Super-Fast Sodium Storage Properties of Nitrogen-Doped Graphene-Based Material Synthesized via Arc-Discharge Method
Abstract
1. Introduction
2. Methods
2.1. Preparation of Artificial Graphene-Based Materials
2.2. Material Characterization
2.3. Electrochemical Properties
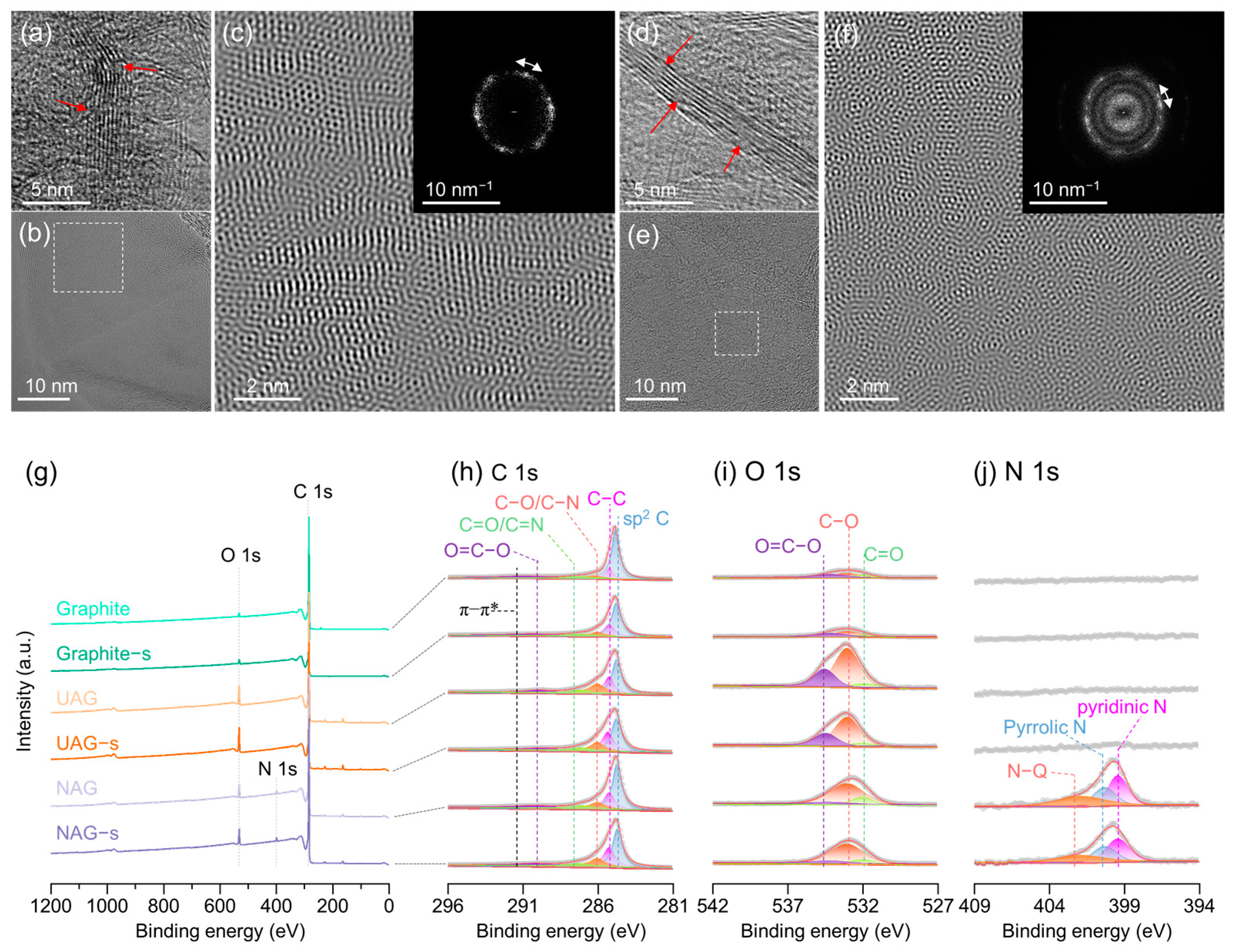
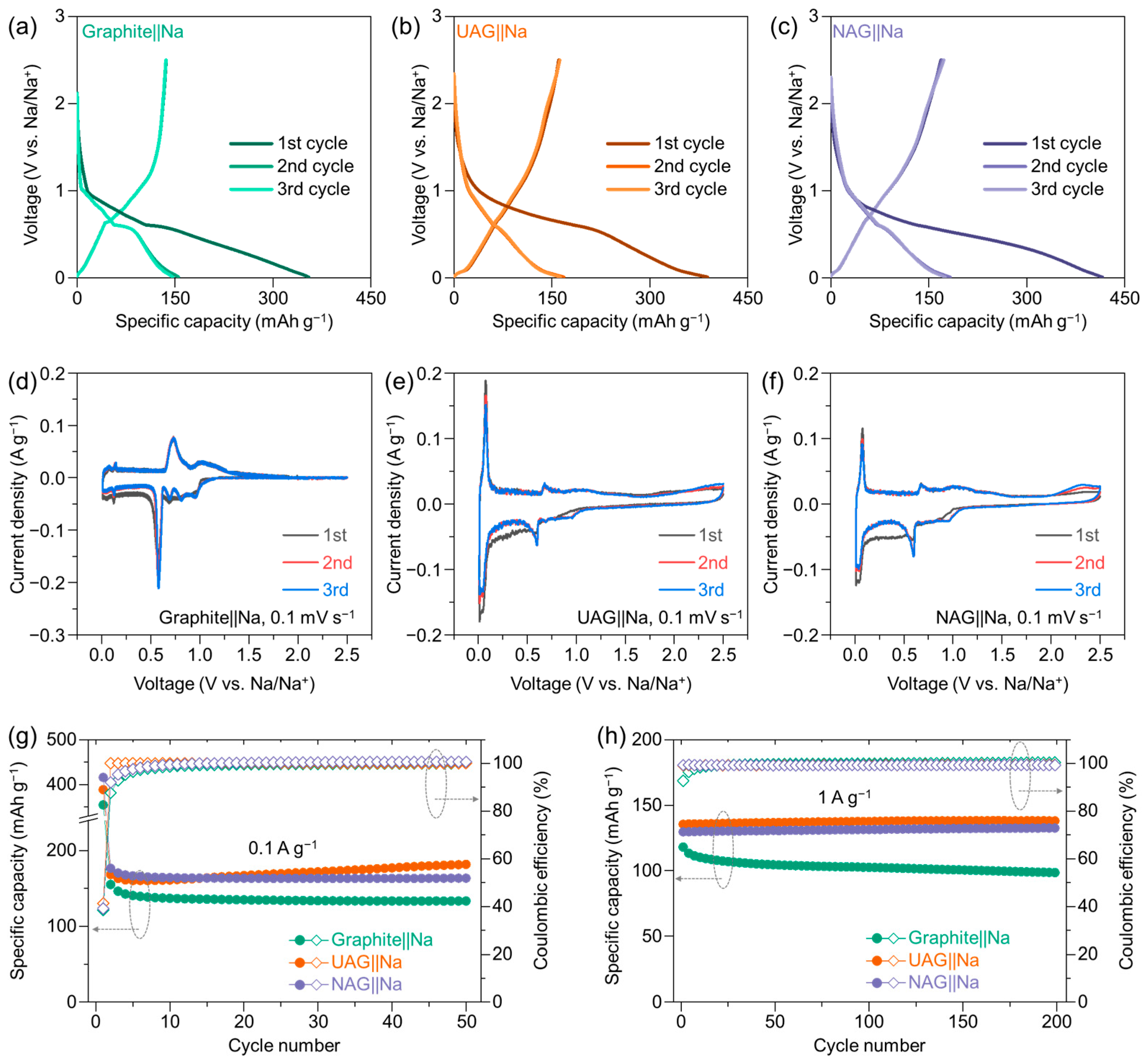
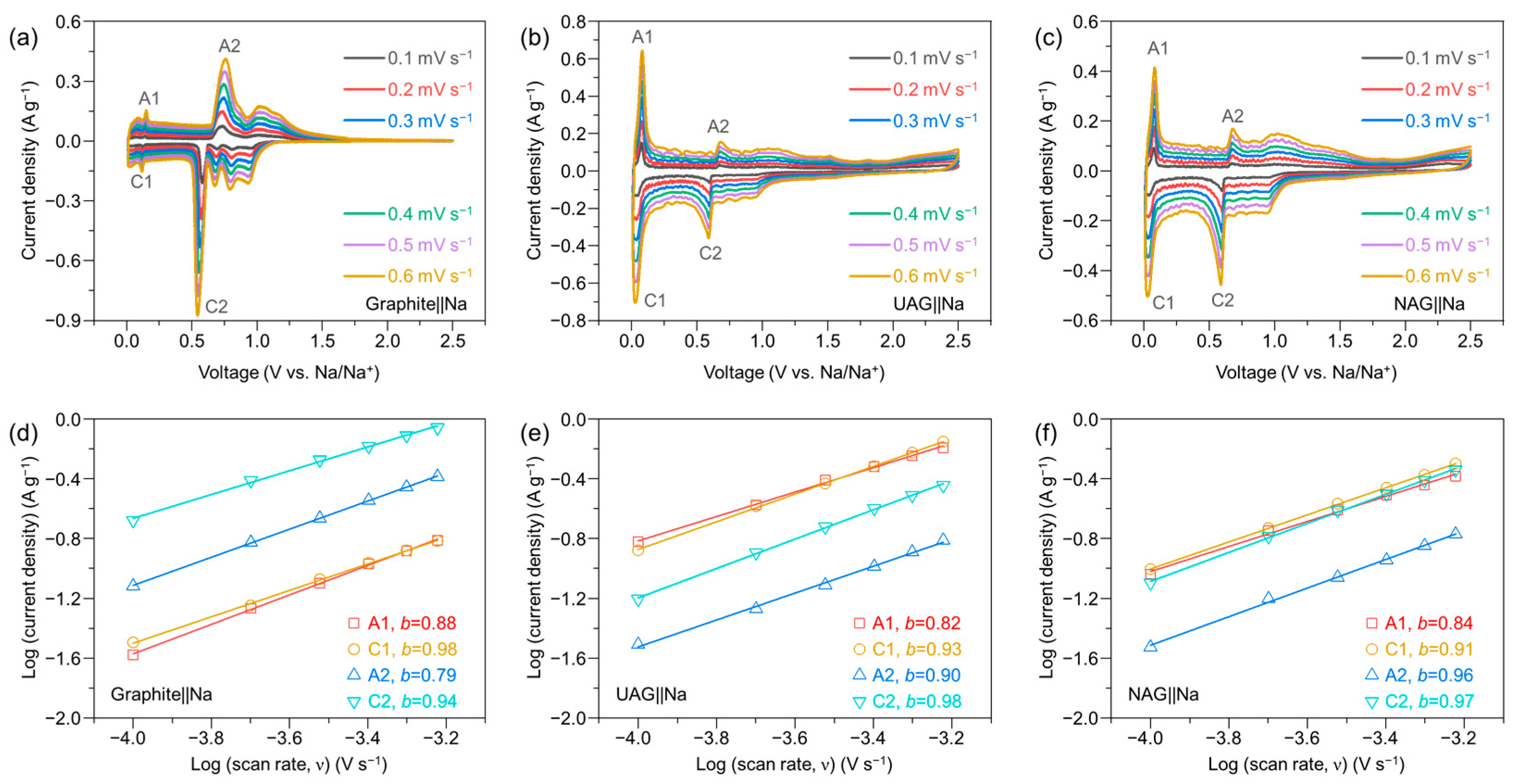
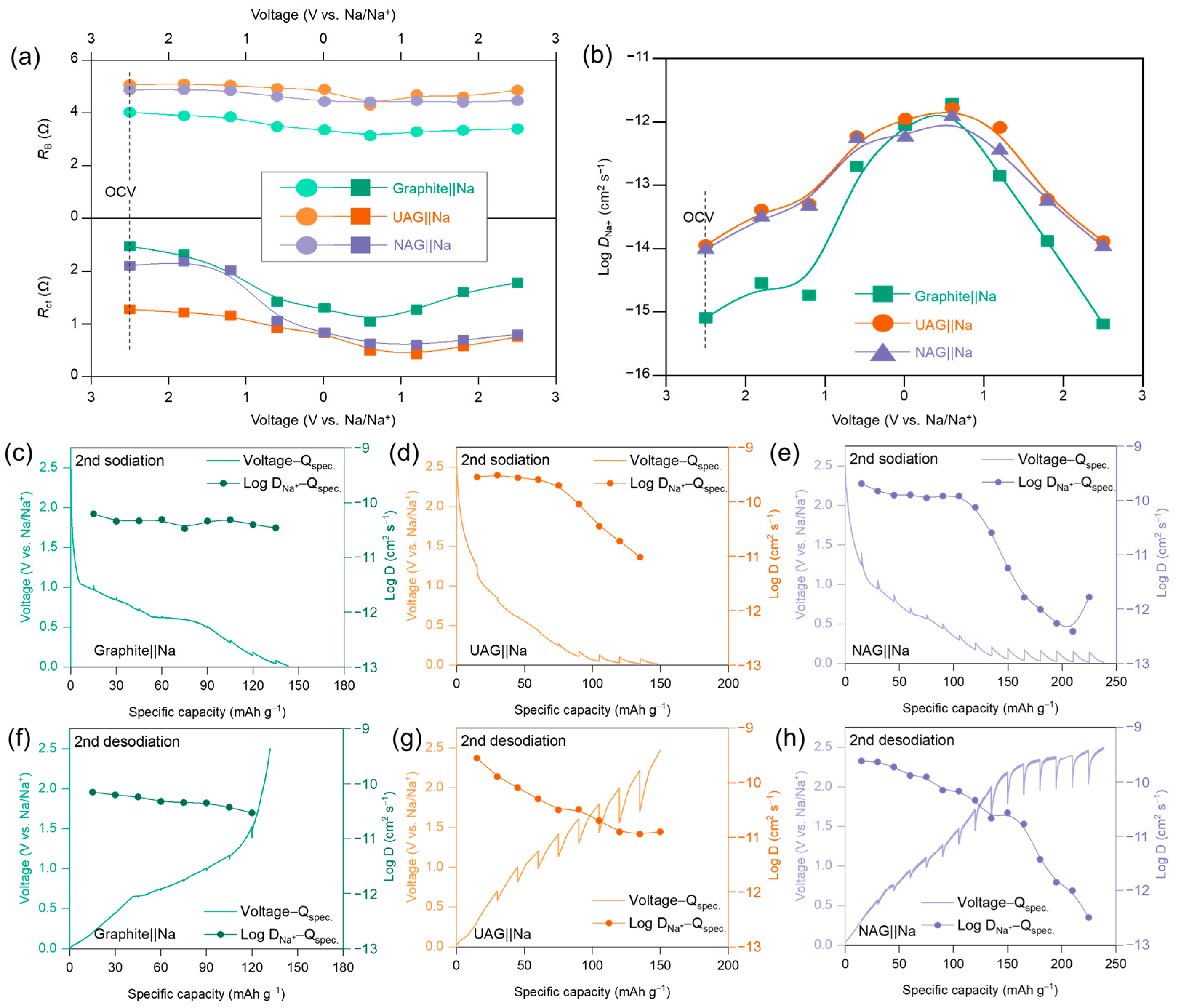
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| UAG | Undoped artificial graphene-based material |
| NAG | Nitrogen-doped artificial graphene-based material |
| LIBs | Lithium-ion batteries |
| SIBs | Sodium-ion batteries |
| PANI | Polyaniline |
| FE-SEM | Field-emission scanning electron microscopy |
| HR-TEM | High-resolution transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| DEGDME | Diethylene glycol dimethyl ether |
| SBR | Styrene butadiene rubber |
| CMC | Carboxymethyl cellulose |
| CV | Cyclic voltammetry |
| GITT | Galvanostatic intermittent titration technique |
| EIS | Electrochemical impedance spectroscopy |
| FFT | Fast Fourier transform |
| IFFT | Inverse fast Fourier transform |
References
- Liu, B.; Zhang, Z.D.; Xu, X. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar]
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614. [Google Scholar] [PubMed]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. Engl. 2018, 57, 102–120. [Google Scholar]
- Masese, T.; Yoshii, K.; Yamaguchi, Y.; Okumura, T.; Huang, Z.D.; Kato, M.; Kubota, K.; Furutani, J.; Orikasa, Y.; Senoh, H.; et al. Rechargeable potassium-ion batteries with honeycomb-layered tellurates as high voltage cathodes and fast potassium-ion conductors. Nat. Commun. 2018, 9, 3823. [Google Scholar]
- Liu, Y.; Tai, Z.; Zhang, J.; Pang, W.K.; Zhang, Q.; Feng, H.; Konstantinov, K.; Guo, Z.; Liu, H.K. Boosting potassium-ion batteries by few-layered composite anodes prepared via solution-triggered one-step shear exfoliation. Nat. Commun. 2018, 9, 3645. [Google Scholar]
- Lin, M.C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 325–328. [Google Scholar]
- Wang, N.; Wan, H.; Duan, J.; Wang, X.; Tao, L.; Zhang, J.; Wang, H. A review of zinc-based battery from alkaline to acid. Mater. Today Adv. 2021, 11, 100149. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 1–11. [Google Scholar]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [PubMed]
- Cohn, A.P.; Muralidharan, N.; Carter, R.; Share, K.; Pint, C.L. Anode-Free Sodium Battery through in Situ Plating of Sodium Metal. Nano Lett. 2017, 17, 1296–1301. [Google Scholar]
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. Energy Chem. 2019, 1, 100012. [Google Scholar]
- Lv, J.; Wang, Q.; OuYang, M.; Cao, Y. Highly Performing Sodium Metal Batteries Reinforced by a Self-Regulated Dual-Layered Solid Electrolyte Interphase via a Metal−Organic Framework. ACS Appl. Mater. Interfaces 2024, 16, 41570–41582. [Google Scholar] [PubMed]
- Wang, X.; Zhang, C.; Sawczyk, M.; Sun, J.; Yuan, Q.; Chen, F.; Mendes, T.C.; Howlett, P.C.; Fu, C.; Wang, Y.; et al. Ultra-stable all-solid-state sodium metal batteries enabled by perfluoropolyether-based electrolytes. Nat. Mater. 2022, 21, 1057–1065. [Google Scholar]
- Jeon, I.; Yang, D.; Yadav, D.; Seo, J.; Zhang, H.; Yin, L.; Ahn, H.S.; Cho, C.R. Sodium storage behavior and long cycle stability of boron-doped carbon nanofibers for sodium-ion battery anodes. Electrochim. Acta 2023, 439, 141730. [Google Scholar]
- Jeon, I.; Kim, T.; Seo, J.; Jeong, I.K.; Lee, J.H.; Park, M.; Park, Y.; Yang, D.; Cho, C.R. Enhanced electrochemical performance and interdiffusion behavior of sodium ions in onion-derived freeze-dried and KOH-activated carbon for sodium-ion battery anodes. Appl. Surf. Sci. 2024, 648, 159023. [Google Scholar]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon. 2019, 145, 67–81. [Google Scholar]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. Engl. 2014, 53, 10169–10173. [Google Scholar] [PubMed]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D Porous γ-Fe2O3 @C Nanocomposite as High-Performance Anode Material of Na-Ion Batteries. Adv. Energy Mater. 2014, 5, 1401123. [Google Scholar]
- Naeyaert, P.J.P.; Avdeev, M.; Sharma, N.; Yahia, H.B.; Ling, C.D. Synthetic, Structural, and Electrochemical Study of Monoclinic Na4Ti5O12 as a Sodium-Ion Battery Anode Material. Chem. Mater. 2014, 26, 7067–7072. [Google Scholar]
- Ma, D.; Li, Y.; Mi, H.; Luo, S.; Zhang, P.; Lin, Z.; Li, J.; Zhang, H. Robust SnO2@x Nanoparticle-Impregnated Carbon Nanofibers with Outstanding Electrochemical Performance for Advanced Sodium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 8901–8905. [Google Scholar] [PubMed]
- Wang, L.; Wei, Z.; Mao, M.; Wang, H.; Li, Y.; Ma, J. Metal oxide/graphene composite anode materials for sodium-ion batteries. Energy Storage Mater. 2019, 16, 434–454. [Google Scholar]
- Zhao, W.; Ma, X.; Wang, X.; Zhou, H.; He, X.; Yao, Y.; Ren, Y.; Luo, Y.; Zheng, D.; Sun, S.; et al. Synergistically Coupling Atomic-Level Defect-Manipulation and Nanoscopic-Level Interfacial Engineering Enables Fast and Durable Sodium Storage. Small 2024, 20, 2311055. [Google Scholar] [CrossRef]
- Vincent, M.; Kumar, S.S.; Kowalski, D. Pseudocapacitive vs. diffusion controlled charge storage in Fe2O3 nanosheet Na-ion battery. Electrochim. Acta 2023, 469, 143161. [Google Scholar]
- Fang, Y.; Luan, D.; Lou, X.W.D. Recent Advances on Mixed Metal Sulfides for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, e2002976. [Google Scholar]
- Xiao, Y.; Lee, S.H.; Sun, Y.K. The Application of Metal Sulfides in Sodium Ion Batteries. Adv. Energy Mater. 2016, 7, 1601329. [Google Scholar]
- Yang, D.; Yadav, D.; Jeon, I.; Seo, J.; Jeong, S.Y.; Cho, C.R. Enhanced High-Rate Capability and Long Cycle Stability of FeS@NCG Nanofibers for Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2022, 14, 44303–44316. [Google Scholar]
- Han, B.; Zou, Y.; Xu, G.; Hu, S.; Kang, Y.; Qian, Y.; Wu, J.; Ma, X.; Yao, J.; Li, T.; et al. Additive stabilization of SEI on graphite observed using cryo-electron microscopy. Energy Environ. Sci. 2021, 14, 4882–4889. [Google Scholar]
- Kim, H.; Hong, J.; Park, Y.U.; Kim, J.; Hwang, I.; Kang, K. Sodium Storage Behavior in Natural Graphite using Ether-based Electrolyte Systems. Adv. Funct. Mater. 2014, 25, 534–541. [Google Scholar]
- Xu, Z.L.; Yoon, G.; Park, K.Y.; Park, H.; Tamwattana, O.; Kim, S.J.; Seong, W.M.; Kang, K. Tailoring sodium intercalation in graphite for high energy and power sodium ion batteries. Nat. Commun. 2019, 10, 2598. [Google Scholar] [PubMed]
- Zhu, J.; Chen, C.; Lu, Y.; Ge, Y.; Jiang, H.; Fu, K.; Zhang, X. Nitrogen-doped carbon nanofibers derived from polyacrylonitrile for use as anode material in sodium-ion batteries. Carbon. 2015, 94, 189–195. [Google Scholar]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar]
- Tian, Y.; Yang, H.; Zeng, Y.; Qi, Y.; Wang, W.; Chen, H.; Yin, W.; Ke, Y.; Jian, Z.; Kan, W.H.; et al. Design of High-Performance Defective Graphite-Type Anodes for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 3854–3861. [Google Scholar]
- Zhou, L.F.; Wang, Y.S.; Jia, H.; Gong, H.; Liu, L.Y.; Du, T. Application of rich-defect expanded graphite with improved ion transport and kinetics for sodium storage at low temperature. Sustain. Energy Fuels 2022, 6, 1727–1732. [Google Scholar]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar]
- Kumar, S.S.A.; Badawi, M.N.; Liew, J.; Prasankumar, T.; Ramesh, K.; Ramesh, S.; Tiong, S.K. High-Performance Sodium-Ion Batteries with Graphene: An Overview of Recent Developments and Design. ChemSusChem 2025, 14, e202400958. [Google Scholar]
- Sun, Z.; Wang, Y.; Jiang, X.; Bando, Y.; Wang, X. 3D network of graphene materials for alkali metal ion batteries. EnergyChem 2025, 7, 100149. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Li, Y.; Shi, X.; Huang, H.; Lu, P.; Qin, J.; Li, Y.; Yin, Q.; Yang, X.; et al. Atomic indium decorated graphene for dendrite-free sodium anodes towards high-energy-density sodium-metal batteries. J. Energy Chem. 2025, 104, 44–51. [Google Scholar]
- Guo, R.; Lv, C.; Xu, W.; Sun, J.; Zhu, Y.; Yang, X.; Li, j.; Sun, J.; Zhang, L.; Yang, D. Effect of Intrinsic Defects of Carbon Materials on the Sodium Storage Performance. Adv. Energy Mater. 2020, 10, 1903652. [Google Scholar]
- Pham, T.V.; Kim, J.G.; Jung, J.Y.; Kim, J.H.; Cho, H.; Seo, T.H.; Lee, H.; Kim, N.D.; Kim, M.J. High areal capacitance of N-doped graphene synthesized by arc discharge. Adv. Funct. Mater. 2019, 29, 1905511. [Google Scholar]
- Kim, C.; Kim, J.-G.; Kim, N.D.; Kim, M.J. Arc discharge synthesis of graphene with enhanced boron doping concentration for electrochemical applications. Appl. Surf. Sci. 2023, 637, 157825. [Google Scholar]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar]
- Quan, Z.; Wang, F.; Wang, Y.; Liu, Z.; Zhang, C.; Qi, F.; Zhang, M.; Ye, C.; Tan, J.; Liu, J. Robust Micro-Sized and Defect-Rich Carbon–Carbon Composites as Advanced Anodes for Potassium-Ion Batteries. Small 2024, 20, 2305841. [Google Scholar]
- Blume, R.; Rosenthal, D.; Tessonnier, J.P.; Li, H.; Knop-Gericke, A.; Schlögl, R. Characterizing graphitic carbon with X-ray photoelectron spectroscopy: A step-by-ste approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar]
- Cabello, M.; Bai, X.; Chyrka, T.; Ortiz, G.F.; Lavela, P.; Alcantara, R.; Tirado, J.L. On the reliability of sodium co-intercalation in expanded graphite prepared by different methods as anodes for sodium-ion batteries. J. Electrochem. Soc. 2017, 164, A3804. [Google Scholar]
- Kondo, T.; Guo, D.; Shikano, T.; Suzuki, T.; Sakurai, M.; Okada, S.; Nakamura, J. Observation of Landau levels on nitrogen-doped flat graphite surfaces without external magnetic fields. Sci. Rep. 2015, 5, 16412. [Google Scholar]
- Wu, J.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-Doped Graphene with Pyridinic Dominance as a Highly Active and Stable Electrocatalyst for Oxygen Reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar]
- Kim, T.; Choi, W.; Shin, H.C.; Choi, J.Y.; Kim, J.M.; Park, M.S.; Yoon, W.S. Applications of voltammetry in lithium ion battery research. J. Electrochem. Sci. Technol. 2020, 11, 14–25. [Google Scholar]
- Yang, X.; Rogach, A.L. Electrochemical techniques in battery research: A tutorial for nonelectrochemists. Adv. Energy Mater. 2019, 9, 1900747. [Google Scholar]
- Cheng, D.; Zhou, X.; Hu, H.; Li, Z.; Chen, J.; Miao, L.; Ye, X.; Zhang, H. Electrochemical storage mechanism of sodium in carbon materials: A study from soft carbon to hard carbon. Carbon 2021, 182, 758–769. [Google Scholar]
- Luo, S.; Liu, F.; Tianxu, W.; Liu, Y.; Zhang, C.; Bie, C.; Liu, M.; Chu, P.K.; Huo, K.; Gao, B. Regeneration of spent graphite via graphite-like turbostratic carbon coating for advanced Li ion battery anode. Energy Storage Mater. 2024, 73, 103833. [Google Scholar]
- Wang, D.; Lian, J.; Wang, Y.; Jia, P.; Gao, F. Turbostratic Lattice and Electronegativity Modification Jointly Enabled an Ultra-High-Rate and Long-Lived Carbon Anode for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 15585–15594. [Google Scholar]
- Aniskevich, Y.; Yu, J.H.; Kim, J.-Y.; Komaba, S.; Myung, S.-T. Tracking Sodium Cluster Dynamics in Hard Carbon with a Low Specific Surface Area for Sodium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2304300. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, I.; Kim, C.; Kang, M.; Kim, H.W.; Chen, H.; Youn, H.S.; Kim, M.J.; Cho, C.-R. Super-Fast Sodium Storage Properties of Nitrogen-Doped Graphene-Based Material Synthesized via Arc-Discharge Method. Batteries 2025, 11, 135. https://doi.org/10.3390/batteries11040135
Jeon I, Kim C, Kang M, Kim HW, Chen H, Youn HS, Kim MJ, Cho C-R. Super-Fast Sodium Storage Properties of Nitrogen-Doped Graphene-Based Material Synthesized via Arc-Discharge Method. Batteries. 2025; 11(4):135. https://doi.org/10.3390/batteries11040135
Chicago/Turabian StyleJeon, Injun, Chunghun Kim, Minseung Kang, Hyun Woo Kim, Hong Chen, Hye Seon Youn, Myung Jong Kim, and Chae-Ryong Cho. 2025. "Super-Fast Sodium Storage Properties of Nitrogen-Doped Graphene-Based Material Synthesized via Arc-Discharge Method" Batteries 11, no. 4: 135. https://doi.org/10.3390/batteries11040135
APA StyleJeon, I., Kim, C., Kang, M., Kim, H. W., Chen, H., Youn, H. S., Kim, M. J., & Cho, C.-R. (2025). Super-Fast Sodium Storage Properties of Nitrogen-Doped Graphene-Based Material Synthesized via Arc-Discharge Method. Batteries, 11(4), 135. https://doi.org/10.3390/batteries11040135






