Styrene and Its Derivatives Used in Proton Exchange Membranes and Anion Exchange Membranes for Fuel Cell Applications: A Review
Abstract
1. Introduction
- Proton exchange membrane fuel cells (PEMFCs);
- Anion exchange membrane fuel cells (AEMFCs);
- Phosphoric acid fuel cells
- Solid acid fuel cells
- Alkaline fuel cells
- Molten carbonate fuel cells
- Microbial fuel cells (MFCs)
- Solid oxide fuel cells (SOFCs)
- Zinc-Air fuel cells (ZAFCs)
- Direct methanol fuel cells (DMFCs)
2. Structure of Nafion and Its Proton Conduction Mechanism
3. Deficiencies of Existing Membrane Materials Used in Fuel Cells
4. Properties of an Ideal PEM
- High mechanical strength and stable porous structure.
- High ion conductivity (usually greater than 0.1 S cm−1) at the required temperature;
- Be electrically insulated to prevent short circuiting.
- High chemical stability against harsh reactive impurities in fuel cells such as radicals, hydrogen peroxide (H2O2), and other agents.
- High tensile strength and durability. Withstand high pressure and maintain structural integrity during hydration and dehydration cycles.
- High protection from fuel crossover.
- High resistance against temperature and ensure thermal stability when operating at high temperature without melting or shrinkage
- The membrane materials should be economical and readily available for manufacturing.
- The membrane should be nontoxic, renewable in nature, and sustainable.
5. Properties of an Ideal AEM
- The membrane should have high ionic conductivity to facilitate the efficient transport of hydroxide ions (OH⁻) from the cathode to the anode.
- The membrane should be an electronic insulator to prevent short-circuiting within the fuel cell.
- The membrane must be chemically stable in high pH environments (typically pH > 13) to withstand the alkaline conditions in AEMFC.
- The membrane should resist degradation from oxidative and reductive environments, especially at the electrodes.
- The membrane should have sufficient mechanical strength to withstand the mechanical stresses during cell assembly and operation.
- The membrane should maintain its shape and size under varying conditions of humidity and temperature to prevent delamination or cracking.
- The membrane should be stable at elevated temperatures (typically up to 80–100 °C) to ensure efficient operation and to accommodate the heat generated during fuel cell operation.
- The membrane should have low permeability to gases (H2 and O2) to prevent crossover, which can lead to mixed potentials and reduced fuel cell efficiency.
- The membrane should maintain adequate hydration to ensure high ionic conductivity without becoming overly swollen, which can compromise mechanical integrity.
- The membrane should facilitate the transport of water produced at the cathode to the anode to maintain proper hydration levels.
- The membrane should be cost effective to produce, as high costs can be a barrier to commercialization.
- The membrane should have a long operational life to reduce the need for frequent replacements, contributing to overall cost effectiveness.
- The membrane should have good interfacial compatibility with the electrodes to ensure low contact resistance and efficient ion transfer.
- The membrane should adhere well to the electrodes to prevent delamination and ensure long-term stability.
- The membrane should be made from non-toxic materials to ensure safety during handling and disposal.
- The membrane should be made from sustainable or recyclable materials to minimize environmental impact.
- The membrane should be easy to fabricate into thin, uniform layers to ensure consistent performance across the fuel cell.
- The membrane should be scalable to large-area production to meet the demands of commercial applications.
6. Styrene
7. Derivatives of Styrene
- Polystyrene (PS)
- Styrene Oxide
- Styrene-Butadiene Rubber (SBR)
- Alpha-Methylstyrene
- Styrene-Acrylic Copolymers
- Divinylbenzene (DVB)
- Styrene Maleic anhydride (SMA)
- Benzaldehyde
- Poly(styrene-b-butadiene-b-styrene) (SBS)
8. Common Uses of Styrene and Its Derivatives
9. Application of Derivatives of Styrene in Membrane of Fuel Cells
9.1. Polystyrene
9.2. Polystyrene-b-polybutadiene-b-polystyrene (SBS)
9.3. Styrene-Ethylene-Butylene-Styrene (SEBS)
9.4. Poly(Styrene Acrylonitrile) [SAN]
10. Conclusions and Future Perspective
10.1. Material Optimization
10.2. Advanced Fabrication Techniques
10.3. Hybrid and Composite Membranes
10.4. Sustainability and Green Chemistry
10.5. Durability Enhancement
10.6. Practical Application in Future Newly Created Fuel Cells
10.7. Performance Check and Validation in Real-World Applications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perera, F. Pollution from Fossil-Fuel Combustion Is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Mahmood Ali, B.; Algburi, S.; Alzoubi, H.M.; Khudhair Al-Jiboory, A.; Zuhair Sameen, A.; Salman, H.M.; Jaszczur, M. The Renewable Energy Role in the Global Energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Gayen, D.; Chatterjee, R.; Roy, S. A Review on Environmental Impacts of Renewable Energy for Sustainable Development. Int. J. Environ. Sci. Technol. 2024, 21, 5285–5310. [Google Scholar] [CrossRef]
- Olabi, A.G.; Elsaid, K.; Obaideen, K.; Abdelkareem, M.A.; Rezk, H.; Wilberforce, T.; Maghrabie, H.M.; Sayed, E.T. Renewable Energy Systems: Comparisons, Challenges and Barriers, Sustainability Indicators, and the Contribution to UN Sustainable Development Goals. Int. J. Thermofluids 2023, 20, 100498. [Google Scholar] [CrossRef]
- Clean Energy Can Fuel the Future—and Make the World Healthier. Nature 2023, 620, 245. [CrossRef]
- Fernandez, M.I.; Go, Y.I.; Wong, D.M.L.; Früh, W.-G. Review of Challenges and Key Enablers in Energy Systems towards Net Zero Target: Renewables, Storage, Buildings, & Grid Technologies. Heliyon 2024, 10, e40691. [Google Scholar] [CrossRef] [PubMed]
- Rehman Asghar, M.; Divya, K.; Su, H.; Xu, Q. Advancement of PVDF and Its Copolymer-Based Proton Exchange Membranes for Direct Methanol Fuel Cells: A Review. Eur. Polym. J. 2024, 213, 113110. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, W.; Su, H.; Zhang, J.; Rhimi, B.; Liu, H.; Xing, L.; Yan, X.; Xu, Q. A Review of Additional Modifications of Additives through Hydrophilic Functional Groups for the Application of Proton Exchange Membranes in Fuel Cells. J. Power Sources 2024, 622, 235353. [Google Scholar] [CrossRef]
- Awan, M.S.U.D.; Anwar, M.T.; Khan, H.I.; Asghar, M.R.; Raza, M.R.; Husnain, N.; Hussain, M.; Rasheed, T. Nanoparticles’ Classification, Synthesis, Characterization and Applications—A Review. Charact. Appl. Nanomater. 2024, 8, 8899. [Google Scholar] [CrossRef]
- Asghar, M.R.; Yu, W.; Zhang, W.; Su, H.; Liu, H.; Xing, L.; Yan, X.; Xu, Q. Improving the Performance of Polyvinylidene Fluoride (PVDF)-Based Proton Exchange Membranes with the Addition of Cellulose Acetate for Direct Methanol Fuel Cells. J. Polym. Res. 2024, 31, 277. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, W.; Su, H.; Zhang, J.; Liu, H.; Xing, L.; Yan, X.; Xu, Q. A Review of Proton Exchange Membranes Modified with Inorganic Nanomaterials for Fuel Cells. Energy Adv. 2025, 4, 185–223. [Google Scholar] [CrossRef]
- Gkionis-Konstantatos, O.; Tavares, L.; Ebel, T. Investigating the Role of Flow Plate Surface Roughness in Polymer Electrolyte Membrane Fuel Cells with the Use of Multiphysics Simulations. Batteries 2024, 10, 276. [Google Scholar] [CrossRef]
- Liang, X.; Yu, W.; Xu, Y.; Shen, X.; Wu, L.; Xu, T. Materials and Advancement for Membrane in Fuel Cells. In Handbook of Energy Materials; Springer Nature Singapore: Singapore, 2022; pp. 1–42. [Google Scholar]
- Gong, A.; Verstraete, D. Fuel Cell Propulsion in Small Fixed-Wing Unmanned Aerial Vehicles: Current Status and Research Needs. Int. J. Hydrogen Energy 2017, 42, 21311–21333. [Google Scholar] [CrossRef]
- Front Matter. In PEM Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2013; p. iii.
- Uralde, J.; Barambones, O.; del Rio, A.; Calvo, I.; Artetxe, E. Rule-Based Operation Mode Control Strategy for the Energy Management of a Fuel Cell Electric Vehicle. Batteries 2024, 10, 214. [Google Scholar] [CrossRef]
- Kumar, P.; Kannaiah, S.K.; Choudhury, S.R.; Rajasekar, N. Genetic Algorithm-Based Modeling of PEM Fuel Cells Suitable for Integration in DC Microgrids. Electr. Power Compon. Syst. 2017, 45, 1152–1160. [Google Scholar] [CrossRef]
- Laurencelle, F.; Chahine, R.; Hamelin, J.; Agbossou, K.; Fournier, M.; Bose, T.K.; Laperrière, A. Characterization of a Ballard MK5-E Proton Exchange Membrane Fuel Cell Stack. Fuel Cells 2001, 1, 66–71. [Google Scholar] [CrossRef]
- Candusso, D.; Harel, F.; Debernardinis, A.; Francois, X.; Pera, M.; Hissel, D.; Schott, P.; Coquery, G.; Kauffmann, J. Characterisation and Modelling of a 5kW PEMFC for Transportation Applications. Int. J. Hydrogen Energy 2006, 31, 1019–1030. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.-G.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M.; et al. Phenylenediamine-Based FeNx/C Catalyst with High Activity for Oxygen Reduction in Acid Medium and Its Active-Site Probing. J. Am. Chem. Soc. 2014, 136, 10882–10885. [Google Scholar] [CrossRef]
- Song, S.; Douvartzides, S.; Tsiakaras, P. Exergy Analysis of an Ethanol Fuelled Proton Exchange Membrane (PEM) Fuel Cell System for Automobile Applications. J. Power Sources 2005, 145, 502–514. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Fan, L.; Du, Q.; Jiao, K. Application Progress of Small-Scale Proton Exchange Membrane Fuel Cell. Energy Rev. 2023, 2, 100017. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An Overview of Proton Exchange Membranes for Fuel Cells: Materials and Manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Qasem, N.A.A. A Recent Overview of Proton Exchange Membrane Fuel Cells: Fundamentals, Applications, and Advances. Appl. Therm. Eng. 2024, 252, 123746. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A Review of High-Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC) System. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Do, H.-Y.; Kim, C.-H.; Han, J.-Y.; Kim, H.-S.; Ryi, S.-K. Low-Temperature Proton-Exchange Membrane Fuel Cell-Grade Hydrogen Production by Membrane Reformer Equipped with Pd-Composite Membrane and Methanation Catalyst on Permeation Stream. J. Memb. Sci. 2021, 634, 119373. [Google Scholar] [CrossRef]
- Scott, K.; Pilditch, S.; Mamlouk, M. Modelling and Experimental Validation of a High Temperature Polymer Electrolyte Fuel Cell. J. Appl. Electrochem. 2007, 37, 1245–1259. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Ma, Z.; Zheng, M.; Song, H. Performance Analysis of a HT-PEMFC System with 6FPBI Membranes Doped with Cross-Linkable Polymeric Ionic Liquid. Int. J. Mol. Sci. 2022, 23, 9618. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H.; Thangarasu, S. Modified Cellulose Proton-Exchange Membranes for Direct Methanol Fuel Cells. Polymers 2023, 15, 659. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Bernier, C.M.I.; Van Herle, J. The Application of Fuel-Cell and Battery Technologies in Unmanned Aerial Vehicles (UAVs): A Dynamic Study. Batteries 2022, 8, 73. [Google Scholar] [CrossRef]
- Sharma, V.; Upadhyay, P.; Rathod, N.H.; Sharma, J.; Mishra, S.; Raj, S.K.; Kishore, V.; Kulshrestha, V. Silica Modified Sulphonated Poly(Ether Ether Ketone) Proton Exchange Membranes for DMFC Application. Int. J. Hydrogen Energy 2023, 48, 37784–37795. [Google Scholar] [CrossRef]
- Dai, C.-A.; Liu, C.-P.; Lee, Y.-H.; Chang, C.-J.; Chao, C.-Y.; Cheng, Y.-Y. Fabrication of Novel Proton Exchange Membranes for DMFC via UV Curing. J. Power Sources 2008, 177, 262–272. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of Cell Performance in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Douglin, J.C.; Varcoe, J.R.; Dekel, D.R. A High-Temperature Anion-Exchange Membrane Fuel Cell. J. Power Sources Adv. 2020, 5, 100023. [Google Scholar] [CrossRef]
- Yassin, K.; Rasin, I.G.; Willdorf-Cohen, S.; Diesendruck, C.E.; Brandon, S.; Dekel, D.R. A Surprising Relation between Operating Temperature and Stability of Anion Exchange Membrane Fuel Cells. J. Power Sources Adv. 2021, 11, 100066. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Kahraman, H.; Akın, Y. Recent Studies on Proton Exchange Membrane Fuel Cell Components, Review of the Literature. Energy Convers. Manag. 2024, 304, 118244. [Google Scholar] [CrossRef]
- Song, S.; He, H.; Chai, S.; Li, H. Advanced Nafion/Nanofiller Composite Proton Exchange Membranes for Fuel Cell Applications. Polymer 2024, 307, 127241. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Li, Y.-C.; Liu, J.; He, J.; Wang, L.-Y.; Lei, J.-D. Recent Developments in High-Performance Nafion Membranes for Hydrogen Fuel Cells Applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Ke, Y.; Yuan, W.; Zhou, F.; Guo, W.; Li, J.; Zhuang, Z.; Su, X.; Lu, B.; Zhao, Y.; Tang, Y.; et al. A Critical Review on Surface-Pattern Engineering of Nafion Membrane for Fuel Cell Applications. Renew. Sustain. Energy Rev. 2021, 145, 110860. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Li, Y. An Overview of the Proton Conductivity of Nafion Membranes through a Statistical Analysis. J. Memb. Sci. 2016, 504, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High Temperature PEM Fuel Cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Kornyshev, A.A.; Kuznetsov, A.M.; Spohr, E.; Ulstrup, J. Kinetics of Proton Transport in Water. J. Phys. Chem. B 2003, 107, 3351–3366. [Google Scholar] [CrossRef]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A Review of Membranes in Proton Exchange Membrane Fuel Cells: Transport Phenomena, Performance and Durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent Approaches to Improve Nafion Performance for Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Chalkova, E.; Pague, M.B.; Fedkin, M.V.; Wesolowski, D.J.; Lvov, S.N. Nafion/TiO2 Proton Conductive Composite Membranes for PEMFCs Operating at Elevated Temperature and Reduced Relative Humidity. J. Electrochem. Soc. 2005, 152, A1035. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Enotiadis, A.; Nicotera, I.; Simari, C.; Charalambopoulou, G.; Giannelis, E.P.; Steriotis, T. Nafion® Nanocomposite Membranes with Enhanced Properties at High Temperature and Low Humidity Environments. Int. J. Hydrogen Energy 2016, 41, 22406–22414. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in Hybrid Composite Nafion®-Based Membranes for Proton Exchange Fuel Cell Application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Ray, A.; Sultana, S.; Paramanik, L.; Parida, K.M. Recent Advances in Phase, Size, and Morphology-Oriented Nanostructured Nickel Phosphide for Overall Water Splitting. J. Mater. Chem. A 2020, 8, 19196–19245. [Google Scholar] [CrossRef]
- Pauer, C.; du Roure, O.; Heuvingh, J.; Liedl, T.; Tavacoli, J. Programmable Design and Performance of Modular Magnetic Microswimmers. Adv. Mater. 2021, 33, 2006237. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Anionic Multiblock Copolymer Membrane Based on Vinyl Addition Polymerization of Norbornenes: Applications in Anion-Exchange Membrane Fuel Cells. J. Memb. Sci. 2019, 570–571, 394–402. [Google Scholar] [CrossRef]
- Cha, M.S.; Park, J.E.; Kim, S.; Han, S.-H.; Shin, S.-H.; Yang, S.H.; Kim, T.-H.; Yu, D.M.; So, S.; Hong, Y.T.; et al. Poly(Carbazole)-Based Anion-Conducting Materials with High Performance and Durability for Energy Conversion Devices. Energy Environ. Sci. 2020, 13, 3633–3645. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Jin, C. Development of a High-Performance Anion Exchange Membrane Using Poly(Isatin Biphenylene) with Flexible Heterocyclic Quaternary Ammonium Cations for Alkaline Fuel Cells. J. Mater. Chem. A 2019, 7, 6883–6893. [Google Scholar] [CrossRef]
- Pham, T.H.; Allushi, A.; Olsson, J.S.; Jannasch, P. Rational Molecular Design of Anion Exchange Membranes Functionalized with Alicyclic Quaternary Ammonium Cations. Polym. Chem. 2020, 11, 6953–6963. [Google Scholar] [CrossRef]
- Sung, S.; Mayadevi, T.S.; Chae, J.E.; Kim, H.-J.; Kim, T.-H. Effect of Increasing Hydrophilic–Hydrophobic Block Length in Quaternary Ammonium-Functionalized Poly(Ether Sulfone) Block Copolymer for Anion Exchange Membrane Fuel Cells. J. Ind. Eng. Chem. 2020, 81, 124–134. [Google Scholar] [CrossRef]
- Wu, X.; Chen, N.; Klok, H.; Lee, Y.M.; Hu, X. Branched Poly(Aryl Piperidinium) Membranes for Anion-Exchange Membrane Fuel Cells. Angew. Chemie Int. Ed. 2022, 61, e202114892. [Google Scholar] [CrossRef]
- Zhang, F.; He, X.; Cheng, C.; Huang, S.; Duan, Y.; Zhu, C.; Guo, Y.; Wang, K.; Chen, D. Bis-Imidazolium Functionalized Self-Crosslinking Block Polynorbornene Anion Exchange Membrane. Int. J. Hydrogen Energy 2020, 45, 13090–13100. [Google Scholar] [CrossRef]
- Harilal; Nayak, R.; Ghosh, P.C.; Jana, T. Cross-Linked Polybenzimidazole Membrane for PEM Fuel Cells. ACS Appl. Polym. Mater. 2020, 2, 3161–3170. [Google Scholar] [CrossRef]
- Maiti, T.K.; Singh, J.; Majhi, J.; Ahuja, A.; Maiti, S.; Dixit, P.; Bhushan, S.; Bandyopadhyay, A.; Chattopadhyay, S. Advances in Polybenzimidazole Based Membranes for Fuel Cell Applications That Overcome Nafion Membranes Constraints. Polymer 2022, 255, 125151. [Google Scholar] [CrossRef]
- Ainla, A.; Brandell, D. Nafion®–Polybenzimidazole (PBI) Composite Membranes for DMFC Applications. Solid State Ionics 2007, 178, 581–585. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-Based Polymers in PEMFC and DMFC Applications: A Review. Int. J. Hydrogen Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; He, H.; Fan, Y.; Li, Y.; Wang, S.; Li, S. Fabrication of an Ultra-Thin and Ordered SPEEK Proton Exchange Membrane by a Langmuir-Blodgett Self-Assembly Process. J. Memb. Sci. 2024, 690, 122196. [Google Scholar] [CrossRef]
- Yang, T. Preliminary Study of SPEEK/PVA Blend Membranes for DMFC Applications. Int. J. Hydrogen Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Ryu, G.Y.; An, S.J.; Yu, S.; Kim, K.J.; Jae, H.; Roh, D.; Chi, W.S. Dual-Sulfonated MOF/Polysulfone Composite Membranes Boosting Performance for Proton Exchange Membrane Fuel Cells. Eur. Polym. J. 2022, 180, 111601. [Google Scholar] [CrossRef]
- Choi, Y.-W.; Jang, S.; Yoon, Y.-G.; Kim, T.-Y. Reinforced Microcomposite Polymer Electrolyte Membrane and Its Characterization Fabricated by a Concise Process for PEMFC. ECS Meet. Abstr. 2018, MA2018-02, 1444. [Google Scholar] [CrossRef]
- Zhu, Y.; Manthiram, A. Synthesis and Characterization of Polysulfone-Containing Sulfonated Side Chains for Direct Methanol Fuel Cells. J. Power Sources 2011, 196, 7481–7487. [Google Scholar] [CrossRef]
- Jang, W.G.; Hou, J.; sik Byun, H. Preparation and Characterization of PVdF Nanofiber Ion Exchange Membrane for the PEMFC Application. Desalin. Water Treat. 2011, 34, 315–320. [Google Scholar] [CrossRef][Green Version]
- Martina, P.; Gayathri, R.; Pugalenthi, M.R.; Cao, G.; Liu, C.; Prabhu, M.R. Nanosulfonated Silica Incorporated SPEEK/SPVdF-HFP Polymer Blend Membrane for PEM Fuel Cell Application. Ionics 2020, 26, 3447–3458. [Google Scholar] [CrossRef]
- Rath, R.; Kumar, P.; Rana, D.; Mishra, V.; Kumar, A.; Mohanty, S.; Nayak, S.K. Sulfonated PVDF Nanocomposite Membranes Tailored with Graphene Oxide Nanoparticles: Improved Proton Conductivity and Membrane Selectivity Thereof. J. Mater. Sci. 2022, 57, 3565–3585. [Google Scholar] [CrossRef]
- Ng, W.K.; Wong, W.Y.; Loh, K.S.; Masdar, M.S.; Shaari, N.; Pang, M.M. A Comprehensive Overview of Polyphenylene Oxide-Based Anion Exchange Membranes from the Perspective of Ionic Conductivity and Alkaline Stability. J. Ind. Eng. Chem. 2024, 138, 49–71. [Google Scholar] [CrossRef]
- Basso Peressut, A.; Montagna, J.; Moretti, P.; Arrigoni, A.; Latorrata, S.; Bertarelli, C.; Dotelli, G. Development and Characterization of Crosslinked PPO-Based Anion Exchange Membranes for AEM Fuel Cells. Solid State Ionics 2023, 394, 116212. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y.; Kang, F.; Liu, C.; Song, W.; Zheng, X.; Liu, X.; Wang, M.; Zhou, X.; Ren, Z.; et al. Poly(Phenylene Oxide)-Based Anion Exchange Membranes Having Linear Cross-Linkers or Star Cross-Linkers. ACS Appl. Energy Mater. 2022, 5, 11613–11623. [Google Scholar] [CrossRef]
- Tuan, C.M.; Patra, A.K.; Kim, D. Chemically Modified Poly(Arylene Ether Ketone)s with Pendant Imidazolium Groups: Anion Exchange Membranes for Alkaline Fuel Cells. Int. J. Hydrogen Energy 2018, 43, 4517–4527. [Google Scholar] [CrossRef]
- Ayaz, S.; Yao, Z.-Y.; Chen, Y.-J.; Yu, H.-Y. Preparation of Poly(Arylene Ether Ketone) Based Anion Exchange Membrane with Pendant Pyrimidinium and Pyridazinium Cation Derivatives for Alkaline Fuel Cell. J. Memb. Sci. 2022, 659, 120778. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, D. Anion Exchange Membrane Prepared from Imidazolium Grafted Poly(Arylene Ether Ketone) with Enhanced Durability for Vanadium Redox Flow Battery. J. Ind. Eng. Chem. 2019, 71, 361–368. [Google Scholar] [CrossRef]
- Zarrin, H.; Jiang, G.; Lam, G.Y.-Y.; Fowler, M.; Chen, Z. High Performance Porous Polybenzimidazole Membrane for Alkaline Fuel Cells. Int. J. Hydrogen Energy 2014, 39, 18405–18415. [Google Scholar] [CrossRef]
- Das, A.; Im, K.S.; Kabir, M.M.; Shon, H.K.; Nam, S.Y. Polybenzimidazole (PBI)-Based Membranes for Fuel Cell, Water Electrolysis and Desalination. Desalination 2024, 579, 117500. [Google Scholar] [CrossRef]
- Guo, M.; Ban, T.; Wang, Y.; Wang, Y.; Zhang, Y.; Zhang, J.; Zhu, X. Exploring Highly Soluble Ether-Free Polybenzimidazole as Anion Exchange Membranes with Long Term Durability. J. Memb. Sci. 2022, 647, 120299. [Google Scholar] [CrossRef]
- Kırlıoğlu, A.C.; Rajabalizadeh Mojarrad, N.; Alkan Gürsel, S.; Güler, E.; Yarar Kaplan, B. New Generation Radiation-Grafted PVDF-g-VBC Based Dual-Fiber Electrospun Anion Exchange Membranes. Int. J. Hydrogen Energy 2024, 51, 1390–1401. [Google Scholar] [CrossRef]
- Gatto, I.; Patti, A.; Carbone, A. Assessment of the FAA3-50 Polymer Electrolyte for Anion Exchange Membrane Fuel Cells. ChemElectroChem 2023, 10, e202201052. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Qu, C.; Ren, J. Poly(Vinylidene Fluoride) Based Anion Conductive Ionomer as a Catalyst Binder for Application in Anion Exchange Membrane Fuel Cell. J. Power Sources 2011, 196, 3099–3103. [Google Scholar] [CrossRef]
- Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M. An Overview of Nanomaterials in Fuel Cells: Synthesis Method and Application. Int. J. Hydrogen Energy 2022, 47, 18468–18495. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Arangadi, A.F.; Velu, S.; Banat, F.; Show, P.L. ZrO2 Incorporated Polysulfone Anion Exchange Membranes for Fuel Cell Applications. Int. J. Hydrogen Energy 2020, 45, 29668–29680. [Google Scholar] [CrossRef]
- Ng, W.; Wong, W.; Rosli, N.; Loh, K. Commercial Anion Exchange Membranes (AEMs) for Fuel Cell and Water Electrolyzer Applications: Performance, Durability, and Materials Advancement. Separations 2023, 10, 424. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, Y.; Tang, J.; Yang, X.; Sun, A.; Lin, B.; Zhu, F.; Jia, H.; Lei, X. Advances in High Performance Anion Exchange Membranes: Molecular Design, Preparation Methods, and Ion Transport Dynamics. J. Environ. Chem. Eng. 2023, 11, 110749. [Google Scholar] [CrossRef]
- Mamlouk, M. Alkaline Anion Exchange Membrane (AEM) Water Electrolysers—Current/Future Perspectives in Electrolysers for Hydrogen. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 473–504. [Google Scholar]
- Wang, B.; Yan, J.; Wang, H.; Li, R.; Fu, R.; Jiang, C.; Nikonenko, V.; Pismenskaya, N.; Wang, Y.; Xu, T. Ionic Liquid-Based Pore-Filling Anion-Exchange Membranes Enable Fast Large-Sized Metallic Anion Migration in Electrodialysis. J. Memb. Sci. 2023, 670, 121348. [Google Scholar] [CrossRef]
- Peltier, C.R.; You, W.; Fackovic Volcanjk, D.; Li, Q.; Macbeth, A.J.; Abruña, H.D.; Coates, G.W. Quaternary Ammonium-Functionalized Polyethylene-Based Anion Exchange Membranes: Balancing Performance and Stability. ACS Energy Lett. 2023, 8, 2365–2372. [Google Scholar] [CrossRef]
- Chu, X.; Liu, L.; Huang, Y.; Guiver, M.D.; Li, N. Practical Implementation of Bis-Six-Membered N-Cyclic Quaternary Ammonium Cations in Advanced Anion Exchange Membranes for Fuel Cells: Synthesis and Durability. J. Memb. Sci. 2019, 578, 239–250. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A Review on Anion Exchange Membranes for Fuel Cells: Anion-Exchange Polyelectrolytes and Synthesis Strategies. Int. J. Hydrogen Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H. Front Matter. In Nomenclature of Organic Chemistry; Royal Society of Chemistry: Cambridge, UK, 2013; pp. P001–P004. [Google Scholar]
- Papaleo, B.; Caporossi, L.; Bernardini, F.; Cristadoro, L.; Bastianini, L.; De Rosa, M.; Capanna, S.; Marcellini, L.; Loi, F.; Battista, G. Exposure to Styrene in Fiberglass-Reinforced Plastic Manufacture. J. Occup. Environ. Med. 2011, 53, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Ramli Sulong, N.H.; Mustapa, S.A.S.; Abdul Rashid, M.K. Application of Expanded Polystyrene (EPS) in Buildings and Constructions: A Review. J. Appl. Polym. Sci. 2019, 136, 47529. [Google Scholar] [CrossRef]
- Nyambara Ngugi, H. Use of Expanded Polystyrene Technology and Materials Recycling for Building Construction in Kenya. Am. J. Eng. Technol. Manag. 2017, 2, 64. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Schaller, D.; Khan, M.M.K.; Hasan, M.M.; Hazrat, M.A. Transport Fuel from Waste Plastics Pyrolysis—A Review on Technologies, Challenges and Opportunities. Energy Convers. Manag. 2022, 258, 115451. [Google Scholar] [CrossRef]
- Gutierrez-Velasquez, E.I.; Monteiro, S.N.; Colorado, H.A. Characterization of Expanded Polystyrene Waste as Binder and Coating Material. Case Stud. Constr. Mater. 2022, 16, e00804. [Google Scholar] [CrossRef]
- Issam, A.M.; Poh, B.T.; Abdul Khalil, H.P.S.; Lee, W.C. Adhesion Properties of Adhesive Prepared from Waste Polystyrene. J. Polym. Environ. 2009, 17, 165–169. [Google Scholar] [CrossRef]
- Winther, T.; Bannerman, J.; Skogstad, H.; Johansson, M.K.G.; Jacobson, K.; Samuelsson, J. Adhesives for Adhering Polystyrene Plastic and Their Long-Term Effect. Stud. Conserv. 2015, 60, 107–120. [Google Scholar] [CrossRef]
- Holme, I. Advances in the Science and Technology of Paints, Inks and Related Coatings. Surf. Coat. Int. Part B Coat. Trans. 2004, 87, 289–298. [Google Scholar] [CrossRef]
- Rehan, Z.A.; Usman, A. Polymeric Paints and Coatings. In Advanced Functional Polymers: Synthesis to Applications; Springer Nature Singapore: Singapore, 2023; pp. 49–76. [Google Scholar]
- McKeen, L.W. Plastics Used in Medical Devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014; pp. 21–53. [Google Scholar]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical Applications of Poly(Styrene-Block-Isobutylene-Block-Styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized Polystyrene Nanoparticles as a Platform for Studying Bio–Nano Interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef]
- He, Z.; Asare-Yeboah, K.; Bi, S. Polystyrene Applications in Organic Electronics. Discov. Electron. 2024, 1, 33. [Google Scholar] [CrossRef]
- He, Z.; Bi, S.; Asare-Yeboah, K. Hybrid System of Polystyrene and Semiconductor for Organic Electronic Applications. Processes 2024, 12, 1944. [Google Scholar] [CrossRef]
- Ashby, M.F. Material Profiles. In Materials and the Environment; Elsevier: Amsterdam, The Netherlands, 2013; pp. 459–595. [Google Scholar]
- Huang, D.; Hwang, J.-Y. Dual Functionalized Graphene Oxide Incorporated with Sulfonated Polyolefin Proton Exchange Membrane for Fuel Cell Application. Solid State Ionics 2023, 392, 116149. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Shahi, V.K. Sulfonated Poly(Styrene-Co-Maleic Anhydride)−Poly(Ethylene Glycol)−Silica Nanocomposite Polyelectrolyte Membranes for Fuel Cell Applications. J. Phys. Chem. B 2007, 111, 12454–12461. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Dhanasekaran, P.; Sasikala, S.; Nagaraju, N.; Bhat, S.D.; Pillai, V.K. Covalent Grafting of Polystyrene Sulfonic Acid on Graphene Oxide Nanoplatelets to Form a Composite Membrane Electrolyte with Sulfonated Poly(Ether Ether Ketone) for Direct Methanol Fuel Cells. J. Memb. Sci. 2020, 595, 117484. [Google Scholar] [CrossRef]
- Shalaby, A.A.; Elmageed, M.H.A.; Malash, G.F.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Špitalský, Z.; Khalifa, R.E. Design of Sulfonated Polystyrene Grafted Cellulose Acetate Membrane for Direct Methanol Fuel Cells. Solid State Ionics 2024, 404, 116420. [Google Scholar] [CrossRef]
- Nakayama, T.; Kajita, T.; Nishimoto, M.; Tanaka, H.; Sato, K.; Marium, M.; Mufundirwa, A.; Iwamoto, H.; Noro, A. Polymer Electrolyte Membranes of Polystyrene with Directly Bonded Alkylenephosphonate Groups on the Side Chains. ACS Appl. Polym. Mater. 2024, 6, 15150–15161. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.F.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanostructured Bacterial Cellulose–Poly(4-Styrene Sulfonic Acid) Composite Membranes with High Storage Modulus and Protonic Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Chen, W.-Y.; Huang, Y.-J.; Cheng, M.-Y.; Yen, Y.-C.; Cheng, C.-C.; Chang, F.-C. Preparation and Characterization of High-Durability Zwitterionic Crosslinked Proton Exchange Membranes. J. Memb. Sci. 2010, 362, 29–37. [Google Scholar] [CrossRef]
- Panawong, C.; Martwiset, S. Synthesis and Characterization of Poly(Styrene Sulfonic Acid-Co-1-Vinylimidazole-Co-Styrene) and Its Blends with Poly(Vinyl Chloride) as Proton Conducting Membranes. Polym. Bull. 2018, 75, 3843–3858. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.S.; Yoon, H.H.; Sharma, C.M.; Das, G.; Yoon, Y.S. Electrospun Poly(Styrene−Co−Vinylbenzyl Chloride−Co−Acrylonitrile) Nanofiber Mat as an Anion Exchange Membrane for Fuel Cell Applications. Polymers 2022, 14, 3236. [Google Scholar] [CrossRef]
- Tian, D.; Park, S.; Jo, S.; Ryu, C.Y.; Ryu, D.Y.; Bae, C. Simultaneous Postfunctionalization and Cross-Linking of Epoxidized Polystyrene-b-Polybutadiene-b-Polystyrene for Anion Exchange Membrane. ACS Appl. Energy Mater. 2024, 7, 6209–6219. [Google Scholar] [CrossRef]
- Turan, S.; Park, S.; Ryu, C.Y.; Ryu, D.Y.; Bae, C. Functionalization of Rubbery Domains in SBS Triblock Copolymers for Anion Exchange Membrane Applications with Enhanced Robustness. J. Memb. Sci. 2024, 700, 122662. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Cai, R.; Wei, X.; Wahid, U.; Zhao, Z.; Liu, W.; Zhang, C. Design and Synthesis of Poly(Styrene-b-(Ethylene-Co-Butadiene)-b-Styrene) Anion Exchange Membranes with High Ion Conductivity. J. Power Sources 2024, 613, 234853. [Google Scholar] [CrossRef]
- Bhavani, P.; Sangeetha, D. Glutaraldehyde Cross-Linked Sulphonated Poly Styrene Ethylene Butylene Poly Styrene Membranes for Methanol Fuel Cells. Int. J. Plast. Technol. 2015, 19, 137–152. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Z.; Liu, W.; Zhang, C. Physically Self-Cross-Linked SEBS Anion Exchange Membranes. Energy Fuels 2020, 34, 16746–16755. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.-H.; Xu, R.-W.; Zhang, S.; Wu, Y.-X. Cross-Linked Quaternized Poly(Styrene-b-(Ethylene-Co-Butylene)-b-Styrene) for Anion Exchange Membrane: Synthesis, Characterization and Properties. ACS Appl. Mater. Interfaces 2016, 8, 20329–20341. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Z.; Zhang, H.; Zhu, H. Study on AEMs with Excellent Comprehensive Performance Prepared by Covalently Cross-Linked p-Triphenyl with SEBS Remotely Grafted Piperidine Cations. ACS Appl. Mater. Interfaces 2024, 16, 7894–7903. [Google Scholar] [CrossRef]
- Min, K.; Lee, Y.; Choi, Y.; Kwon, O.J.; Kim, T.-H. High-Performance Anion Exchange Membranes Achieved by Crosslinking Two Aryl Ether-Free Polymers: Poly(Bibenzyl N-Methyl Piperidine) and SEBS. J. Memb. Sci. 2022, 664, 121071. [Google Scholar] [CrossRef]
- Wang, F.; Kong, D.; Zhang, S.; Zhu, H. Poly(Styrene-b (Ethylene-Co-Butylene)-b-Styrene) Cross-Linked Anion Exchange Membranes Grafted with Caged Double Cations and Poly (m-Triphenylene-Piperidine). ACS Appl. Polym. Mater. 2024, 6, 5039–5048. [Google Scholar] [CrossRef]
- Sung, S.; Chae, J.E.; Min, K.; Kim, H.-J.; Nam, S.Y.; Kim, T.-H. Preparation of Crosslinker-Free Anion Exchange Membranes with Excellent Physicochemical and Electrochemical Properties Based on Crosslinked PPO-SEBS. J. Mater. Chem. A 2021, 9, 1062–1079. [Google Scholar] [CrossRef]
- Silva, A.L.A.; Takase, I.; Pereira, R.P.; Rocco, A.M. Poly(Styrene-Co-Acrylonitrile) Based Proton Conductive Membranes. Eur. Polym. J. 2008, 44, 1462–1474. [Google Scholar] [CrossRef]
- Sarkar, A.; Bidu, J.M.; Panda, J.; Kwon, Y.J.; Bak, S.; Cho, K.Y.; Byun, S.; Cheong, J.Y. Applications of Electrospinning for Fuel Cell and Electrolysis Cell Applications in Hydrogen Technologies. Energy Rev. 2025, 4, 100119. [Google Scholar] [CrossRef]
- Golubkov, S.S.; Morozova, S.M. Recent Progress of 3D Printing of Polymer Electrolyte Membrane-Based Fuel Cells for Clean Energy Generation. Polymers 2023, 15, 4553. [Google Scholar] [CrossRef]
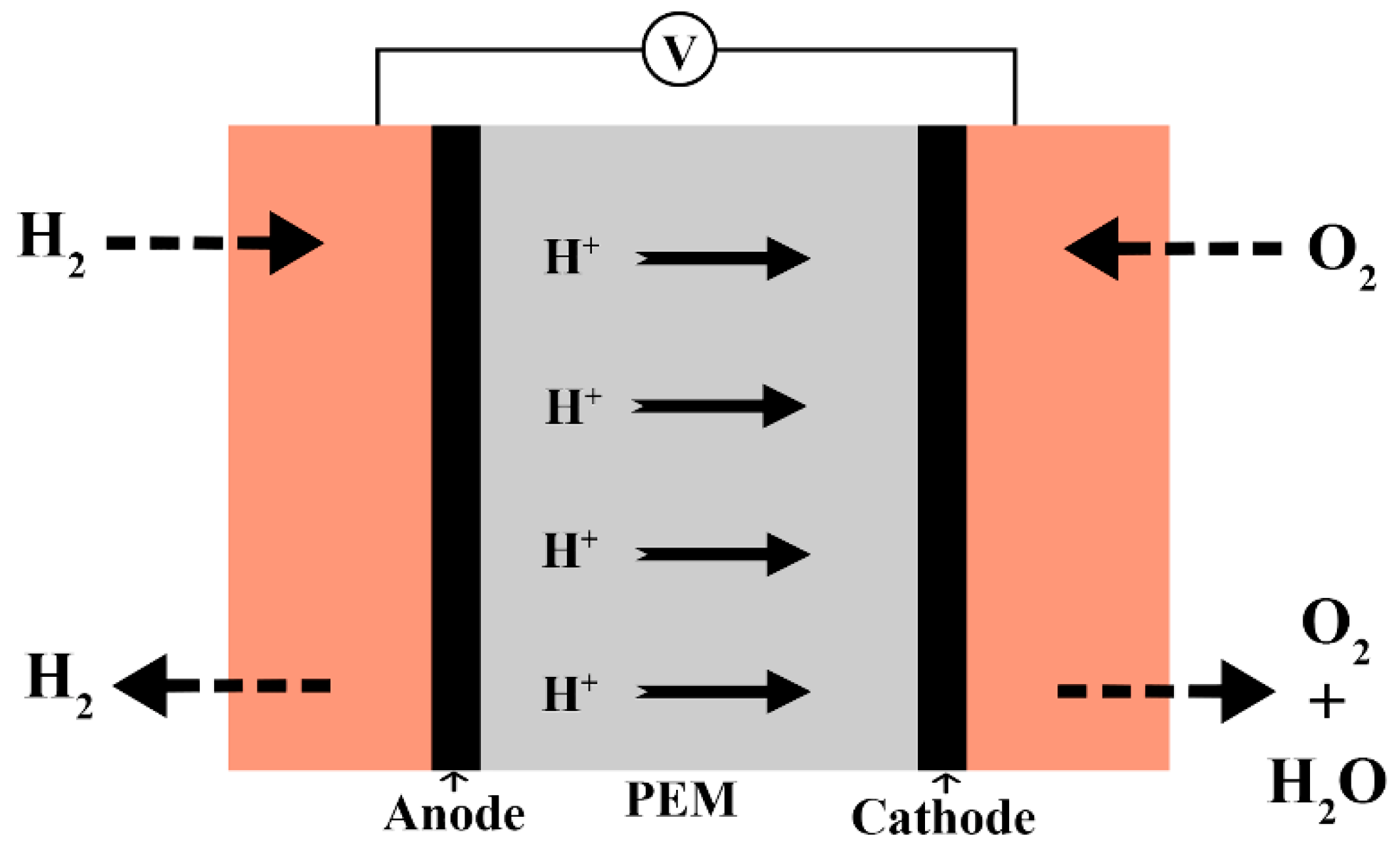
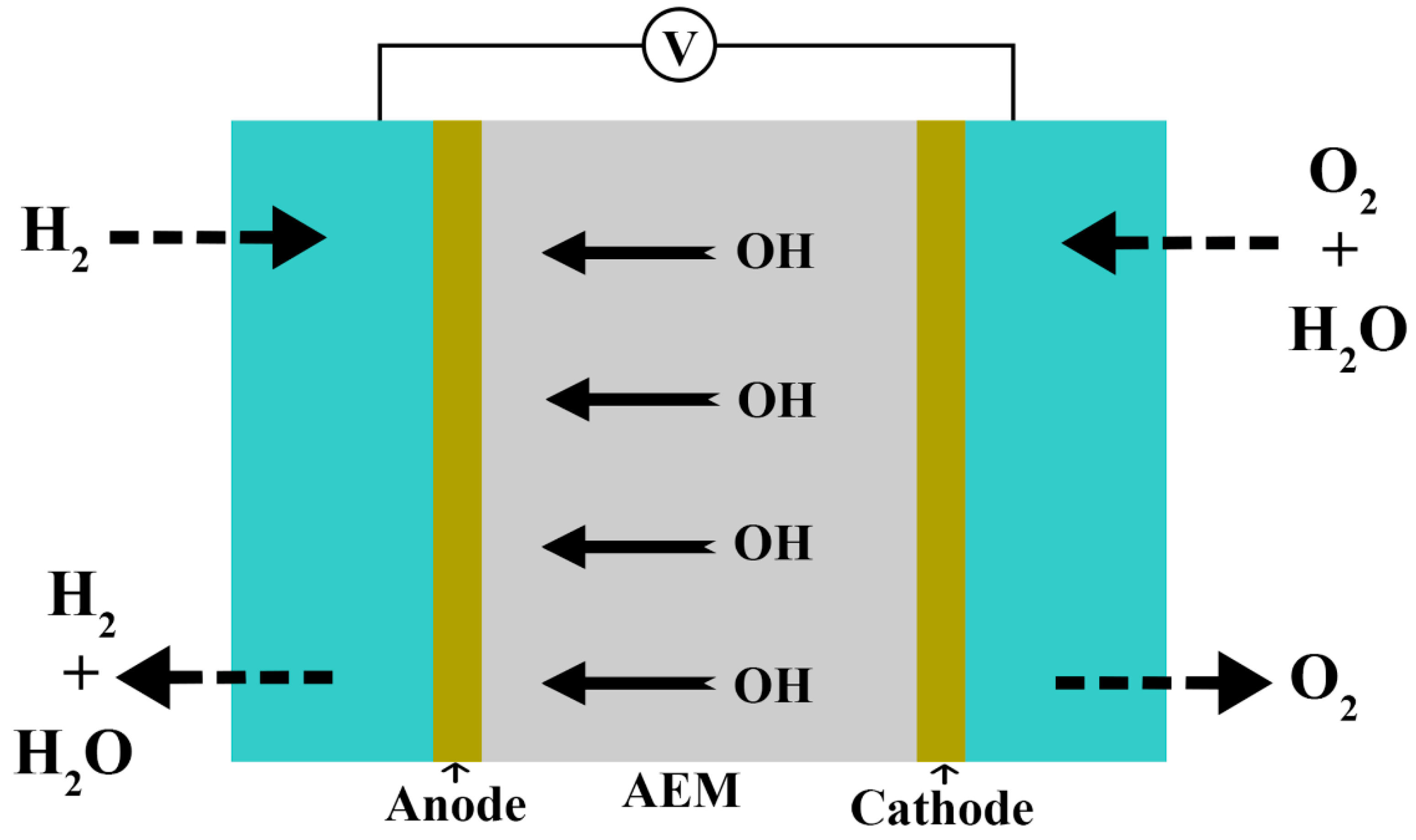
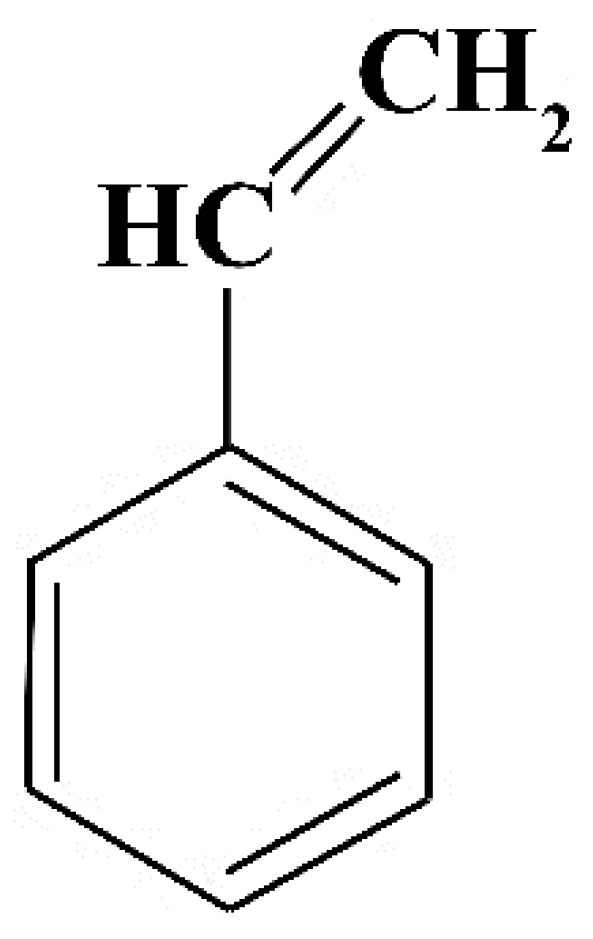
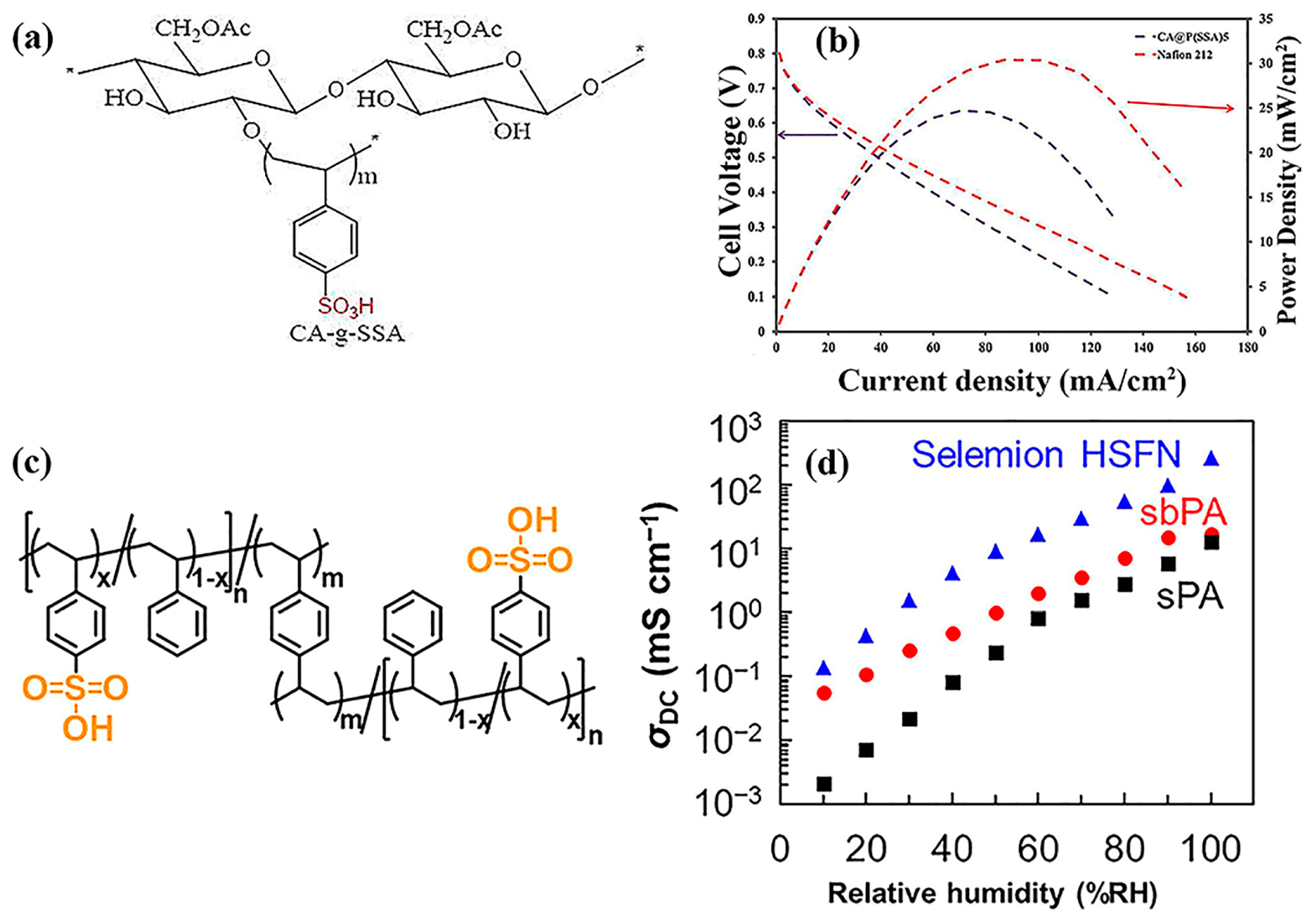


| Characteristics | Physical State | Density (ρ) | Boiling Point (°C) | Melting Point (°C) | Solubility |
|---|---|---|---|---|---|
| Values | Colorless/light yellow | 0.91 | 145 | −30 °C | Water: 0% Organic solvents (alcohol, ether, etc.): 100% |
| Types of Materials | Types of Fuel Cell | Ion Conductivity (mS cm−1) | Current Density (mA cm−2) | Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|
| styrene−co−vinylbenzyl chloride−co−acrylonitrile copolymer | AEMFC | 214 | - | - | [119] |
| poly(styrene sulfonic acid-co-1-vinylimidazole-co-styrene) | PEMFC | 7.8 × 10−2 | - | - | [118] |
| NaSS-4VP | PEMFC | 71 | - | - | [117] |
| SSA/cellulose acetate | DMFC | 4.77 | - | 24.6 | [114] |
| Polystyrene/graphene oxide | DMFC | 73 | 170 | [113] | |
| Polystyrene/PE/graphene oxide | DMFC | - | - | 78 | [110] |
| Types of Materials | Types of Fuel Cell | Ion Conductivity (mS cm−1) | Current Density (mA cm−2) | Power Density (mW cm−2) | Ref. |
|---|---|---|---|---|---|
| SBS-TMA | AEMFC | 100 | - | - | [120] |
| h-SBS | AEMFC | 93 | - | - | [121] |
| SEBS-PS | - | 190 | 2.09 | 1000 | [122] |
| SPSEBS-glutaraldehyde | DMFC | 10 | 200 | 68/56 | [123] |
| SEBS-PEB | AEMFC | 81 | 320 | 320 | [124] |
| Amine-SEBS | AEMFC | 20 | [125] | ||
| PTP-SEBS | AEMFC | 102.02 | [126] | ||
| PBB-SEBS | AEMFC | 146.25 | - | - | [127] |
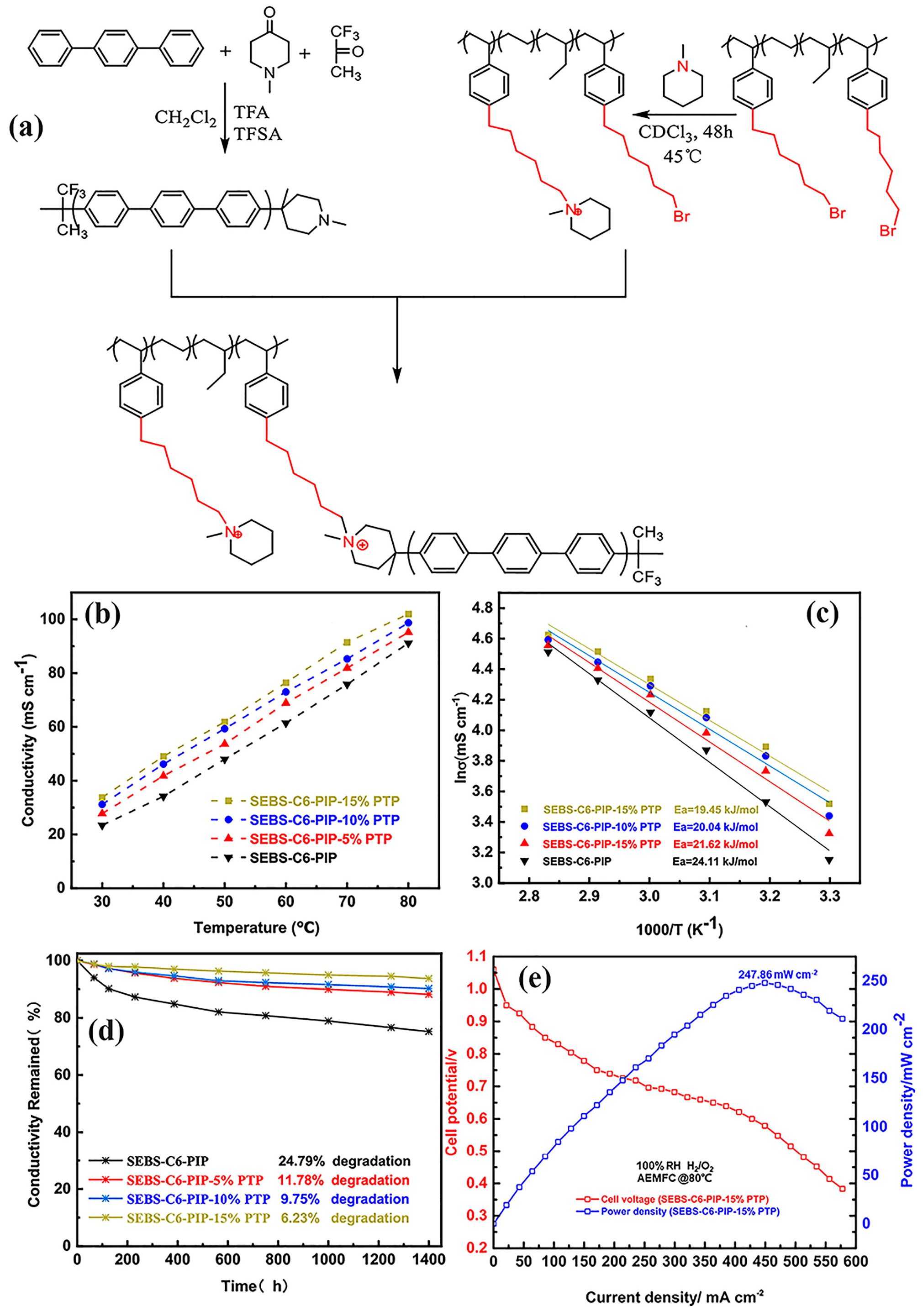
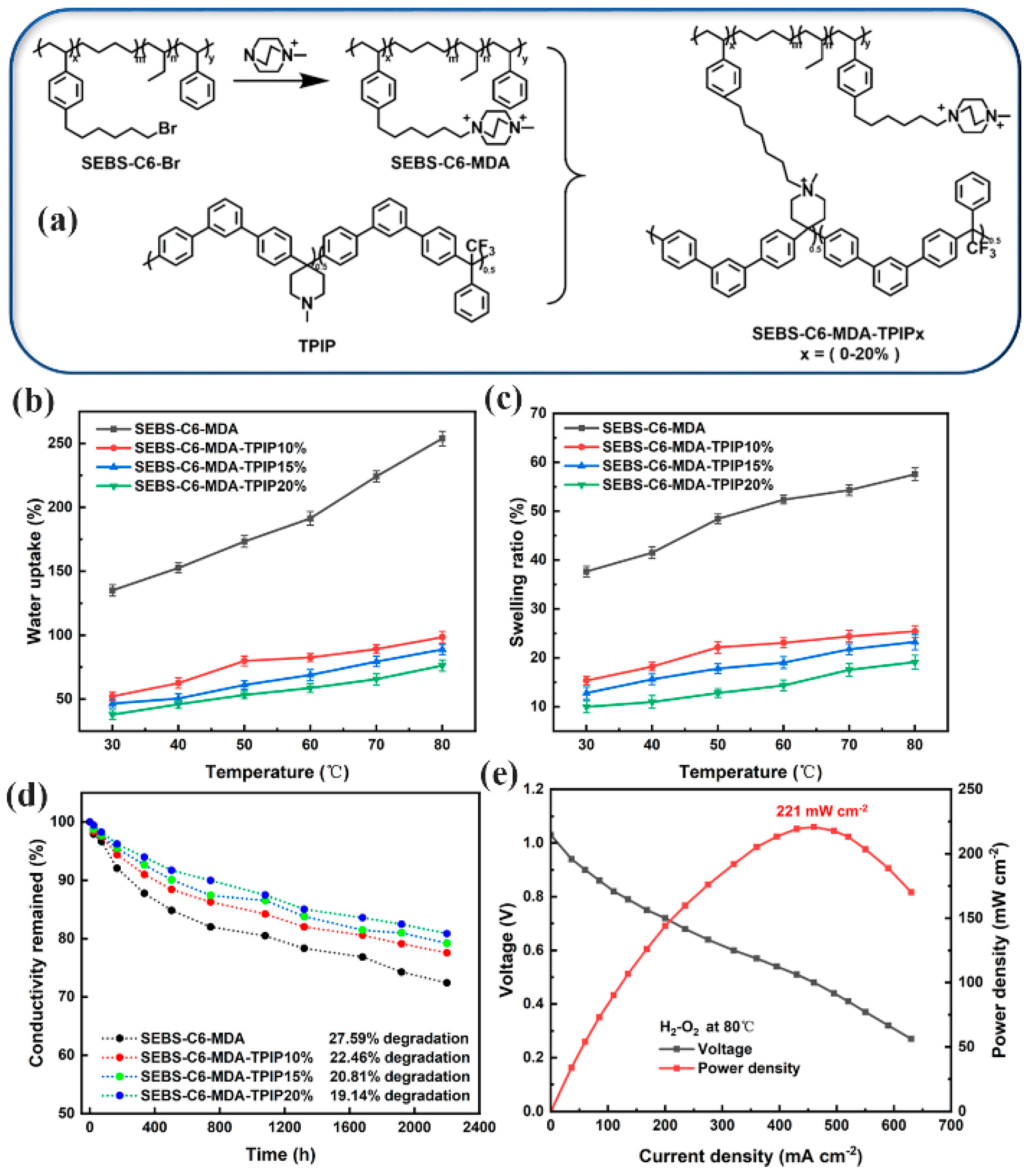
| SEBS-PTPIP | AEMFC | 86.76 | 460 | 221 | [128] |
| PPO-SEBS | AEMFC | - | - | 405 | [129] |
| PSAN/H3PO4 | PEMFC | 10 | - | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, M.R.; Zahid, A.; Su, H.; Divya, K.; Anwar, M.T.; Xu, Q. Styrene and Its Derivatives Used in Proton Exchange Membranes and Anion Exchange Membranes for Fuel Cell Applications: A Review. Batteries 2025, 11, 134. https://doi.org/10.3390/batteries11040134
Asghar MR, Zahid A, Su H, Divya K, Anwar MT, Xu Q. Styrene and Its Derivatives Used in Proton Exchange Membranes and Anion Exchange Membranes for Fuel Cell Applications: A Review. Batteries. 2025; 11(4):134. https://doi.org/10.3390/batteries11040134
Chicago/Turabian StyleAsghar, Muhammad Rehman, Ayesha Zahid, Huaneng Su, Kumar Divya, Muhammad Tuoqeer Anwar, and Qian Xu. 2025. "Styrene and Its Derivatives Used in Proton Exchange Membranes and Anion Exchange Membranes for Fuel Cell Applications: A Review" Batteries 11, no. 4: 134. https://doi.org/10.3390/batteries11040134
APA StyleAsghar, M. R., Zahid, A., Su, H., Divya, K., Anwar, M. T., & Xu, Q. (2025). Styrene and Its Derivatives Used in Proton Exchange Membranes and Anion Exchange Membranes for Fuel Cell Applications: A Review. Batteries, 11(4), 134. https://doi.org/10.3390/batteries11040134









