On the Morphological Evolution with Cycling of a Ball-Milled Si Slag-Based Electrode for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Preparation, Cell Assembling and Cycling
2.3. Electrochemical Dilatometry
2.4. Operando Optical Microscopy
2.5. X-Ray Diffraction (XRD) and Wide-Angle X-Ray Scattering (WAXS)
2.6. Scanning Electron Microscopy (SEM)
2.6.1. Two-Dimensional BIB-SEM Analyses
2.6.2. Three-Dimensional FIB-SEM Analyses
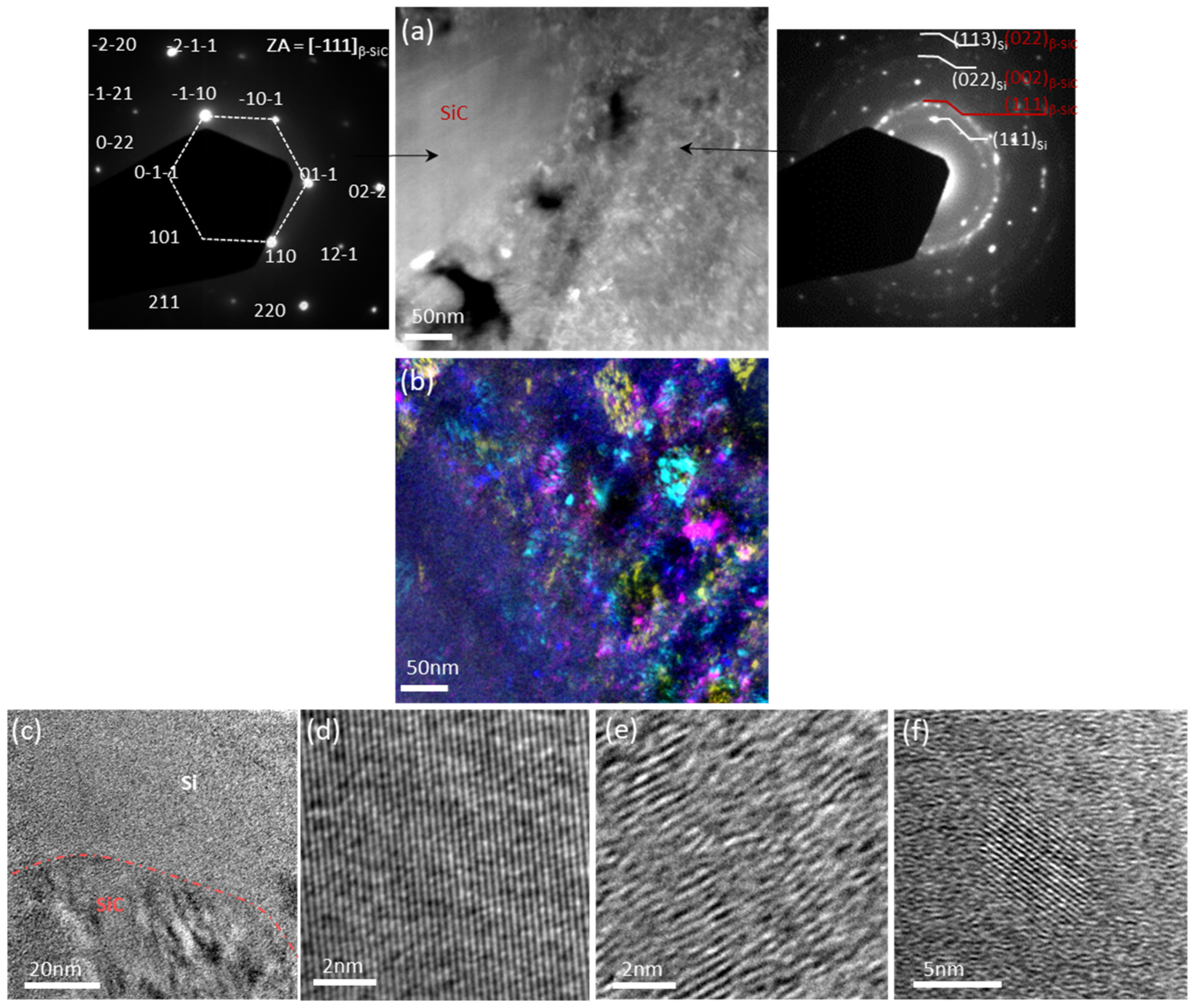
2.7. Transmission Electron Microscopy (TEM)
3. Results and Discussion
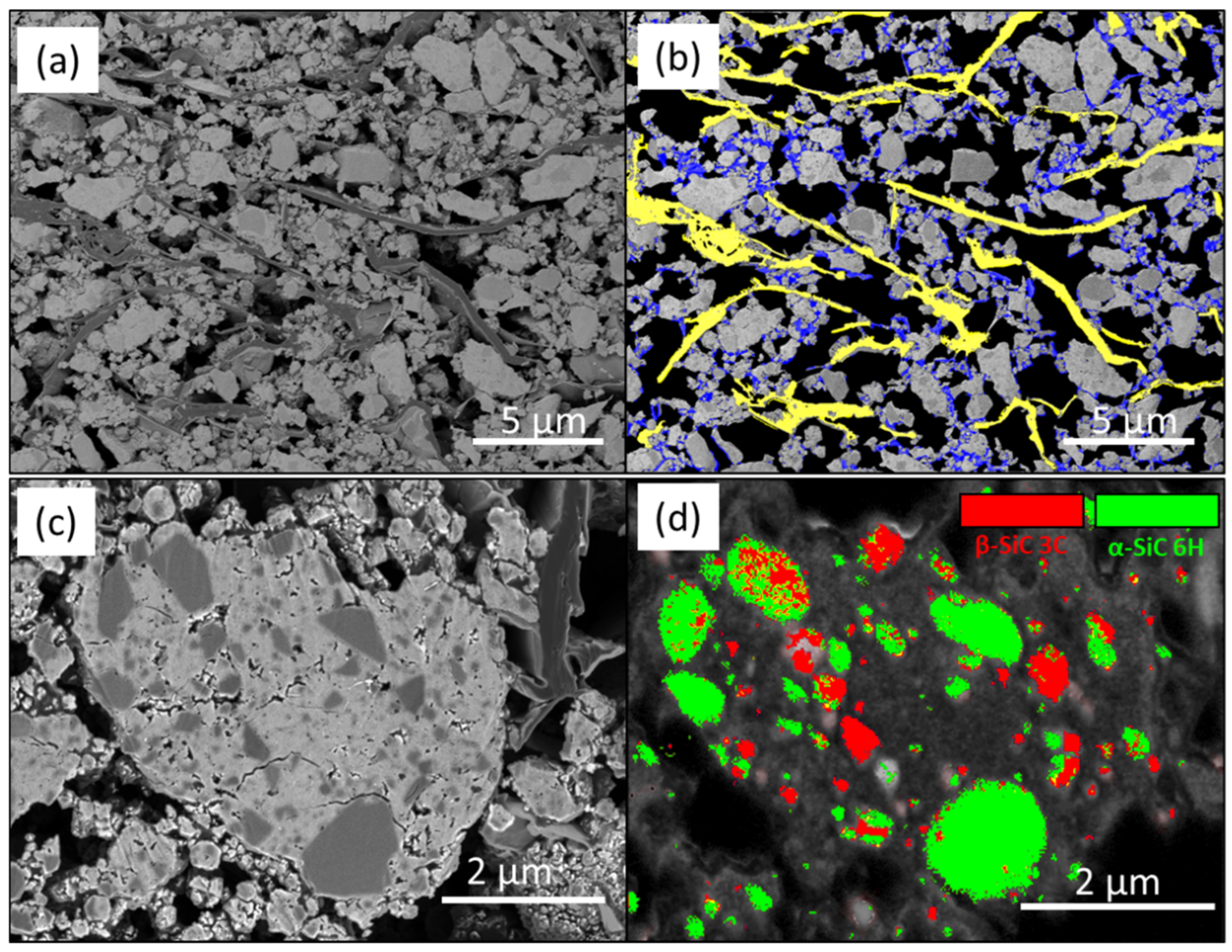
3.1. Microstructure of the Pristine Electrode
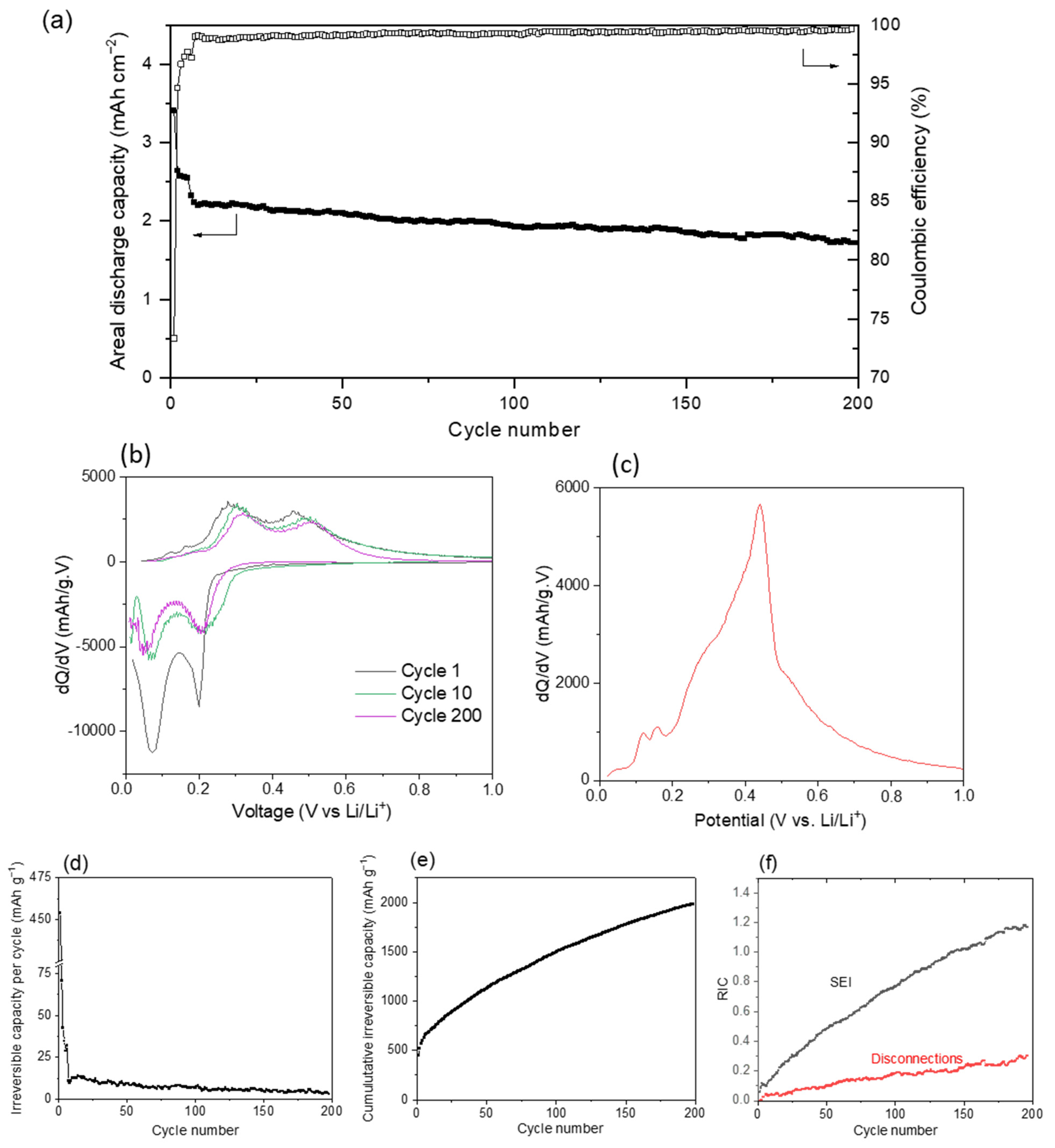
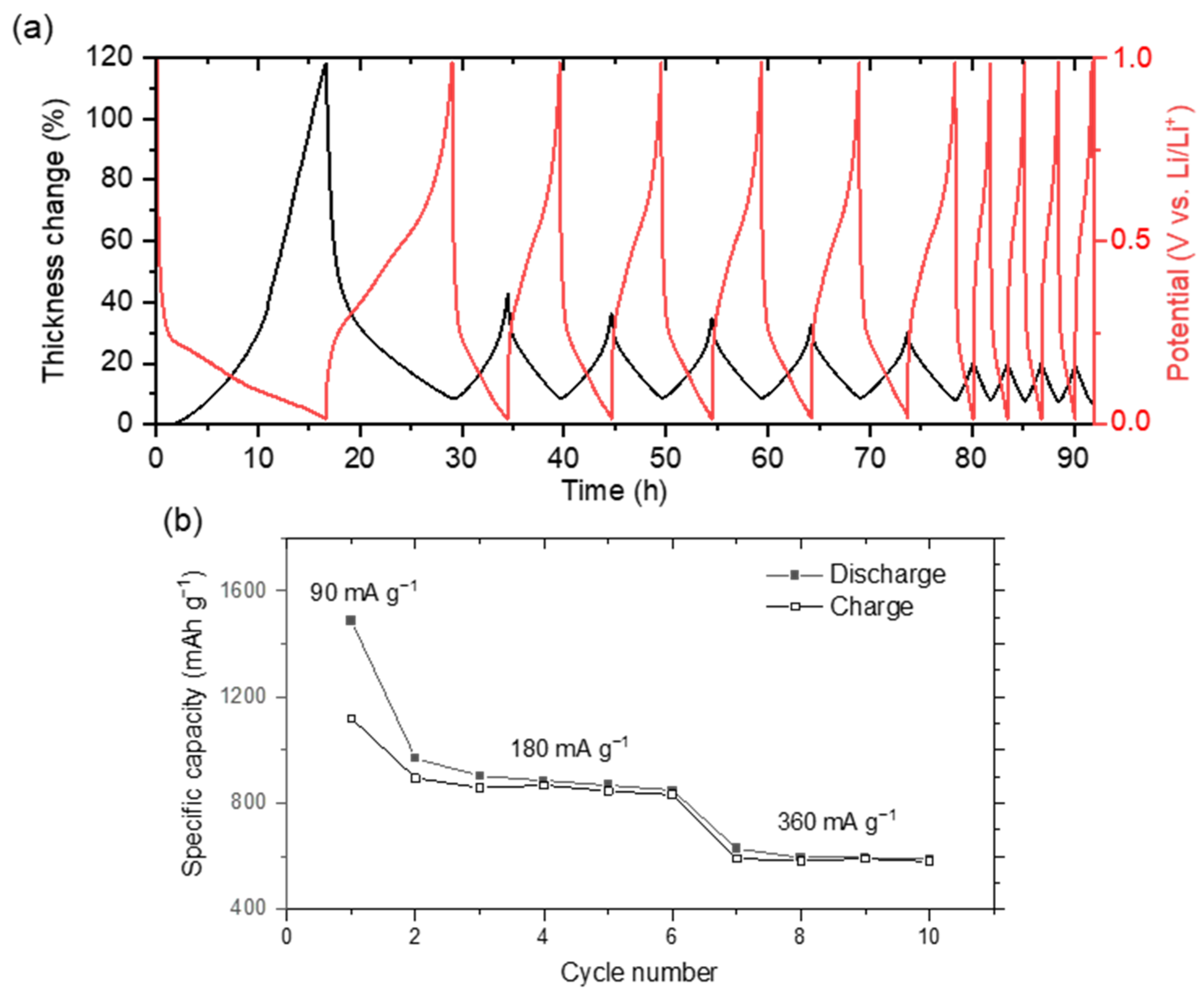
3.2. Electrochemical Performance
3.3. Microstructural and Morphological Evolution of the Electrode During Cycling
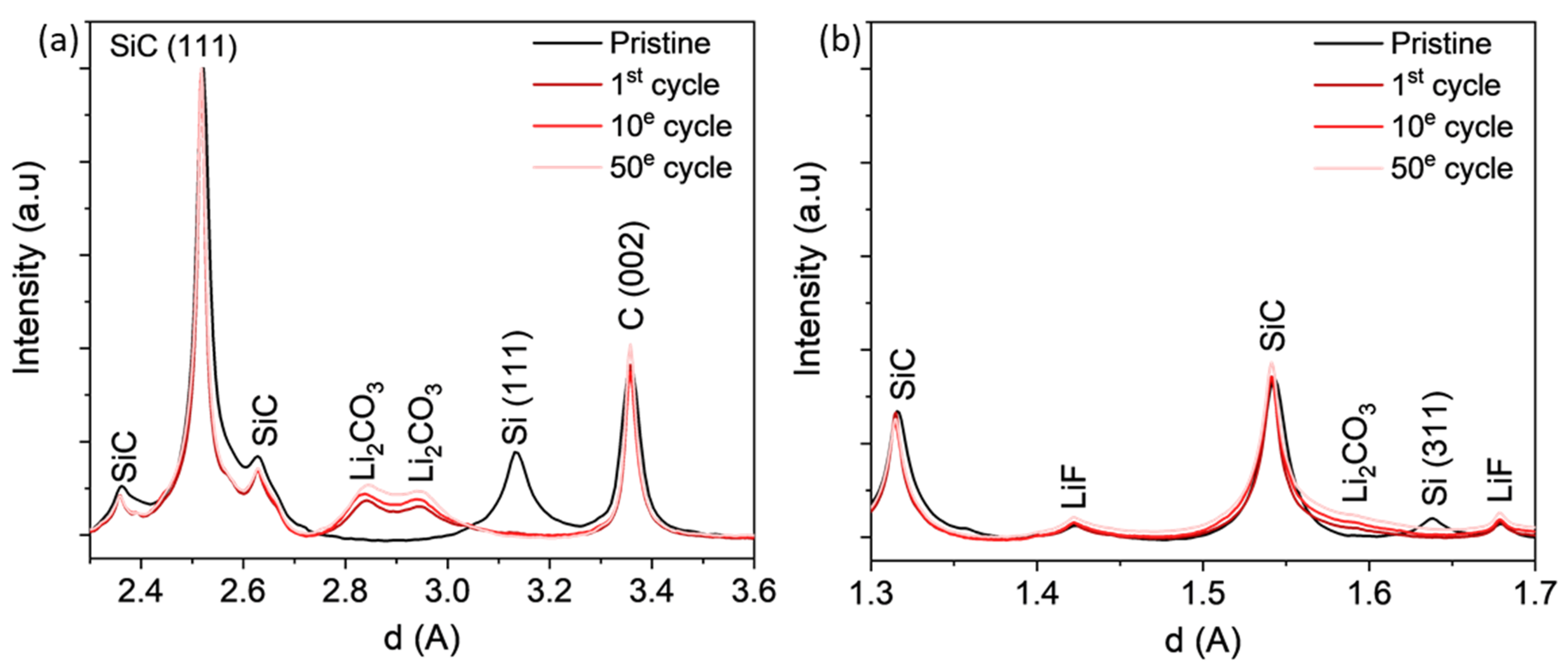
3.3.1. WAXS Analysis
3.3.2. Operando Dilatometry
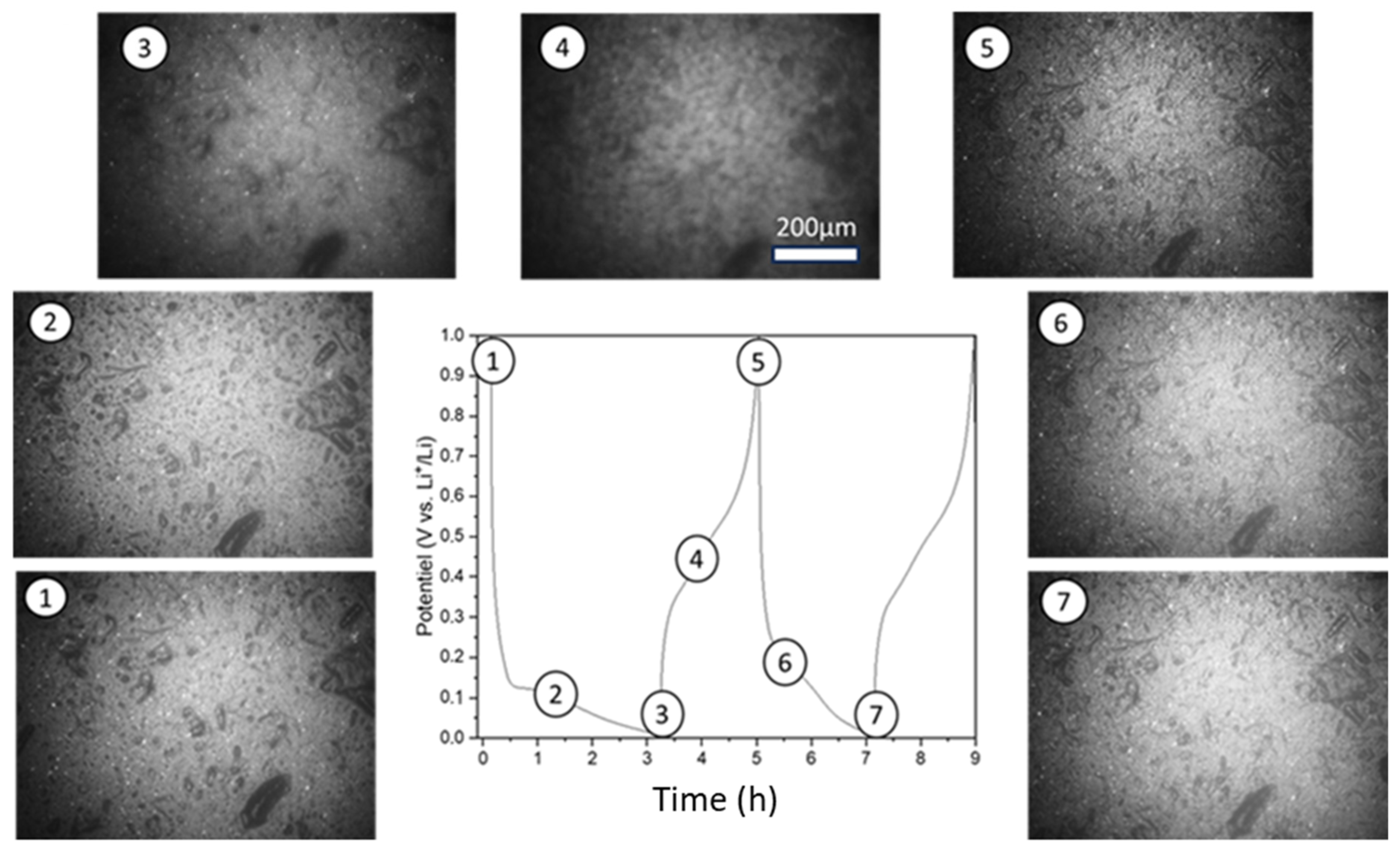
3.3.3. Operando Optical Microscopy
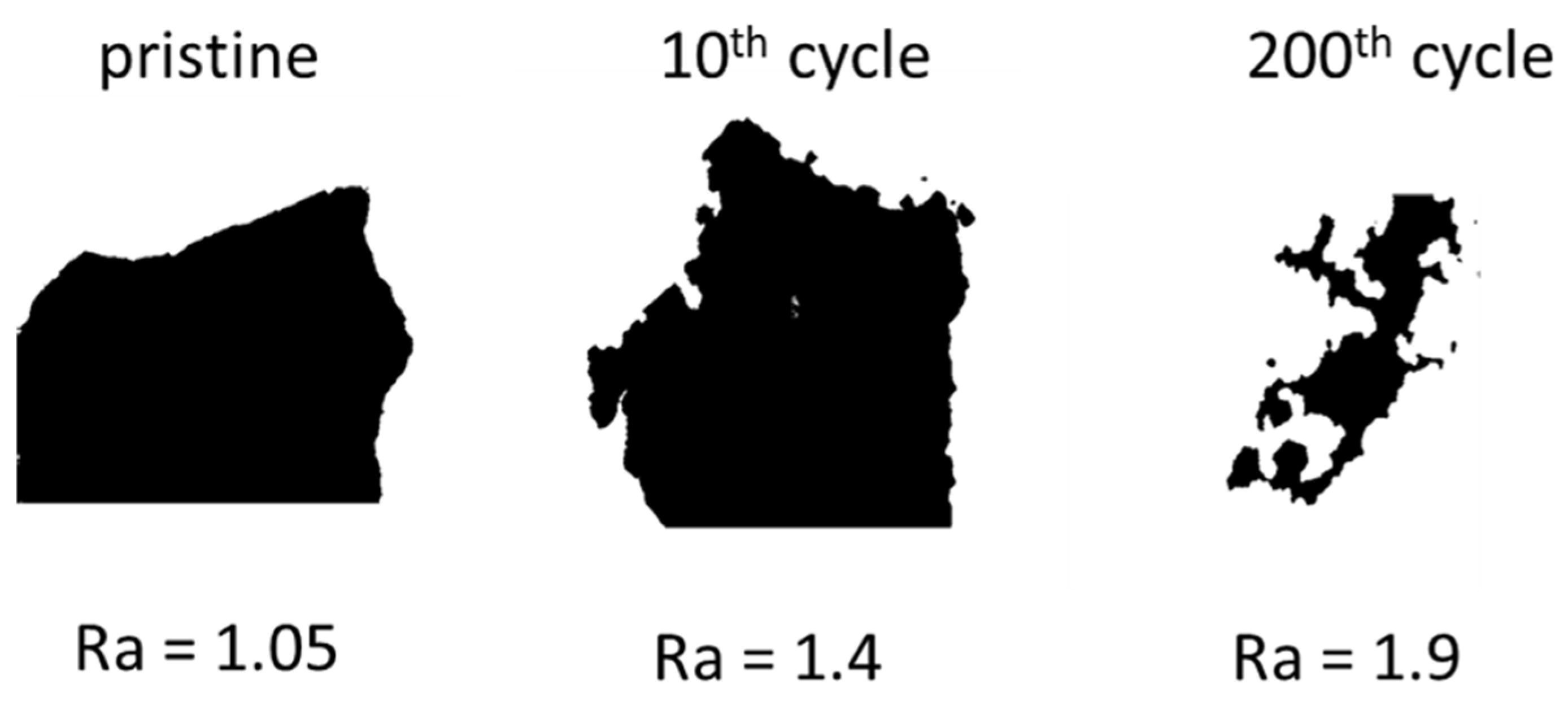
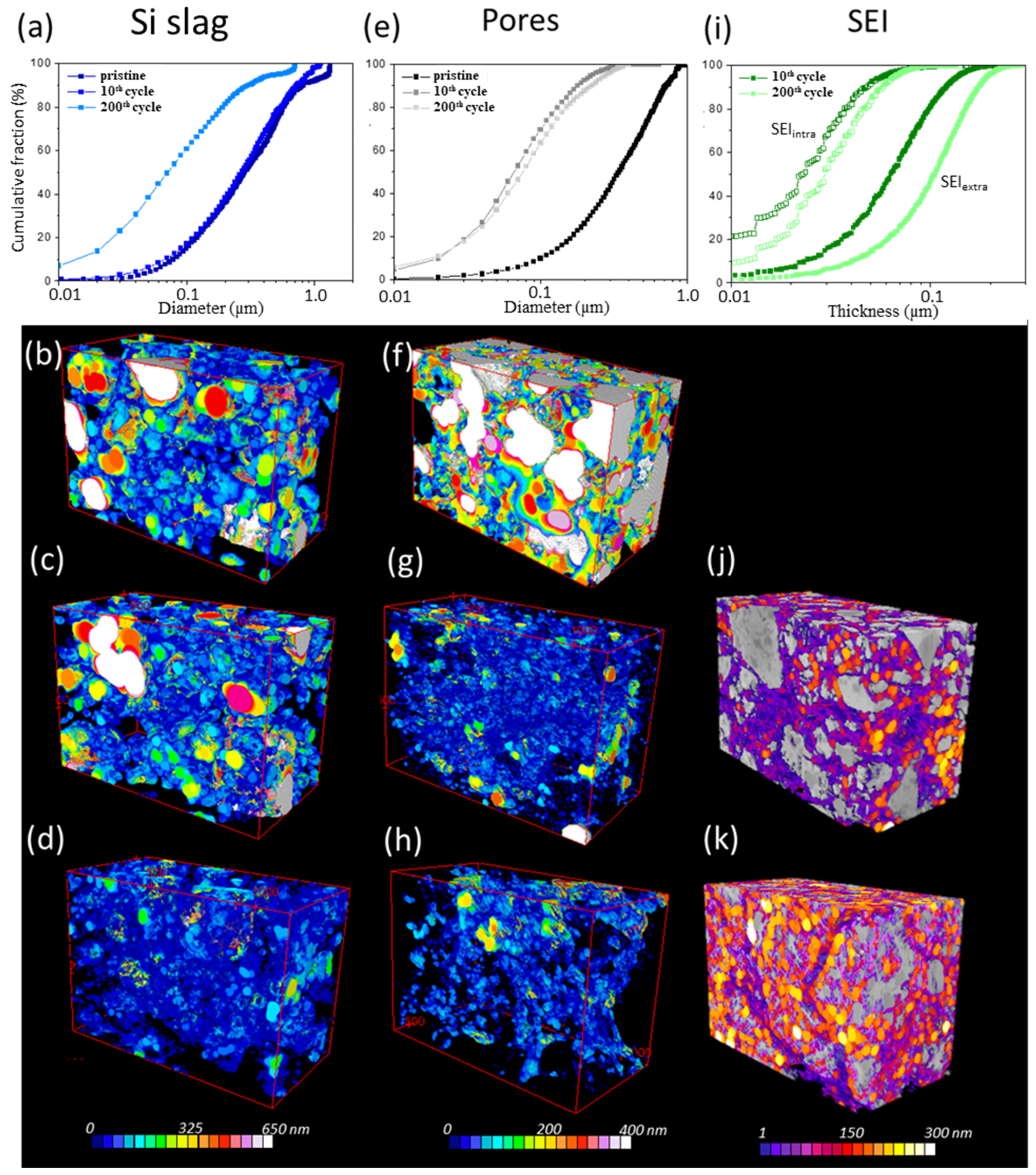
3.3.4. BIB-SEM Analyses
3.3.5. Three-Dimensional FIB-SEM Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feyzi, E.; Kumar, A.M.R.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A comprehensive review of silicon anodes for high-energy lithium-ion batteries: Challenges, latest developments, and perspectives. Next Energy 2024, 5, 100176. [Google Scholar] [CrossRef]
- Shahverdi, A.; Carabin, P. Silica to High Purity Silicon Production Process. Patent Application WO 2017/024378 A1, 19 February 2017. [Google Scholar]
- Heitz, A.; Vanpeene, V.; Herkendaal, N.; Soucy, P.; Douillard, T.; Roué, L. Transforming silicon slag into high-capacity anode material for lithium-ion batteries. Battery Energy 2022, 1, 20220016. [Google Scholar] [CrossRef]
- Xu, C.; Xia, T.; Li, X.; Zhang, A.; Chen, Y.; Wang, C.; Lin, R.; Li, Z.; Dai, P.; Zhou, Y.; et al. Covalent Binding of Holey Si–SiC Layer on Graphene Aerogel with Enhanced Lithium Storage Kinetics and Capability. Surf. Coat. Technol. 2021, 420, 127336. [Google Scholar] [CrossRef]
- Du, J.; Zhu, R.; Chen, Q.; Xie, J.; Xian, H.; Zhang, J.; Zhu, J. In Situ Synthesis of Stable Silicon Carbide-Reinforced Silicon Nanosheets from Organoclay for High-Performance Lithium-Ion Battery Anodes. Appl. Surf. Sci. 2023, 617, 156566. [Google Scholar] [CrossRef]
- Yan, X.; Hu, L.; He, X.; Zhang, J.; Zhang, W.; Gan, Y.; Xia, Y.; Huang, H. Ball-Milling-Triggered Synthesis of Si/C/SiC@MCMB Composites from Carbon Dioxide for Improved Lithium Storage Capability. Energy Fuels 2023, 37, 746–753. [Google Scholar] [CrossRef]
- Gautam, M.; Kumar Mishra, G.; Furquan, M.; Bhawana, K.; Kumar, D.; Mitra, S. Design of Low-Stress Robust Silicon and Silicon-Carbide Anode with High Areal Capacity and High Energy Density for Next-Generation Lithium-Ion Batteries. Chem. Eng. J. 2023, 472, 144916. [Google Scholar] [CrossRef]
- Furquan, M.; Jangid, M.K.; Khatribail, A.R.; Vijayalakshmi, S.; Mukhopadhyay, A.; Mitra, S. Mechanical and Electrochemical Stability Improvement of SiC-Reinforced Silicon-Based Composite Anode for Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12613–12626. [Google Scholar] [CrossRef]
- Ngo, D.T.; Le, H.T.T.; Pham, X.-M.; Park, C.-N.; Park, C.-J. Facile Synthesis of Si@SiC Composite as an Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 32790–32800. [Google Scholar] [CrossRef]
- Park, S.; Sung, J.; Chae, S.; Hong, J.; Lee, T.; Lee, Y.; Cha, H.; Kim, S.Y.; Cho, J. Scalable Synthesis of Hollow β-SiC/Si Anodes via Selective Thermal Oxidation for Lithium-Ion Batteries. ACS Nano 2020, 14, 11548–11557. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Xiao, Z.; Lei, C.; Zhang, C.; Lin, X.; Shen, B.; Zhang, R.; Wei, F. Silicon Carbide as a Protective Layer to Stabilize Si-Based Anodes by Inhibiting Chemical Reactions. Nano Lett. 2019, 19, 5124–5132. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Ren, J.; Wu, Y.; Yu, N.; Feng, A.; Huang, Z.; Jia, Z.; Wu, G. A Sandwich-Like Si/SiC/Nanographite Sheet as a High-Performance Anode for Lithium-Ion Batteries. Dalton Trans. 2019, 48, 17683. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, G.; Dou, F.; Zhuang, X.; Wang, Q.; Lu, M.; Shi, L.; Zhang, D. In Situ Growth of Silicon Carbide Interface Enhances the Long Life and High Power of the Mulberry-Like Si-Based Anode for Lithium-Ion Batteries. J. Energy Storage 2020, 32, 101856. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chen, X.; Yang, P.; An, M. Facile Preparation of Hollow Si/SiC/C Yolk-Shell Anode by One-Step Magnesiothermic Reduction. Ceram. Int. 2019, 45, 17040–17047. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H. Sequential-Template Synthesis of Hollowed Carbon Polyhedron@SiC@Si for Lithium-Ion Battery with High Capacity and Electrochemical Stability. Appl. Surf. Sci. 2020, 514, 145920. [Google Scholar] [CrossRef]
- Tzeng, Y.; He, J.-L.; Jhan, C.-Y.; Wu, Y.-H. Effects of SiC and Resorcinol–Formaldehyde (RF) Carbon Coatings on Silicon-Flake-Based Anode of Lithium-Ion Battery. Nanomaterials 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, P.; Liao, M.; Lu, X.; Shen, G.; Zhong, C.; Zhang, M.; Huang, Q.; Su, Z. SiO Anode with Long-Term Cycling Ability Enhanced by a SiC Transition Layer Based on Polymethylsilane in LIBs. Appl. Surf. Sci. 2024, 653, 159308. [Google Scholar] [CrossRef]
- Choi, J.-H.; Choi, S.; Cho, J.S.; Kim, H.-K.; Jeong, S.M. Efficient Synthesis of High Areal Capacity Si@Graphite@SiC Composite Anode Material via One-Step Electro-Deoxidation. J. Alloys Compd. 2022, 896, 163010. [Google Scholar] [CrossRef]
- Liang, J.; Wang, W.; Yang, W.; Zhang, Z.; Zhang, X.; Jung, Y.; Dong, X. Facile Synthesis of Ceramic SiC-Based Nanocomposites and the Superior Electrochemical Lithiation/Delithiation Performances. Mater. Chem. Phys. 2020, 243, 122618. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Qin, C.; Li, Y.; Yang, Y. Synthesis of Free-Standing N-Doping Si/SiC/C Composite Nanofiber Film as Superior Lithium-Ion Batteries Anode. Mater. Lett. 2022, 306, 130895. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, H.; Hoeppener, S.; Friebe, C.; Wang, J.; Chanteux, G.; Xie, D.; Lu, Y.; Vlad, A.; Schubert, U.S.; et al. Hollow Porous Silicon Nanospheres with 3D SiC@C Coating as High-Performance Anodes. Mater. Des. 2023, 226, 111624. [Google Scholar] [CrossRef]
- Tranchot, A.; Idrissi, H.; Thivel, P.X.; Roué, L. Impact of the Slurry pH on the Expansion/Contraction Behavior of Silicon/Carbon/Carboxymethylcellulose Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1020–A1026. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Pierre, W.; Wenxing, Z. A variational model for multiplicative structured noise removal. J. Math. Imaging Vis. 2017, 57, 43–55. [Google Scholar]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Kreshuk, A.; Zhang, C. Machine Learning: Advanced Image Segmentation Using Ilastik. Methods Mol. Biol. 2019, 2040, 449–463. [Google Scholar]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystal. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Gauthier, R.; Scott, B.; Benett, J.C.; Salehabadi, M.; Wang, J.; Sainuddin, T.; Obrovac, M.N. The Amorphization of Crystalline Silicon by Ball Milling. Heliyon 2024, 10, e34881. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Wang, W.; Zhang, Q. Formation and Quantitative Analysis of Internal Structure of Si Nanoparticles Developed via Bead-Milling. AIP Adv. 2021, 11, 075101. [Google Scholar] [CrossRef]
- Timmons, A.; Todd, A.D.W.; Mead, S.D.; Carey, G.H.; Sanderson, R.J.; Mar, R.E.; Dahn, J.R. Studies of Si1-xCx Electrode Materials Prepared by High-Energy Mechanical Milling and Combinatorial Sputter Deposition. J. Electrochem. Soc. 2007, 154, 865–874. [Google Scholar] [CrossRef]
- Li, J.; Dahn, J.R. An In Situ X-Ray Diffraction Study of the Reaction of Li with Crystalline Si. J. Electrochem. Soc. 2007, 154, 156–161. [Google Scholar] [CrossRef]
- Iaboni, D.S.M.; Obrovac, M.N. Li15Si4 Formation in Silicon Thin Film Negative Electrodes. J. Electrochem. Soc. 2016, 163, 255–261. [Google Scholar] [CrossRef]
- Gauthier, M.; Mazouzi, D.; Reyter, D.; Moreau, P.; Lestriez, B.; Guyomard, D.; Roué, L. A Low-Cost and High-Performance Si-Based Electrode for Li-Ion Batteries. Energy Environ. Sci. 2013, 6, 2145–2155. [Google Scholar] [CrossRef]
- Nguyen, B.P.N.; Gaubicher, J.; Lestriez, B. Analogy Between Electrochemical Behaviour of Thick Silicon Granular Electrodes for Lithium Batteries and Fine Soils Micromechanics. Electrochim. Acta 2014, 120, 319–326. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Wu, X.; Song, K.; Ren, B.; Li, T.; Zhang, S. Effect of N/P Ratios on the Performance of LiNi0.8Co0.15Al0.05O2||SiOx/Graphite Lithium-Ion Batteries. J. Power Sources 2019, 439, 227056. [Google Scholar] [CrossRef]
- Klett, M.; Gilbert, J.A.; Pupek, K.Z.; Trask, S.E.; Abraham, D.P. Layered Oxide, Graphite and Silicon-Graphite Electrodes for Lithium-Ion Cells: Effect of Electrolyte Composition and Cycling Windows. J. Electrochem. Soc. 2017, 164, 6095–6102. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Krause, L.J.; Jensen, L.D.; Huynh, C.; Triemert, M.; Bowen, E.L.; Thorson, J. Design of Positive Electrodes for Li-Ion Full Cells with Silicon. J. Electrochem. Soc. 2018, 165, 2968–2977. [Google Scholar] [CrossRef]
- Song, B.F.; Dhanabalan, A.; Biswal, S.L. Evaluating the Capacity Ratio and Prelithiation Strategies for Extending Cyclability in Porous Silicon Composite Anodes and Lithium Iron Phosphate Cathodes for High Capacity Lithium-Ion Batteries. J. Energy Storage 2020, 28, 101268. [Google Scholar] [CrossRef]
- Karkar, Z.; Mazouzi, D.; Reale Hernandez, C.; Guyomard, D.; Roué, L.; Lestriez, B. Threshold-like Dependence of Silicon-Based Electrode Performance on Active Mass Loading and Nature of Carbon Conductive Additive. Electrochim. Acta 2016, 215, 276–288. [Google Scholar] [CrossRef]
- Huet, L.; Mazouzi, D.; Moreau, P.; Dupré, N.; Paris, M.; Mittelette, S.; Laurencin, D.; Devic, T.; Roué, L.; Lestriez, B. Coordinatively Cross-Linked Binders for Silicon-Based Electrodes for Li-Ion Batteries: Beneficial Impact on Mechanical Properties and Electrochemical Performance. ACS Appl. Mater. Interfaces 2023, 15, 15509–15524. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, 103–108. [Google Scholar] [CrossRef]
- Hernandez, C.R.; Etiemble, A.; Mazouzi, D.; Karkar, Z.; Maire, E.; Guyomard, D.; Lestriez, B.; Roué, L. A Facile and Very Effective Method to Enhance the Mechanical Strength and the Cyclability of Si-Based Electrodes for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701787. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Berhaut, C.L.; Dominguez, D.Z.; De Vito, E.; Tardif, S.; Pouget, S.; Lyonnard, S.; Jouneau, P.-H. Nano-Architectured Composite Anode Enabling Long-Term Cycling Stability for High-Capacity Lithium-Ion Batteries. Small 2020, 16, 1906812. [Google Scholar] [CrossRef]
- Wetjen, M.; Solchenbach, S.; Pritzl, D.; Hou, J.; Tileli, V.; Gasteiger, H.A. Morphological Changes of Silicon Nanoparticles and the Influence of Cutoff Potentials in Silicon-Graphite Electrodes. J. Electrochem. Soc. 2018, 165, 1503–1514. [Google Scholar] [CrossRef]
- Kim, I.-S.; Blomgren, G.E.; Kumta, P.N. Si–SiC Nanocomposite Anodes Synthesized Using High-Energy Mechanical Milling. J. Power Sources 2004, 130, 275–280. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, J.-G.; Wang, X.; Chui, Y.-S.; Wu, Q.-H.; Chen, X.; Zhang, W. Graphene Encapsulated and SiC Reinforced Silicon Nanowires as an Anode Material for Lithium-Ion Batteries. Nanoscale 2013, 5, 8689–8694. [Google Scholar] [CrossRef] [PubMed]
- Lipson, A.L.; Chattopadhyay, S.; Karmel, H.J.; Fister, T.T.; Emery, J.D.; Dravid, V.P.; Thackeray, M.M.; Fenter, P.A.; Bedzyk, M.J.; Hersam, M.C. Enhanced Lithiation of Doped 6H Silicon Carbide (0001) via High Temperature Vacuum Growth of Epitaxial Graphene. J. Phys. Chem. C 2012, 116, 20949–20957. [Google Scholar] [CrossRef]
- Virdis, S.; Vetter, U.; Ronning, C.; Kröger, H.; Hofsäss, H. Lattice Site Location of Ion-Implanted 8Li in Silicon Carbide. J. Appl. Phys. 2002, 91, 1046. [Google Scholar] [CrossRef]
- Sri Devi Kumari, T.; Jeyakumar, D.; Prem Kumar, T. Nano Silicon Carbide: A New Lithium-Insertion Anode Material on the Horizon. RSC Adv. 2013, 3, 15028. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhang, X.; Guo, G.; Hu, J.; Dong, A.; Yang, D. Bowl-Like 3C-SiC Nanoshells Encapsulated in Hollow Graphitic Carbon Spheres for High-Rate Lithium-Ion Batteries. Chem. Mater. 2016, 28, 1179–1186. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.-J.; Gu, H.; Fan, H.; Yang, G.; Ignaszak, A.; Tang, X.; Liu, D.; Zhang, J. Interfacial Coupled Design of Epitaxial Graphene@SiC Schottky Junction with Built-in Electric Field for High-Performance Anode of Lithium-Ion Batteries. Nano Energy 2020, 77, 105092. [Google Scholar] [CrossRef]
- Bijoy, T.K.; Karthikeyan, J.; Murugan, P. Exploring the Mechanism of Spontaneous and Lithium-Assisted Graphitic Phase Formation in SiC Nanocrystallites of a High Capacity Li-Ion Battery Anode. J. Phys. Chem. C 2017, 121, 15106–15113. [Google Scholar] [CrossRef]
- Nandan, R.; Takamori, N.; Higashimine, K.; Badam, R.; Matsumi, N. Confronting the Issues Associated with the Practical Implementation of Zinc Blende-Type SiC Anodes for Efficient and Reversible Storage of Lithium Ions. ACS Appl. Energy Mater. 2024, 7, 2088–2100. [Google Scholar] [CrossRef]




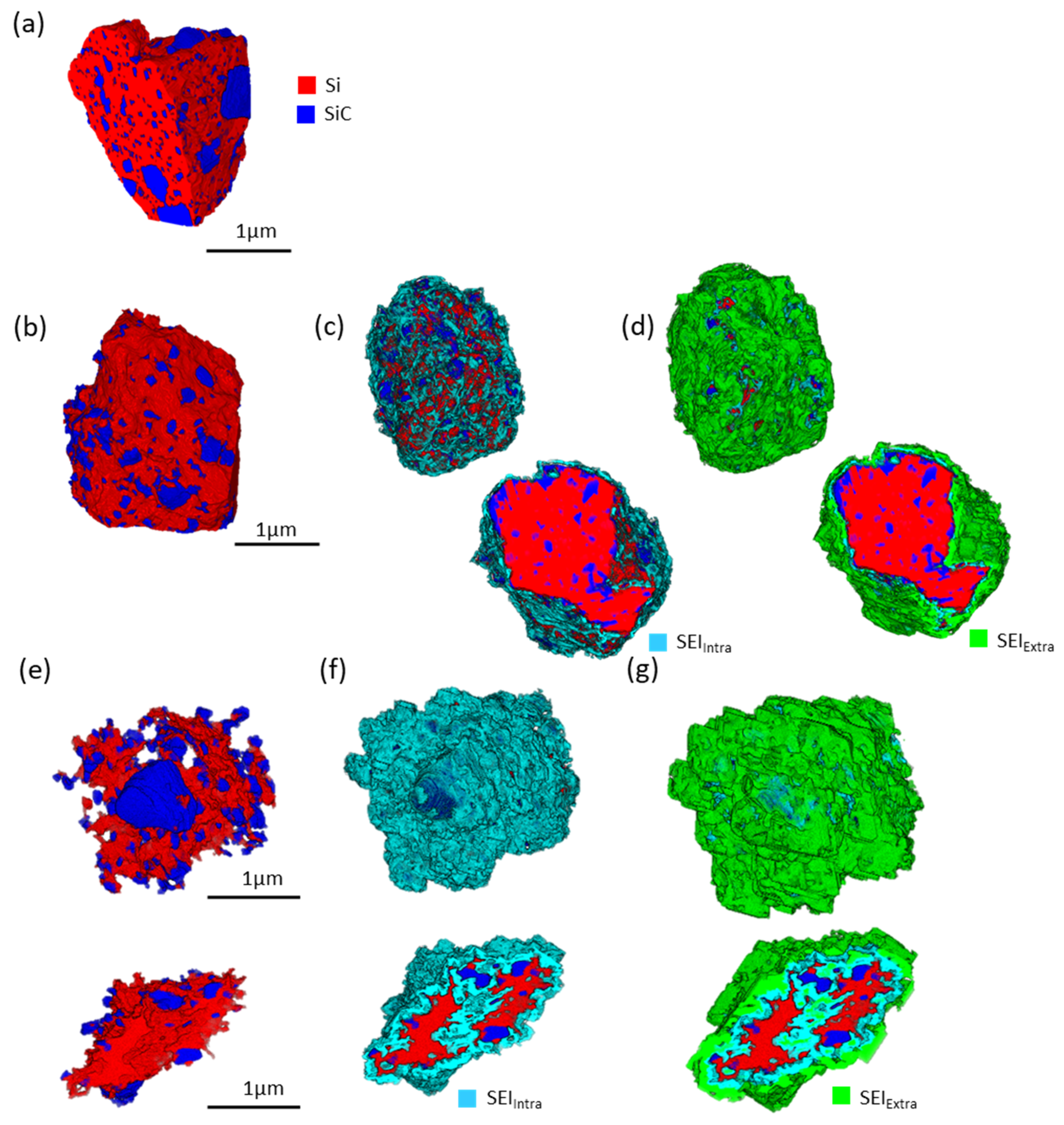
| Si | SiC | Si + SiC | GnP | Pores | SEIintra | SEIextra | SEI Total | |
|---|---|---|---|---|---|---|---|---|
| Pristine | 24.9 | 12.7 | 37.6 | 5.8 | 56.6 | 0 | 0 | 0 |
| 10th cycle | 22.6 | 15.0 | 37.6 | 6.7 | 18.7 | 10.2 | 26.8 | 37.0 |
| 200th cycle | 5.4 | 6.1 | 11.5 | 8.5 | 8.9 | 29.5 | 41.6 | 71.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitz, A.; Vanpeene, V.; Quéméré, S.; Herkendaal, N.; Douillard, T.; Martens, I.; Mirolo, M.; Roué, L. On the Morphological Evolution with Cycling of a Ball-Milled Si Slag-Based Electrode for Li-Ion Batteries. Batteries 2025, 11, 151. https://doi.org/10.3390/batteries11040151
Heitz A, Vanpeene V, Quéméré S, Herkendaal N, Douillard T, Martens I, Mirolo M, Roué L. On the Morphological Evolution with Cycling of a Ball-Milled Si Slag-Based Electrode for Li-Ion Batteries. Batteries. 2025; 11(4):151. https://doi.org/10.3390/batteries11040151
Chicago/Turabian StyleHeitz, Alexandre, Victor Vanpeene, Samuel Quéméré, Natalie Herkendaal, Thierry Douillard, Isaac Martens, Marta Mirolo, and Lionel Roué. 2025. "On the Morphological Evolution with Cycling of a Ball-Milled Si Slag-Based Electrode for Li-Ion Batteries" Batteries 11, no. 4: 151. https://doi.org/10.3390/batteries11040151
APA StyleHeitz, A., Vanpeene, V., Quéméré, S., Herkendaal, N., Douillard, T., Martens, I., Mirolo, M., & Roué, L. (2025). On the Morphological Evolution with Cycling of a Ball-Milled Si Slag-Based Electrode for Li-Ion Batteries. Batteries, 11(4), 151. https://doi.org/10.3390/batteries11040151







