1. Introduction
Ethylene carbonate (EC) is a polar solvent with a dielectric constant of 89.8 at 25 °C, a dipole moment of 4.61D, and a melting point of 36.5 °C [
1]. EC is used as a plasticizer, crosslinking agent, additive for lubricants, and as a (co-)solvent in the pharmaceutical industry. Moreover, EC is used in cleaning agents, agrochemicals, polymer processing, and as a functional fluid [
2]. The biggest global market share for EC in the industry is its application as an electrolyte solvent in Li-ion batteries [
3,
4]. The electrolyte in Li-ion batteries is typically a mixture of polar (i.e., EC, propylene carbonate) and non-polar (i.e., dimethyl carbonate, ethyl methyl carbonate, and diethyl carbonate) solvents to dissociate the conductive salt while maintaining low viscosity [
3]. The recycling of the electrolyte from spent Li-ion batteries remains a challenge for the Li-ion battery industry [
5]. Literature suggests different approaches to recover the electrolyte from spent LiBs. Among them are vaporization processes, organic solvent extraction, and supercritical CO
2 (scCO
2) extraction [
5,
6,
7,
8,
9]. The drawback of vaporization processes is that the conductive salt, LiPF
6, decomposes to toxic gases such as hydrofluoric acid and organo-fluorophosphates [
10].
ScCO
2 extraction has high potential to recover the electrolyte [
5,
7]. Compared to conventional extraction techniques, sCO
2 extraction is superior in terms of sustainability aspects as CO
2 is non-toxic, non-carcinogenic, non-flammable, thermodynamically stable, abundant, and can be recycled back into the process [
11]. Moreover, it possesses excellent mass transfer characteristics owed to its liquid-like densities, gas-like viscosities, negligible surface tension, and high diffusion coefficient [
12]. Any contamination of the sample matrix during the extraction process is suppressed in scCO
2 extraction processes as the solvent can be quickly removed by depressurization. Another advantage is that CO
2 can be regenerated into the process after separation from the solute [
11]. The solvent characteristics of scCO
2 can be easily fine-tuned by changing pressure or temperature conditions. Generally, an increase in pressure at a given temperature leads to an increase in solvent density, by which the solvent power increases as the probability of solvent–solute interactions increases. The effect of temperature at a given pressure is somewhat more complex, and its tendency is dependent on the cross-over pressure. Below the cross-over pressure, the solvation power decreases with an increase in the temperature due to the solvent density decrease. Above the cross-over pressure, the density effects become less dominant as the solvent changes are no longer remarkable, and solute vapor pressure effects become dominant. Consequently, the solubility increases with temperature [
12,
13].
ScCO
2 is classified as a non-dipolar solvent and is thus effective in dissolving non-polar, low-molecular-weight compounds. Literature shows that non-polar electrolyte solvents were successfully extracted using scCO
2 [
14,
15]. EC is more challenging to be extracted in scCO
2 due to its high polarity. However, EC contains a CO
2-phillic carbonyl group, which improves the solvation of polar compounds in scCO
2 [
16,
17]. Knowledge of the solute solubility is a key factor in designing an extraction process as the extraction rate of a solute from a certain matrix is governed by the solubility and mass-transfer, i.e., partitioning and diffusion [
18,
19]. The process conditions leading to high solubility can be used for the extraction of the solute, and the low-solubility conditions can be then used for the separation of the extracted substance from the CO
2 [
11]. A study investigated the solubility of CO
2 in EC at different temperatures (18–58 °C) at atmospheric pressure and reported a decrease in the CO
2 mole fraction with temperature from 0.00622 to 0.00274 [
20]. However, there is a lack of phase equilibria data or solubility data for EC in scCO
2 conditions in the literature.
In this work, the solubility of EC in scCO2 at different pressure conditions (80 to 160 bar) at isothermal temperatures (40 °C and 60°) was studied. The retrieved solubility data of EC in scCO2 will be a key factor in the design of a scCO2 extraction process to recover the electrolyte from spent Li-ion batteries.
2. Materials and Methods
2.1. Materials
Liquid carbon dioxide (CO2) with a purity of ≥99.99% (H2O ≤ 5 ppm w/w) was purchased from Air Liquide (Paris, France). Ethylene carbonate (>98%) was purchased from Merck Millipore.
2.2. Experimental
The solubility of EC in supercritical CO
2 was isothermally (40 °C and 60 °C) determined at different pressure conditions (80 to 160 bar) using the gravimetrical flow-through method. Thereby, the equilibrium chamber was loaded with an adequate amount of solute. ScCO
2 is passed from top to bottom in a continuous flow, and the solubilized solute is trapped in the collection vial by reducing the pressure. The solubility was then determined using the weight of the trapped amount and the total mass of passed CO
2 [
21].
The experimental set-up is illustrated in
Figure 1. Pure EC (1.00 ± 0.05 g) was placed inside a stainless-steel equilibrium chamber (7.5 mL), which was leak-tight closed using wrenches. The equilibrium chamber was then thermally stabilized to the desired temperature using water pipes connected to an external water bath (Model F12 & ED; Julabo, Seelbach, Germany). The temperature in the equilibrium chamber was monitored using a thermocouple connected to a temperature logger and the pressure using a pressure gauge. The equilibrium chamber was pressurized using a syringe pump connected to a heating jacket (ISCO 260D; Teledyne ISCO, Lincoln, NE, USA), and the system was equilibrated for 7.5 min before the CO
2 flow was started. A CO
2 flow was initiated using a metering valve and was adjusted to the desired flow rate, 1500 ± 300 mL/min, which was measured at depressurized conditions at 1 bar and 22 ± 1 °C. The flowrate was adjusted to be as low as possible to achieve saturation. The effluent of the equilibrium chamber was filtered using a stainless-steel filter (0.5 μm) before passing through the metering valve to prevent the leak of undissolved EC. The flow was kept constant for 5 min, and the solubilized EC was collected in two successive collection vials to maximize the collection yield. After 5 min, the collection vials were weighted using a precision scale (resolution 0.01 mg and linearity ±0.1 mg), and meanwhile, the equilibrium chamber was held in static pressure for 5 min. The procedure was repeated several times until the collected EC amount approached the input weight. At all pressure and temperature conditions, the experimental runs were conducted three times.
A proof-of-concept experiment was conducted to extract EC from Li-ion battery black mass. The extraction chamber was loaded with the sample (5.5 ± 0.3 g). After an equilibration time of 5 min, a constant flow of CO
2 was applied (1500 ± 300 mL/min measured at depressurized conditions, 1 bar, 22 ± 1 °C). The extracts were absorbed in a glass vial filled with acetonitrile (4 mL). Two approaches were compared. In the first approach, an extraction time of 45 min was selected using 140 bar and 40 °C. In the second approach, three subsequent extraction steps were used for the extraction of the EC from the same black mass sample. The first step involved dynamic extraction for 30 min at 80 bar and 40 °C, then the pressure was raised to 100 bar in the second extraction step for 30 min, and finally raised to 140 bar for another 30 min. Then, the extract solution was analyzed using gas chromatography coupled with mass spectrometry (GC-MS). To determine the extraction yield of EC, the content of EC in the black mass was analyzed before the scCO
2 extraction process. Therefore, a procedure developed by Peschel et al. was adopted [
22]. The black mass sample (5.2 ± 0.02 g) was mixed with acetonitrile (5 mL) inside a plastic vial (50 mL). Then, the vial was shaken for 5 min, and the extraction solution was filtered. Afterward, the solution was analyzed using GC-MS.
2.3. Calculation of the Solubility and Molar Fraction Solubility
To calculate the solubility of EC in CO2, the cumulative collected amount of EC (g) was plotted against the amount of consumed CO2 (g).
The solubility of EC at the corresponding scCO
2 condition was then determined using the slope coefficient of the linear regression of data points within the linear range of the extraction curve. The amount of consumed CO
2 was calculated based on the following equation (Equation (1)):
where V is the consumed CO
2 in ml during the extraction period of 5 min given by the pressurized syringe pump at the corresponding pressure condition, and ρ is the density (g/mL) at the process condition taken from the NIST Chemistry Webbook [
23]. As the CO
2 flowrate fluctuated during the extraction period of 5 min, the consumed CO
2 volume differed slightly between the serial runs.
The molar fraction solubility of EC (x
EC) was calculated according to Equation (2).
where S is the determined solubility, given in g/g, MM
EC is the molar mass of EC (88.06 g/mol), and MM
CO2 is the molar mass of CO
2 (44.01 g/mol).
2.4. Chrastil Model
The measured solubility data were modeled using the Chrastil model, as it requires only information about the density of the supercritical CO
2, temperature, and experimental solubility data. The semi-empirical model was derived based on the formation of a solvato complex at equilibrium between the solute and scCO
2 molecules. It relates solute solubility to temperature and the pure supercritical fluid solvent density up to a solute concentration of 200 g/L. (Equation (3)) [
24].
where S is the solubility of the solute in kg/m
3, ρ is the density of the solvent in kg/m
3, and T is the operating temperature in K. A, B, and k are the adjustable model parameters and can be determined using linear regression of the experimental data by plotting the natural logarithm of S against the natural logarithm of ρ. Constant A is a function of the enthalpy of solvation (ΔH
sol), the enthalpy of vaporization (ΔH
vap), and the universal gas constant, denoted as R (A = (ΔH
vap + ΔH
solv)/R). B is a function of the association number and molecular weights of the solute and supercritical fluid. k is the average equilibrium association number of the pseudo solvato-complex compound.
2.5. Measurement and Characterization
Universal attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR, Spectrum Two, Perkin Elmer, Hägersten, Sweden) was used to characterize the collected EC and initial EC, which served as a reference. The ATR-FTIR spectra were recorded in a range between 4000 cm−1 and 500 cm−1, with a resolution of 2 cm−1 and a total of 4 scans.
X-ray diffraction analysis (XRD, Bruker D8 Discover, EIGER2R 500 K detector, Dectris, Baden, Switzerland) was performed to analyze potential structural changes of the initial and collected EC at different conditions. The pattern was recorded in a 2Ɵ range of 10° to 55° using the characteristic Kα wavelength of 1.5406 Å provided by a Cu radiation source. The operating voltage and current were set to 40 kV and 40 mA and the operational speed of 15 rpm. The sample preparation for the XRD measurement included grinding of the samples to approximately the same particle size (evaluated by eyesight), and then the sample holder was filled and pressed with a glass piece.
Scanning electron microscopy (SEM, Quanta 200 ESEM, FEI, Lausanne, Switzerland) with 5 kV high voltage and ETD detector was used to study the surface morphology of the samples. The collected and initial EC was placed on a graphite substrate and coated with a gold film (5 nm) using a sputter (EM ACE 600, Leica, Wetzlar, Germany).
GC-MS (GC-2030 NX, GCMS-QP2020 NX, Shimadzu, Kyoto, Japan) was used to quantitatively analyze the extract solutions. Split injection (1:20) with a purge flow of 3 mL/min was used for sample injection into the column (Zebron ZB-5MS column, 30 m × 250 μm × 0.25 μm). The injection and transfer line temperature were set to 270 °C and 230 °C, respectively. Helium was used as carrier gas at a flowrate of 1 mL/min. The GC oven temperature was maintained at 40 °C for 1 min and then ramped at 20 °C/min to 260 °C, which was held for 2 min. MS conditions were electron impact, ionization source temperature of 230 °C, 70 eV filament voltage, and mass range of 30–300 m/z. The integrated area of EC was used for quantification.
3. Results and Discussion
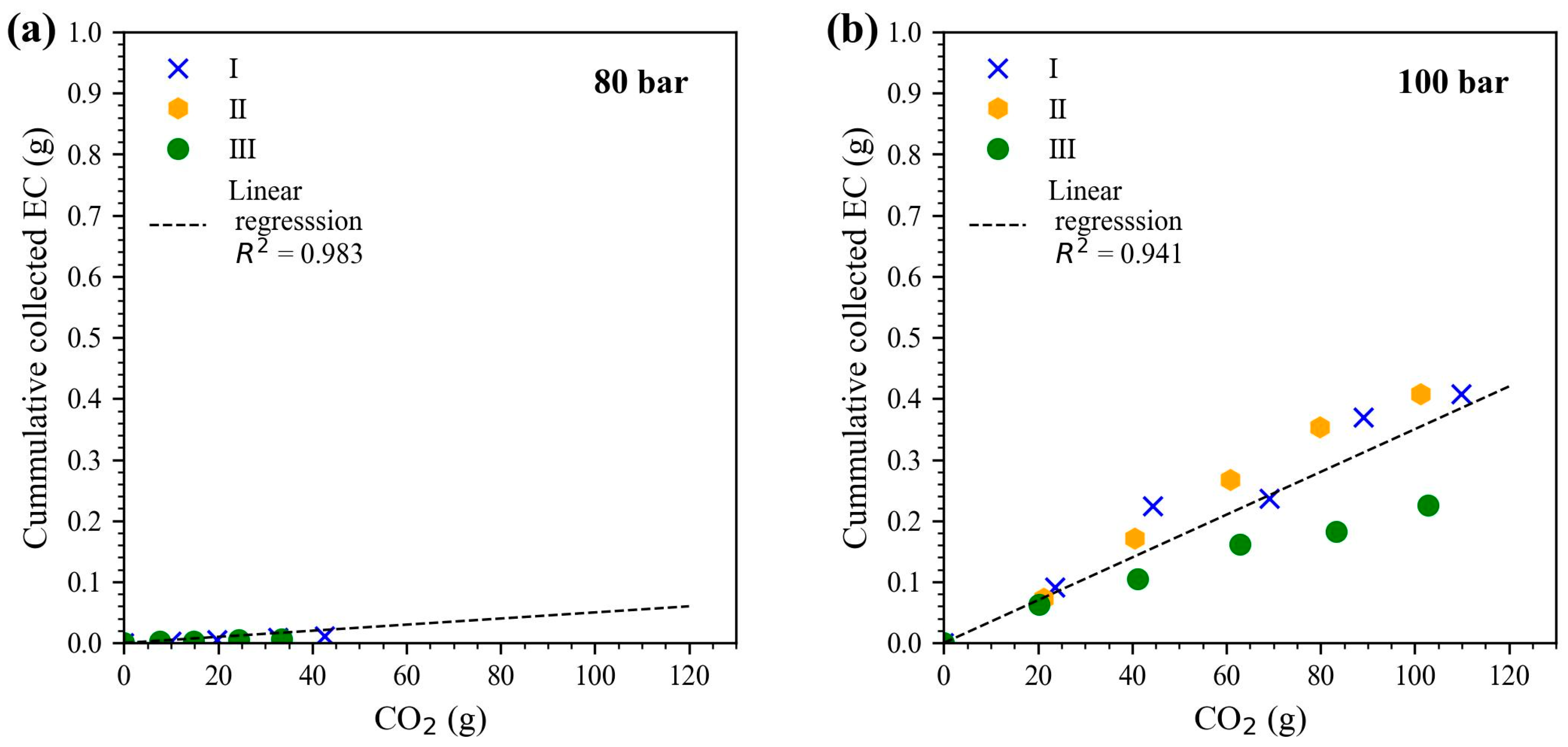
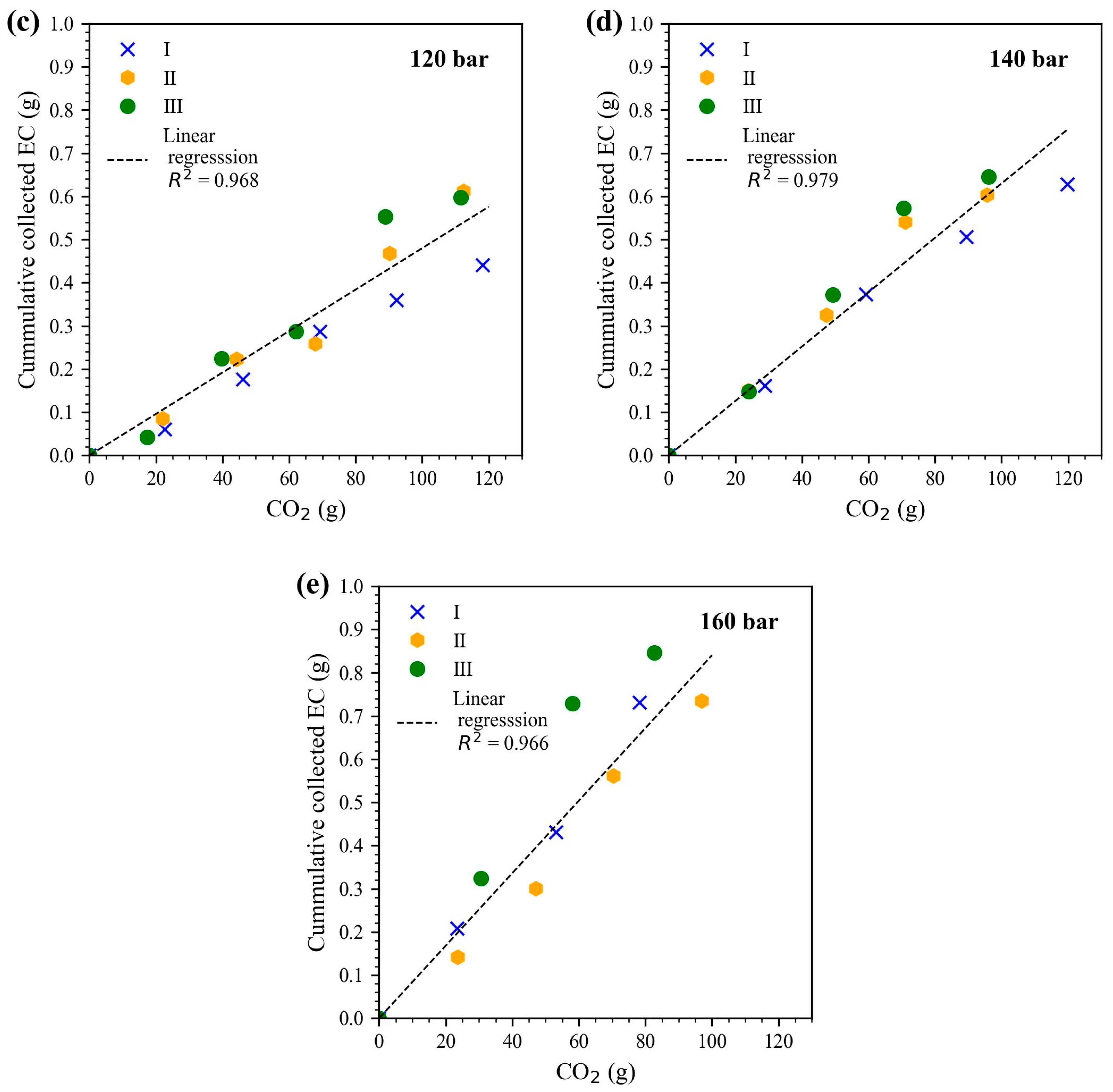
The solubility of EC in scCO
2 was studied at different isothermal (40 °C and 60 °C) pressure conditions (80, 100, 120, 140, and 160 bar). Therefore, the cumulative collected EC (g) was plotted over the CO
2 amount (g). The solubility of EC in CO
2 was determined based on the linear slope. The results at the different pressure conditions for 40 °C are plotted in
Figure 2. The corresponding solubility data are given in
Table 1. The respective plots used for the isothermal condition at 60 °C are plotted in
Figure S1 in the Supplementary Material.
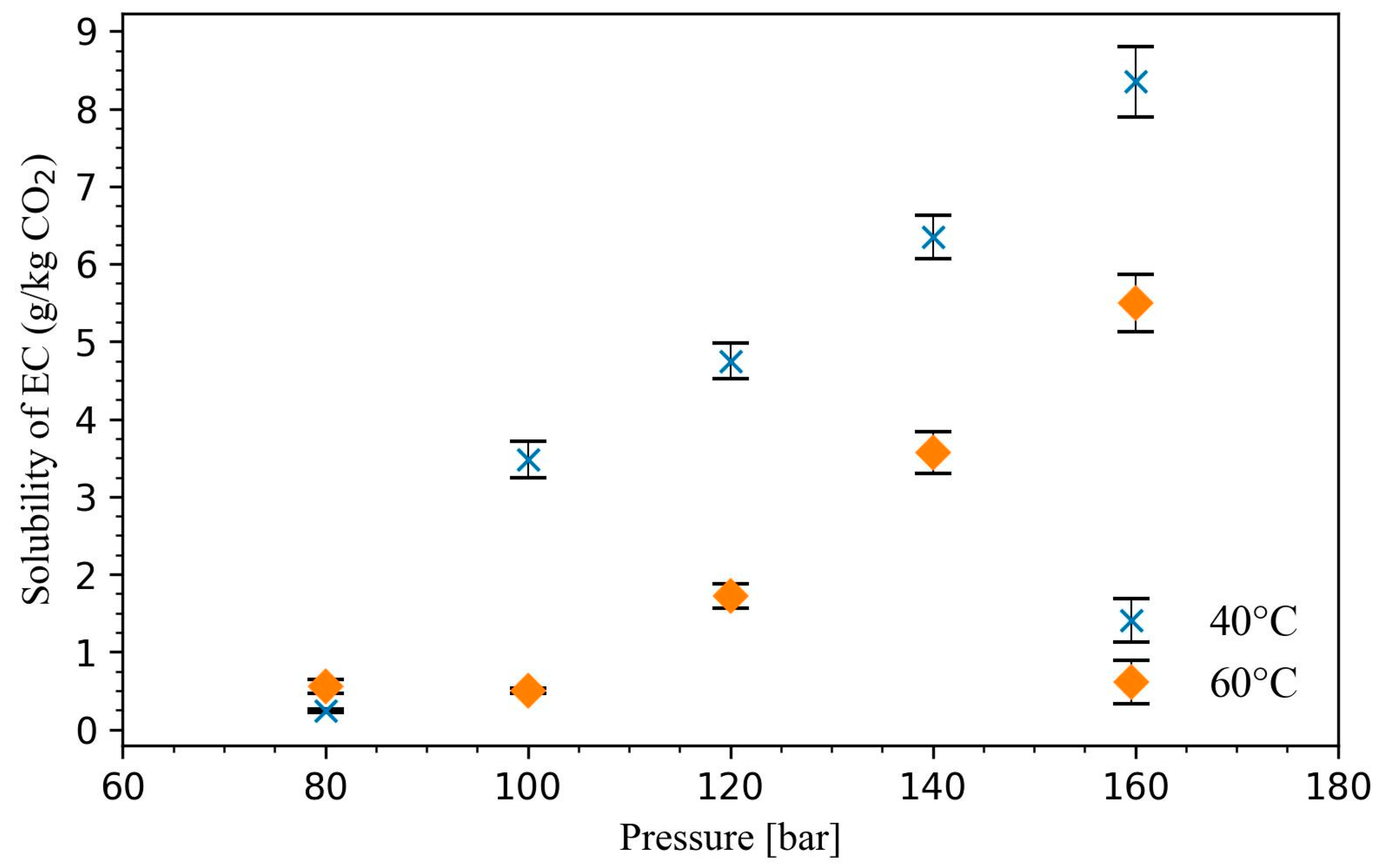
The mole fraction solubility of EC in supercritical CO
2 is plotted in
Figure 3. The solubility of EC in scCO
2 increases with pressure at a constant temperature in the range from 0.24 g/kg CO
2 to 8.35 g/kg CO
2. The increase in solubility under increased pressure can be linked to the increased likelihood of specific solute–solvent interactions caused by the increased density of CO
2 [
12]. The solubility at 40 °C was higher than at 60 °C measured at the same pressure conditions. This indicates that the studied pressure range was below the cross-over pressure, meaning that the CO
2 density was the dominating factor in dissolving EC [
12,
13]. The solubility at 80 bar and 40 °C is very low compared to the solubility at 140 bar and 40 °C. Thus, for the design of an extraction process for EC recycling, 140 bar and 40 °C can be used for the extraction of the solute, while the low solubility condition at 80 bar and 40 °C can be used for the separation of the extracted substance from the CO
2 [
11].
As there is a lack in the literature of solubility data of EC in scCO
2, the mole fraction solubility was compared to propylene carbonate (PC) in CO
2. PC is another cyclic carbonate used in Li-ion batteries. The vapor phase composition and the calculated corresponding mole fraction solubility values reported by Hongling et al. and Rubin et al. are presented in
Table S1 in the Supplementary Material [
25,
26]. It can be observed that the mole fraction solubility of PC in CO
2 is a magnitude higher compared to EC at similar pressure and temperature conditions. It is believed that the higher solubility of PC in CO
2 is due to its lower polarity. The vapor phase fraction reported by Hongling et al. fluctuated in the pressure range between 20 bar and 130 bar. Thus, a clear trend in terms of pressure and temperature cannot be observed. However, according to the study by Rubin et al., the mole fraction solubility decreases with raising temperature, which was also observed for EC.
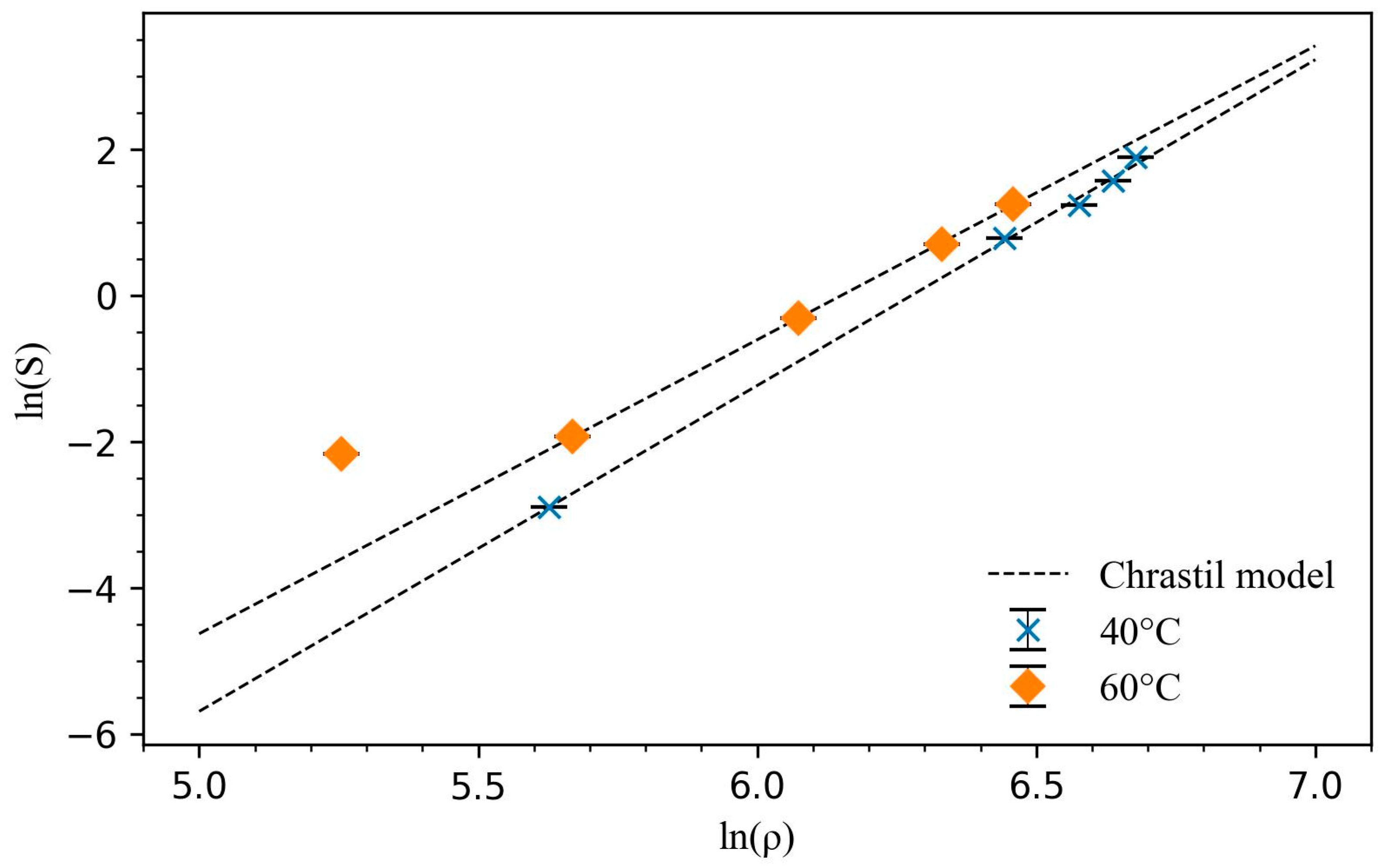
The experimental solubility data fitted well to the Chrastil model, as seen in
Figure 4, and the fitting parameters are presented in
Table 2. The average equilibrium association number of the pseudo solvato-complex compound determined from the Chrastil model is quite similar for 40 °C and 60 °C, at 4.28 and 4.02, respectively. This indicates that, on average, four CO
2 molecules are used to dissolve one molecule of EC. The CO
2 molecule was reported to form stable Lewis acid–Lewis base interactions with the CO
2-phillic carbonyl oxygen in other esters [
16]. The constant A is a magnitude higher at 60 °C compared to 40 °C, suggesting a higher solvation and vaporization enthalpy.
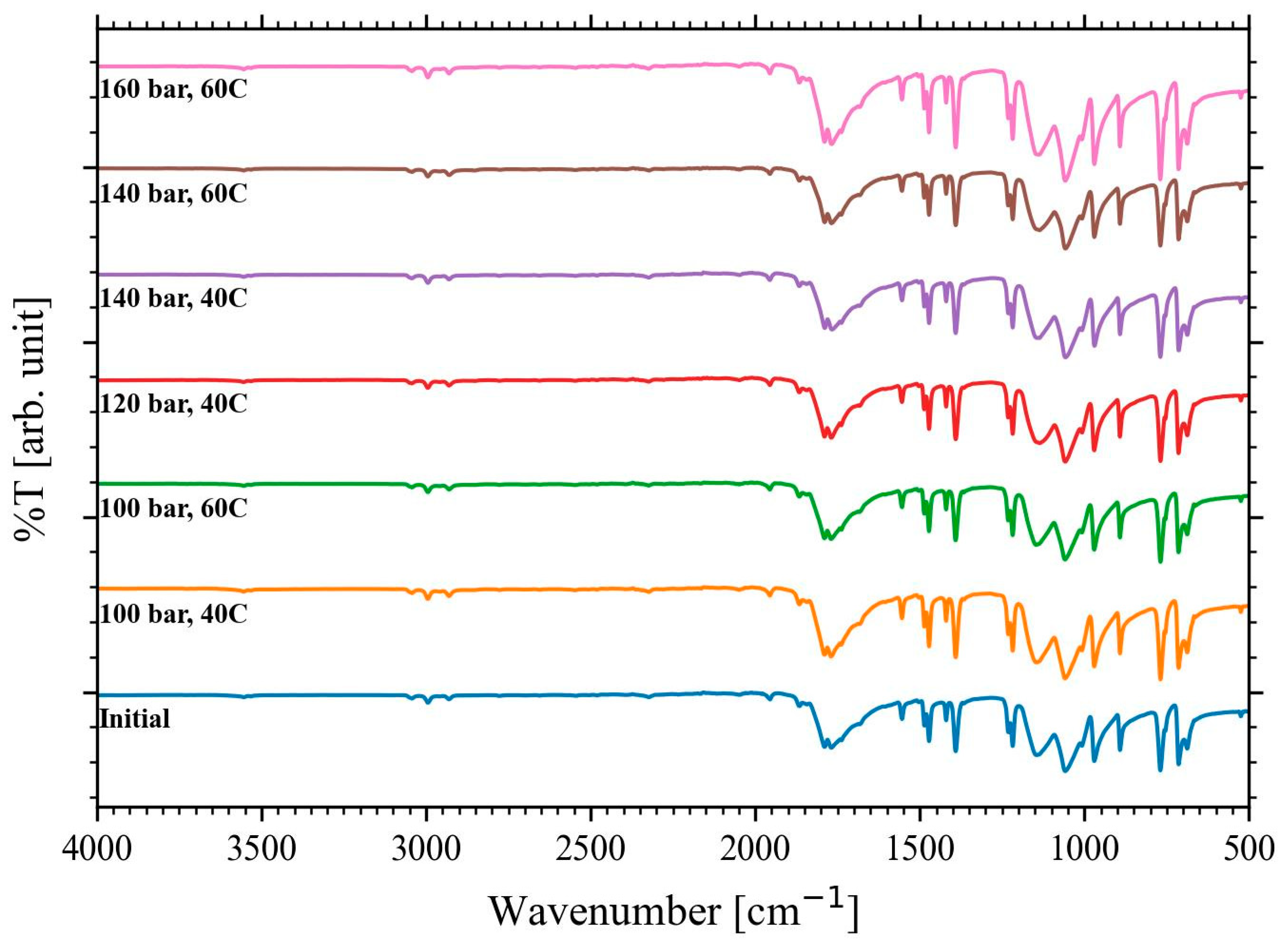
The collected EC was analyzed using FTIR to study any compositional change occurring after the scCO
2 dissolution.
Figure 5 shows the spectra between 4000 and 500 cm
−1 at the different pressure and temperature conditions and the initial EC as a reference. The initial EC spectra match the collected EC sample without any clear peak shifts. Thus, it can be concluded that the scCO
2 extraction at the studied conditions did not affect the chemical bonding of the molecule.
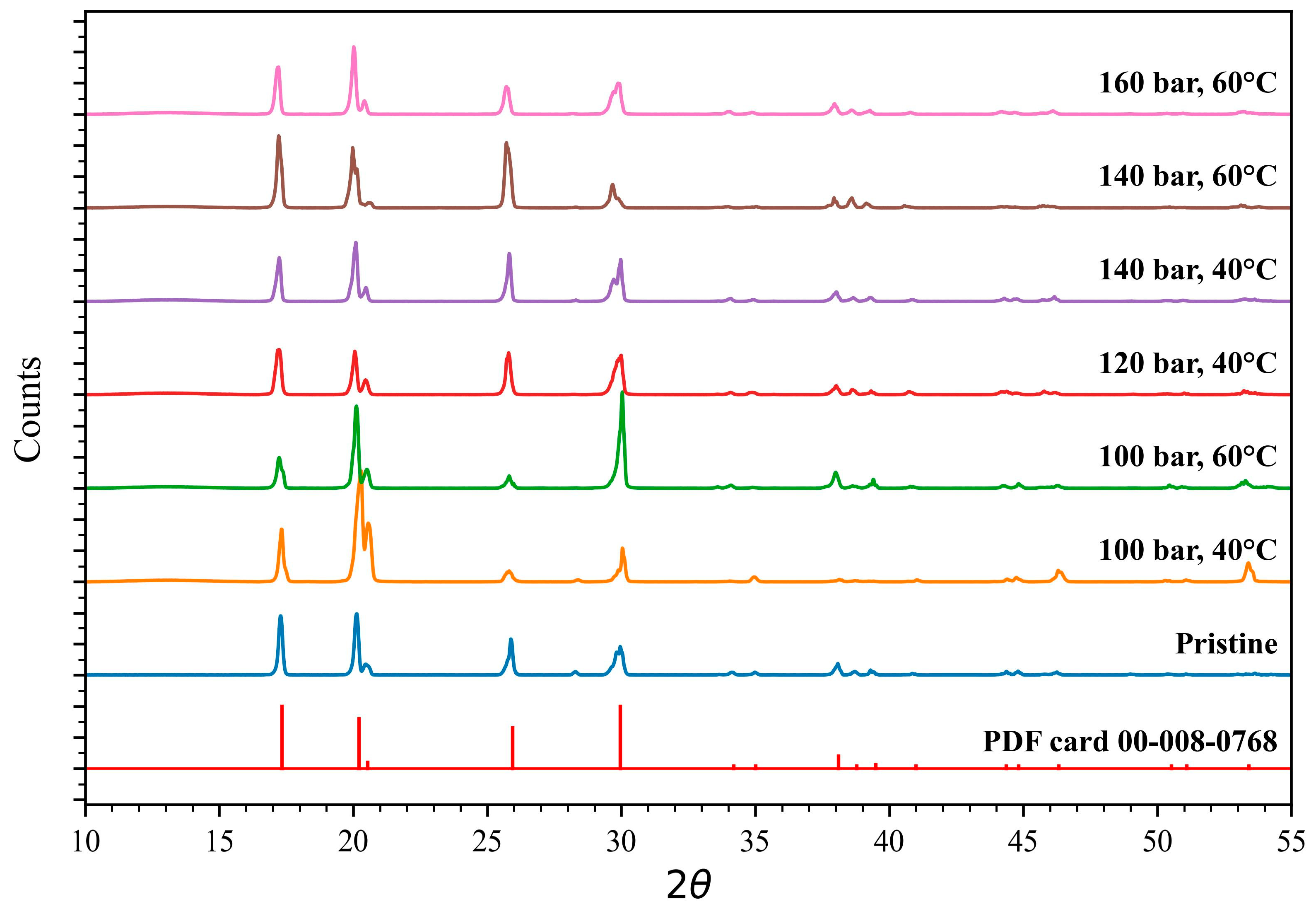
XRD analysis results are plotted in
Figure 6. The overlap of the peak position of the initial EC and the collected EC, which represent recovered material, indicates that the monoclinic crystal structure is stable during the processing and, generally, independent from the pressure and temperature conditions. However, random selective orientation of the crystal planes is observable in respect to the different peak intensities and shapes. The random crystal orientation may arise from the XRD sample preparation or the recrystallization process after dissolution in EC. The crystal orientation during the recrystallization process can be altered by several factors such as nucleation, growth rates, solution concentration, pre-and post-expansion pressure, and temperature [
27].
ScCO
2 can be applied in micronization processes to produce powder and composites while controlling their morphology and particle size distribution [
28]. The properties of the obtained product depend on the phase equilibria, thermodynamic behavior of the system, fluid dynamics, mass transfer, and nucleation growth kinetics [
11]. The particle size of the collected EC appeared to be smaller than the initial EC observed by eyesight. Determination of the changes in particle diameter and particle size distribution after EC collection was not part of this study. However, micronization of the EC due to the rapid expansion of the effluent in the collection process used in the RESS process (the rapid expansion of supercritical solutions) can be expected [
29]. SEM images were taken from the initial EC and the collected EC at 140 bar and 40 °C and are given in
Figure 7. According to the SEM images, the EC morphology of EC was not altered during the process. Similar findings were observed by Yim et al., as they dissolved polyvinylidene fluoride, which is a common binder for Li-ion batteries [
30].
In a previous research study, the non-polar electrolyte solvents dimethyl carbonate and ethyl methyl carbonate were selectively extracted from Li-ion battery pouch cells using different pressures (60 to 120 bar) and temperature conditions (29 °C), while EC remained in the LiB electrode stack [
14]. An experiment was conducted to validate that EC can be successfully recovered from LiB waste material. Therefore, two approaches were used. In the first approach, dynamic scCO
2 extraction at 140 bar and 40 °C was applied for 45 min to extract the residual electrolyte in industrial Li-ion battery black mass. In the second approach, three subsequent steps were used for the extraction of the EC. First, the electrolyte was extracted for 30 min at 80 bar and 40 °C. The pressure was raised to 100 bar in the second extraction step for 30 min and finally raised to 140 bar for another 30 min using the same black mass. The result of the extraction yields for the different steps is plotted in
Figure 8. It can be observed that an extraction yield of 90% was reached using approach 1. Approach 2 indicates the impact of the different solubilities on the extraction of EC from the LiB black mass. During the first step at 80 bars, the extraction yield of EC was low, only 4.5%. The extraction yield increased to 38% by raising the pressure to 100 bar and finally exceeding 93% at 140 bar. This proves that EC can be successfully recovered from Li-ion battery waste using pure scCO
2 extraction at 140 bar and 40 °C. However, further research is required to study the mass transfer characteristics during the extraction process.