Evaluation of Lithium Battery Cycle Aging Based on Temperature Increase During Charging
Abstract
1. Introduction
2. Methodology
2.1. Experimental Subject
2.2. Experimental Content and Procedures
2.2.1. Cycle Life Aging Test
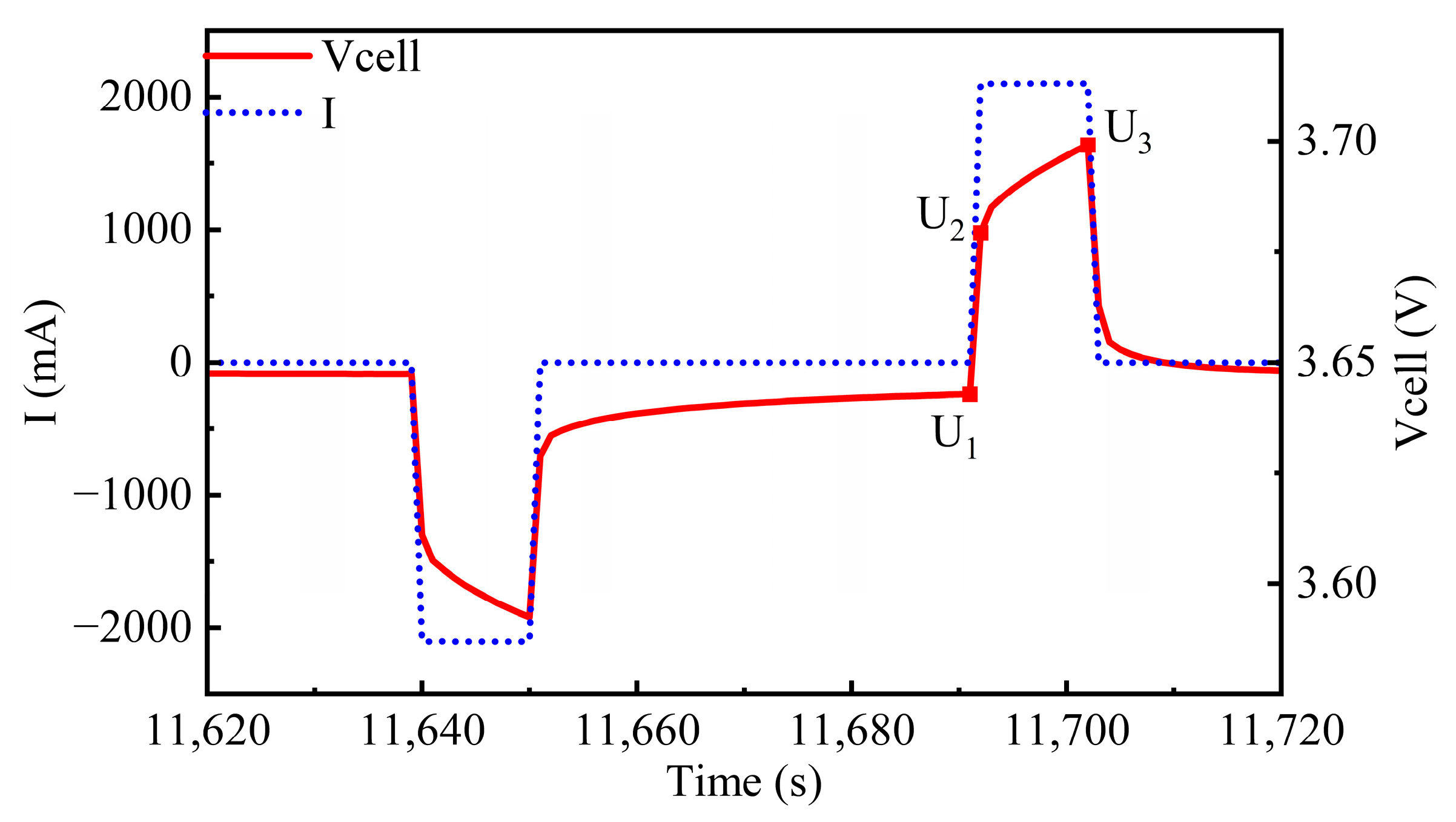
2.2.2. DCIR Test
2.2.3. Temperature Distribution Test at Different Locations
2.2.4. Battery Disassembly
3. Results and Discussion
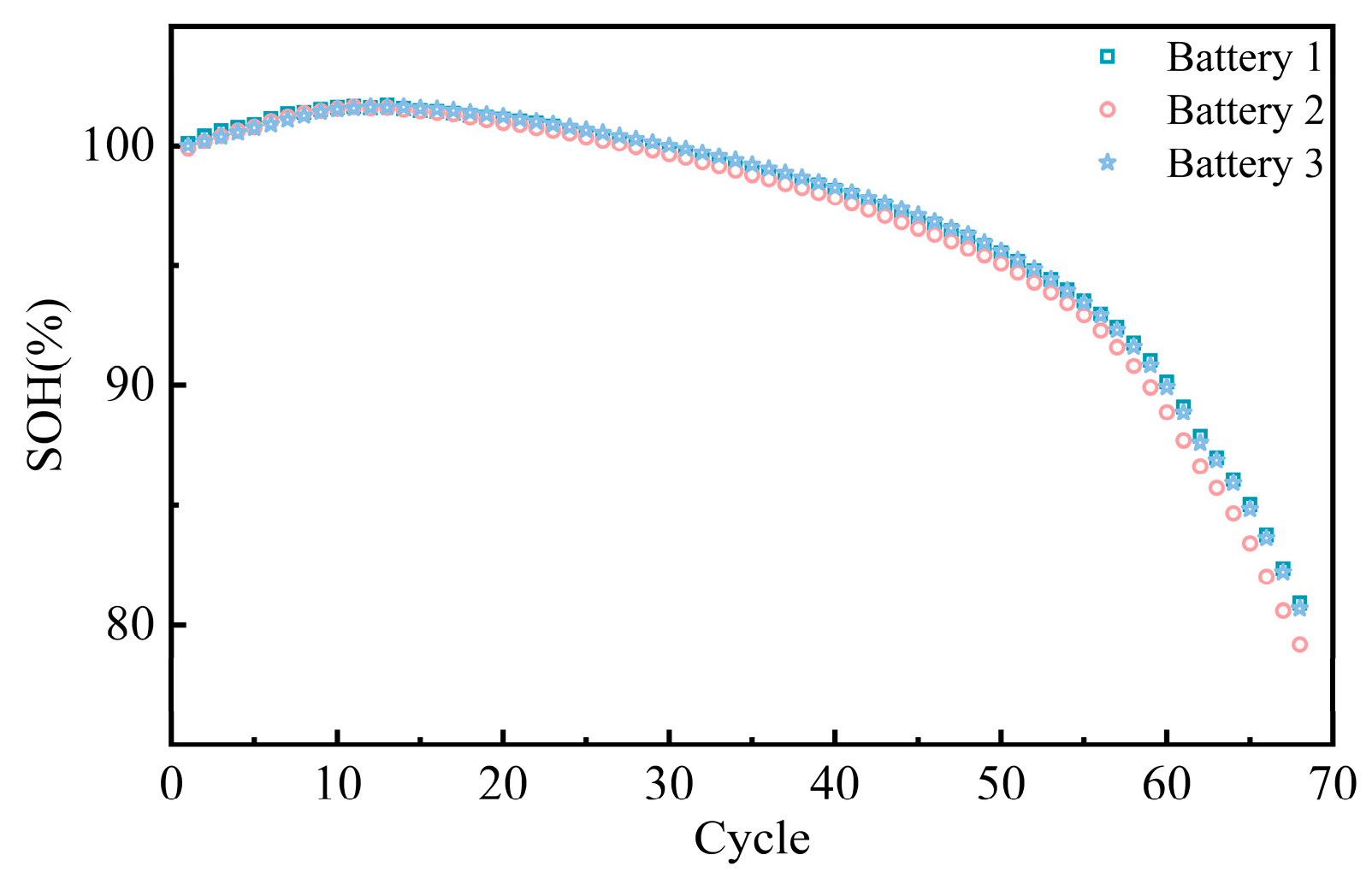
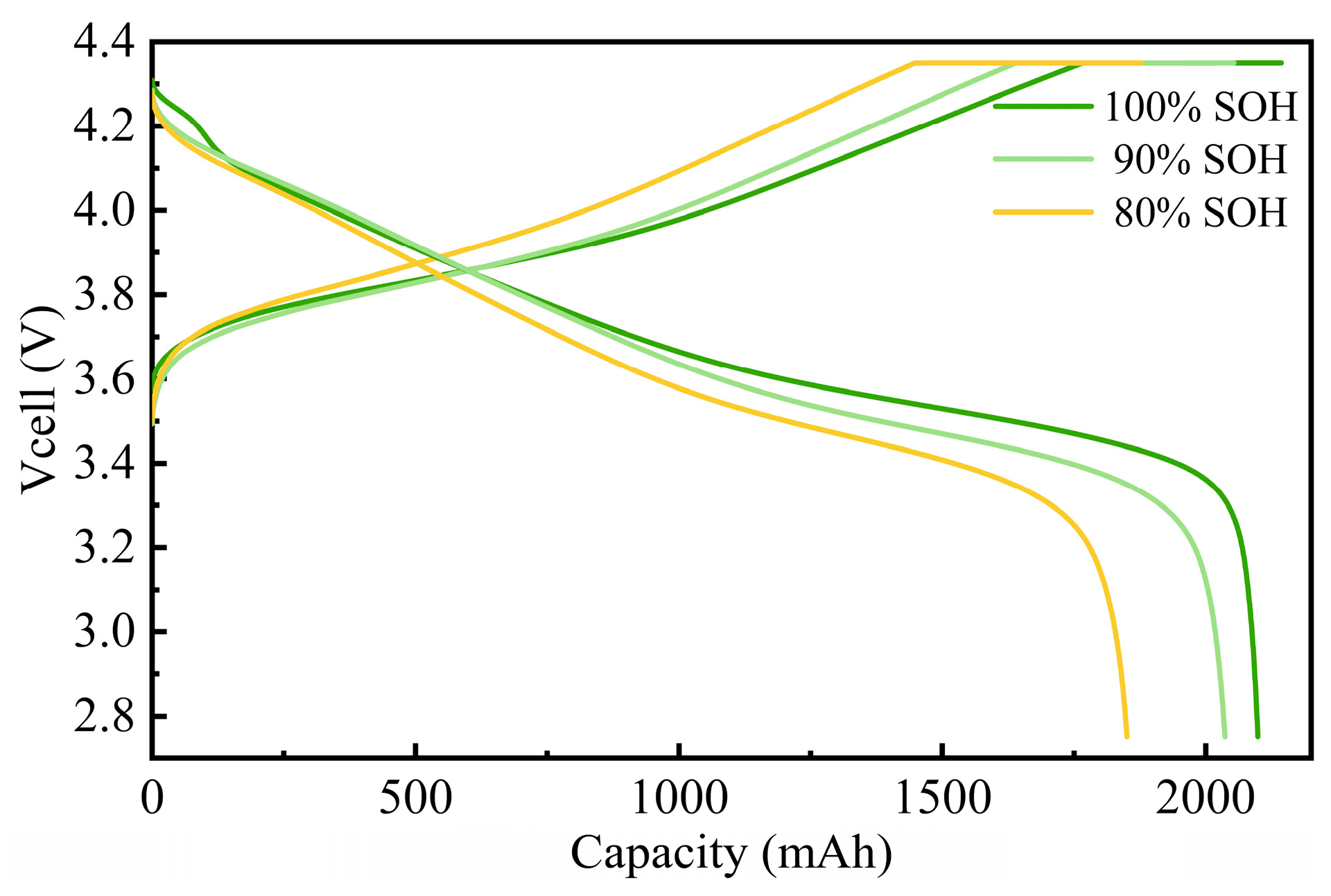
3.1. Impact of Cycle Aging on Battery Performance
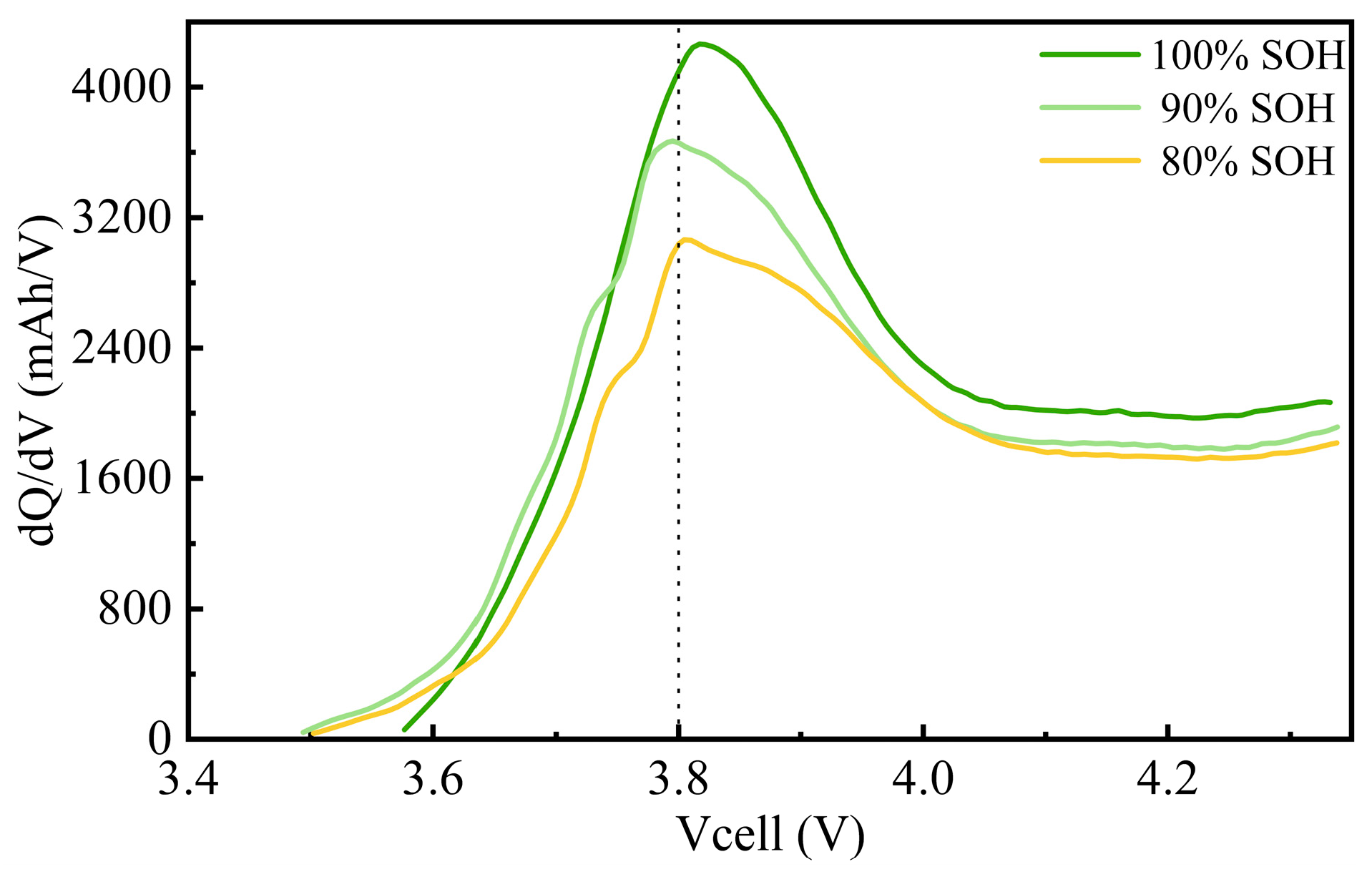
3.1.1. Changes in Charge–Discharge Performance
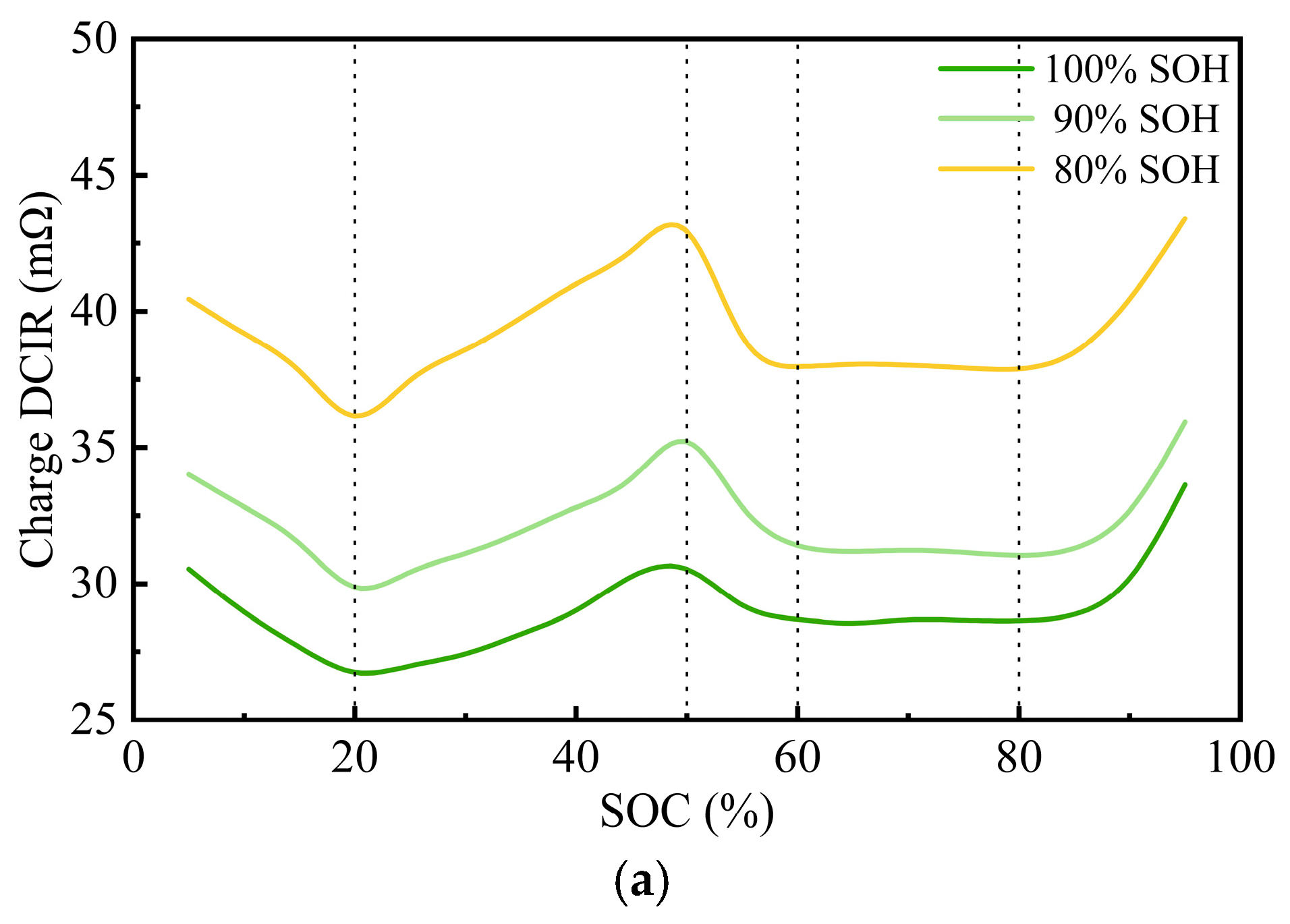
3.1.2. Variations in the DCIR
3.2. Analysis of Temperature Increase Differences at Different Locations
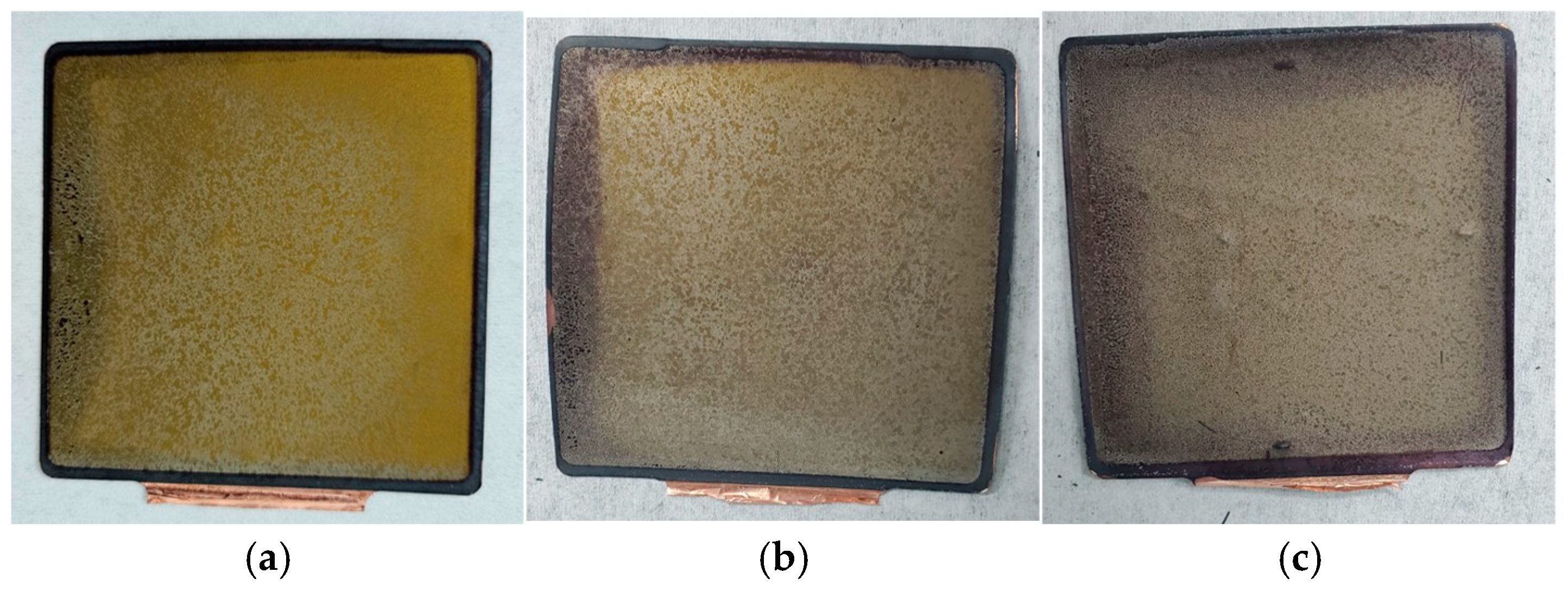
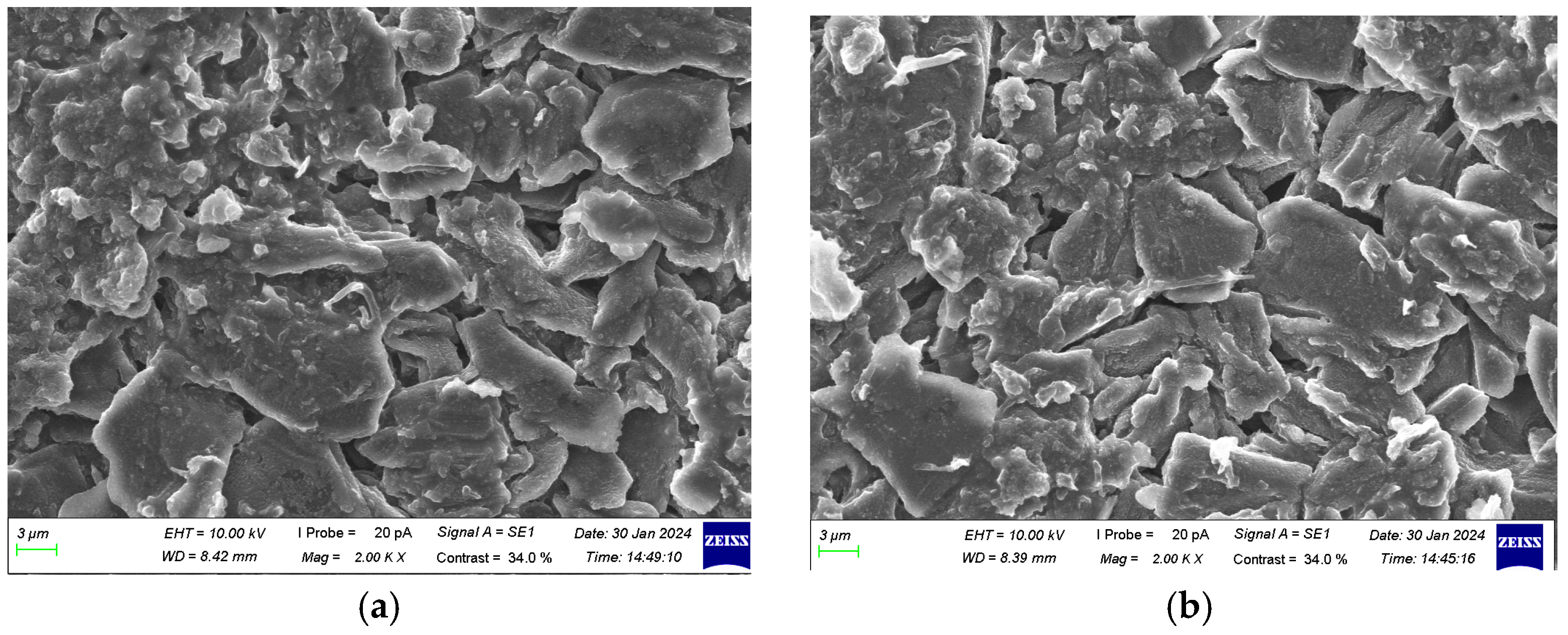
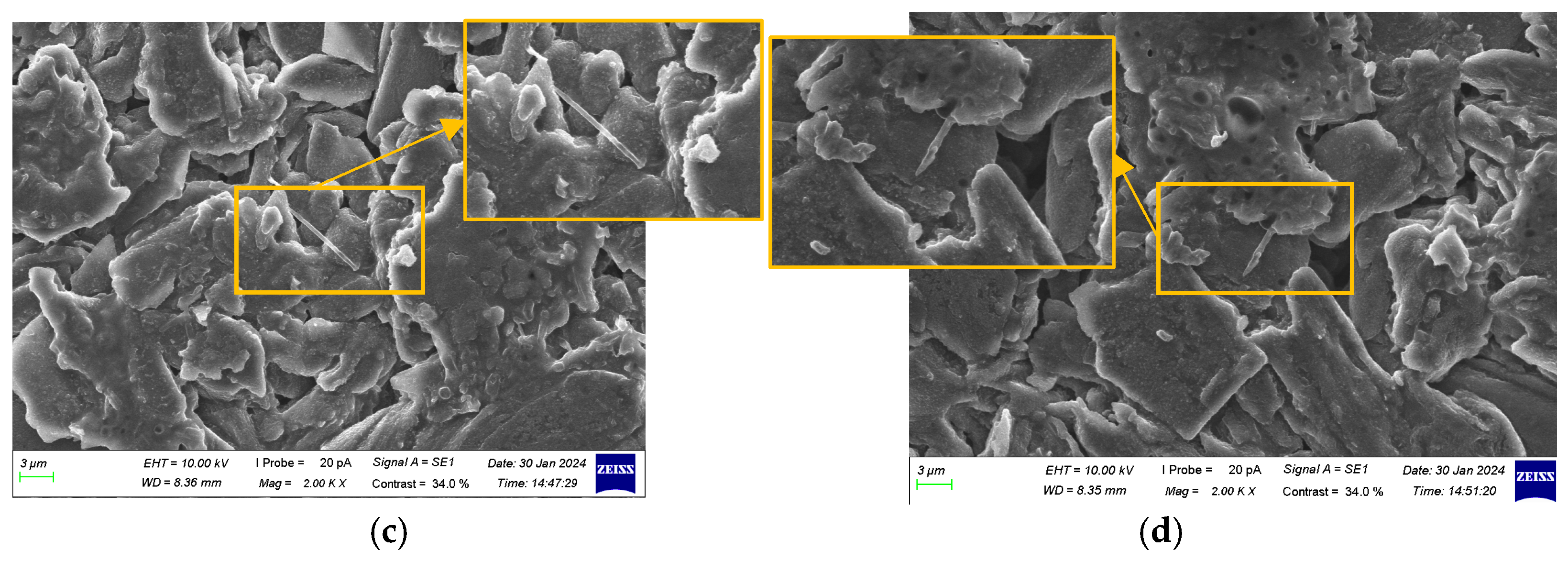
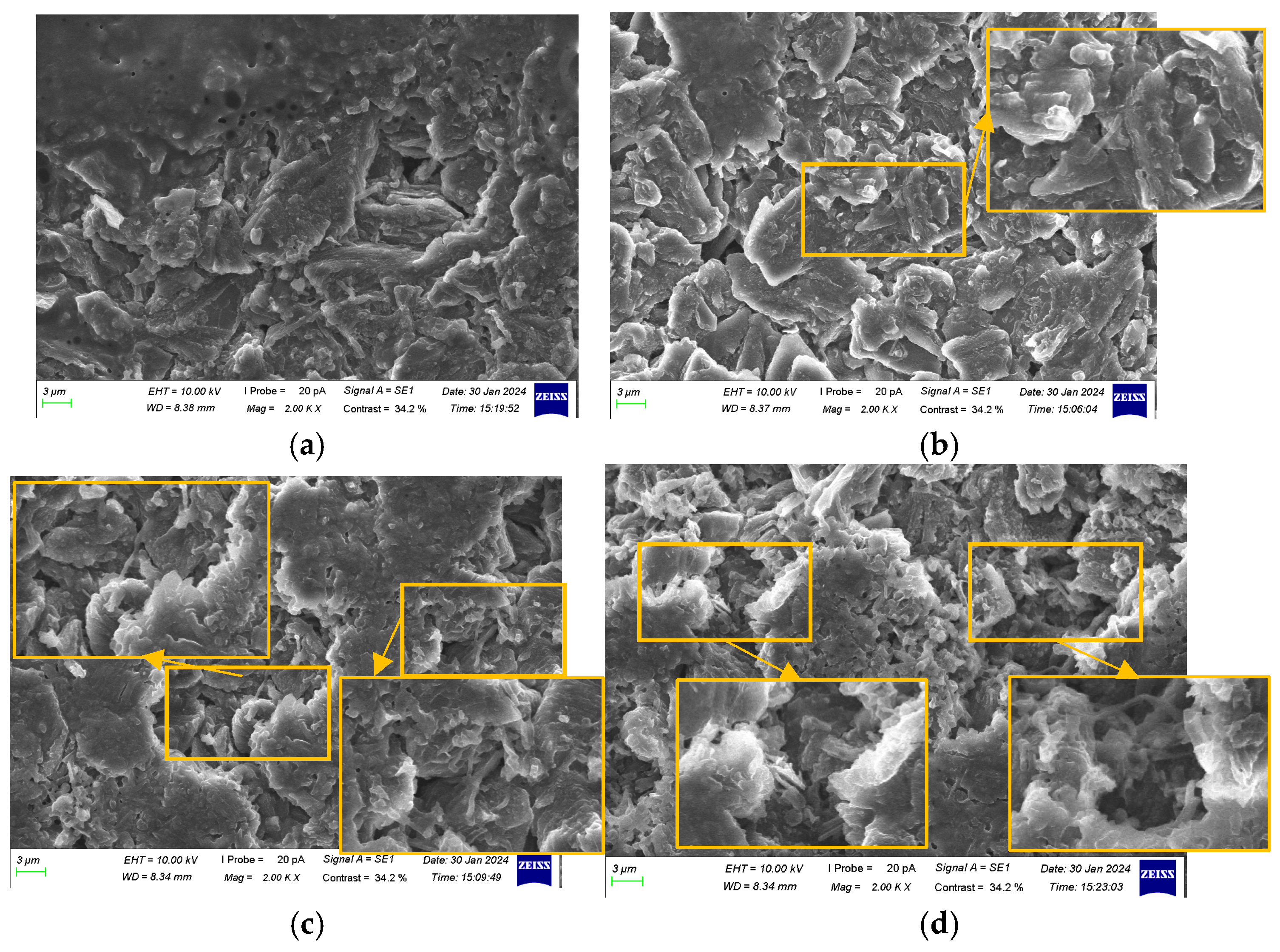
3.3. Morphological Characteristic Analysis of Anode Electrodes at Different SOH Levels
4. Conclusions
- Due to the relatively high 1.8 C charging rate, the transport rate of lithium ions between the cathode and anode was limited. Even if lithium plating occurred during charging, the reaction time was insufficient to reveal characteristic peaks, making it difficult to observe the lithium plating peak (Peak III) in the differential capacity curve. This suggests that, under the current experimental conditions, ICA analysis may not be sensitive enough to directly detect lithium plating-related degradation mechanisms.
- During aging, the temperature at the center of the surface remained consistently high, while the side edges and the center exhibited a relatively uniform thermal distribution. The temperature increase at the negative terminal was smaller, and the temperature increase difference between the center and terminal regions became more pronounced as the battery aged.
- Repeated cycling caused the SEI layer to repeatedly break and reform, gradually thickening. The layered structure of graphite particles underwent fragmentation, accompanied by the accumulation of by-products, leading to browning on the anode surface. The ability of the anode to intercalate lithium ions gradually declined, with lithium dendrites first appearing at the center of the surface and the negative terminal and progressively increasing across all locations of the anode.
- Combining temperature increase data with anode aging observations and monitoring temperature increase during charging show promise for assessing battery aging. As the battery aged, the temperature increase at the center of the surface became more significant, accompanied by increased lithium dendrite formation. While the side edges also experienced temperature increases, lithium dendrite formation was less pronounced compared to the center, but severe anode degradation was observed. The negative terminal showed smaller temperature increases and fewer lithium dendrites. In contrast, the temperature increase behavior at the positive terminal was less consistent, making it less suitable for aging assessment through temperature monitoring.
- The findings of this paper primarily pertain to large-format batteries with pouch battery architecture, where the planar dimensions significantly exceed the transverse dimension. In such batteries, the in-plane distribution of current may be equally important, or even more critical, than the electrochemical behavior in the through-plane direction (i.e., perpendicular to the current collectors). Extrapolation of these results to other battery geometries, such as cylindrical or prismatic formats, requires further investigation.
- While the findings of this paper cannot be directly applied to determine the SOH, such as providing a real-time value within a battery management system (BMS), they can nonetheless contribute to improving the selection methods for retired batteries intended for second-life applications. In such cases, experiments can be conducted under controlled conditions (outside the vehicle and within a laboratory setting), thereby minimizing the influence of external factors such as ambient temperature, thermal management systems, and other environmental variables.
- 7.
- Integration with real-world operational data: Future studies will incorporate field data from batteries operating under dynamic load conditions to refine the temperature–SOH correlation model.
- 8.
- Validation across different battery chemistries and configurations: Additional experiments will be conducted on various battery chemistries, pack configurations, and charge–discharge current rates to assess the generalizability and robustness of the proposed method.
- 9.
- Experimental validation under different ambient temperatures: Since the current study was conducted at a fixed ambient temperature (25 °C), future work will include tests under varying ambient conditions to better understand the influence of environmental temperature on battery temperature increase and its correlation with the SOH.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Li, M.; El-Hady, D.A.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Pigłowska, M.; Marcinek, M.; Marczewski, M.; Wieczorek, W. Challenges for Safe Electrolytes Applied in Lithium-Ion Cells—A Review. Materials 2021, 14, 6783. [Google Scholar] [CrossRef]
- Rahman, T.; Alharbi, T. Exploring Lithium-Ion Battery Degradation: A Concise Review of Critical Factors, Impacts, Data-Driven Degradation Estimation Techniques, and Sustainable Directions for Energy Storage Systems. Batteries 2024, 10, 220. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Yann Liaw, B.; Metzler, V.; Zhang, J. A Review of Lithium Deposition in Lithium-Ion and Lithium Metal Secondary Batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Alipour, M.; Ziebert, C.; Conte, F.V.; Kizilel, R. A Review on Temperature-Dependent Electrochemical Properties, Aging, and Performance of Lithium-Ion Cells. Batteries 2020, 6, 35. [Google Scholar] [CrossRef]
- Mao, N. Experimental Investigation of the Thermal Runaway Mechanisms of Lithium-Ion Batteries Under Overcharge and Other Conditions. Ph.D. Thesis, University of Surrey, Guildford, UK, 2023. [Google Scholar]
- Yang, Y.; Wang, R.; Shen, Z.; Yu, Q.; Xiong, R.; Shen, W. Towards a Safer Lithium-Ion Batteries: A Critical Review on Cause, Characteristics, Warning and Disposal Strategy for Thermal Runaway. Adv. Appl. Energy 2023, 11, 100146. [Google Scholar] [CrossRef]
- Hu, X.; Gao, F.; Xiao, Y.; Wang, D.; Gao, Z.; Huang, Z.; Ren, S.; Jiang, N.; Wu, S. Advancements in the Safety of Lithium-Ion Battery: The Trigger, Consequence and Mitigation Method of Thermal Runaway. Chem. Eng. J. 2024, 481, 148450. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, A. Experimental and Numerical Investigation of Thermal Performance of an Air-Cooled Battery Module Under High Ambient Temperature Conditions. J. Therm. Sci. Eng. Appl. 2023, 15, 091006. [Google Scholar]
- Xu, X.M.; He, R. Review on the Heat Dissipation Performance of Battery Pack with Different Structures and Operation Conditions. Renew. Sustain. Energy Rev. 2014, 29, 301–315. [Google Scholar]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal Behavior of Small Lithium-Ion Battery During Rapid Charge and Discharge Cycles. J. Power Sources 2006, 158, 535–542. [Google Scholar] [CrossRef]
- Cheng, J.; Shuai, S.; Zhao, R.; Tang, Z. Numerical Analysis of Heat-Pipe-Based Battery Thermal Management System for Prismatic Lithium-Ion Batteries. J. Therm. Sci. Eng. Appl. 2022, 14, 081008. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Song, L.; Xiang, L. Development of a Theoretically Based Thermal Model for Lithium Ion Battery Pack. J. Power Sources 2013, 223, 155–164. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; He, W.; Wang, J. Thermal Issues about Li-Ion Batteries and Recent Progress in Battery Thermal Management Systems: A Review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar]
- Wang, Q.K.; He, Y.J.; Shen, J.N.; Hu, X.S.; Ma, Z.F. State of Charge-Dependent Polynomial Equivalent Circuit Modeling for Electrochemical Impedance Spectroscopy of Lithium-Ion Batteries. IEEE Trans. Power Electron. 2017, 33, 8449–8460. [Google Scholar] [CrossRef]
- Castaings, A.; Lhomme, W.; Trigui, R.; Bouscayrol, A. Comparison of Energy Management Strategies of a Battery/Supercapacitors System for Electric Vehicle under Real-Time Constraints. Appl. Energy 2016, 163, 190–200. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Jiang, Y. Aging Mechanisms under Different State-of-Charge Ranges and the Multi-Indicators System of State-of-Health for Lithium-Ion Battery with Li(NiMnCo)O2 Cathode. J. Power Sources 2018, 400, 641–656. [Google Scholar]
- DOE/ID-11069; FreedomCAR Battery Test Manual for Power-Assist Hybrid Electric Vehicles. Idaho National Laboratory: Idaho Falls, ID, USA, 2003.
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Sta-niewicz, R.J. Main Aging Mechanisms in Li Ion Batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, X.; Zhao, W.; Qiu, Y.; Liu, M.; Ye, F.; Xu, Y. Liquid-Phase Electrochemical Scanning Electron Microscopy for in Situ Investigation of Lithium Dendrite Growth and Dissolution. Adv. Mater. 2017, 29, 1606187. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, M.; Sohn, C.-H.; Ali, H.M. Understanding Wetting Behavior in Electrode-Electrolyte Interface Formation and Its Sensitivity to Electrode-Current Collector Interaction: A Lattice Boltzmann Method Approach. J. Therm. Anal. Calorim. 2024, 149, 5443–5456. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S. Regulating the Performance of Lithium-Ion Battery Focus on the Electrode-Electrolyte Interface. Front. Chem. 2020, 8, 821. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, T.; Wang, Z.; Cai, Q. A Systematic Investigation of Internal Physical and Chemical Changes of Lithium-Ion Batteries During Overcharge. J. Mater. Chem. A Mater. Energy Sustain. 2022, 518, 230767. [Google Scholar] [CrossRef]
- Dubarry, M.; Truchot, C.; Cugnet, M.; Bor Yann Liaw, K.; Gering, S.; Sazhin, D.; Jamison, C. Evaluation of Commercial Lithium-Ion Cells Based on Composite Positive Electrode for Plug-in Hybrid Electric vehicle Applications. Part I: Initial Characterizations. J. Power Sources 2011, 196, 10328–10335. [Google Scholar] [CrossRef]
- Li, Y.; Abdel-Monem, M.; Gopalakrishnan, R.; Berecibar, M.; Nanini-Maury, E.; Omar, N.; van den Bossche, P.; Van Mierlo, J. A Quick On-Line State of Health Estimation Method for Li-Ion Battery with Incremental Capacity Curves Processed by Gaussian Filter. J. Power Sources 2018, 373, 40–53. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li Plating as Unwanted Side Reaction in Commercial Li-Ion Cells—A Review. J. Power Sources 2018, 384, 107–124. [Google Scholar]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.-M. Comparison of Modeling Predictions with Experimental Data from Plastic Lithium Ion Cells. J. Electrochem. Soc. 1996, 143, 1890. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and Optimization of the Dual Lithium Ion Insertion Cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef]
- Davis, A.L.; Goel, V.; Liao, D.W.; Main, M.N.; Kazyak, E.; Lee, J.; Thornton, K.; Dasgupta, N.P. Rate Limitations in Composite Solid-State Battery Electrodes: Revealing Heterogeneity with Operando Microscopy. ACS Energy Lett. 2021, 6, 2993–3003. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A Comparative Study of Equivalent Circuit Models for Li-Ion Batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A Comprehensive Review of Lithi-Um-Ion Batteries Used in Hybrid and Electric Vehicles at Cold Temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Taheri, P.; Mansouri, A.; Mar-Yam, Y.; Bahrami, M. Theoretical Analysis of Potential and Current Distributions in Planar Electrodes of Lithi-Um-Ion Batteries. Electrochim. Acta 2014, 133, 197–208. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Z.; Wang, X.; Jia, L.; Yang, L. A Pseudo Three-Dimensional Electrochemical-Thermal Model of a Prismatic LiFePO4 Battery During Discharge Process. Energy 2015, 80, 303–317. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A Review of Ni-Based Layered Oxides for Re-chargeable Li-Ion Batteries. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 874–901. [Google Scholar] [CrossRef]
- Deich, T.; Storch, M.; Steiner, K.; Bund, A. Effects of Module Stiffness and Initial Compression on Lithium-Ion Cell Aging. J. Power Sources 2021, 506, 230163. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress Evolution and Capacity Fade in Constrained Lithium-Ion Pouch Cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. State of Health and Charge Measurements in Lithium-Ion Batteries Using Mechanical Stress. J. Power Sources 2014, 269, 7–14. [Google Scholar] [CrossRef]






| Parameter | Specification |
|---|---|
| Cathode Material Anode Material | NCM622 Graphite |
| Rated Capacity | 2.1 Ah |
| Nominal Voltage | 3.8 V |
| Operating Voltage | 2.75~4.35 V |
| Dimensions | 126 mm × 96 mm |
| Mass | 43 g |
| Step | Procedure | Termination Condition |
|---|---|---|
| 1 | Constant-Voltage Charging at 4.35 V with 1.8 C Current | Charging Current ≤ 0.05 C |
| 2 | Resting | t = 0.5 h |
| 3 | Constant Current Discharge at 1 C | Terminal Voltage ≤ 2.75 V |
| 4 | Resting | t = 1 h |
| 5 | Repeat Steps 1 to 4 | SOH Reduced to 90%, 80% |
| Step | Procedure | Setup Conditions |
|---|---|---|
| 1 | CC-CV Charging | Charge 5% SOC |
| 2 | Resting | t = 1 h |
| 3 | Pulse Discharge | I = 1 C, t = 10 s |
| 4 | Resting | t = 40 s |
| 5 | Pulse Charging | I = 1 C, t = 10 s |
| 6 | Resting | t = 1 h |
| 7 | Repeat Steps 1 to 6 | Cycle 18 Times Until Fully Charged |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.; Zhu, Y.; d’Apolito, L.; Shen, S. Evaluation of Lithium Battery Cycle Aging Based on Temperature Increase During Charging. Batteries 2025, 11, 150. https://doi.org/10.3390/batteries11040150
Hong H, Zhu Y, d’Apolito L, Shen S. Evaluation of Lithium Battery Cycle Aging Based on Temperature Increase During Charging. Batteries. 2025; 11(4):150. https://doi.org/10.3390/batteries11040150
Chicago/Turabian StyleHong, Hanchi, Yu Zhu, Luigi d’Apolito, and Shuiwen Shen. 2025. "Evaluation of Lithium Battery Cycle Aging Based on Temperature Increase During Charging" Batteries 11, no. 4: 150. https://doi.org/10.3390/batteries11040150
APA StyleHong, H., Zhu, Y., d’Apolito, L., & Shen, S. (2025). Evaluation of Lithium Battery Cycle Aging Based on Temperature Increase During Charging. Batteries, 11(4), 150. https://doi.org/10.3390/batteries11040150






