Improved Self-Assembled Silicon-Based Graphite Composite Anodes for Commercially Viable High-Energy-Density Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing Ni–Mo Binary Catalyst for the Synthesis of CNFs via Chemical Vapor Deposition
2.2. Synthesis of the Si@G/CNF/Graphite Composites
2.3. Material Characterization
2.4. Cell Fabrication and Characterization
3. Results and Discussion
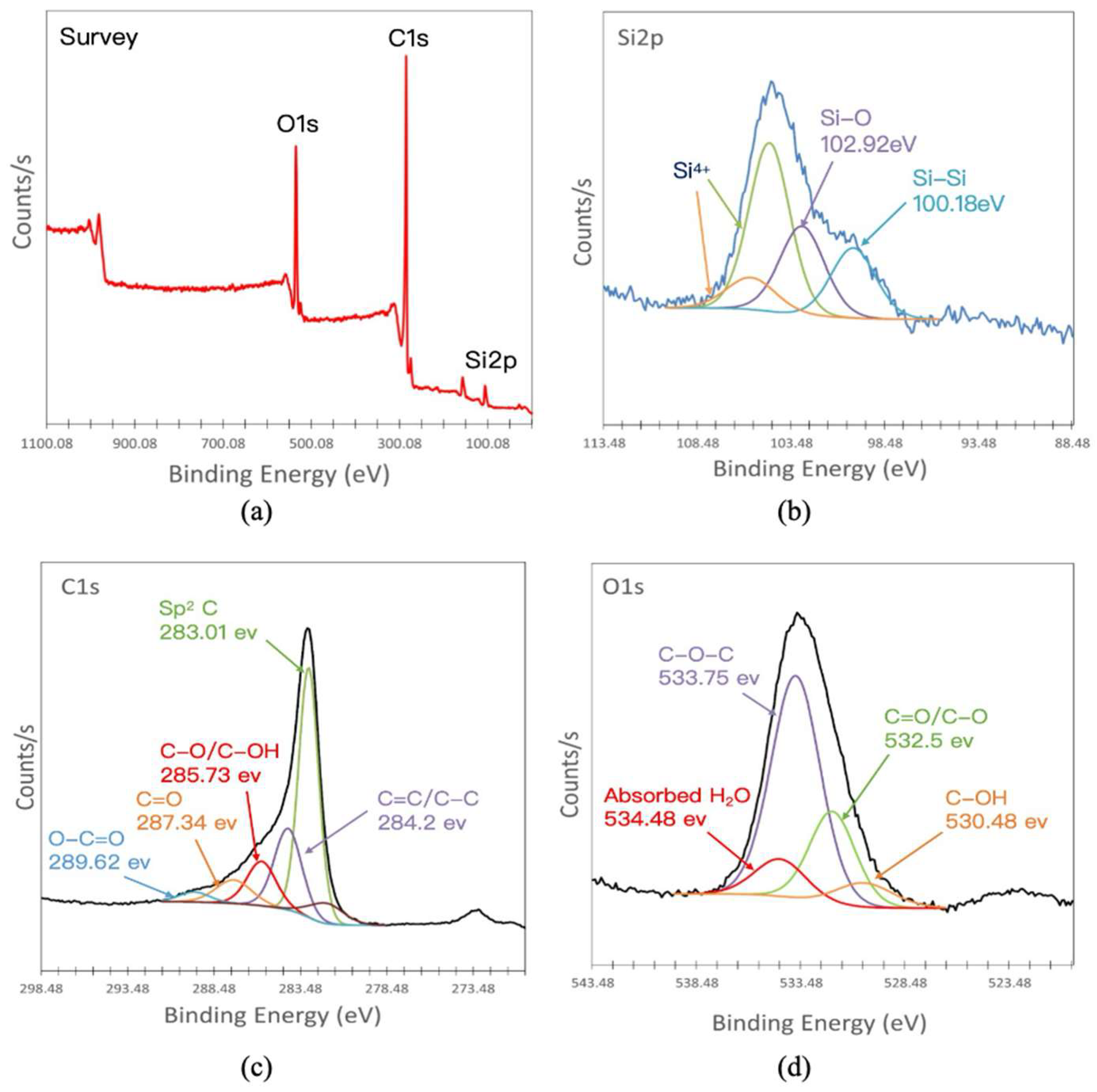
3.1. Structural and Morphological Characterizations of the Si@1-G/CNF/Graphite Composites
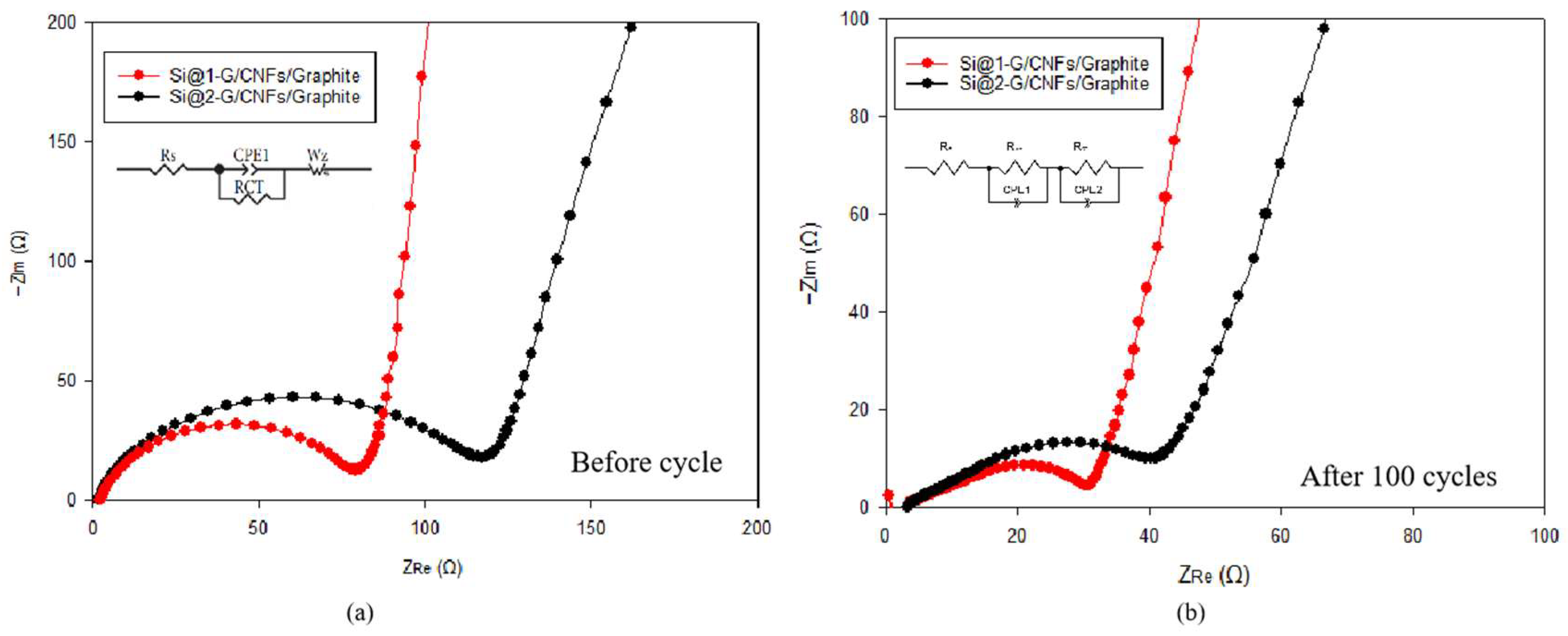
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIBs | Lithium-ion batteries |
| SEI | Solid electrolyte interface |
| CFTs | Carbon nanofibers |
| CVD | Chemical vapor deposition |
| GO | Graphene oxide |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| PVDF | Polyvinylidene fluoride |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| RCT | Charge transfer resistance |
| ICE | Initial Coulombic efficiency |
| CE | Coulombic efficiency |
References
- Mishra, G.K.; Gautam, M.; Bhawana, K.; Ghosh, J.; Mitra, S. High energy density lithium-ion pouch cell with modified high voltage lithium cobalt oxide cathode and graphite anode: Prototype stabilization, electrochemical and thermal study. J. Power Sources 2023, 580, 233395. [Google Scholar] [CrossRef]
- Tubtimkuna, S.; Sawangphruk, M.; Duriyasart, F. Effect of electrolyte additives on cycling performance of 18650 graphite//nmc811 Li-ion batteries. ECS Trans. 2020, 97, 155–166. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building safe lithium-ion batteries for electric vehicles: A review. Electrochem. Energ. Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting issues of the practical implementation of Si anode in high-energy lithium-ion batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Lavigne Philippot, M.; Costa, D.; Cardellini, G.; De Sutter, L.; Smekens, J.; Van Mierlo, J.; Messagie, M. Life cycle assessment of a lithium-ion battery with a silicon anode for electric vehicles. J. Energy Storage 2023, 60, 106635. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, C.; Li, S.; Qiu, L.; Yang, Z.; Zhong, Y.; Zhong, B.; Song, Y.; Wang, G.; Liu, Y. A unique structure of highly stable interphase and self-consistent stress distribution radial-gradient porous for silicon anode. Adv. Funct. Mater. 2022, 32, 2107897. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Yuan, L.; You, C.; Wu, J.; Liu, L.; Ye, J.; Wu, Y.; Fu, L. Recent advances in modification strategies of silicon-based lithium-ion batteries. Nano Res. 2023, 16, 3781–3803. [Google Scholar] [CrossRef]
- Nuhu, B.A.; Bamisile, O.; Adun, H.; Abu, U.O.; Cai, D. Effects of transition metals for silicon-based lithium-ion battery anodes: A comparative study in electrochemical applications. J. Alloys 2023, 933, 167737. [Google Scholar] [CrossRef]
- Wang, J.; Huang, W.; Kim, Y.S.; Jeong, Y.K.; Kim, S.C.; Heo, J.; Lee, H.K.; Liu, B.; Nah, J.; Cui, Y. Scalable synthesis of nanoporous silicon microparticles for highly cyclable lithium-ion batteries. Nano Res. 2020, 13, 1558–1563. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, J.; Chen, F.; Xiao, J.; Zhao, Y.; Zhang, Y.; Kong, D.; Weng, Z.; Wu, S.; Yang, Q. Liquid metal remedies silicon microparticulates toward highly stable and superior volumetric lithium storage. Adv. Energy Mater. 2022, 12, 2103565. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Shao, R.; Wu, J.; Jiang, R.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Gautam, M.; Mishra, G.K.; Bhawana, K.; Kalwar, C.S.; Dwivedi, D.; Yadav, A.; Mitra, S. Relationship between silicon percentage in graphite anode to achieve high-energy-density lithium-ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 45809–45820. [Google Scholar] [CrossRef]
- Zhang, W.; Weng, Y.; Shen, W.; Lv, R.; Kang, F.; Huang, Z.-H. Scalable synthesis of lotus-seed-pod-like Si/siox@cnf: Applications in freestanding electrode and flexible full lithium-ion batteries. Carbon 2020, 158, 163–171. [Google Scholar] [CrossRef]
- Yan, Z.; Yi, S.; Li, X.; Jiang, J.; Yang, D.; Du, N. A scalable silicon/graphite anode with high silicon content for high-energy lithium-ion batteries. Mater. Today Energy 2023, 31, 101225. [Google Scholar] [CrossRef]
- Moyassari, E.; Roth, T.; Kücher, S.; Chang, C.-C.; Hou, S.-C.; Spingler, F.B.; Jossen, A. The role of silicon in silicon-graphite composite electrodes regarding specific capacity, cycle stability, and expansion. J. Electrochem. Soc. 2022, 169, 010504. [Google Scholar] [CrossRef]
- Gautam, M.; Mishra, G.K.; Bhawana, K.; Kalwar, C.S.; Mitra, S. Enhancing electrochemical stability of silicon-carbon and nmc based lithium-ion batteries through the synergistic impact of charge balance and direct-contact pre-lithiation strategy. J. Electrochem. Soc. 2024, 171, 050526. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Mu, G.; Ma, C.; Mu, D.; Wu, F. Recent progress and perspectives on silicon anode: Synthesis and prelithiation for libs energy storage. J. Energy Chem. 2022, 64, 615–650. [Google Scholar] [CrossRef]
- Li, X.; Yan, P.; Xiao, X.; Woo, J.H.; Wang, C.; Liu, J.; Zhang, J.-G. Design of porous si/c–graphite electrodes with long cycle stability and controlled swelling. Energy Environ. Sci. 2017, 10, 1427–1434. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Wu, W.; Cai, Y.; Zhang, Y. Nodes-connected silicon-carbon nanofibrous hybrids anodes for lithium-ion batteries. Appl. Surf. Sci. 2021, 548, 148944. [Google Scholar] [CrossRef]
- Wiggers, H.; Sehlleier, Y.H.; Kunze, F.; Xiao, L.; Schnurre, S.M.; Schulz, C. Self-assembled nano-silicon/graphite hybrid embedded in a conductive polyaniline matrix for the performance enhancement of industrial applicable lithium-ion battery anodes. Solid State Ion. 2020, 344, 115117. [Google Scholar] [CrossRef]
- Cong, R.; Jo, M.; Martino, A.; Park, H.-H.; Lee, H.; Lee, C.-S. Three-dimensional network of nitrogen-doped carbon matrix-encapsulated si nanoparticles/carbon nanofibers hybrids for lithium-ion battery anodes with excellent capability. Sci. Rep. 2022, 12, 16002. [Google Scholar] [CrossRef]
- Meng, W.-J.; Han, X.-Y.; Hou, Y.-L.; Xie, Y.; Zhang, J.; He, C.-J.; Zhao, D.-L. Defect-repaired reduced graphene oxide caging silicon nanoparticles for lithium-ion anodes with enhanced reversible capacity and cyclic performance. Electrochim. Acta 2021, 382, 138271. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Song, J.; Zhang, Y.; Shi, Q.; Wang, J.; Tian, F.; Yuan, S.; Su, Z.; Zhou, C. Functionalization-assistant ball milling towards Si/graphene anodes in high performance Li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Liu, X.; Du, Y.; Hu, L.; Zhou, X.; Li, Y.; Dai, Z.; Bao, J. Understanding the effect of different polymeric surfactants on enhancing the silicon/reduced graphene oxide anode performance. J. Phys. Chem. C 2015, 119, 5848–5854. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, Y.; Yang, B.; Chen, W.; Ding, J. Embedded Si/graphene composite fabricated by magnesium-thermal reduction as anode material for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 1186. [Google Scholar] [CrossRef]
- Liu, W.; Xu, H.; Qin, H.; Lv, Y.; Zhu, G.; Lei, X.; Lin, F.; Zhang, Z.; Wang, L. Rapid coating of asphalt to prepare carbon-encapsulated composites of nano-silicon and graphite for lithium battery anodes. J. Mater. Sci. 2020, 55, 4382–4394. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, C.; Chang, H.; Jang, H.D. Preparation of Silicon-carbon-graphene composites and their application to lithium ion secondary battery. Aerosol Air Qual. Res. 2022, 22, 220009. [Google Scholar] [CrossRef]
- Duan, H.; Xu, H.; Wu, Q.; Zhu, L.; Zhang, Y.; Yin, B.; He, H. Silicon/graphite/amorphous carbon as anode materials for lithium secondary batteries. Molecules 2023, 28, 464. [Google Scholar] [CrossRef]
- Lei, G.; Huaj, G.; Zhix, W. A facile synthesis of graphite/silicon/graphene spherical composite anode for lithium-ion batteries. Electrochim. Acta 2013, 104, 117–123. [Google Scholar]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a high-power Si@graphite anode for lithium ion batteries through a wet ball milling process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.H.; Kim, S.Y.; Kim, J.H.; Kim, Y.A.; Yang, C.-M. Robust core–shell carbon-coated silicon-based composite anode with electrically interconnected spherical framework for lithium-ion battery. Int. J. Energy Res. 2023, 2023, 6874429. [Google Scholar] [CrossRef]
- Roland, A.; Fullenwarth, J.; Ledeuil, J.; Martinez, H.; Louvain, N.; Monconduit, L. How carbon coating or continuous carbon pitch matrix influence the silicon electrode/electrolyte interfaces and the performance in Li-ion batteries. Battery Energy 2022, 1, 1002. [Google Scholar] [CrossRef]
- Su, M.; Wang, Z.; Guo, H.; Li, X.; Huang, S.; Xiao, W.; Gan, L. Enhancement of the cyclability of a Si/graphite@graphene composite as anode for lithium-ion batteries. Electrochim. Acta 2014, 116, 230–236. [Google Scholar] [CrossRef]
- Nayak, S.K.; Mohanty, S.; Nayak, S.K. A new way synthesis of expanded graphite as a thermal filler to enhance the thermal conductivity of DGEBA resin as thermal interface material. Adv. Polym. Sci. 2020, 32, 506–523. [Google Scholar] [CrossRef]
- Chu, Z.; Zhao, X.; Wang, Q.; Bao, T.; Li, H.; Cao, Y.; Zhang, B.; Cao, J.; Si, W. Preparation of a flexible reduced graphene oxide-si composite film and its application in high-performance lithium ion batteries. Crystals 2023, 13, 547. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Liu, J.; Wang, Y.; Hu, J. Facile preparation of reduced graphene by optimizing oxidation condition and further reducing the exfoliated products. J. Mater. Res. 2017, 32, 383–391. [Google Scholar] [CrossRef]
- Kareem, A.A. Enhanced thermal and electrical properties of epoxy/carbon fiber–silicon carbide composites. Adv. Compos. Lett. 2020, 1, 29. [Google Scholar] [CrossRef]
- Sun, Z.G.; Wang, S.J.; Qiao, X.J.; Li, Y.; Zheng, W.H.; Bai, P.Y. Synthesis and microwave absorbing properties of SIC nanowires. Appl. Phys. A 2018, 124, 1007. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H. Minimizing the volume expansion by a self-standing reduced graphene oxide/silicon nanoparticles/copper mesh hybrid electrodes for enhanced lithium-ion batteries. J. Energy Storage 2023, 64, 107202. [Google Scholar] [CrossRef]
- Chae, C.; Choi, W.; Ji, S.; Lee, S.S.; Kim, J.-K.; Choi, S.; Kang, Y.; Choi, Y.; Kim, D.Y.; Jeong, S. Electrostatically assembled silicon–carbon composites employing amine-functionalized carbon intra-interconnections for lithium-ion battery anodes. ACS Appl. Energy Mater. 2019, 2, 1868–1875. [Google Scholar] [CrossRef]
- Xin, L.; Kun, L.; Man, Y.; Jiapeng, Z.; Haiyan, L.; Ang, L.; Xiaohong, C.; Huaihe, S. Graphene-doped silicon-carbon materials with multi-interface structures for lithium-ion battery anodes. J. Colloid Interface Sci. 2024, 667, 470–477. [Google Scholar]
- Woo Sung, C.; Heon-Cheol, S.; Ji Man, K.; Jae-Young, C.; Won-Sub, Y. Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar]
- Haiping, S.; Xinrui, L.; Changwei, L.; Yazhuo, S.; Honglai, L. Scalable synthesis of micrometer-sized porous silicon/carbon composites for high-stability lithium-ion battery anodes. Chem. Eng. J. 2023, 451, 138394. [Google Scholar]
- Angelica, M.; Ruye, C.; Minsang, J.; Hyun-Ho, P.; Hochun, L.; Chang-Seop, L. Characteristics and Electrochemical Performance of Hydroxyl-Functionalized Graphene Quantum Dot-Coated Si Nanoparticles/Reduced. J. Nanomater. 2023, 6353894, 23. [Google Scholar]







| No. | Sample Code Si@n-G/CNF/Graphite | Si (wt%) | GO (wt%) | CNF (wt%) | Graphite (wt%) | Conducting Carbon (wt%) | Binder (wt%) |
|---|---|---|---|---|---|---|---|
| 1 | Si@1-G/CNF/graphite | 15 | 15 | 15 | 45 | 5 | 5 |
| 2 | Si@2-G/CNF/graphite | 10 | 10 | 10 | 60 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, R.; Jeong, D.-E.; Jung, Y.-Y.; Park, H.-H.; Jeon, J.; Lee, H.; Lee, C.-S. Improved Self-Assembled Silicon-Based Graphite Composite Anodes for Commercially Viable High-Energy-Density Lithium-Ion Batteries. Batteries 2025, 11, 115. https://doi.org/10.3390/batteries11030115
Cong R, Jeong D-E, Jung Y-Y, Park H-H, Jeon J, Lee H, Lee C-S. Improved Self-Assembled Silicon-Based Graphite Composite Anodes for Commercially Viable High-Energy-Density Lithium-Ion Batteries. Batteries. 2025; 11(3):115. https://doi.org/10.3390/batteries11030115
Chicago/Turabian StyleCong, Ruye, Da-Eun Jeong, Ye-Yeong Jung, Hyun-Ho Park, Jiyun Jeon, Hochun Lee, and Chang-Seop Lee. 2025. "Improved Self-Assembled Silicon-Based Graphite Composite Anodes for Commercially Viable High-Energy-Density Lithium-Ion Batteries" Batteries 11, no. 3: 115. https://doi.org/10.3390/batteries11030115
APA StyleCong, R., Jeong, D.-E., Jung, Y.-Y., Park, H.-H., Jeon, J., Lee, H., & Lee, C.-S. (2025). Improved Self-Assembled Silicon-Based Graphite Composite Anodes for Commercially Viable High-Energy-Density Lithium-Ion Batteries. Batteries, 11(3), 115. https://doi.org/10.3390/batteries11030115









