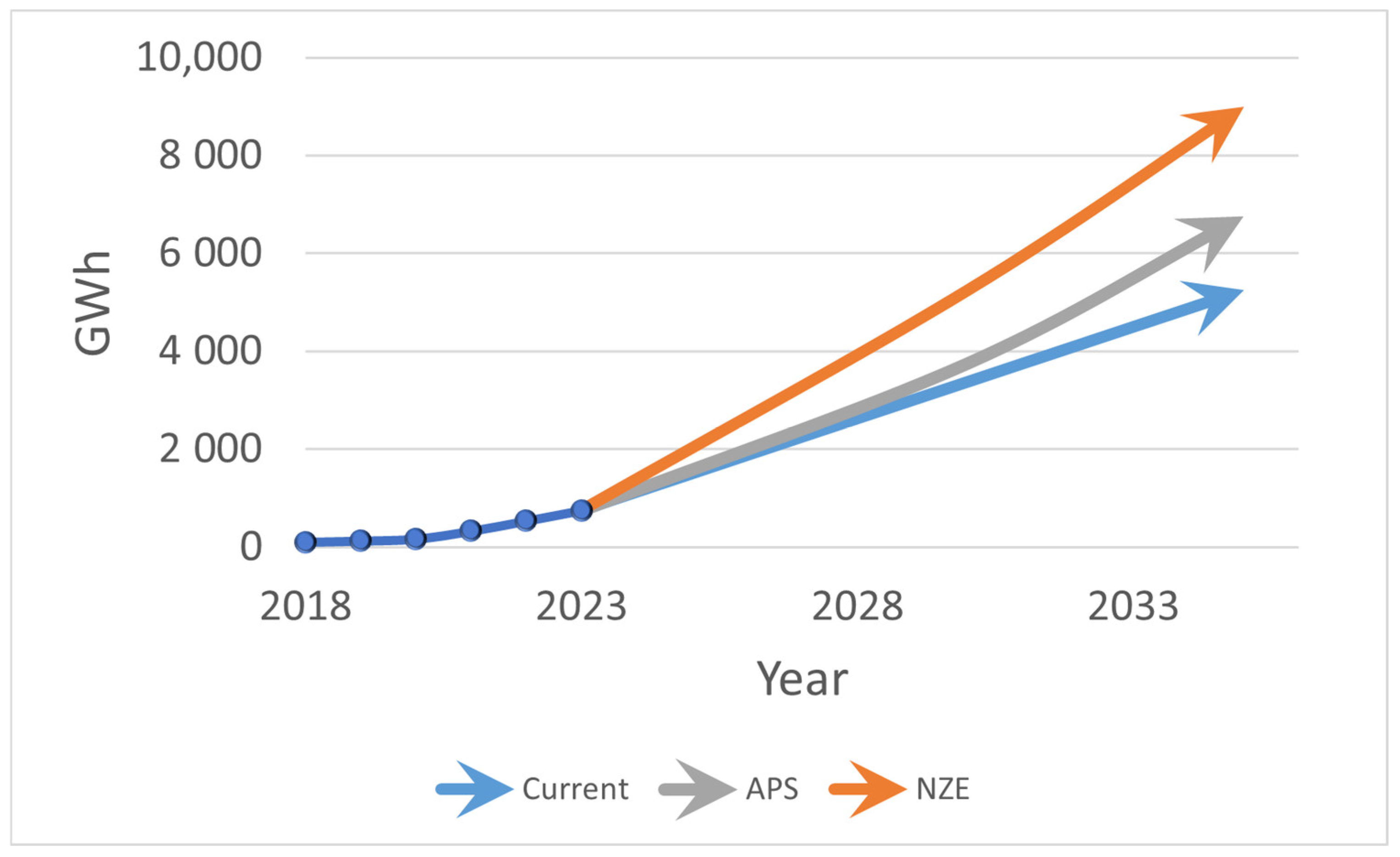
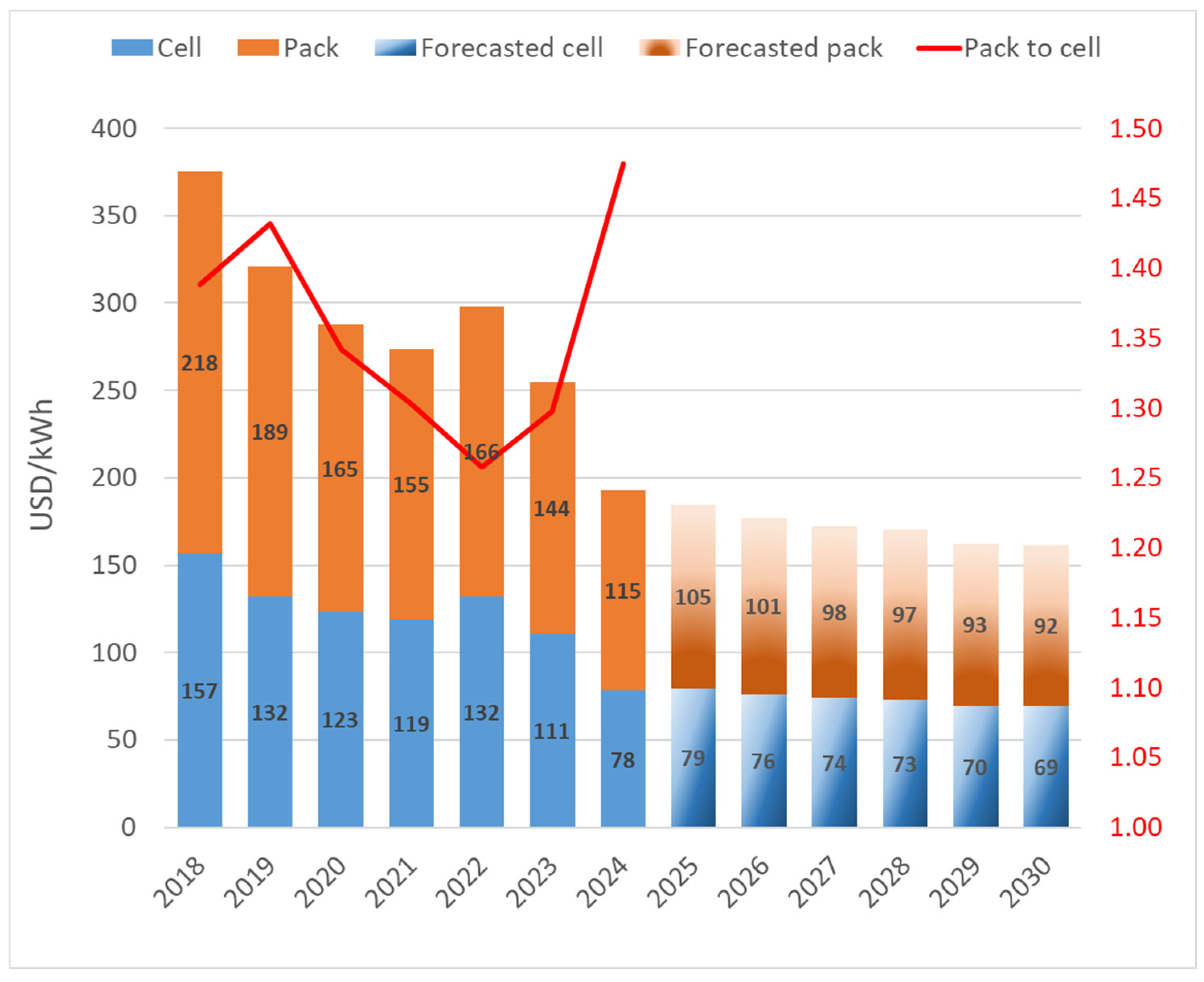
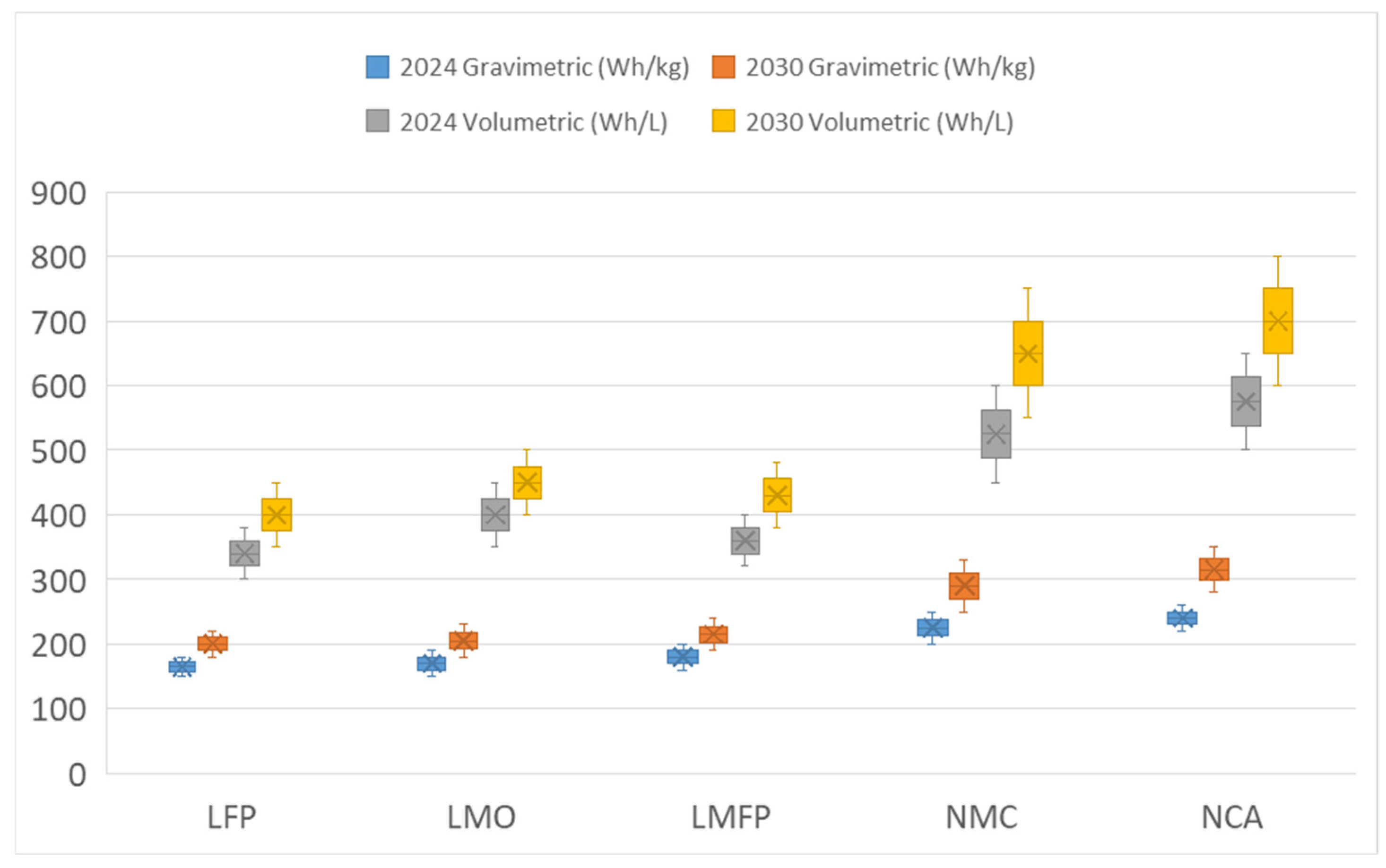
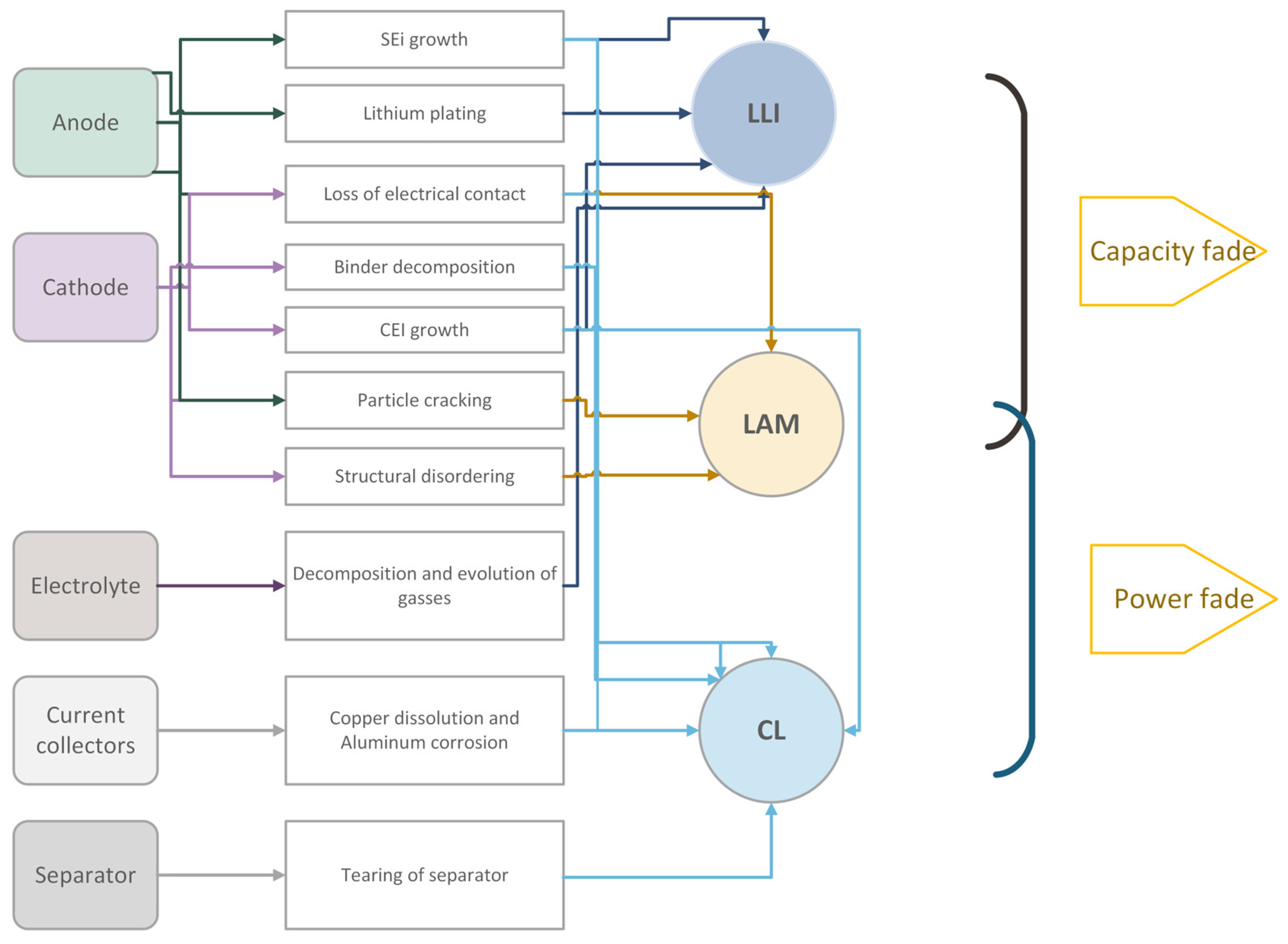
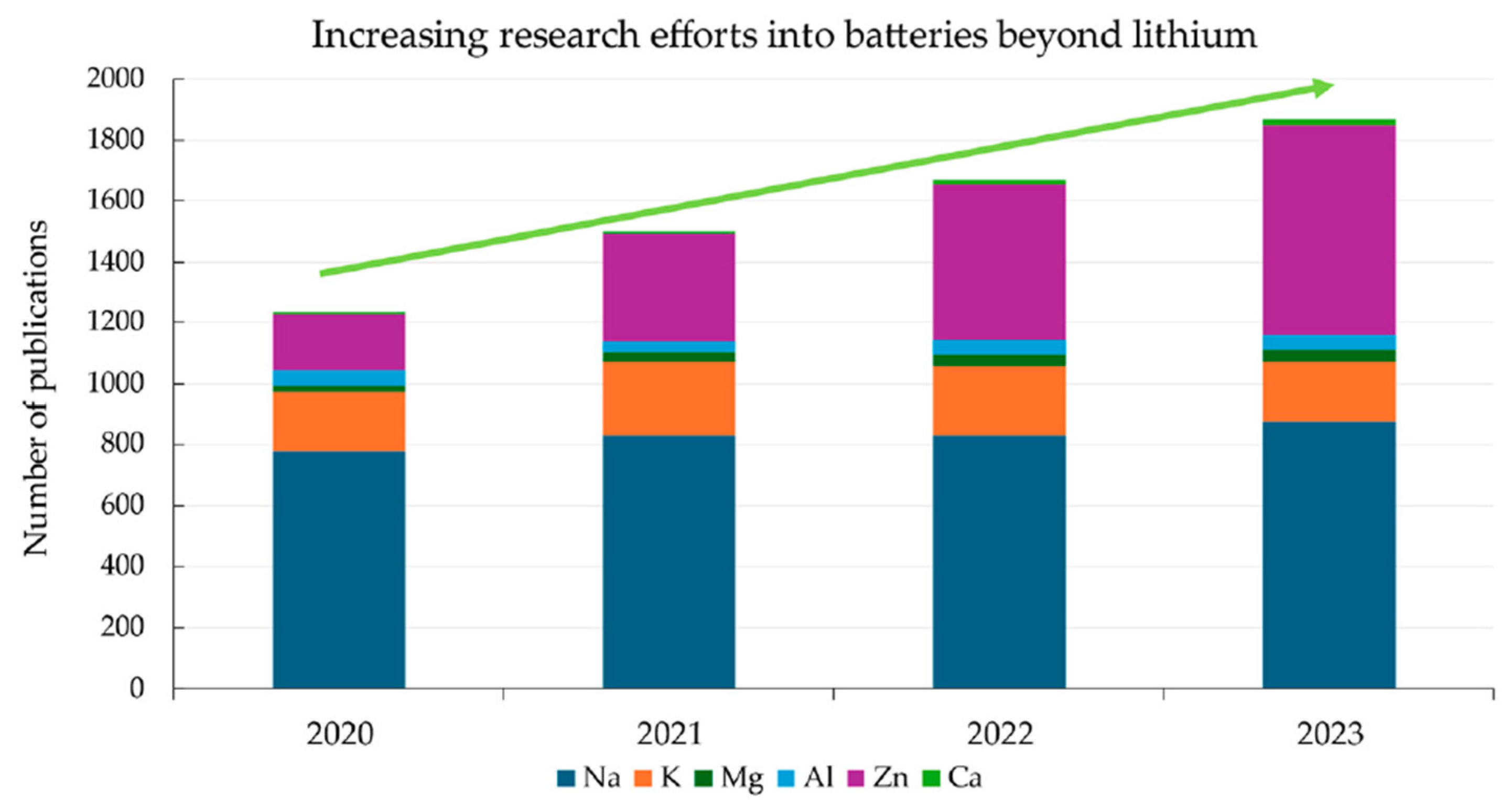
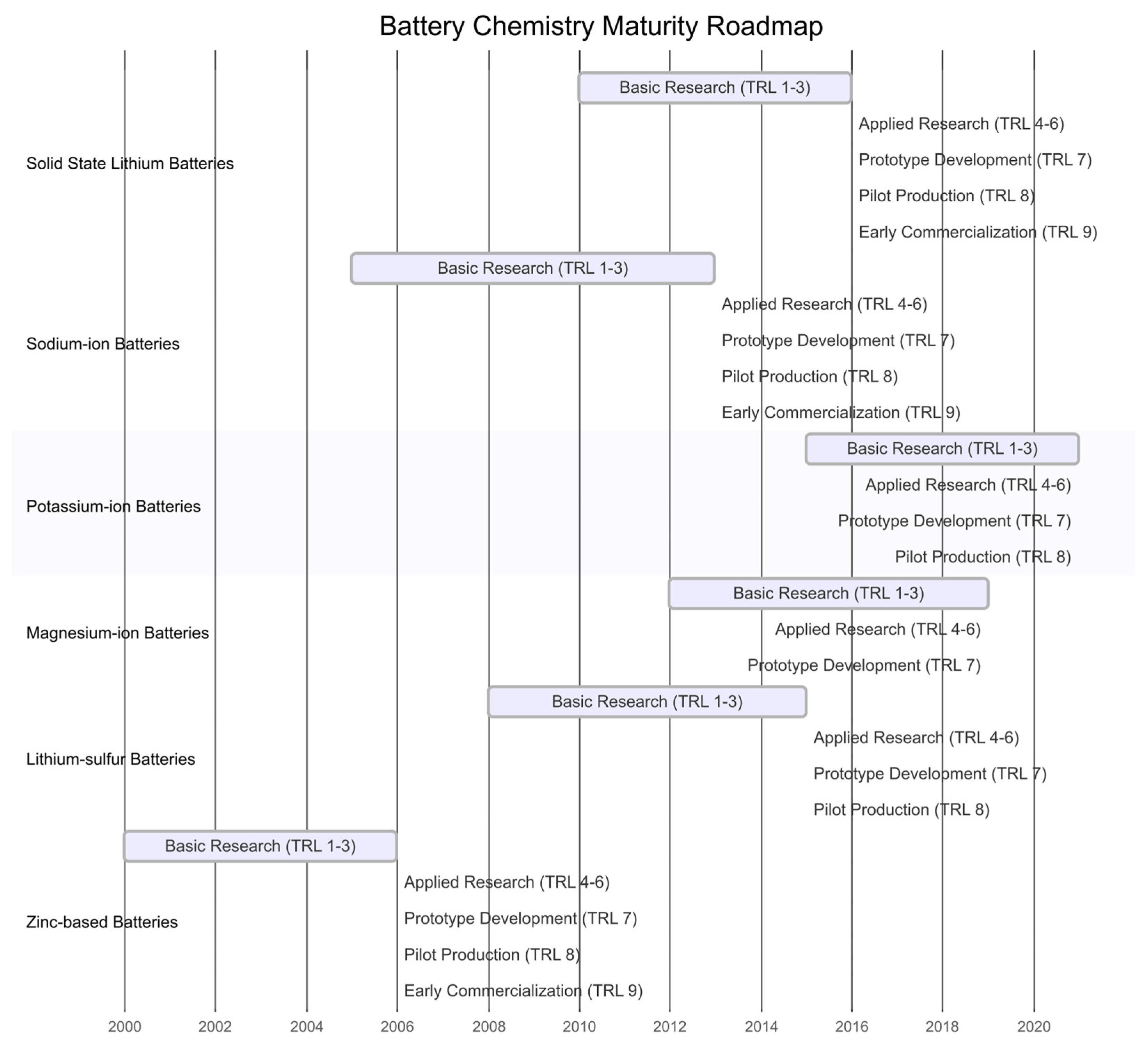

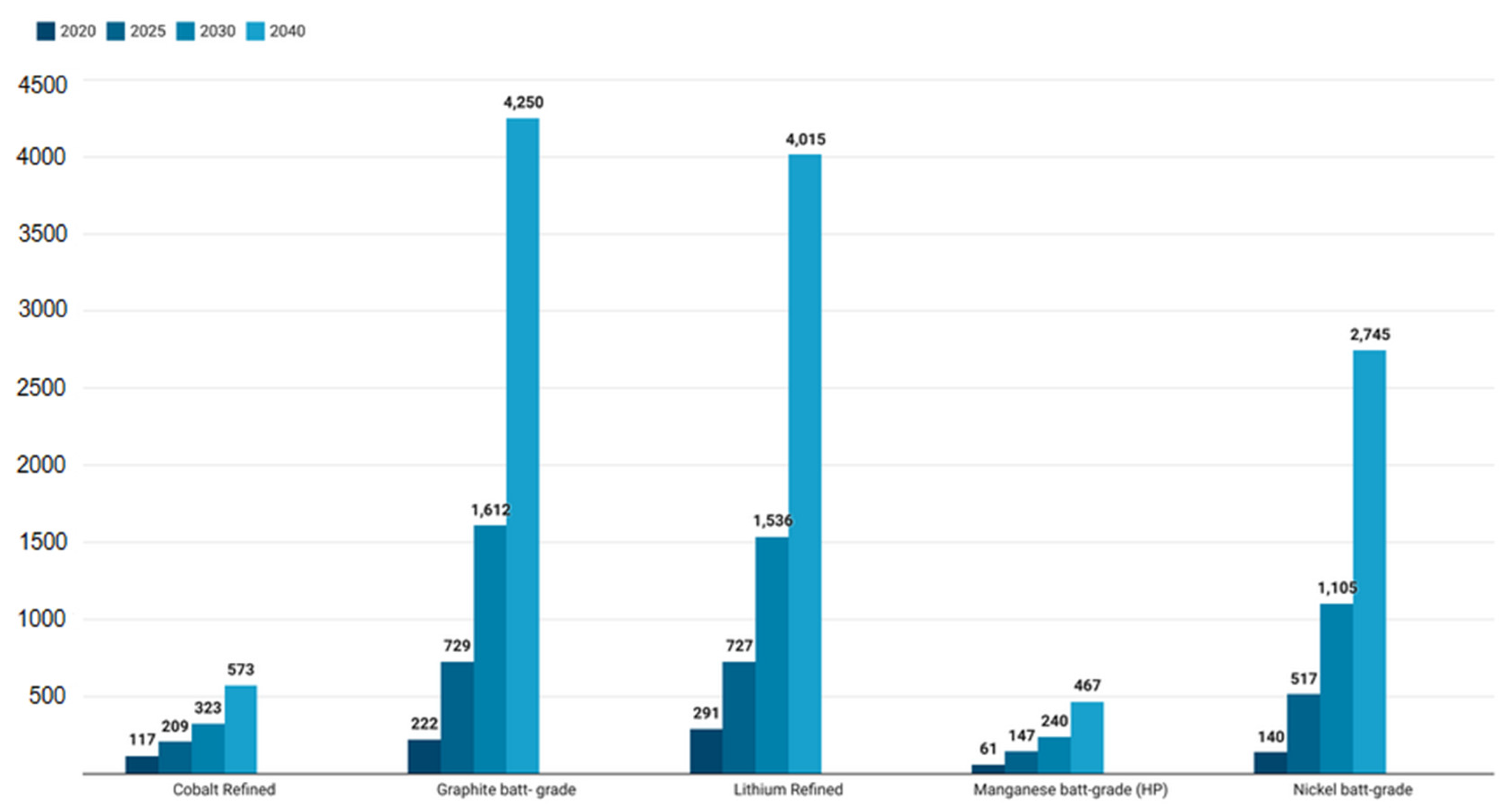

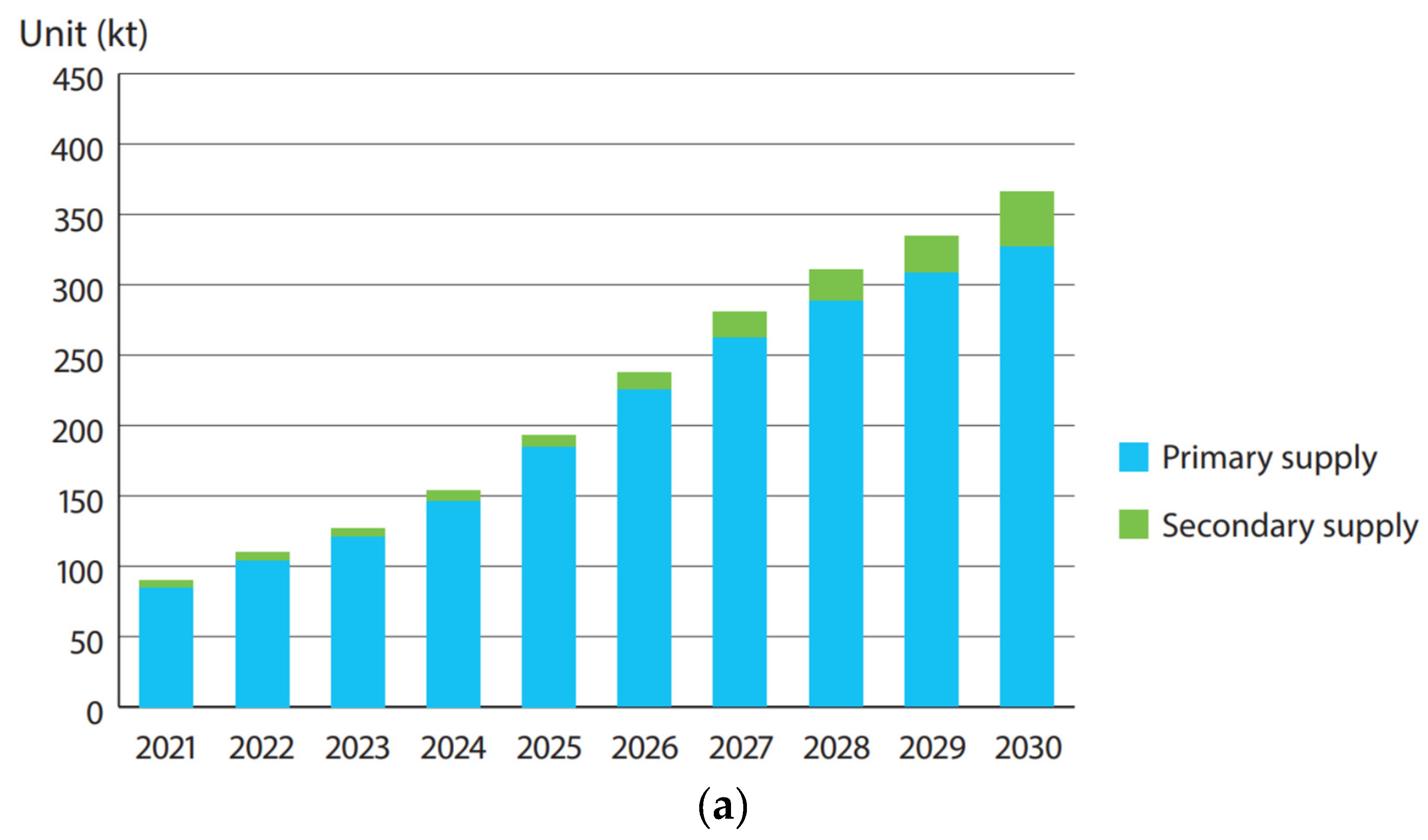
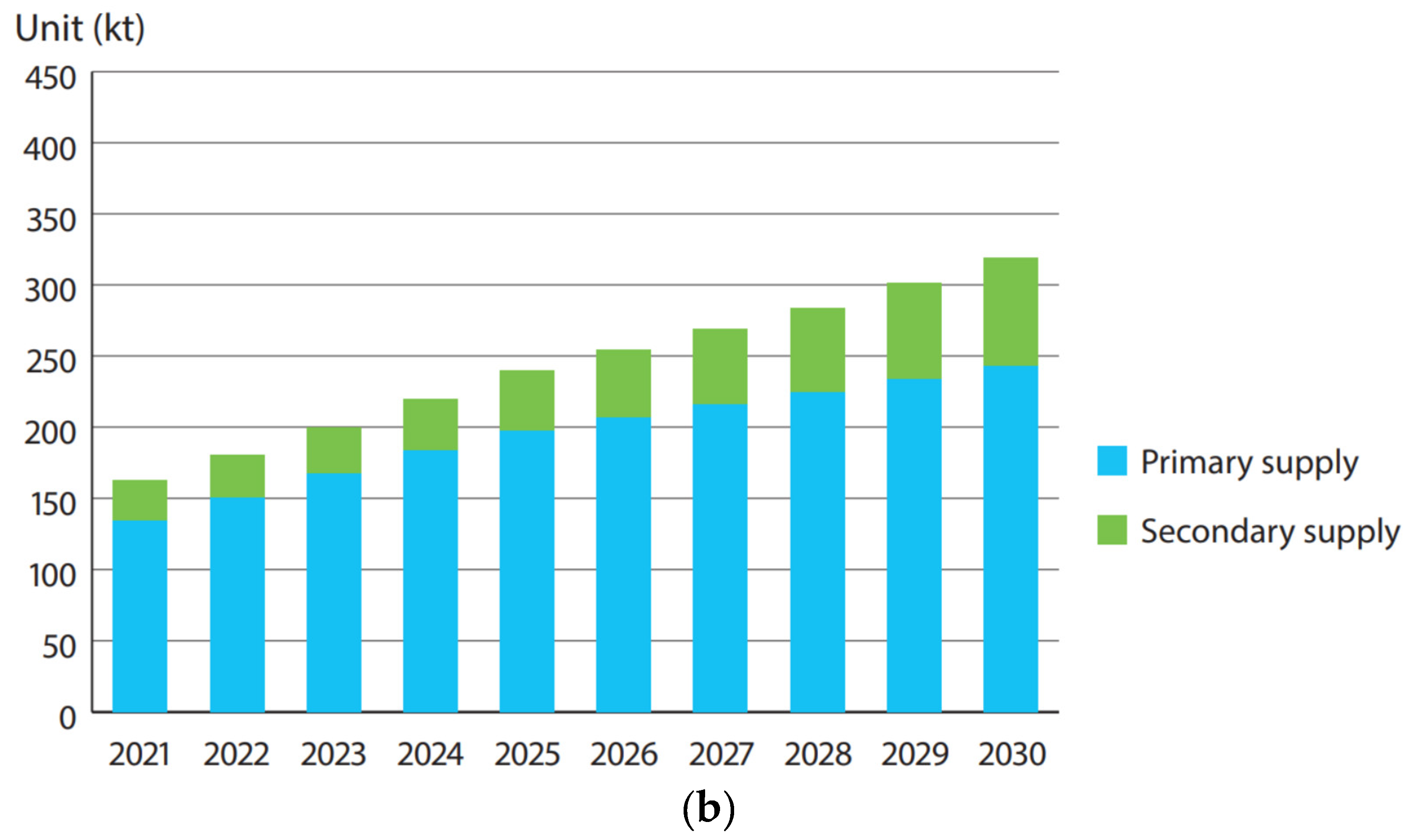
| Country | Main Regulations | Main Focus | Main Action | References |
|---|---|---|---|---|
| EU | Batteries Regulation (2023/1542) | Sustainability; safety; labeling, collection, and recycling of all battery types | Substance restrictions, carbon footprint declarations, recycled content requirements, and battery passports for traceability | [35] |
| China | EV battery safety standards (GB38031-2025) | Safety; promotion of technological standards | Prevention of fire and explosion after thermal runaway | [36] |
| US | Mercury-Containing and Rechargeable Battery Management Act; Inflation Reduction Act | Recycling; battery collection and labeling guidelines; safety; domestic battery production | Multi-faceted approach involving federal and state regulations, as well as voluntary standards | [37,38] |
| Parameters at Cell Level | Current 2020–2025 | 2030–2035 | Source |
|---|---|---|---|
| Specific energy | 160–290 | 275–450 | [64,74,75,76] |
| Energy density Wh/l | 450–730 | 750–1000 | [55,56,57] |
| Continuous specific power—discharge W/kg | 340–750 | 800–1750 | [74,76] |
| Continuous power density—discharge W/L | 1000–1500 | 2000–3850 | [74,76] |
| Charging rate C (1/h) | 2–3 | 6–3.5 | [55,56,57] |
| Cost EUR/kWh | 60–200 | 40–100 | [74,76] |
| Hazard level | <=4 | <=3 | [74,76] |
| Parameters at Pack Level | Current 2020–2025 | 2030–2035 | Source |
|---|---|---|---|
| Specific energy Wh/kg | 90–180 | 190–360 | [74,76] |
| Energy density Wh/l | 250–400 | 450–750 | [74,76] |
| Continuous specific power—discharge W/kg | 525 | 800–1400 | [74] |
| Continuous power density—discharge W/L | 900 | 1650–2600 | [74] |
| Cost EUR/kWh | 90–286 | 65–120 | [74,76] |
| External Stress | Induced Degradation Mechanism | Aging Effect |
|---|---|---|
| Low temperature | Lithium plating and dendrites formation | Conductivity loss/capacity fade/short circuit |
| High temperature | Electrolyte and binder decomposition SEI film growth and decomposition | Capacity/power fade |
| Low voltage/SOC | Corrosion of current collectors Transition metal dissolution Loss of electric contact Lithium plating and dendrites formation SEI and CEI growth | Conductivity loss/capacity fade/power fade |
| High voltage/SOC | Electrolyte and binder decomposition SEI film growth and decomposition Graphite exfoliation Lithium plating | Capacity/power fade |
| High current | SEI film growth and decomposition Graphite exfoliation Structural disordering and particle cracking Loss of electric contact | Conductivity loss/capacity fade/power fade |
| Cathode | Anode | Operating Voltage (V) | Energy Density (Wh/kg) | Power Density (W/kg) | Fast-Charging | Lifespan (Cycles) |
|---|---|---|---|---|---|---|
| LFP | C or Si-C | 2.5–3.6 | 90–190 | 247 | 3 C | 2000–4000 |
| NMC | C or Si-C | 3–4.2 | 130–280 | 300–800 | 0.7–1 C | 1000–2000 |
| NMC | LTO | 1.5–2.8 | 70–90 | 2200 | 10 C | 3000–10,000 |
| LMO | C or Si-C | 3–4.2 | 100–185 | 925 | 0.7–1 C | 300–1000 |
| LMO | LTO | 1.5–2.8 | 70–90 | 3600 | 5 C | 3000–7000 |
| NCA | C or Si-C | 3–4.2 | 175–300 | 670 | 0.7–2 C | 500–1000 |
| Battery Chemistry | Cost | Energy Density | Specific Power | Lifespan | Safety | Thermal Stability | Overall Suitability for EVs |
|---|---|---|---|---|---|---|---|
| LFP | Low to Med | Moderate | Good | Excellent | Excellent | Very Good | Good for mass-market EVs, especially where cost and longevity are prioritized. |
| LMFP | Medium | Moderate to High | Good to Very Good | Very Good to Excellent | Good | Good to Very Good | Promising for mid-range EVs, potentially bridging the gap between LFP and NMC. |
| LMO | Low to Med | Low to Moderate | High | Moderate | Good | Good | Niche applications (e.g., some hybrids, power-focused EVs if lifespan is acceptable). |
| LMNO | High | High | Good to Very Good | Potentially Good | Moderate | Moderate | Potential for future high-performance EVs if safety and cost challenges are overcome. |
| LCO | High | High | Moderate | Moderate | Poor to Med | Poor to Moderate | Limited suitability for EVs due to cost, safety concerns, and lifespan. |
| NCA | Medium to High | Very High | High | Good | Moderate | Moderate | Well-suited for premium, long-range EVs where performance is a key factor. |
| Cathode | Anode | Specific Energy [Wh/kg] | Specific Capacity [mAh/g] | Authors |
|---|---|---|---|---|
| PTCDA | 3D-NTC | 187 | 241 | [347] |
| PTCDA | (BiO2)CO3 | 732.8 | - | [348] |
| KFeC2O4F | Soft Carbon | 235 | 112 | [349] |
| KMgHCF | Graphite and K2TP | 214.8 | 83 | [345] |
| Cathode | Anode | Specific Energy [Wh/kg] | Specific Capacity [mAh/g] | Source |
|---|---|---|---|---|
| NaV2O2(PO4)2F @rGO | Mg0.79NaTi2(PO4)3/C | 76 | - | [360] |
| Te @CSs | Mg metal | 337 | 387 | [361] |
| Mg0.75V10O24·4H2O | PTCDA | 67 | - | [362] |
| Energy Density (Wh/kg) | Capacity Density (mAh/g) | Average Discharge Voltage (V) | Cell Characteristics | Source |
|---|---|---|---|---|
| 395 | - | - | 9.5 Ah pouch cell | [369] |
| >550 (pack), up to 700 (cell) | - | - | Low electrolyte-to-sulfur ratio pouch cells | [370] |
| - | >250 (pouch cell), >3 mAh/cm2 | - | Catholyte, sulfur-free carbon nanofiber cathode | [371] |
| 2500 (theoretical) | 1672 (theoretical) | ~2.1 | Theoretical values | [372] |
| ~542.7 (estimated All-solid-state) | - | ~2.0 | All-solid-state, 90% sulfur utilization, 60 wt% sulfur in cathode | [373] |
| 2400 (theoretical with Li anode) | 1675 (theoretical cathode), 3860 (theoretical anode) | - | Theoretical values | [374] |
| Technology | Energy Density (Wh/kg) | Energy Density (Wh/L) | Power Density (W/kg) | Charging Speed (Typical) | Cycle Life (Typical) | Safety Characteristics | Estimated Cost (per kWh) | Estimated Commercialization Timeline for Automotive |
|---|---|---|---|---|---|---|---|---|
| Solid-State | 200–500+ | 400–800+ | Up to 1000+ | 10–30 min | 1000–5000+ | Non-flammable solid electrolyte, reduced thermal runaway | Higher | Late 2020s–Mid 2030s |
| Silicon Anode (Li-ion) | 250–400+ | 500–800+ | Up to 1000+ | 10–30 min | 300–1000+ | Similar to Li-ion | Similar | Late 2020s onwards |
| Sodium-Ion | 100–160 | 200–300 | Up to 500 | 30–60 min | 2000–5000+ | Lower thermal runaway risk, better low-temp perf. | Lower | 2025 onwards |
| Lithium-Sulfur | 300–600+ | 300–500+ | Up to 500 | Varies | 100–500+ | Lithium metal anode poses dendrite risk | Lower | 2030 and beyond |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrenacci, N.; Vitiello, F.; Boccaletti, C.; Vellucci, F. Powering the Future Smart Mobility: A European Perspective on Battery Storage. Batteries 2025, 11, 185. https://doi.org/10.3390/batteries11050185
Andrenacci N, Vitiello F, Boccaletti C, Vellucci F. Powering the Future Smart Mobility: A European Perspective on Battery Storage. Batteries. 2025; 11(5):185. https://doi.org/10.3390/batteries11050185
Chicago/Turabian StyleAndrenacci, Natascia, Francesco Vitiello, Chiara Boccaletti, and Francesco Vellucci. 2025. "Powering the Future Smart Mobility: A European Perspective on Battery Storage" Batteries 11, no. 5: 185. https://doi.org/10.3390/batteries11050185
APA StyleAndrenacci, N., Vitiello, F., Boccaletti, C., & Vellucci, F. (2025). Powering the Future Smart Mobility: A European Perspective on Battery Storage. Batteries, 11(5), 185. https://doi.org/10.3390/batteries11050185










