Design and Validation of Anode-Free Sodium-Ion Pouch Cells Employing Prussian White Cathodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Assembly of Pouch Cells
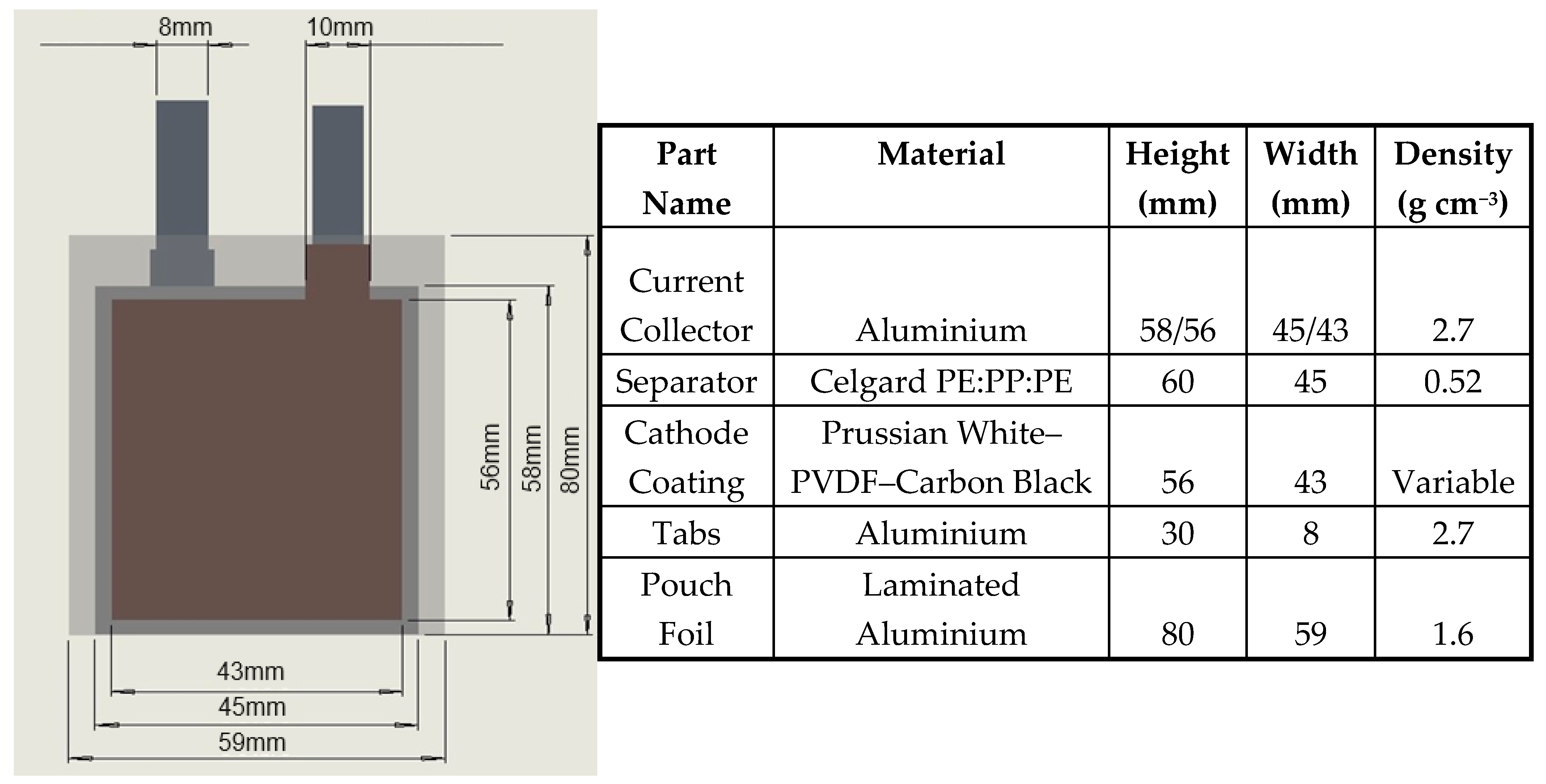
2.2. Building the Pouch Cells: 3D Model and Material Properties
2.3. Cell Model Calculations
3. Results and Discussion
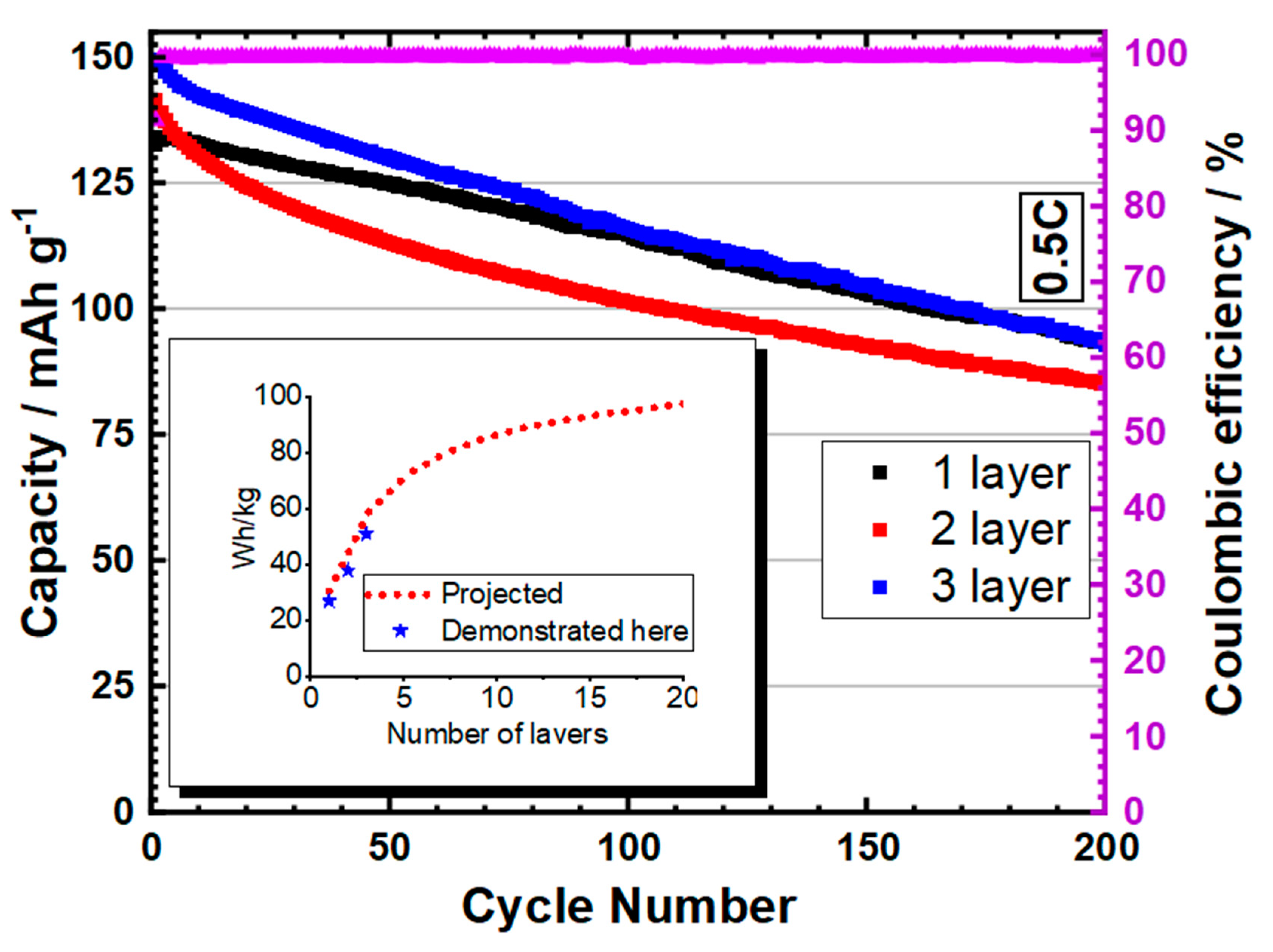
3.1. Pouch Cell Results
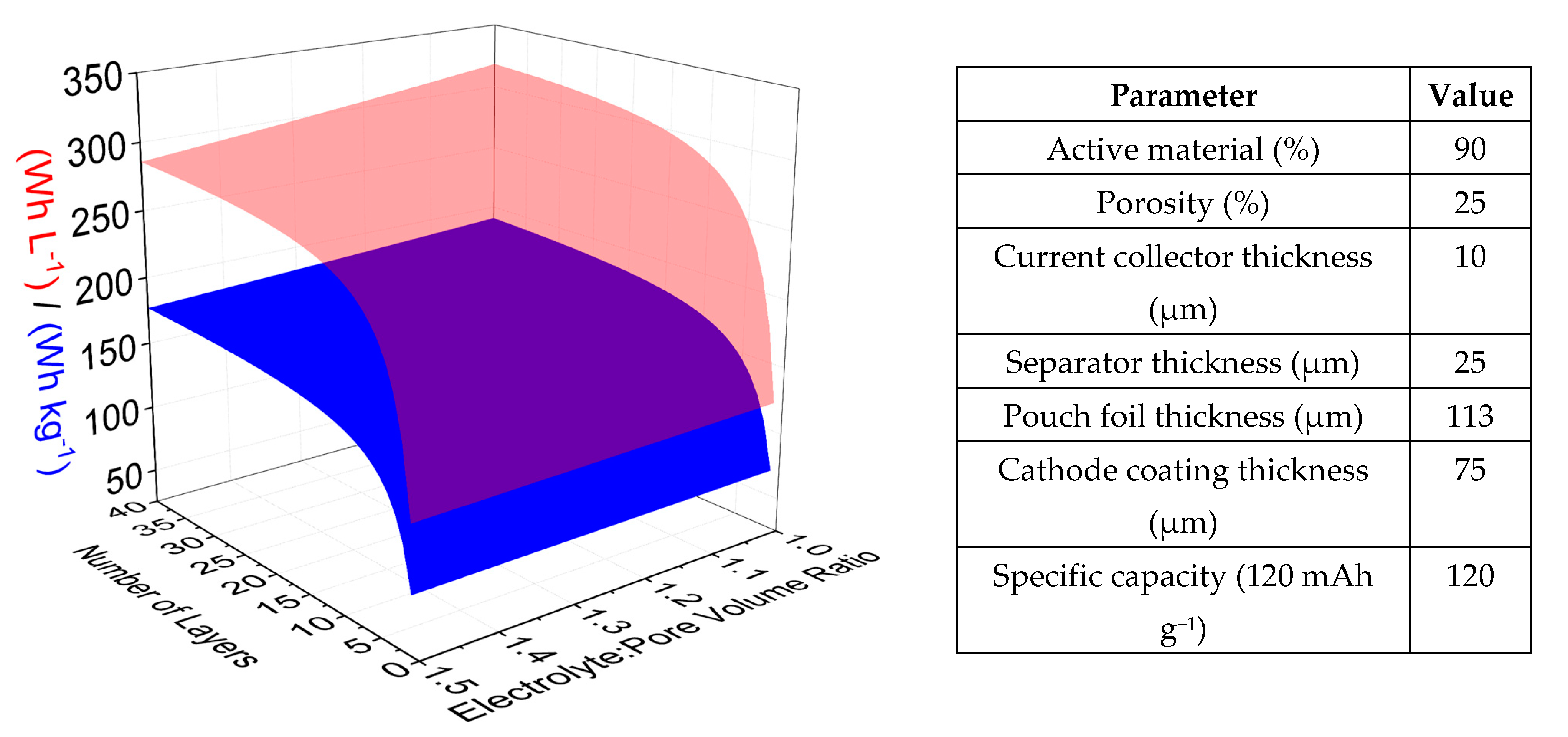
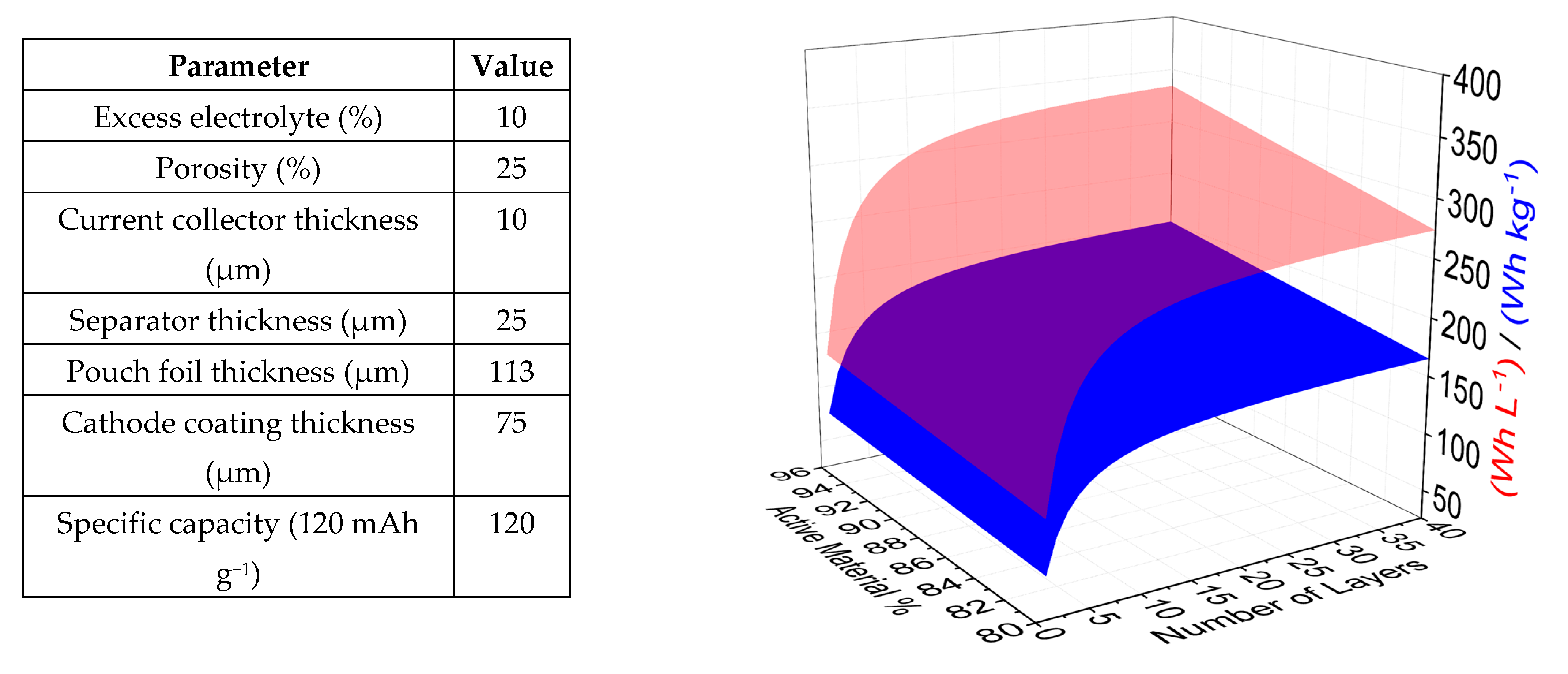
3.2. Modelling Expected Multilayer Pouch Cell Performance
- A reduced separator thickness (from 25 µm to 10 µm) would decrease the volume and weight of the cell due to the combined effect of the thickness and the lower amount of electrolyte required to wet the separator pores. However, mechanical stability and fire resistance would become increasingly problematic (+19 Wh kg−1/41 Wh L−1);
- Pouch foil thickness may be reduced (from 113 µm to 68 µm) (+6 Wh kg−1/9 Wh L−1);
- Ultrathin aluminium (6 µm) or aluminium/PET composite films (8 µm) as substrate materials for both electrodes should be investigated in place of the 10-micron aluminium used here (+21 Wh kg−1/10 Wh L−1).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Chao, D.; Li, W.; Zhao, D. Recent advances in hard carbon anodes with high initial Coulombic efficiency for sodium-ion batteries. Nano Mater. Sci. 2023, 5, 189–201. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Lin, D.; Wang, D.-W.; Li, B.; Lv, W.; Sun, S.; He, Y.-B.; Kang, F.; Yang, Q.-H.; et al. Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes. Nat. Commun. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; He, Y.; Xiong, P.; Zhao, X.; Xu, K.; Xu, Y. Long cycle life and high rate sodium-ion chemistry for hard carbon anodes. Energy Storage Mater. 2018, 13, 274–282. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Lu, Y.; Li, B.; Chen, L.; Hu, Y.-S. High-temperature treatment induced carbon anode with ultrahigh Na storage capacity at low-voltage plateau. Sci. Bull. 2018, 63, 1125–1129. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Xu, B. Facile synthesis of high performance hard carbon anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 20560–20566. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, F.; Xi, L.; Zeng, J.; Song, X.; Wan, X.; Zhao, J.; Liu, J. Recent Progress of Promising Cathode Candidates for Sodium-Ion Batteries: Current Issues, Strategy, Challenge, and Prospects. Small Struct. 2023, 4, 2300150. [Google Scholar] [CrossRef]
- Nielsen, I.; Dzodan, D.; Ojwang, D.O.; Henry, P.F.; Ulander, A.; Ek, G.; Häggström, L.; Ericsson, T.; Boström, H.L.B.; Brant, W.R. Water driven phase transitions in Prussian white cathode materials. J. Phys. Energy 2022, 4, 044012. [Google Scholar] [CrossRef]
- Bauer, A.; Song, J.; Vail, S.; Pan, W.; Barker, J.; Lu, Y. The Scale-up and Commercialization of Nonaqueous Na-Ion Battery Technologies. Adv. Energy Mater. 2018, 8, 1702869. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of high energy density sodium-ion batteries: Faradion’s journey and outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.-F. Past and Present of LiFePO4: From Fundamental Research to Industrial Applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef]
- Pampel, F.; Pischinger, S.; Teuber, M. A systematic comparison of the packing density of battery cell-to-pack concepts at different degrees of implementation. Results Eng. 2022, 13, 100310. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, C.; Zhang, N.; Liu, J.; Li, H.; Davey, K.; Qiao, S.-Z. Prospects for practical anode-free sodium batteries. Mater. Today 2024, 73, 260–274. [Google Scholar] [CrossRef]
- Dahunsi, O.J.; Gao, S.; Kaelin, J.; Li, B.; Abdul Razak, I.B.; An, B.; Cheng, Y. Anode-free Na metal batteries developed by nearly fully reversible Na plating on the Zn surface. Nanoscale 2023, 15, 3255–3262. [Google Scholar] [CrossRef]
- Wang, H.; Tan, H.; Luo, X.; Wang, H.; Ma, T.; Lv, M.; Song, X.; Jin, S.; Chang, X.; Li, X. The progress on aluminum-based anode materials for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 25649–25662. [Google Scholar] [CrossRef]
- Cohn, A.P.; Muralidharan, N.; Carter, R.; Share, K.; Pint, C.L. Anode-Free Sodium Battery through in Situ Plating of Sodium Metal. Nano Lett. 2017, 17, 1296–1301. [Google Scholar] [CrossRef]
- Mazzali, F.; Orzech, M.W.; Adomkevicius, A.; Pisanu, A.; Malavasi, L.; Deganello, D.; Margadonna, S. Designing a High-Power Sodium-Ion Battery by in Situ Metal Plating. ACS Appl. Energy Mater. 2019, 2, 344–353. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Weng, S.; Ding, F.; Qi, X.; Lu, J.; Li, Y.; Zhang, X.; Rong, X.; Lu, Y.; et al. Interfacial engineering to achieve an energy density of over 200 Wh kg−1 in sodium batteries. Nat. Energy 2022, 7, 511–519. [Google Scholar] [CrossRef]
- Cohn, A.P.; Metke, T.; Donohue, J.; Muralidharan, N.; Share, K.; Pint, C.L. Rethinking sodium-ion anodes as nucleation layers for anode-free batteries. J. Mater. Chem. A 2018, 6, 23875–23884. [Google Scholar] [CrossRef]
- Ma, B.; Lee, Y.; Bai, P. Dynamic Interfacial Stability Confirmed by Microscopic Optical Operando Experiments Enables High-Retention-Rate Anode-Free Na Metal Full Cells. Adv. Sci. 2021, 8, 2005006. [Google Scholar] [CrossRef]
- Lim, C.Q.X.; Tan, Z.-K. Prussian White with Near-Maximum Specific Capacity in Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 6214–6220. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, H.; Ran, F. Fast-charging cathode materials for lithium & sodium ion batteries. Mater. Today 2023, 63, 360–379. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.; Wray, L.A.; Hossain, M.A.; Chuang, Y.-D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.-J.; et al. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef]
- Maddar, F.M.; Walker, D.; Chamberlain, T.W.; Compton, J.; Menon, A.S.; Copley, M.; Hasa, I. Understanding dehydration of Prussian white: From material to aqueous processed composite electrodes for sodium-ion battery application. J. Mater. Chem. A 2023, 11, 15778–15791. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian Blue Analogues for Sodium-Ion Batteries: Past, Present, and Future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, M.; Hou, Z.; Li, T.; Zhang, X.; Gao, Y.; Peng, J.; Li, Y.; Wang, J.-G. Medium-mediated high-crystalline Prussian blue toward exceptionally boosted sodium energy storage. Energy Storage Mater. 2024, 70, 103411. [Google Scholar] [CrossRef]
- Ericsson, T.; Häggström, L.; Ojwang, D.O.; Brant, W.R. Investigation of Valence Mixing in Sodium-Ion Battery Cathode Material Prussian White by Mössbauer Spectroscopy. Front. Energy Res. 2022, 10, 909549. [Google Scholar] [CrossRef]
- Brant, W.R.; Mogensen, R.; Colbin, S.; Ojwang, D.O.; Schmid, S.; Häggström, L.; Ericsson, T.; Jaworski, A.; Pell, A.J.; Younesi, R. Selective Control of Composition in Prussian White for Enhanced Material Properties. Chem. Mater. 2019, 31, 7203–7211. [Google Scholar] [CrossRef]
- Hartmann, L.; Deshmukh, J.; Zhang, L.; Buechele, S.; Metzger, M. Reversing the Chemical and Structural Changes of Prussian White After Exposure to Humidity to Enable Aqueous Electrode Processing for Sodium-ion Batteries. J. Electrochem. Soc. 2023, 170, 030540. [Google Scholar] [CrossRef]
- Sayahpour, B.; Li, W.; Bai, S.; Lu, B.; Han, B.; Chen, Y.-T.; Deysher, G.; Parab, S.; Ridley, P.; Raghavendran, G.; et al. Quantitative analysis of sodium metal deposition and interphase in Na metal batteries. Energy Environ. Sci. 2024, 17, 1216–1228. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Singh, A.; Ren, D.; Hou, Z.; Ouyang, M. Effect of external pressure and internal stress on battery performance and lifespan. Energy Storage Mater. 2022, 52, 395–429. [Google Scholar] [CrossRef]
- Willow, A.; Hussein, H.E.M.; Vajirakaphan, S.; Chasri, A.; Margadonna, S. Improving In-Situ Sodium Metal Plating on Copper Foil Through Optimization of Mechanical Pressure: Towards High-Performance Anode-Free Sodium Ion Batteries. Front. Energy Res. 2022, 10, 888321. [Google Scholar] [CrossRef]
- Louli, A.J.; Eldesoky, A.; Weber, R.; Genovese, M.; Coon, M.; deGooyer, J.; Deng, Z.; White, R.T.; Lee, J.; Rodgers, T.; et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Beuse, T.; Fingerle, M.; Wagner, C.; Winter, M.; Börner, M. Comprehensive Insights into the Porosity of Lithium-Ion Battery Electrodes: A Comparative Study on Positive Electrodes Based on LiNi0.6Mn0.2Co0.2O2 (NMC622). Batteries 2021, 7, 70. [Google Scholar] [CrossRef]
- Primo, E.N.; Chouchane, M.; Touzin, M.; Vazquez, P.; Franco, A.A. Understanding the calendering processability of Li(Ni0.33Mn0.33Co0.33)O2-based cathodes. J. Power Sources 2021, 488, 229361. [Google Scholar] [CrossRef]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3.xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, J.; Sun, Y.; Cui, Y. A Highly Reversible Room-Temperature Sodium Metal Anode. ACS Cent. Sci. 2015, 1, 449–455. [Google Scholar] [CrossRef]
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709. [Google Scholar] [CrossRef]
- Baumgart, S.; Sotoudeh, M.; Groß, A. Rhombohedral (R) Prussian White as Cathode Material: An Ab-initio Study. Batter. Supercaps 2023, 6, e202300294. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, H.; Yang, Q.-H.; He, P.; Zhou, H. Building a Beyond Concentrated Electrolyte for High-Voltage Anode-Free Rechargeable Sodium Batteries. Angew. Chem. Int. Ed. 2022, 61, e202200410. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Wu, F.; Zarrabeitia, M.; Geiger, D.; Kaiser, U.; Varzi, A.; Passerini, S. Sodiophilic Current Collectors Based on MOF-Derived Nanocomposites for Anode-Less Na-Metal Batteries. Adv. Energy Mater. 2022, 12, 2202293. [Google Scholar] [CrossRef]
- Li, T.; Sun, J.; Gao, S.; Xiao, B.; Cheng, J.; Zhou, Y.; Sun, X.; Jiang, F.; Yan, Z.; Xiong, S. Superior Sodium Metal Anodes Enabled by Sodiophilic Carbonized Coconut Framework with 3D Tubular Structure. Adv. Energy Mater. 2021, 11, 2003699. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Liu, Y.; Wu, Q.; Cheng, S.; Xie, J. Space-Confined Guest Synthesis to Fabricate Sn-Monodispersed N-Doped Mesoporous Host toward Anode-Free Na Batteries. Adv. Mater. 2023, 35, 2301967. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Chen, Z.-N.; Zhang, R.; He, W.; Li, K.; Yan, S.; Cui, J.; Fang, X.; Yan, J.; et al. Robust Anode-Free Sodium Metal Batteries Enabled by Artificial Sodium Formate Interface. Adv. Energy Mater. 2023, 13, 2204125. [Google Scholar] [CrossRef]
- Wu, J.; Lin, C.; Liang, Q.; Zhou, G.; Liu, J.; Liang, G.; Wang, M.; Li, B.; Hu, L.; Ciucci, F.; et al. Sodium-rich NASICON-structured cathodes for boosting the energy density and lifespan of sodium-free-anode sodium metal batteries. InfoMat 2022, 4, e12288. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Ren, Z.; Wang, J.; Luo, J.; Wang, Y.; Tao, X.; Liu, T. Anode-Free Sodium Metal Pouch Cell Using Cu3P Nanowires In Situ Grown on Current Collector. Adv. Mater. 2024, 36, 2310347. [Google Scholar] [CrossRef]
- Zhuang, R.; Zhang, X.; Qu, C.; Xu, X.; Yang, J.; Ye, Q.; Liu, Z.; Kaskel, S.; Xu, F.; Wang, H. Fluorinated porous frameworks enable robust anode-less sodium metal batteries. Sci. Adv. 2023, 9, eadh8060. [Google Scholar] [CrossRef]
- Lee, M.E.; Kwak, H.W.; Kwak, J.H.; Jin, H.-J.; Yun, Y.S. Catalytic Pyroprotein Seed Layers for Sodium Metal Anodes. ACS Appl. Mater. Interfaces 2019, 11, 12401–12407. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Liu, S.; Jiang, Y.; Shen, D.; Kang, T.; Tong, Z.; Wu, D.; Li, X.; Lee, C.-S. 3D Ag@C Cloth for Stable Anode Free Sodium Metal Batteries. Small Methods 2021, 5, 2001050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xing, G.; Jin, L.; Lu, Y.; Qin, N.; Gao, S.; Zheng, J.P. Strategies and Challenge of Thick Electrodes for Energy Storage: A Review. Batteries 2023, 9, 151. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willow, A.; Orzech, M.; Kiani, S.; Reynolds, N.; Houchell, M.; Omisore, O.; Tehrani, Z.; Margadonna, S. Design and Validation of Anode-Free Sodium-Ion Pouch Cells Employing Prussian White Cathodes. Batteries 2025, 11, 97. https://doi.org/10.3390/batteries11030097
Willow A, Orzech M, Kiani S, Reynolds N, Houchell M, Omisore O, Tehrani Z, Margadonna S. Design and Validation of Anode-Free Sodium-Ion Pouch Cells Employing Prussian White Cathodes. Batteries. 2025; 11(3):97. https://doi.org/10.3390/batteries11030097
Chicago/Turabian StyleWillow, Ashley, Marcin Orzech, Sajad Kiani, Nathan Reynolds, Matthew Houchell, Olutimilehin Omisore, Zari Tehrani, and Serena Margadonna. 2025. "Design and Validation of Anode-Free Sodium-Ion Pouch Cells Employing Prussian White Cathodes" Batteries 11, no. 3: 97. https://doi.org/10.3390/batteries11030097
APA StyleWillow, A., Orzech, M., Kiani, S., Reynolds, N., Houchell, M., Omisore, O., Tehrani, Z., & Margadonna, S. (2025). Design and Validation of Anode-Free Sodium-Ion Pouch Cells Employing Prussian White Cathodes. Batteries, 11(3), 97. https://doi.org/10.3390/batteries11030097







